Abstract
Objective
To determine the natural history of cirrhosis from parenteral nutrition-associated liver disease (PNALD) after resolution of cholestasis with fish oil (FO) therapy
Background
Historically, cirrhosis from PNALD resulted in end-stage liver disease (ESLD), often requiring transplantation for survival. With FO therapy, most children now experience resolution of cholestasis and rarely progress to ESLD. However, outcomes for cirrhosis after resolution of cholestasis are unknown and patients continue to be considered for liver/multivisceral transplantation.
Methods
Prospectively collected data was reviewed for children with cirrhosis due to PNALD who had resolution of cholestasis after treatment with FO from 2004 to 2012. Outcomes evaluated included need for liver/multivisceral transplantation, mortality, and the clinical progression of liver disease.
Results
Fifty-one patients with cirrhosis from PNALD were identified, with 76% demonstrating resolution of cholestasis after FO therapy. The mean direct bilirubin decreased from 6.4 ± 4 mg/dL to 0.2 ± 0.1 mg/dL (p <0.001) 12 months after resolution of cholestasis, with a mean time to resolution of 74 days. None of the patients required transplantation or died from ESLD. Pediatric End-Stage Liver Disease (PELD) scores decreased from 16 ± 4.6 to −1.2 ± 4.6, 12 months after resolution of cholestasis (p <0.001). In children who remained PN-dependent, the PELD score remained normal throughout the follow-up period.
Conclusions
Cirrhosis from PNALD may be stable rather than progressive once cholestasis resolves with FO therapy. Furthermore, these patients may not require transplantation and show no clinical evidence of liver disease progression, even when persistently PN-dependent.
Introduction
Parenteral nutrition (PN) is a life-saving therapy for children with intestinal failure due to insufficient bowel length or function. However, long-term PN use carries the risk of developing a life-threatening liver disease, characterized initially by intrahepatic cholestasis 1. Parenteral nutrition-associated liver disease (PNALD) is often progressive and its severity is largely related to the duration of PN therapy. Histologic evidence of cholestasis can be seen within 2 weeks of initiating PN, with progression to worsening fibrosis and ultimately cirrhosis over the course of several months 2–4. In Zambrano et al.’s review of 24 autopsies from children with PNALD, fibrosis was observed in 71% of patients, with the most severe cases in infants who received PN for greater than 6 weeks. Cirrhosis was present in 13% of the infants, with 66% of these patients having received PN for greater than 12 weeks 5.
The incidence of PNALD in the 1990s was as high as 85% in children with intestinal failure receiving long-term PN 6. Without transition to full enteral feeding or small bowel and/or liver transplantation, cholestasis may progress to cirrhosis and end-stage liver disease (ESLD), with death from sepsis or portal hypertension 7. For children treated between 2000 and 2004, the Pediatric Intestinal Failure Consortium reported the incidence of PNALD at 74.4%, with a mortality rate of 27% over 72 months. Intestinal transplantation was performed in 26% of patients, with a 17% mortality rate after transplantation 8.
Over the last 10 years, new insights into the pathophysiology of PNALD have provided the impetus for alternative treatments in children unable to be weaned from PN. Although the pathogenesis of PNALD is multifactorial, considerable evidence has emerged implicating harmful components present within parenteral soybean oil (SO) (Intralipid, Fresenius Kabi AB, Uppsala, Sweden). High levels of phytosterols (plant-based steroid compounds) and pro-inflammatory mediators derived from omega-6 polyunsaturated fatty acids may be responsible for the liver injury 9–14.
