Abstract
Myocardial infarction is a major cause of death and disability worldwide and myocardial infarct size is a major determinant of prognosis. Early and successful restoration of myocardial reperfusion following an ischemic event is the most effective strategy to reduce final infarct size and improve clinical outcome, but reperfusion may induce further myocardial damage itself. Development of adjunctive therapies to limit myocardial reperfusion injury beyond opening of the coronary artery gains increasing attention. A vast number of experimental studies have shown cardioprotective effects of ischemic and pharmacological conditioning, but despite decades of research, the translation into clinical effects has been challenging. Recently published clinical studies, however, prompt optimism as novel techniques allow for improved clinical applicability. Cyclosporine A, the GLP-1 analogue exenatide and rapid cooling by endovascular infusion of cold saline all reduce infarct size and may confer clinical benefit for patients admitted with acute myocardial infarcts. Equally promising, three follow-up studies of the effect of remote ischemic conditioning (RIC) show clinical prognostic benefit in patients undergoing coronary surgery and percutaneous coronary intervention. The discovery that RIC can be performed noninvasively using a blood pressure cuff on the upper arm to induce brief episodes of limb ischemia and reperfusion has facilitated the translation of RIC into the clinical arena. This review focus on novel advances in adjunctive therapies in relation to acute and elective coronary procedures.
Keywords: Myocardial infarction, Primary percutaneous intervention, Coronary artery by-pass graft, Ischemia-reperfusion injury, Ischemic preconditioning, Remote ischemic conditioning, Cyclosporine, Cooling, Exenatide
Core tip: Patients with ischemic heart disease have a high risk of developing myocardial infarction, which is associated with considerable morbidity and mortality. Limiting the detrimental consequences of myocardial infarction is a major focus of cardiovascular research. Recent clinical studies suggest that novel adjunctive therapy with pharmacological and ischemic conditioning reduce ischemia-reperfusion injury in patients during coronary procedures. In three independent randomized trials, remote ischemic conditioning (RIC) improves clinical outcome in patients undergoing acute or elective percutaneous intervention or coronary artery by-pass surgery. RIC can be performed safely and non-invasively by intermittent inflation of a blood-pressure cuff on the upper arm and is easily applicable in most clinical settings.
INTRODUCTION
Heart disease and stroke are the leading causes of death worldwide[1,2]. Since 1990, more people have died from coronary artery disease than any other death cause[3,4].
In China, a staggering 230 million are estimated to suffer from cardiovascular disease, and three million Chinese die of cardiovascular disease annually, accounting for 41% of all deaths[5,6]. In the United States alone, cardiovascular diseases, including ischemic heart disease and stroke, account for more than one-third deaths and an estimated 900000 heart attacks and 800000 strokes occur each year. In the remaining parts of the world, from the Sub-Saharan developing countries over booming South America to affluent areas in Europe and Asia, similar patterns are seen[7,8].
Globally, socio-demographic factors, unhealthy life style, escalating obesity and suboptimal control of risk factors are likely to further aggravate the disease burden over the coming decades[9]. In the Western world, nearly half of the male and a third of the female population will develop coronary artery disease[10]. Partly driven by urbanization and adoption of Western life style, China undergoes a transition towards a similar health statistic[11].
The pandemic of cardiovascular disease has immense negative effects on global population health and life expectancy. While attempts to modify risk factor and life style related growth in cardiovascular disease are important and have been successful in some parts of the world[8], improved treatment of acute and chronic cardiovascular disease is also crucial to alleviate the disease burden.
This review will focus on novel advances in the treatment of coronary artery disease, particularly the recent reports of successful adjunctive therapy in relation to elective percutaneous coronary intervention (PCI), coronary artery by-pass graft surgery (CABG), and acute angioplasty (primary PCI) for ST-elevation myocardial infarction (STEMI).
PROTECTING THE HEART AGAINST ISCHEMIA-REPERFUSION INJURY
Ischemia-reperfusion injury is the essence of myocardial infarction in relation to acute coronary events, but ischemia-reperfusion injury also occurs during planned procedures such as elective PCI and CABG, although usually to a lesser extent. As the term implies, not just the ischemia itself but also the following reperfusion harms the myocardium. Although reperfusion ultimately saves the ischemic myocardium and it may seem paradoxical that reperfusion induces myocardial injury, several biological phenomena explain for this effect [for detailed reviews, please see Hausenloy et al[12] (2013) and Heusch et al[13] (2013)]. Of potential clinical importance, ischemic and pharmacological conditioning of the myocardium can modify reperfusion injury and significantly reduce the tissue damage (Figure 1).
Figure 1.
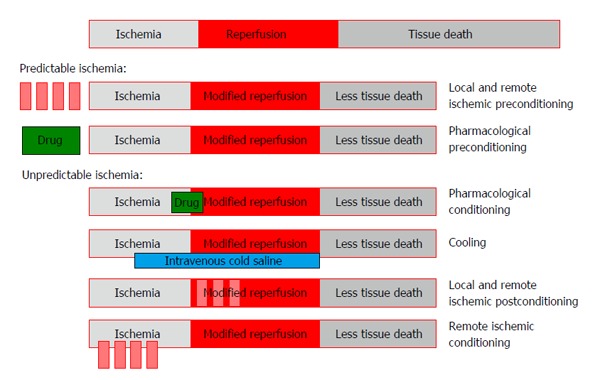
Overview of interventional strategies for achieving cardioprotection as adjunct to thrombolysis or primary percutaneous coronary intervention, see text for details.
LOCAL ISCHEMIC CONDITIONING
Local ischemic preconditioning, induced by brief periods of ischemia before a sustained ischemic insult, has long been known to afford potent protection against ischemia-reperfusion injury[14]. However, the technique has inherent limitations as it requires interruption of blood flow to the target organ and, thus, can only be achieved in the operating room or during coronary angioplasty. Furthermore, additional time for the preconditioning procedure is required during surgery or during intervention. Preconditioning itself might cause deterioration of organ function or cause complications, such as emboli of atheroma, because of the intermittent aortic clamping or intermittent coronary balloon inflation. Hence, local ischemic preconditioning has not found widespread clinical use.
However, by instead applying the local ischemic conditioning stimulus after the ischemic event (e.g., at the time of reperfusion in primary PCI), so-called ischemic postconditioning, most of these obstacles for clinical use are overcome. In an experimental setting, ischemic postconditioning inhibits ischemia-reperfusion injury almost as efficiently as ischemic preconditioning[15,16]. Some clinical studies suggest that local ischemic postconditioning reduces myocardial injury in patients undergoing primary PCI for acute myocardial infarction[17,18], but another recently published trial did not confirm this effect[19]. Furthermore, a large-scale trial of 700 patients admitted with STEMI randomized to either standard primary PCI or primary PCI followed by postconditioning, failed to show any effect on myocardial reperfusion and clinical endpoints[20].
REMOTE ISCHEMIC CONDITIONING
Remote ischemic conditioning (RIC) by repeated short-lasting ischemia in a distant tissue-mostly achieved by intermittent interruption of circulation in a limb-has recently emerged as a promising adjunctive therapy to avoid organ damage, thereby improving the outcomes of well-established therapies.