As a result, many institutions have reduced the administered dose of parenteral SO from 2–3 g/kg/day to 1 g/kg/day to try to decrease the incidence of PNALD. This strategy has produced mixed results. Sanchez et al. compared the incidence of PNALD in 132 children treated with standard SO doses (2.1 ± 0.39 g/kg/day) between 2005 and 2008 and 82 children treated with reduced SO doses (1.25 ± 0.38 g/kg/day) from 2009–2012. The incidence of PNALD was observed to decrease from 43% to 22% for standard and reduced lipid doses, respectively 15. In contrast, a study found no significant difference in the incidence of PNALD among children treated between 2007 and 2011 with standard SO dosing compared to reduced SO dosing (43.8% versus 51.7%, p=0.61) 16. However, considering the potential benefit, children with intestinal failure are commonly treated with reduced SO dosing.
Unfortunately, limited treatment options are available for children who develop PNALD despite lipid restriction strategies. In 2004, parenteral fish oil (FO) (Omegaven, Fresenius Kabi, Bad Homburg v.d.h, Germany) was introduced in the United States for compassionate use as a treatment for PNALD. The initial experience reported by Gura et al. demonstrated that children treated with parenteral FO experienced earlier resolution of cholestasis, exhibited no biochemical evidence of essential fatty acid deficiency, and had a markedly lower mortality rate and need for liver transplantation than a historical cohort of children treated with parenteral soybean oil (2 deaths and zero transplants in FO group versus 7 deaths and 2 liver transplants in the SO group) 17. The use of parenteral FO for the treatment of PNALD has since been adopted by multiple institutions with similar improvements in clinical outcomes 18–21. In the largest and most recent of these studies, by Premkumar et al., 57 children with PNALD were treated with parenteral FO. Cholestasis resolved in 82.5% of treated children, with a median time to resolution of 35 days 21.
With the advent of parenteral FO, children with cirrhosis from PNALD are able to remain on PN without progression to ESLD. However, the long-term outcomes for these children after resolution of cholestasis is unknown. Small case series have reported histologic evidence of persistent or progressive hepatic fibrosis in PN-dependent patients, despite resolution of cholestasis 22,23. Furthermore, studies have indicated that histologic evidence of liver fibrosis and cirrhosis may persist and even progress despite advancement to complete enteral feeding 24–26. Persistent elevation in alanine aminotransferase (ALT) has also been reported in patients despite full enteral feeding 27. These data have raised concerns that liver injury may continue to progress in children with PNALD despite resolution of cholestasis. However, these biochemical and histologic findings have not been correlated with long-term patient outcomes.
Children continue to be considered for liver transplantation despite a paucity of data regarding the natural history of cirrhosis in children with PNALD after resolution of biochemical cholestasis. The objective of this study is to determine the need for liver and/or multivisceral organ transplantation and mortality in children with cirrhosis from PNALD after resolution of cholestasis with parenteral FO therapy. Attention is also paid to non-cholestatic markers of the progression of liver disease, particularly in patients with persistent PN-dependence.
Materials and Methods
Patients
In this retrospective review of prospectively collected data, medical records from 2004 to 2012 were reviewed for all patients under the age of 12 years treated with parenteral FO at Boston Children’s Hospital (BCH) for a diagnosis of PNALD. PNALD was defined as a direct bilirubin (DB) level greater than 2 mg/dL for at least two weeks with no other diagnoses of liver dysfunction (i.e. biliary atresia, inborn errors of metabolism). FO monotherapy was administered at a dose of 1g/kg/day as previously described 17. FO lipid emulsion is administered separately from PN due to a lack of compatibility data regarding use of FO as part of a total nutrient admixture (Mirtallo et al., 2004). For children receiving FO, monthly laboratory studies are required to be in compliance with the study protocol at BCH for the compassionate use of FO through the Food and Drug Administraton (FDA) (IND 73,488). Laboratory tests include: hepatic panel (aspartate aminotransferase (AST), ALT, DB, total bilirubin (TB), alkaline phosphatase (AP)), complete blood count (CBC), International Normalized Ratio (INR), and serum fatty acid profiles. Patients with a concurrent diagnosis of cirrhosis due to PNALD were then identified. Cirrhosis was defined by the evidence of bridging fibrosis (METAVIR Stage F3) or cirrhosis (METAVIR Stage F4) on histologic analysis by a board certified pathologist for patients who underwent liver biopsy 29. For patients without a liver biopsy, cirrhosis was defined by the evidence of hepatofugal flow, or reversal of portal blood flow, on duplex ultrasound 30. Resolution of cholestasis was defined as a DB less than 2 mg/dL.
Study Endpoints
The primary end points of this study were the incidence of multivisceral or hepatic transplantation, and/or mortality in children with cirrhosis due to PNALD who experienced resolution of cholestasis after treatment with FO. Secondary endpoints included recurrent cholestasis, hepatic injury, hepatic synthetic function, growth, and presence of symptomatic cirrhosis due to PNALD requiring medical or surgical therapy. All available patient information from the date of initiation of FO through January 1, 2012 was reviewed.
Electronic medical records and archived charts were reviewed for each patient to identify the date that FO was initiated and the date that cholestasis resolved, defined as the date of the first DB under 2 mg/dL with all subsequent DB values under 2 mg/dL. Records were reviewed at 1 month, 2 months, 6 months, and 12 months after resolution of cholestasis for all patients. For children with continued PN-dependence receiving FO, additional chart review was performed from one year after resolution of cholestasis at 6-month intervals until January 1, 2012 (i.e. 18 months, 24 months, 30 months, etc.). For children who advanced to full enteral feeds, laboratory studies were required for two months after weaning from PN, in compliance with the study protocol. As a result, gaps in data exist for children who successfully advanced to enteral feeds within 12 months of initiating FO.
To determine the presence of cholestasis, extent of hepatic injury, and status of hepatic synthetic function, the following laboratory values were recorded from the medical record: DB, ALT, AST, AP, INR, albumin, platelet count, and creatinine. From these data, the Pediatric End-stage Liver Disease (PELD) score was calculated using the formula currently used by the United Network for Organ Sharing (UNOS):
The PELD score is a validated predictor of mortality from ESLD and one of the main criteria used in evaluation for possible liver transplantation 31.
AST to platelet ratio index (APRI) was calculated using the formula described by Mangus et al.:
The APRI correlates with histologic evidence of fibrosis and cirrhosis on liver biopsy in children with intestinal failure 32.
Patient growth over the study period was measured, with height, weight, and head circumference reviewed at each time point and age-adjusted z-scores calculated according to World Health Organization standards. In addition, charts were reviewed for the inpatient administration or outpatient prescription of medications commonly used to treat symptoms or complications of cirrhosis. Medications of interest included diuretics (furosemide, chlorthiazide, spironolactone), nitrogen-reducing agents (rifaximin, neomycin), and anti-pruritic agents (cholestyramine). When one of these medications was identified, the chart was reviewed for the associated medical indication for use of the drug.
Statistical Analysis
Continuous data are presented as mean ± standard deviation or median and inter-quartile range (IQR), and categorical data as frequency and percent of total. A Generalized Estimating Equation (GEE) was used to compare mean outcome over time, assuming normality and the identity link function 33,34. The covariance was modeled as AR(1). A within-subject effect was specified in the model to account for unequal spacing of the visits. Pair-wise comparisons of the index visit with each successive follow-up visit was specified a priori, and evaluated with a Wald chi-square test. All pairwise comparisons were adjusted with a Bonferroni correction to reduce Type I error. Statistical analysis was performed for all end points at 12 months. Raw data for DB and PELD score are reported for patients with persistent PN-dependence beyond 12 months with the associated number of patients at each long-term time point. Analysis was limited by low numbers of patients with long-term PN-dependence. All analysis results and figures were produced using SAS® software, Version 9.3 of the SAS System for Windows (Cary, NC).