From the site of the remote stimulus, through humoral[21] and neuronal[22,23] pathways, RIC activates several protective mechanisms in the target organ similar to those activated by local preconditioning. They include the reperfusion-injury salvage kinase[24] and survivor activating factor enhancement[25,26] signaling pathways. Furthermore, RIC modifies systemic inflammatory response[27,28], prevents endothelial dysfunction[29] and platelet activation[30] following ischemia-reperfusion injury.
In experimental studies, RIC has been shown to afford protection against ischemia-reperfusion in the liver[31], lung[32], kidney[33], brain[34], and heart[29].
The ability to induce organ protection by a simple, non-invasive stimulus has facilitated the translation of RIC into the clinical setting. In patients, RIC can be induced by 3-4 cycles of inflation (ischemia) and deflation (reperfusion) of a standard blood pressure cuff placed on a limb. Following the original description of the method in 1997[35] and its translation to humans in 2002[29], multiple randomized clinical trials have shown that RIC affords organ protection in many clinical ischemia-reperfusion syndromes, including the kidney[33,36], brain[37], and heart[38-41].
COOLING
Moderate hypothermia induced prior to reperfusion reduces infarct size in animal models[42-44]. A clinical pilot study has suggested that patients admitted with anterior STEMI who are rapidly cooled to a body temperature below 35 °C by the combination of cold saline infusion together with an endovascular cooling catheter before primary PCI develop smaller infarcts[45]. However, difficulties in applying the technique in the clinical setting without delaying treatment together with inconsistent results cause controversy about the clinical value and applicability[46], although a recent pooled analysis of two clinical trials indicate a potential beneficial effect[47]. Most recently, the CHILL-MI study, using a similar cooling technique as in the initial pilot study, showed that while cooling did not have a general cardioprotective effect, it seems to reduce infarct size in patients with anterior STEMI admitted for primary PCI within four hours of symptom onset. In addition, cooling caused a significant reduction in heart failure events[48]. A possible explanation for an overall lack of cardioprotective effect in the CHILL-MI study may be the fact that cooling below 35 °C was only achieved in 76% of the patients, and that sufficient cooling may be crucial for achieving cardioprotective effects.
PHARMACOLOGICAL CONDITIONING
The increasing insight into the mechanistic pathways involved in local and remote ischemic conditioning has encouraged identification of potential targets for pharmacological intervention against ischemia-reperfusion injury. A vast number of pharmacological agents have been shown to afford cardioprotection in experimental models, including adenosine[49], erythropoietin[50], rotigaptide[51], statins[52], atrial natriuretic peptide[53], glucose-insulin-potassium[54], P-selectin antagonist[55] cyclosporine[56], exenatide[57] and metoprolol[58]. A larger number of these agents have been tested in clinical studies (Table 1) with ambiguous results, the most promising being cyclosporine[64], exenatide[67] and metoprolol[75], all of which seem to consistently provide cardioprotection in the clinical setting. For a comprehensive review, please see Kloner (2013)[76]. However, an important limitation-and a potential explanation for the lack of success-of pharmacological conditioning with some drugs, is that most agents act through a single signaling pathway in the complex and interactive system of protective mechanisms activated by ischemic conditioning and cooling[13].
Table 1.
Clinical studies using pharmacological adjunctive therapy in patients with acute myocardial infarction
| Intervention | n | Outcome | |
| Adenosine | |||
| Mahaffey et al[59], 1999 (AMISTAD) | Infusion of adenosine for 3 h as adjunct to thrombolysis | 236 | Reduction in infarct size |
| Kloner et al[60], 2006 (AMISTAD II) | Infusion of adenosine for 3 h | 2118 | No difference in death or heart failure |
| Atrial natriuretic peptide | |||
| Kitakaze et al[61], 2007 (J-WIND) | Infusion of atrial natriuretic peptide for 3 d | 569 | Reduction in creatine kinase, increase in LVEF |
| Atorvastatin | |||
| Kim et al[62], 2010 (STATIN-STEMI) | Oral atorvastatin 80 mg before primary PCI | 171 | No difference in death, revascularization or infarct size |
| Hahn et al[63], 2011 | Oral atorvastatin 80 mg before primary PCI | 173 | No difference in infarct size |
| Cyclosporine A | |||
| Piot et al[64], 2008 | Infusion of cyclosporine before primary PCI | 58 | Reduction in infarct size |
| Ghaffari et al[65], 2013 | Infusion of cyclosporine as adjunct to thrombolysis | 101 | No difference in infarct size, death, heart failure or LVEF |
| Erythropoietin | |||
| Voors et al[66], 2010 | Single dose erythropoietin | 529 | No difference in LVEF or infarct size |
| Exenatide | |||
| Lønborg et al[67], 2012 | Infusion of exenatide for 6 h | 105 | Reduction in infarct size |
| Bernink et al[68], 2012 (EXAMI) | Loading dose of exenatide before PCI followed by infusion for 72 h | 39 | No difference in left ventricular function or infarct size |
| Woo et al[69], 2013 | Subcutaneously and intravenous exenatide before primary PCI followed by twice daily subcutaneous injection for 2 d | 58 | Reduction in infarct size and improvement of LVEF |
| Glucose-insulin-potassium | |||
| Mehta et al[70], 2005 (CREATE-ECLA) | Infusion of glucose-insulin-potassium for 24 h | 20201 | No difference in mortality |
| Selker et al[71], 2012 (IMMEDIATE) | Out-of-hospital infusion of GIK | 357 | Reduced mortality among patients with cardiac arrest |
| δ-protein kinase C inhibitor | |||
| Bates et al[72], 2008 | 2 doses of KAI-9803 | 154 | No difference in infarct size |
| Lincoff et al 2012 (PROTECTION-AMI) | Infusion of delcasertib for 24 h | 1083 | No difference in infarct size |
| P-selectin antagonist | |||
| Mertens et al[73], 2006 (PSALM) | Infusion of recombinant P-selectin glycoprotein ligand-immunoglobulin as adjunct to thrombolysis | 88 | No difference in ST-segment resolution or LVEF |
| Tardif et al[74], 2013 (SELECT-ACS) | Infusion of inclacumab before PCI in NSTEMI patients | 322 | Reduction in troponin I and creatine kinase |
| Metoprolol | |||
| Ibanez et al[75], 2013 (METOCARD-CNIC) | Infusion of metoprolol before primary PCI | 220 | Reduction in infarcts size and improvement of LVEF |
LVEF: Left ventricular ejection fraction; PCI: Percutaneous coronary intervention; CK: Creatine kinase; NSTEMI: Non-ST-elevation myocardial infarction.
Cyclosporine
Cyclosporine, a widely used immunosuppressant drug, is believed to facilitate its cardioprotective effects by inhibition of mitochondrial permeability transition pore opening, thus preventing mitochondrial destruction[77]. In a study by Piot et al[64], administration of cyclosporine at the time of reperfusion in STEMI patients treated with primary PCI, was associated with a reduction in infarct size measured by creatine kinase and troponin I release. In a subgroup analysis, a similar reduction in infarct size was demonstrated on day 5 with cardiac magnetic resonance imaging (CMR). In a follow-up study, Mewton et al[78] found that this infarct-sparing effect of cyclosporine was persistent at 6 mo. However, in a more recent study, no effect was shown of early cyclosporine administration as an adjunct to thrombolysis in STEMI patients in relation to infarct size, left ventricular function, heart failure or death[65].