Results
Patient Demographics
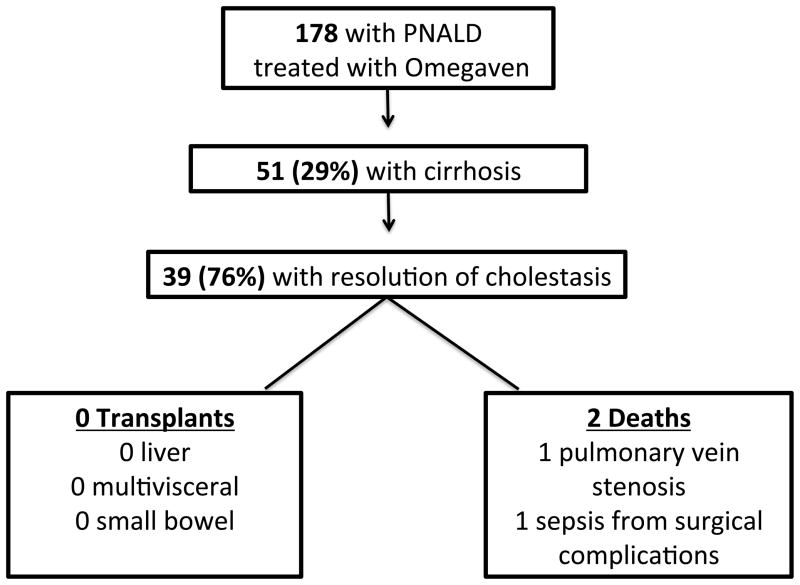
A total of 178 patients treated with FO for PNALD from January 1, 2004 to January 1, 2012 were identified. Fifty-one of the 178 (29%) patients carried a concurrent diagnosis of cirrhosis from PNALD. Of the 51 patients with cirrhosis, 39 (76%) experienced resolution of cholestasis after initiation of FO [Figure 1]. Median time to resolution of cholestasis was 74 days, with an interquartile range (IQR) of 39–123 days. Females comprised 39% of the population. The majority of patients were of preterm birth and of low birth weight, with a median weight of 1250 grams [Table 1].
Figure 1.
Table 1.
| Patient Group | Number of Patients (Percent) | Median PN Duration in months (IQR) |
|---|---|---|
| All Patients | 37 | 11.6 (4.0–35.5) |
| Full Enteral Nutrition | 20 (54) | 4.8 (2.5–11) |
| Continued PN + Omegaven | 13 (35) | 31.9 (17.5–58.4) |
| Continued PN + Intralipid | 4 (11) | 40.9 (25.4–53.5) |
| Patient Group | Number of Patients (Percent) | Median Value (IQR) |
| Male Gender | 25 (61) | - |
| Preterm Birth (<37 weeks) | 35 (92) | - |
| Gestational Age (weeks) | - | 29 (5 – 34) |
| Birth Weight (grams) | - | 1250 (801–2200) |
| Age at Omegaven Initiation (months) | - | 4 (2 – 6) |
Fifty-one percent of patients achieved enteral autonomy, defined as tolerance of full enteral nutrtion for at least 2 months, within 12 months after resolution of cholestasis. Of the patients who remained PN-dependent, 13/17 (76%) continued to receive FO with PN. Four patients were transitioned back to SO after resolution of cholestasis to accommodate the need for the convenience of total nutrient admixtures, which cannot currently be compounded using FO. The duration of PN-dependence ranged from 1 month to 9 years, with a median of 2.7 years [Table 1].
Transplantation and Mortality
Thirty-nine patients with cirrhosis due to PNALD had resolution of biochemical cholestasis with FO therapy. None of the 39 patients have required liver or multivisceral transplantation to date, with a follow-up range of 1 to 9 years. Two of the 39 patients died after initiation of FO. One patient died 3 months after initiation of FO due to complications associated with severe pulmonary stenosis and heart failure. Another patient died 3 months after initiation of FO from sepsis with multi-organ failure due to surgical complications after intervention for a small bowel obstruction. Neither death was related to liver failure or central venous catheter-associated sepsis.
Hepatic Injury
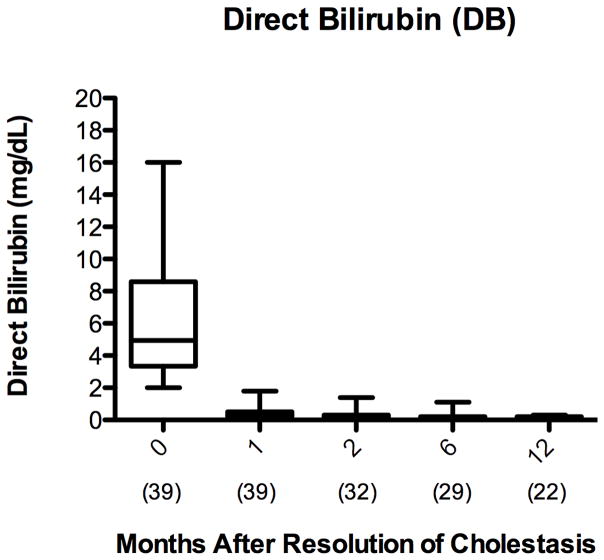
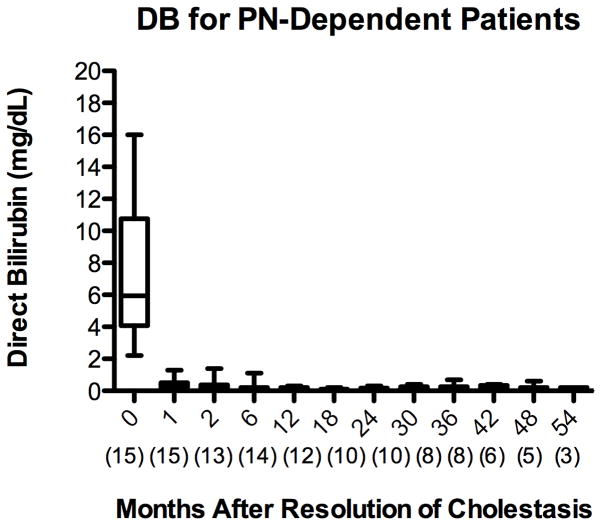
Several biochemical markers were assessed to determine the extent of hepatic injury in children with cirrhosis from PNALD after resolution of cholestasis. Resolution of cholestasis was defined as a DB less than 2 mg/dL. In this patient cohort, the DB decreased from a mean of 6.4 ± 4 mg/dL at initiation of FO to 0.2 ± 0.1 mg/dL 12 months (p <0.001) after the resolution of cholestasis [Figure 2A]. In the patients who remained PN-dependent, the DB remained below 2 mg/dL for the remainder of the follow-up period after resolution of cholestasis [Figure 2B].
Figure 2.