Exenatide
Exenatide, a glucagon-like peptide-1 analog, is primarily used as an anti-diabetic drug for patients with type 2 diabetes. However, in addition to its beneficial metabolic effect, exenatide is believed to induce cardioprotection through activation of ischemia-reperfusion injury survival pathways[79]. Lønborg et al[67] found that in STEMI patients, a 6 h infusion of exenatide started prior to primary PCI was associated with increased myocardial salvage measured by CMR. This increase in myocardial salvage from exenatide infusion translated to a reduction in final infarct size, although reserved for patients with short system delay (< 132 min from first medical contact to first balloon inflation)[80]. In a recent study by Woo et al[69], subcutaneous injection together with intravenous infusion of exenatide as adjunct to primary PCI followed by twice daily subcutaneous injections of exenatide for another two days, was associated with both a reduction in infarct size and improvement of left ventricular function.
Metoprolol
Most randomized clinical trials investigating potential infarct sparing effects of betablockers in STEMI patients have been conducted in the pre-reperfusion era, and only a few studies have evaluated the cardioprotective effect of beta-blockage as an adjunct to thrombolysis or primary PCI. However, recently, the METOCARD-CNIC trial demonstrated that intravenous metoprolol administrated to STEMI patients prior to primary PCI was associated with significantly smaller infarct size measured by CMR compared to primary PCI treatment alone. In addition, early metoprolol administration increased left ventricular function[75].
IMPROVING THE OUTCOME OF MYOCARDIAL INFARCTION IN PATIENTS
The translation of cardioprotective strategies to counter the detrimental consequences of ischemia-reperfusion injury is still in its infancy, and large-scale multicenter trials to show real-world clinical impact are lacking. However, recently published long-term clinical data on the use of RIC provide reason for optimism about a prognostic benefit of adjunctive therapy beyond opening the coronary artery.
REMOTE ISCHEMIC CONDITIONING IN PREDICTABLE ISCHEMIA
Predictable cardiac ischemia-reperfusion injury occurs in both elective PCI and CABG, and procedural tissue injury-as measured by biomarkers-is correlated to clinical outcome. Mid-scale clinical studies have shown that RIC applied prior to CABG[39,81] and PCI[38] reduces surrogate markers of myocardial injury, but until recently, the clinical relevance of these findings was questionable. However, two recent publications strongly suggest, that RIC should find a place as standard adjunctive therapy in elective PCI and CABG.
In a single center, randomized controlled trial, Davies et al[82] investigated the long-term clinical outcomes of 192 patients undergoing elective coronary angioplasty randomized to RIC or standard treatment. While their original study showed a significant reduction in troponin release in the RIC group[38], the follow-up study revealed that this translated into a reduced major adverse cardiac and cerebrovascular events (MACCE) rate up to 6 years after the coronary intervention.
In another single center, double-blind trial, Thielmann et al[83] studied 329 low-risk patients undergoing elective isolated on-pump first-time CABG randomized to either standard CABG or CABG preceded by RIC. Besides reduced perioperative troponin I release as also shown previously by others[84], the authors found a reduction in all-cause and cardiac mortality as well as MACCE in the intervention group during the follow-up period that was a mean of 1.5 year. During the follow-up period, MACCE occurred 23 times in the control group vs 8 times in the RIC group (P = 0.011). The authors observed 11 deaths in the control group and only 3 deaths in the RIC group (P = 0.046). The combined endpoint (death, MACCE and repeat revascularization) yielded a HR of 0.38 (0.21-0.70) in favor of RIC. Interestingly, Thielmann et al[83] also found that RIC reduced the occurrence of sepsis, stroke and non-cardiac deaths, which adds to the speculation that RIC could confer systemic beneficial effects beyond the organ exposed to ischemia-reperfusion injury.
REMOTE ISCHEMIC CONDITIONING IN UNPREDICTABLE ISCHEMIA
In unpredictable ischemic events, like myocardial infarction and stroke, rapid restoration of blood flow to the ischemic territory has been the primary focus. Optimization of prehospital admission logistics to reduce any delay improves outcome[85] and decreases mortality[86]. While acute thrombolysis and primary PCI have improved outcome, recent studies show that further injury occurs early after reperfusion and can continue long afterwards[87,88] emphasizing the need for therapies limiting clinical reperfusion injury in acute ischemic events.
In a study of 333 patients admitted with STEMI for primary PCI and randomized to either standard treatment or RIC performed in the ambulance during transportation to primary angioplasty, we showed, that RIC improves myocardial salvage index (0.75 in the RIC group vs 0.55 in the control group, P = 0.033) as measured by single-photon emission computed tomography[40]. Recently, Sloth et al[89] published 4-year follow-up data on our original study, showing that the improved myocardial salvage translates into clinical prognostic benefit, as MACCE occurred for 17 (13.5%) patients in the RIC treated group compared with 32 (25.6%) patients in the control group, yielding a HR of 0.49 (95%CI: 0.27-0.89, P = 0.018). Furthermore, only 5 deaths (4%) occurred in the intervention group compared with 15 (12%) in the control group, yielding a HR of 0.32 (95%CI: 0.12-0.88, P = 0.027) (Figure 2)[89]. Specific evaluation of death causes suggested a reduction in both cardiac and non-cardiac mortality, although only the latter was statistically significantly reduced (and most likely arose by chance). However, even when excluding non-cardiac deaths, MACCE was still reduced in the RIC group.
Figure 2.
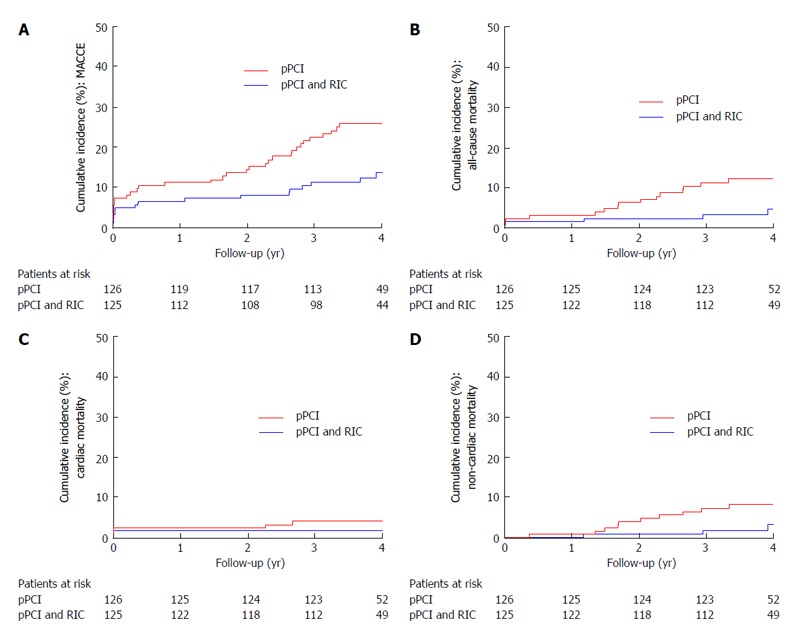
Effect of remote ischemic conditioning on long-term clinical outcome in patients with ST-elevation myocardial infarction treated with primary percutaneous coronary intervention. Cumulative incidence (%). A: Of major adverse cardiac and cerebrovascular events (MACCE) by year since randomization (per-protocol analysis). P = 0.010; B: Of all-cause mortality by year since randomization (per-protocol analysis). P = 0.019; C: Of cardiac mortality (%) by year since randomization (per-protocol analysis). P = 0.248; D: Of non-cardiac mortality (%) by year since randomization (per-protocol analysis). P = 0.045. Modified from Sloth et al[89]. Eur Heart J (2014) 35: 168-175. pPCI: Primary percutaneous interven- tion; RIC: Remote ischemic conditioning.