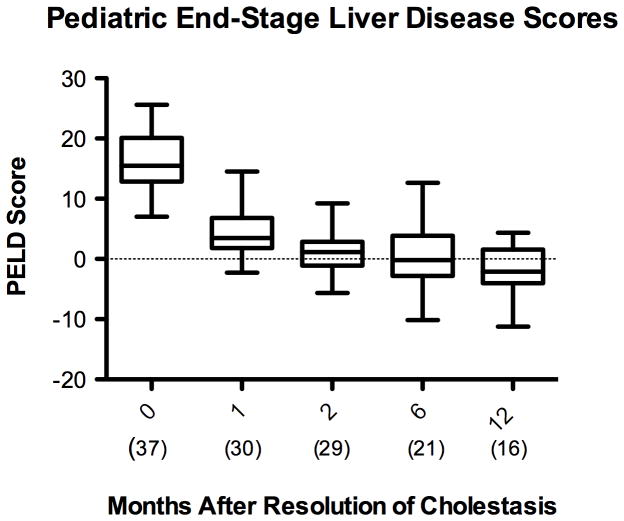
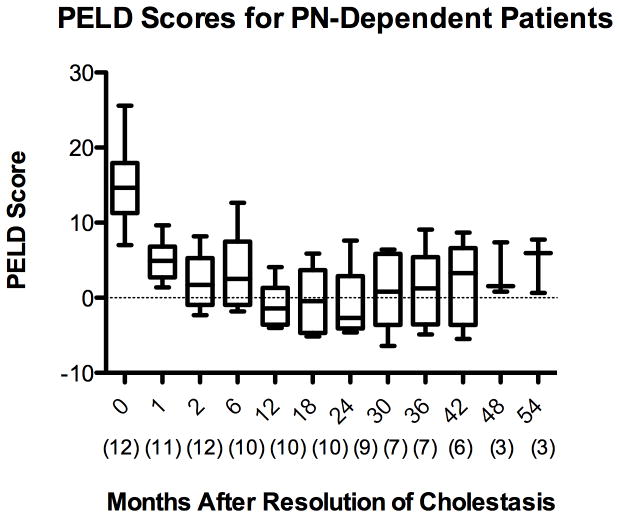
The PELD score is a validated predictor of mortality from ESLD and one of the main criteria used in evaluation for possible liver transplantation 31. The American Association for the Study of Liver Disease practice guidelines describe a PELD score of 15 as a criterion for initiating evaluation for liver transplantation 35. The PELD score in the study population decreased from 16 ± 4.6 at initiation of FO to −1.2 ± 4.6, 12 months after resolution of cholestasis (p <0.001) [Figure 3A]. For the small number of patients with persistent PN-dependence, the PELD score remained in the normal range for the remainder of the follow-up period after resolution of cholestasis [Figure 3B].
Figure 3.
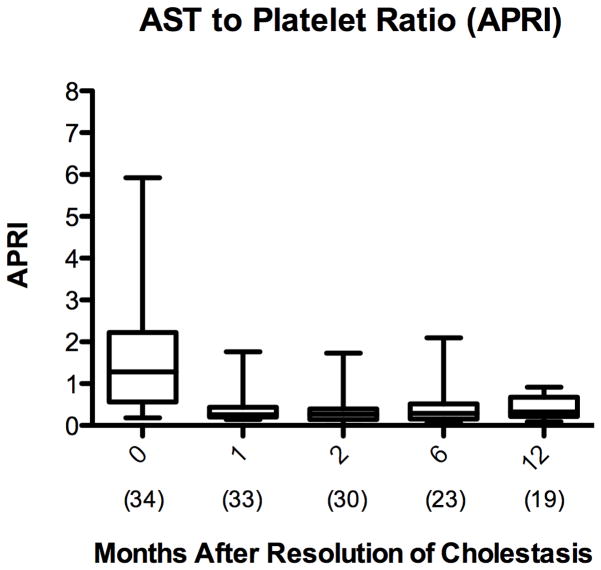
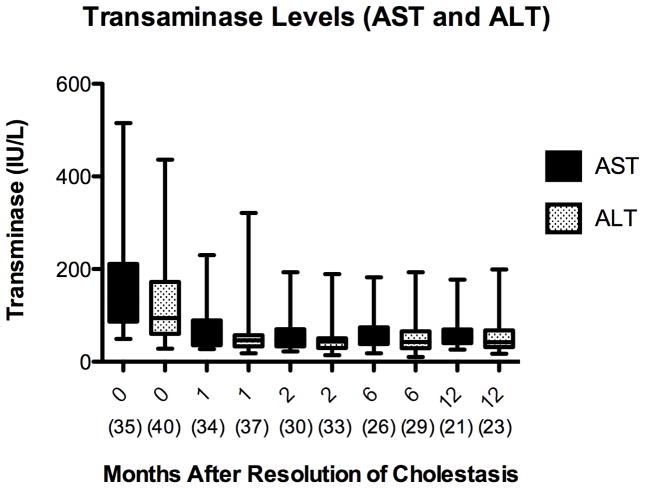
The APRI is a validated, non-invasive predictor of the progression of liver injury, with values greater than 1.5 correlating with biopsies demonstrating bridging fibrosis (METAVIR F3) and greater than 2 correlating with biopsies demonstrating cirrhosis (METAVIR F4) 36,37. Mean APRI decreased from 1.9 ± 1.8 at initiation of FO to 0.5 ± 0.3 at 12 months after cholestasis resolution (p <0.001) [Figure 4A]. Transaminases also decreased after resolution of cholestasis and remained low. AST and ALT decreased from 162 IU/L and 126 IU/L at initiation of FO to 72 IU/L and 62 IU/L at 12 months after cholestasis resolution, respectively (p <0.001) [Figure 4B].