CONCLUSION
The globally increasing burden of cardiovascular disease calls for improved prevention and treatment. Acute and chronic coronary artery disease constitute the leading death cause in the World, and adjunctive therapies to limit the morbidity and mortality related to myocardial infarction may have major impact on global health. Pharmacological adjunctive therapy and rapid cooling decrease infarct size in some clinical studies but have yet to prove convincing clinical benefit. Remote ischemic conditioning-a low-cost, non-invasive and easily applicable adjunctive therapy-may confer prognostic benefit for patients undergoing coronary artery by-pass surgery and elective and acute percutaneous coronary interventions. Large-scale studies with clinical endpoints, such as the ERICCA trial (ClinicalTrials.gov NCT01247545), the RIPHeart-study (ClinicalTrials.gov NCT01067703) and the CONDI 2 trial (ClinicalTrials.gov NCT01857414) are, however, needed to confirm the clinical effect, before RIC should be applied as standard adjunctive therapy. Similarly, as an adjunctive therapy to primary PCI the CIRCUS trial (Clinicaltrials.gov NCT01502774) will clarify the potential clinical benefit of cyclosporine A, and the DANAMI-3 trial (Clinicaltrials.gov NCT01435408) the potential clinical efficacy of ischemic postconditioning.
Footnotes
P- Reviewers: Caceres-Loriga FM, de Carvalho ACC S- Editor: Wen LL L- Editor: A E- Editor: Liu SQ
References
- 1.Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990-2020: Global Burden of Disease Study. Lancet. 1997;349:1498–1504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- 2.Wang H, Dwyer-Lindgren L, Lofgren KT, Rajaratnam JK, Marcus JR, Levin-Rector A, Levitz CE, Lopez AD, Murray CJ. Age-specific and sex-specific mortality in 187 countries, 1970-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2071–2094. doi: 10.1016/S0140-6736(12)61719-X. [DOI] [PubMed] [Google Scholar]
- 3.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, et al. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21–181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 4.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, et al. Executive summary: heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121:948–954. doi: 10.1161/CIRCULATIONAHA.109.192666. [DOI] [PubMed] [Google Scholar]
- 5.Moran A, Gu D, Zhao D, Coxson P, Wang YC, Chen CS, Liu J, Cheng J, Bibbins-Domingo K, Shen YM, et al. Future cardiovascular disease in china: markov model and risk factor scenario projections from the coronary heart disease policy model-china. Circ Cardiovasc Qual Outcomes. 2010;3:243–252. doi: 10.1161/CIRCOUTCOMES.109.910711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith SC, Zheng ZJ. The impending cardiovascular pandemic in China. Circ Cardiovasc Qual Outcomes. 2010;3:226–227. doi: 10.1161/CIRCOUTCOMES.110.957183. [DOI] [PubMed] [Google Scholar]
- 7.Murray CJ, Lopez AD. Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet. 1997;349:1269–1276. doi: 10.1016/S0140-6736(96)07493-4. [DOI] [PubMed] [Google Scholar]
- 8.WHO. Cardiovascular diseases - Fact sheet N°317. Available from: http://www.who.int/mediacentre/factsheets/fs317/en/
- 9.Wildman RP, Gu D, Muntner P, Wu X, Reynolds K, Duan X, Chen CS, Huang G, Bazzano LA, He J. Trends in overweight and obesity in Chinese adults: between 1991 and 1999-2000. Obesity (Silver Spring) 2008;16:1448–1453. doi: 10.1038/oby.2008.208. [DOI] [PubMed] [Google Scholar]
- 10.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, et al. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang G, Wang Y, Zeng Y, Gao GF, Liang X, Zhou M, Wan X, Yu S, Jiang Y, Naghavi M, et al. Rapid health transition in China, 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet. 2013;381:1987–2015. doi: 10.1016/S0140-6736(13)61097-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Invest. 2013;123:92–100. doi: 10.1172/JCI62874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heusch G. Cardioprotection: chances and challenges of its translation to the clinic. Lancet. 2013;381:166–175. doi: 10.1016/S0140-6736(12)60916-7. [DOI] [PubMed] [Google Scholar]
- 14.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 15.Kin H, Zhao ZQ, Sun HY, Wang NP, Corvera JS, Halkos ME, Kerendi F, Guyton RA, Vinten-Johansen J. Postconditioning attenuates myocardial ischemia-reperfusion injury by inhibiting events in the early minutes of reperfusion. Cardiovasc Res. 2004;62:74–85. doi: 10.1016/j.cardiores.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 16.Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, Vinten-Johansen J. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285:H579–H588. doi: 10.1152/ajpheart.01064.2002. [DOI] [PubMed] [Google Scholar]
- 17.Staat P, Rioufol G, Piot C, Cottin Y, Cung TT, L’Huillier I, Aupetit JF, Bonnefoy E, Finet G, André-Fouët X, et al. Postconditioning the human heart. Circulation. 2005;112:2143–2148. doi: 10.1161/CIRCULATIONAHA.105.558122. [DOI] [PubMed] [Google Scholar]
- 18.Lønborg J, Kelbaek H, Vejlstrup N, Jørgensen E, Helqvist S, Saunamäki K, Clemmensen P, Holmvang L, Treiman M, Jensen JS, et al. Cardioprotective effects of ischemic postconditioning in patients treated with primary percutaneous coronary intervention, evaluated by magnetic resonance. Circ Cardiovasc Interv. 2010;3:34–41. doi: 10.1161/CIRCINTERVENTIONS.109.905521. [DOI] [PubMed] [Google Scholar]
- 19.Freixa X, Bellera N, Ortiz-Pérez JT, Jiménez M, Paré C, Bosch X, De Caralt TM, Betriu A, Masotti M. Ischaemic postconditioning revisited: lack of effects on infarct size following primary percutaneous coronary intervention. Eur Heart J. 2012;33:103–112. doi: 10.1093/eurheartj/ehr297. [DOI] [PubMed] [Google Scholar]
- 20.Hahn JY, Song YB, Kim EK, Yu CW, Bae JW, Chung WY, Choi SH, Choi JH, Bae JH, An KJ, et al. Ischemic postconditioning during primary percutaneous coronary intervention: the effects of postconditioning on myocardial reperfusion in patients with ST-segment elevation myocardial infarction (POST) randomized trial. Circulation. 2013;128:1889–1896. doi: 10.1161/CIRCULATIONAHA.113.001690. [DOI] [PubMed] [Google Scholar]
- 21.Shimizu M, Tropak M, Diaz RJ, Suto F, Surendra H, Kuzmin E, Li J, Gross G, Wilson GJ, Callahan J, et al. Transient limb ischaemia remotely preconditions through a humoral mechanism acting directly on the myocardium: evidence suggesting cross-species protection. Clin Sci (Lond) 2009;117:191–200. doi: 10.1042/CS20080523. [DOI] [PubMed] [Google Scholar]
- 22.Lim SY, Yellon DM, Hausenloy DJ. The neural and humoral pathways in remote limb ischemic preconditioning. Basic Res Cardiol. 2010;105:651–655. doi: 10.1007/s00395-010-0099-y. [DOI] [PubMed] [Google Scholar]
- 23.Loukogeorgakis SP, Panagiotidou AT, Broadhead MW, Donald A, Deanfield JE, MacAllister RJ. Remote ischemic preconditioning provides early and late protection against endothelial ischemia-reperfusion injury in humans: role of the autonomic nervous system. J Am Coll Cardiol. 2005;46:450–456. doi: 10.1016/j.jacc.2005.04.044. [DOI] [PubMed] [Google Scholar]
- 24.Hausenloy DJ, Iliodromitis EK, Andreadou I, Papalois A, Gritsopoulos G, Anastasiou-Nana M, Kremastinos DT, Yellon DM. Investigating the signal transduction pathways underlying remote ischemic conditioning in the porcine heart. Cardiovasc Drugs Ther. 2012;26:87–93. doi: 10.1007/s10557-011-6364-y. [DOI] [PubMed] [Google Scholar]
- 25.Heusch G, Musiolik J, Kottenberg E, Peters J, Jakob H, Thielmann M. STAT5 activation and cardioprotection by remote ischemic preconditioning in humans: short communication. Circ Res. 2012;110:111–115. doi: 10.1161/CIRCRESAHA.111.259556. [DOI] [PubMed] [Google Scholar]
- 26.Tamareille S, Mateus V, Ghaboura N, Jeanneteau J, Croué A, Henrion D, Furber A, Prunier F. RISK and SAFE signaling pathway interactions in remote limb ischemic perconditioning in combination with local ischemic postconditioning. Basic Res Cardiol. 2011;106:1329–1339. doi: 10.1007/s00395-011-0210-z. [DOI] [PubMed] [Google Scholar]
- 27.Shimizu M, Saxena P, Konstantinov IE, Cherepanov V, Cheung MM, Wearden P, Zhangdong H, Schmidt M, Downey GP, Redington AN. Remote ischemic preconditioning decreases adhesion and selectively modifies functional responses of human neutrophils. J Surg Res. 2010;158:155–161. doi: 10.1016/j.jss.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 28.Konstantinov IE, Arab S, Kharbanda RK, Li J, Cheung MM, Cherepanov V, Downey GP, Liu PP, Cukerman E, Coles JG, et al. The remote ischemic preconditioning stimulus modifies inflammatory gene expression in humans. Physiol Genomics. 2004;19:143–150. doi: 10.1152/physiolgenomics.00046.2004. [DOI] [PubMed] [Google Scholar]
- 29.Kharbanda RK, Mortensen UM, White PA, Kristiansen SB, Schmidt MR, Hoschtitzky JA, Vogel M, Sorensen K, Redington AN, MacAllister R. Transient limb ischemia induces remote ischemic preconditioning in vivo. Circulation. 2002;106:2881–2883. doi: 10.1161/01.cir.0000043806.51912.9b. [DOI] [PubMed] [Google Scholar]
- 30.Pedersen CM, Cruden NL, Schmidt MR, Lau C, Bøtker HE, Kharbanda RK, Newby DE. Remote ischemic preconditioning prevents systemic platelet activation associated with ischemia-reperfusion injury in humans. J Thromb Haemost. 2011;9:404–407. doi: 10.1111/j.1538-7836.2010.04142.x. [DOI] [PubMed] [Google Scholar]
- 31.Lai IR, Chang KJ, Chen CF, Tsai HW. Transient limb ischemia induces remote preconditioning in liver among rats: the protective role of heme oxygenase-1. Transplantation. 2006;81:1311–1317. doi: 10.1097/01.tp.0000203555.14546.63. [DOI] [PubMed] [Google Scholar]
- 32.Jan WC, Chen CH, Tsai PS, Huang CJ. Limb ischemic preconditioning mitigates lung injury induced by haemorrhagic shock/resuscitation in rats. Resuscitation. 2011;82:760–766. doi: 10.1016/j.resuscitation.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 33.Ali ZA, Callaghan CJ, Lim E, Ali AA, Nouraei SA, Akthar AM, Boyle JR, Varty K, Kharbanda RK, Dutka DP, et al. Remote ischemic preconditioning reduces myocardial and renal injury after elective abdominal aortic aneurysm repair: a randomized controlled trial. Circulation. 2007;116:I98–105. doi: 10.1161/circulationaha.106.679167. [DOI] [PubMed] [Google Scholar]
- 34.Hahn CD, Manlhiot C, Schmidt MR, Nielsen TT, Redington AN. Remote ischemic per-conditioning: a novel therapy for acute stroke? Stroke. 2011;42:2960–2962. doi: 10.1161/STROKEAHA.111.622340. [DOI] [PubMed] [Google Scholar]
- 35.Birnbaum Y, Hale SL, Kloner RA. Ischemic preconditioning at a distance: reduction of myocardial infarct size by partial reduction of blood supply combined with rapid stimulation of the gastrocnemius muscle in the rabbit. Circulation. 1997;96:1641–1646. doi: 10.1161/01.cir.96.5.1641. [DOI] [PubMed] [Google Scholar]
- 36.Kadkhodaee M, Seifi B, Najafi A, Sedaghat Z. First report of the protective effects of remote per- and postconditioning on ischemia/reperfusion-induced renal injury. Transplantation. 2011;92:e55. doi: 10.1097/TP.0b013e31823411f8. [DOI] [PubMed] [Google Scholar]
- 37.Hougaard KD, Hjort N, Zeidler D, Sørensen L, Nørgaard A, Hansen TM, von Weitzel-Mudersbach P, Simonsen CZ, Damgaard D, Gottrup H, et al. Remote ischemic perconditioning as an adjunct therapy to thrombolysis in patients with acute ischemic stroke: a randomized trial. Stroke. 2014;45:159–167. doi: 10.1161/STROKEAHA.113.001346. [DOI] [PubMed] [Google Scholar]
- 38.Hoole SP, Heck PM, Sharples L, Khan SN, Duehmke R, Densem CG, Clarke SC, Shapiro LM, Schofield PM, O’Sullivan M, et al. Cardiac Remote Ischemic Preconditioning in Coronary Stenting (CRISP Stent) Study: a prospective, randomized control trial. Circulation. 2009;119:820–827. doi: 10.1161/CIRCULATIONAHA.108.809723. [DOI] [PubMed] [Google Scholar]