Figure 4.
Hepatic Synthetic Function
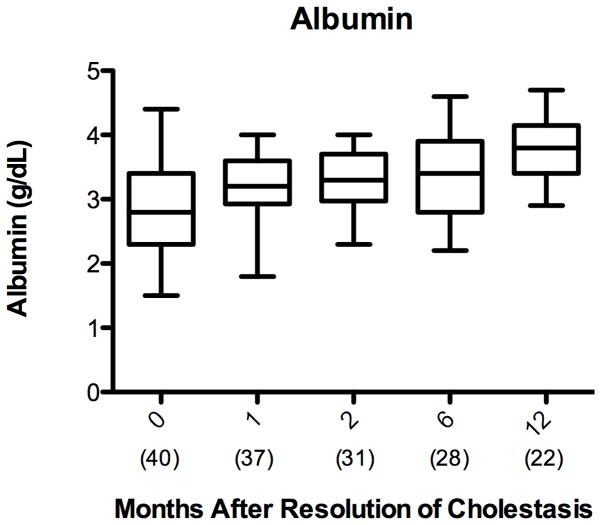
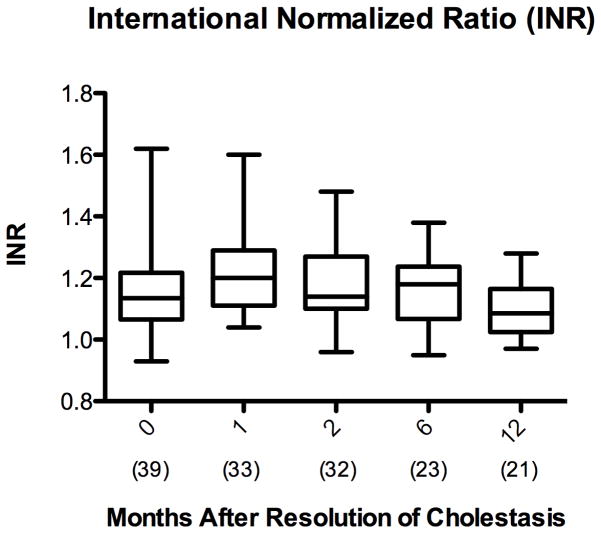
Albumin and INR are sensitive markers of hepatic synthetic function and are incorporated in the PELD score as previously described 38. Independently, albumin increased in the patients with cirrhosis from PNALD from 2.9 ± 0.7 g/dL at initiation of FO to 3.7 ± 0.5 g/dL at 12 months after cholestasis resolution (p< 0.001) [Figure 5A]. Mean INR remained below 2 throughout the period from initiation of FO through 12 months after resolution of cholestasis [Figure 5B].
Figure 5.
Patient Growth
Weight, height, and head circumference were compiled to calculate age-adjusted z-scores for patients with cirrhosis due to PNALD before and after resolution of cholestasis with FO therapy. The z-score provides an estimate of the deviation of an individual’s growth from the growth of others of the same age in the population. Negative and positive z-scores indicate the number of standard deviations below and above the mean, respectively, for individuals of the same age 39. Age-adjusted z-scores for weight and height trended upward from −2.03 ± 2.07 and −2.21 ± 2.29 at initiation of FO to −1.09 ± 1.05 and −1.52 ± 1.86 at 12 months after resolution, respectively (p=0.07, p=0.68). Age-adjusted weight for height z-score also trended upward from −1.28 ± 1.93 at initiation to −0.44 ± 1.06 (p=0.21).