- 39.Thielmann M, Kottenberg E, Boengler K, Raffelsieper C, Neuhaeuser M, Peters J, Jakob H, Heusch G. Remote ischemic preconditioning reduces myocardial injury after coronary artery bypass surgery with crystalloid cardioplegic arrest. Basic Res Cardiol. 2010;105:657–664. doi: 10.1007/s00395-010-0104-5. [DOI] [PubMed] [Google Scholar]
- 40.Bøtker HE, Kharbanda R, Schmidt MR, Bøttcher M, Kaltoft AK, Terkelsen CJ, Munk K, Andersen NH, Hansen TM, Trautner S, et al. Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomised trial. Lancet. 2010;375:727–734. doi: 10.1016/S0140-6736(09)62001-8. [DOI] [PubMed] [Google Scholar]
- 41.Cheung MM, Kharbanda RK, Konstantinov IE, Shimizu M, Frndova H, Li J, Holtby HM, Cox PN, Smallhorn JF, Van Arsdell GS, et al. Randomized controlled trial of the effects of remote ischemic preconditioning on children undergoing cardiac surgery: first clinical application in humans. J Am Coll Cardiol. 2006;47:2277–2282. doi: 10.1016/j.jacc.2006.01.066. [DOI] [PubMed] [Google Scholar]
- 42.Dave RH, Hale SL, Kloner RA. Hypothermic, closed circuit pericardioperfusion: a potential cardioprotective technique in acute regional ischemia. J Am Coll Cardiol. 1998;31:1667–1671. doi: 10.1016/s0735-1097(98)00129-6. [DOI] [PubMed] [Google Scholar]
- 43.Yang X, Liu Y, Yang XM, Hu F, Cui L, Swingle MR, Honkanen RE, Soltani P, Tissier R, Cohen MV, et al. Cardioprotection by mild hypothermia during ischemia involves preservation of ERK activity. Basic Res Cardiol. 2011;106:421–430. doi: 10.1007/s00395-011-0165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maeng M, Mortensen UM, Kristensen J, Kristiansen SB, Andersen HR. Hypothermia during reperfusion does not reduce myocardial infarct size in pigs. Basic Res Cardiol. 2006;101:61–68. doi: 10.1007/s00395-005-0550-7. [DOI] [PubMed] [Google Scholar]
- 45.Götberg M, Olivecrona GK, Koul S, Carlsson M, Engblom H, Ugander M, van der Pals J, Algotsson L, Arheden H, Erlinge D. A pilot study of rapid cooling by cold saline and endovascular cooling before reperfusion in patients with ST-elevation myocardial infarction. Circ Cardiovasc Interv. 2010;3:400–407. doi: 10.1161/CIRCINTERVENTIONS.110.957902. [DOI] [PubMed] [Google Scholar]
- 46.Tissier R, Cohen MV, Downey JM. Does mild hypothermia protect against reperfusion injury? The debate continues. Basic Res Cardiol. 2011;106:691–695. doi: 10.1007/s00395-011-0194-8. [DOI] [PubMed] [Google Scholar]
- 47.Erlinge D, Götberg M, Grines C, Dixon S, Baran K, Kandzari D, Olivecrona GK. A pooled analysis of the effect of endovascular cooling on infarct size in patients with ST-elevation myocardial infarction. EuroIntervention. 2013;8:1435–1440. doi: 10.4244/EIJV8I12A217. [DOI] [PubMed] [Google Scholar]
- 48.Erlinge D, Götberg M, Lang I, Holzer M, Noc M, Clemmensen P, Jensen U, Metzler B, James S, Bötker HE, et al. Rapid endovascular catheter core cooling combined with cold saline as an adjunct to percutaneous coronary intervention for the treatment of acute myocardial infarction. The CHILL-MI trial: a randomized controlled study of the use of central venous catheter core cooling combined with cold saline as an adjunct to percutaneous coronary intervention for the treatment of acute myocardial infarction. J Am Coll Cardiol. 2014;63:1857–1865. doi: 10.1016/j.jacc.2013.12.027. [DOI] [PubMed] [Google Scholar]
- 49.Toombs CF, McGee S, Johnston WE, Vinten-Johansen J. Myocardial protective effects of adenosine. Infarct size reduction with pretreatment and continued receptor stimulation during ischemia. Circulation. 1992;86:986–994. doi: 10.1161/01.cir.86.3.986. [DOI] [PubMed] [Google Scholar]
- 50.Wright GL, Hanlon P, Amin K, Steenbergen C, Murphy E, Arcasoy MO. Erythropoietin receptor expression in adult rat cardiomyocytes is associated with an acute cardioprotective effect for recombinant erythropoietin during ischemia-reperfusion injury. FASEB J. 2004;18:1031–1033. doi: 10.1096/fj.03-1289fje. [DOI] [PubMed] [Google Scholar]
- 51.Hennan JK, Swillo RE, Morgan GA, Keith JC, Schaub RG, Smith RP, Feldman HS, Haugan K, Kantrowitz J, Wang PJ, et al. Rotigaptide (ZP123) prevents spontaneous ventricular arrhythmias and reduces infarct size during myocardial ischemia/reperfusion injury in open-chest dogs. J Pharmacol Exp Ther. 2006;317:236–243. doi: 10.1124/jpet.105.096933. [DOI] [PubMed] [Google Scholar]
- 52.Lefer AM, Campbell B, Shin YK, Scalia R, Hayward R, Lefer DJ. Simvastatin preserves the ischemic-reperfused myocardium in normocholesterolemic rat hearts. Circulation. 1999;100:178–184. doi: 10.1161/01.cir.100.2.178. [DOI] [PubMed] [Google Scholar]
- 53.Rastegar MA, Végh A, Papp JG, Parratt JR. Atrial natriuretic peptide reduces the severe consequences of coronary artery occlusion in anaesthetized dogs. Cardiovasc Drugs Ther. 2000;14:471–479. doi: 10.1023/a:1007828804553. [DOI] [PubMed] [Google Scholar]
- 54.Hess ML, Okabe E, Poland J, Warner M, Stewart JR, Greenfield LJ. Glucose, insulin, potassium protection during the course of hypothermic global ischemia and reperfusion: a new proposed mechanism by the scavenging of free radicals. J Cardiovasc Pharmacol. 1984;5:35–43. doi: 10.1097/00005344-198301000-00005. [DOI] [PubMed] [Google Scholar]
- 55.Kumar A, Villani MP, Patel UK, Keith JC, Schaub RG. Recombinant soluble form of PSGL-1 accelerates thrombolysis and prevents reocclusion in a porcine model. Circulation. 1999;99:1363–1369. doi: 10.1161/01.cir.99.10.1363. [DOI] [PubMed] [Google Scholar]
- 56.Massoudy P, Zahler S, Kupatt C, Reder E, Becker BF, Gerlach E. Cardioprotection by cyclosporine A in experimental ischemia and reperfusion--evidence for a nitric oxide-dependent mechanism mediated by endothelin. J Mol Cell Cardiol. 1997;29:535–544. doi: 10.1006/jmcc.1996.0297. [DOI] [PubMed] [Google Scholar]
- 57.Timmers L, Henriques JP, de Kleijn DP, Devries JH, Kemperman H, Steendijk P, Verlaan CW, Kerver M, Piek JJ, Doevendans PA, et al. Exenatide reduces infarct size and improves cardiac function in a porcine model of ischemia and reperfusion injury. J Am Coll Cardiol. 2009;53:501–510. doi: 10.1016/j.jacc.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 58.Ibanez B, Prat-González S, Speidl WS, Vilahur G, Pinero A, Cimmino G, García MJ, Fuster V, Sanz J, Badimon JJ. Early metoprolol administration before coronary reperfusion results in increased myocardial salvage: analysis of ischemic myocardium at risk using cardiac magnetic resonance. Circulation. 2007;115:2909–2916. doi: 10.1161/CIRCULATIONAHA.106.679639. [DOI] [PubMed] [Google Scholar]
- 59.Mahaffey KW, Puma JA, Barbagelata NA, DiCarli MF, Leesar MA, Browne KF, Eisenberg PR, Bolli R, Casas AC, Molina-Viamonte V, et al. Adenosine as an adjunct to thrombolytic therapy for acute myocardial infarction: results of a multicenter, randomized, placebo-controlled trial: the Acute Myocardial Infarction STudy of ADenosine (AMISTAD) trial. J Am Coll Cardiol. 1999;34:1711–1720. doi: 10.1016/s0735-1097(99)00418-0. [DOI] [PubMed] [Google Scholar]
- 60.Kloner RA, Forman MB, Gibbons RJ, Ross AM, Alexander RW, Stone GW. Impact of time to therapy and reperfusion modality on the efficacy of adenosine in acute myocardial infarction: the AMISTAD-2 trial. Eur Heart J. 2006;27:2400–2405. doi: 10.1093/eurheartj/ehl094. [DOI] [PubMed] [Google Scholar]
- 61.Kitakaze M, Asakura M, Kim J, Shintani Y, Asanuma H, Hamasaki T, Seguchi O, Myoishi M, Minamino T, Ohara T, et al. Human atrial natriuretic peptide and nicorandil as adjuncts to reperfusion treatment for acute myocardial infarction (J-WIND): two randomised trials. Lancet. 2007;370:1483–1493. doi: 10.1016/S0140-6736(07)61634-1. [DOI] [PubMed] [Google Scholar]
- 62.Kim JS, Kim J, Choi D, Lee CJ, Lee SH, Ko YG, Hong MK, Kim BK, Oh SJ, Jeon DW, et al. Efficacy of high-dose atorvastatin loading before primary percutaneous coronary intervention in ST-segment elevation myocardial infarction: the STATIN STEMI trial. JACC Cardiovasc Interv. 2010;3:332–339. doi: 10.1016/j.jcin.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 63.Hahn JY, Kim HJ, Choi YJ, Jo SH, Kim HJ, Lee S, Ahn KJ, Song YB, Choi JH, Choi SH, et al. Effects of atorvastatin pretreatment on infarct size in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Am Heart J. 2011;162:1026–1033. doi: 10.1016/j.ahj.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 64.Piot C, Croisille P, Staat P, Thibault H, Rioufol G, Mewton N, Elbelghiti R, Cung TT, Bonnefoy E, Angoulvant D, et al. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N Engl J Med. 2008;359:473–481. doi: 10.1056/NEJMoa071142. [DOI] [PubMed] [Google Scholar]
- 65.Ghaffari S, Kazemi B, Toluey M, Sepehrvand N. The effect of prethrombolytic cyclosporine-A injection on clinical outcome of acute anterior ST-elevation myocardial infarction. Cardiovasc Ther. 2013;31:e34–e39. doi: 10.1111/1755-5922.12010. [DOI] [PubMed] [Google Scholar]
- 66.Voors AA, Belonje AM, Zijlstra F, Hillege HL, Anker SD, Slart RH, Tio RA, van ‘t Hof A, Jukema JW, Peels HO, et al. A single dose of erythropoietin in ST-elevation myocardial infarction. Eur Heart J. 2010;31:2593–2600. doi: 10.1093/eurheartj/ehq304. [DOI] [PubMed] [Google Scholar]
- 67.Lønborg J, Vejlstrup N, Kelbæk H, Bøtker HE, Kim WY, Mathiasen AB, Jørgensen E, Helqvist S, Saunamäki K, Clemmensen P, et al. Exenatide reduces reperfusion injury in patients with ST-segment elevation myocardial infarction. Eur Heart J. 2012;33:1491–1499. doi: 10.1093/eurheartj/ehr309. [DOI] [PubMed] [Google Scholar]
- 68.Bernink FJ, Timmers L, Diamant M, Scholte M, Beek AM, Kamp O, Marques KM, Denham RN, Chen WJ, Doevendans PA, et al. Effect of additional treatment with EXenatide in patients with an Acute Myocardial Infarction: the EXAMI study. Int J Cardiol. 2013;167:289–290. doi: 10.1016/j.ijcard.2012.09.204. [DOI] [PubMed] [Google Scholar]
- 69.Woo JS, Kim W, Ha SJ, Kim JB, Kim SJ, Kim WS, Seon HJ, Kim KS. Cardioprotective effects of exenatide in patients with ST-segment-elevation myocardial infarction undergoing primary percutaneous coronary intervention: results of exenatide myocardial protection in revascularization study. Arterioscler Thromb Vasc Biol. 2013;33:2252–2260. doi: 10.1161/ATVBAHA.113.301586. [DOI] [PubMed] [Google Scholar]
- 70.Mehta SR, Yusuf S, Díaz R, Zhu J, Pais P, Xavier D, Paolasso E, Ahmed R, Xie C, Kazmi K, et al. Effect of glucose-insulin-potassium infusion on mortality in patients with acute ST-segment elevation myocardial infarction: the CREATE-ECLA randomized controlled trial. JAMA. 2005;293:437–446. doi: 10.1001/jama.293.4.437. [DOI] [PubMed] [Google Scholar]
- 71.Selker HP, Beshansky JR, Sheehan PR, Massaro JM, Griffith JL, D’Agostino RB, Ruthazer R, Atkins JM, Sayah AJ, Levy MK, et al. Out-of-hospital administration of intravenous glucose-insulin-potassium in patients with suspected acute coronary syndromes: the IMMEDIATE randomized controlled trial. JAMA. 2012;307:1925–1933. doi: 10.1001/jama.2012.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bates E, Bode C, Costa M, Gibson CM, Granger C, Green C, Grimes K, Harrington R, Huber K, Kleiman N, et al. Intracoronary KAI-9803 as an adjunct to primary percutaneous coronary intervention for acute ST-segment elevation myocardial infarction. Circulation. 2008;117:886–896. doi: 10.1161/CIRCULATIONAHA.107.759167. [DOI] [PubMed] [Google Scholar]
- 73.Mertens P, Maes A, Nuyts J, Belmans A, Desmet W, Esplugas E, Charlier F, Figueras J, Sambuceti G, Schwaiger M, et al. Recombinant P-selectin glycoprotein ligand-immunoglobulin, a P-selectin antagonist, as an adjunct to thrombolysis in acute myocardial infarction. The P-Selectin Antagonist Limiting Myonecrosis (PSALM) trial. Am Heart J. 2006;152:125.e1–125.e8. doi: 10.1016/j.ahj.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 74.Tardif JC, Tanguay JF, Wright SS, Duchatelle V, Petroni T, Grégoire JC, Ibrahim R, Heinonen TM, Robb S, Bertrand OF, Cournoyer D, Johnson D, Mann J, Guertin MC, L’Allier PL. Effects of the P-selectin antagonist inclacumab on myocardial damage after percutaneous coronary intervention for non-ST-segment elevation myocardial infarction: results of the SELECT-ACS trial. J Am Coll Cardiol. 2013;61:2048–2055. doi: 10.1016/j.jacc.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 75.Ibanez B, Macaya C, Sánchez-Brunete V, Pizarro G, Fernández-Friera L, Mateos A, Fernández-Ortiz A, García-Ruiz JM, García-Álvarez A, Iñiguez A, et al. Effect of early metoprolol on infarct size in ST-segment-elevation myocardial infarction patients undergoing primary percutaneous coronary intervention: the Effect of Metoprolol in Cardioprotection During an Acute Myocardial Infarction (METOCARD-CNIC) trial. Circulation. 2013;128:1495–1503. doi: 10.1161/CIRCULATIONAHA.113.003653. [DOI] [PubMed] [Google Scholar]
- 76.Kloner RA. Current state of clinical translation of cardioprotective agents for acute myocardial infarction. Circ Res. 2013;113:451–463. doi: 10.1161/CIRCRESAHA.112.300627. [DOI] [PubMed] [Google Scholar]
- 77.Gerczuk PZ, Kloner RA. An update on cardioprotection: a review of the latest adjunctive therapies to limit myocardial infarction size in clinical trials. J Am Coll Cardiol. 2012;59:969–978. doi: 10.1016/j.jacc.2011.07.054. [DOI] [PubMed] [Google Scholar]
- 78.Mewton N, Croisille P, Gahide G, Rioufol G, Bonnefoy E, Sanchez I, Cung TT, Sportouch C, Angoulvant D, Finet G, et al. Effect of cyclosporine on left ventricular remodeling after reperfused myocardial infarction. J Am Coll Cardiol. 2010;55:1200–1205. doi: 10.1016/j.jacc.2009.10.052. [DOI] [PubMed] [Google Scholar]
- 79.Hausenloy DJ, Yellon DM. Taking lizard saliva to heart. Eur Heart J. 2012;33:1426–1430. doi: 10.1093/eurheartj/ehr382. [DOI] [PubMed] [Google Scholar]
- 80.Lønborg J, Kelbæk H, Vejlstrup N, Bøtker HE, Kim WY, Holmvang L, Jørgensen E, Helqvist S, Saunamäki K, Terkelsen CJ, et al. Exenatide reduces final infarct size in patients with ST-segment-elevation myocardial infarction and short-duration of ischemia. Circ Cardiovasc Interv. 2012;5:288–295. doi: 10.1161/CIRCINTERVENTIONS.112.968388. [DOI] [PubMed] [Google Scholar]
- 81.Venugopal V, Hausenloy DJ, Ludman A, Di Salvo C, Kolvekar S, Yap J, Lawrence D, Bognolo J, Yellon DM. Remote ischaemic preconditioning reduces myocardial injury in patients undergoing cardiac surgery with cold-blood cardioplegia: a randomised controlled trial. Heart. 2009;95:1567–1571. doi: 10.1136/hrt.2008.155770. [DOI] [PubMed] [Google Scholar]
- 82.Davies WR, Brown AJ, Watson W, McCormick LM, West NE, Dutka DP, Hoole SP. Remote ischemic preconditioning improves outcome at 6 years after elective percutaneous coronary intervention: the CRISP stent trial long-term follow-up. Circ Cardiovasc Interv. 2013;6:246–251. doi: 10.1161/CIRCINTERVENTIONS.112.000184. [DOI] [PubMed] [Google Scholar]
- 83.Thielmann M, Kottenberg E, Kleinbongard P, Wendt D, Gedik N, Pasa S, Price V, Tsagakis K, Neuhäuser M, Peters J, et al. Cardioprotective and prognostic effects of remote ischaemic preconditioning in patients undergoing coronary artery bypass surgery: a single-centre randomised, double-blind, controlled trial. Lancet. 2013;382:597–604. doi: 10.1016/S0140-6736(13)61450-6. [DOI] [PubMed] [Google Scholar]
- 84.Hausenloy DJ, Mwamure PK, Venugopal V, Harris J, Barnard M, Grundy E, Ashley E, Vichare S, Di Salvo C, Kolvekar S, et al. Effect of remote ischaemic preconditioning on myocardial injury in patients undergoing coronary artery bypass graft surgery: a randomised controlled trial. Lancet. 2007;370:575–579. doi: 10.1016/S0140-6736(07)61296-3. [DOI] [PubMed] [Google Scholar]
- 85.Terkelsen CJ, Jensen LO, Tilsted HH, Trautner S, Johnsen SP, Vach W, Bøtker HE, Thuesen L, Lassen JF. Health care system delay and heart failure in patients with ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention: follow-up of population-based medical registry data. Ann Intern Med. 2011;155:361–367. doi: 10.7326/0003-4819-155-6-201109200-00004. [DOI] [PubMed] [Google Scholar]
- 86.Sørensen JT, Terkelsen CJ, Nørgaard BL, Trautner S, Hansen TM, Bøtker HE, Lassen JF, Andersen HR. Urban and rural implementation of pre-hospital diagnosis and direct referral for primary percutaneous coronary intervention in patients with acute ST-elevation myocardial infarction. Eur Heart J. 2011;32:430–436. doi: 10.1093/eurheartj/ehq437. [DOI] [PubMed] [Google Scholar]
- 87.Heusch G. Postconditioning: old wine in a new bottle? J Am Coll Cardiol. 2004;44:1111–1112. doi: 10.1016/j.jacc.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 88.Ovize M, Baxter GF, Di Lisa F, Ferdinandy P, Garcia-Dorado D, Hausenloy DJ, Heusch G, Vinten-Johansen J, Yellon DM, Schulz R. Postconditioning and protection from reperfusion injury: where do we stand? Position paper from the Working Group of Cellular Biology of the Heart of the European Society of Cardiology. Cardiovasc Res. 2010;87:406–423. doi: 10.1093/cvr/cvq129. [DOI] [PubMed] [Google Scholar]
- 89.Sloth AD, Schmidt MR, Munk K, Kharbanda RK, Redington AN, Schmidt M, Pedersen L, Sørensen HT, Bøtker HE. Improved long-term clinical outcomes in patients with ST-elevation myocardial infarction undergoing remote ischaemic conditioning as an adjunct to primary percutaneous coronary intervention. Eur Heart J. 2014;35:168–175. doi: 10.1093/eurheartj/eht369. [DOI] [PubMed] [Google Scholar]