Clinically Significant Cirrhosis
To determine the clinical impact of cirrhosis due to PNALD, patient charts were reviewed for evidence of in or outpatient administration of medications commonly used in the treatment of cirrhosis. Categories of medications included diuretics, nitrogen-reducing agents, and anti-pruritic agents. None of the 39 patients who experienced resolution of cholestasis after initiation of FO required these medications for hepatic indications. Eight patients (20%) required the use of diuretics for the management of chylous ascites from abdominal surgical complications, pulmonary edema, and chronic lung disease. Three patients (8%) required nitrogen-reducing agents for preoperative bowel preparation and the treatment of small bowel bacterial overgrowth in the setting of intestinal failure. Two patients (5%) were treated with cholestyramine, and in both instances it was used as an ingredient in a paste applied to treat diaper dermatitis (Table 2).
Table 2.
| Medication Type | Medication | Hepatic Indication | Non-hepatic Indication |
|---|---|---|---|
| Diuretics | Furosemide | None | N= 1: Chylous Ascites N= 3: Pulmonary edema |
| Spironolactone | None | N= 1: Chylous Ascites | |
| Chlorthiazide | None | N= 1: Pulmonary edema N=2: Chronic lung disease |
|
| Nitrogen- Reducing | Rifaximin | None | N=2: Bacterial Overgrowth |
| Neomycin | None | N= 1: Bowel Preparation | |
| Anti-pruritic | Cholestyramine | None | N=2: Diaper dermatitis |
Discussion
The incidence of and mortality from PNALD have decreased significantly over the last decade. Historically, children who progressed to cirrhosis from PNALD and were unable to wean to enteral nutrition frequently required liver transplantation or succumbed to ESLD 6,7. With the introduction of reduced SO lipid dosing and parenteral FO therapy, mortality from PNALD has decreased for most children, from approximately 25% prior to 2004 to less than 10% in recent years 15,18–20.
However, mortality remains high in children who develop cirrhosis and ESLD from PNALD. Kaufman et al. showed that between 2003 and 2007, 36% of infants aged 3 to 6 months with DB levels sustained above 6 mg/dL developed liver failure within a cohort of patients that did not receive FO therapy 40. In Premkumar et al.’s report of 57 children with PNALD from 2007 to 2011, 6 children (17%) died due to liver disease. Half of those children had signs of ESLD despite reduced SO doses and without receiving FO therapy 21.
Unfortunately, the management of children with cirrhosis due to PNALD has been largely institution-specific due to limited studies evaluating treatments and outcomes in this population. Furthermore, the natural history of cirrhosis in children with PNALD who do respond to FO therapy with resolution of cholestasis is currently unknown and children continue to be considered for liver transplantation. This report is the first to focus on this high-risk cohort and describe the response to FO therapy in the short- and long-term with attention to the need for liver or multivisceral transplantation, mortality, and clinical evidence of liver disease progression.
Between 2004 and 2012, 39 of 51 (76%) of children with cirrhosis due to PNALD at BCH experienced resolution of biochemical cholestasis with FO therapy and none have required liver transplantation or have died from liver failure to date. Twelve children did not achieve resolution of biochemical cholestasis, with 5 children requiring liver transplantation and 8 deaths. Eleven of the 12 children who did not respond to FO therapy were delayed transfers from outside hospitals and consequently experienced delays in therapy. Of the children who did respond to FO therapy, none have had a recurrence of cholestasis over the follow-up period of 1 to 9 years, including the children who remained PN-dependent for over 1 year. Within 1 year of treatment, 54% of these children were transitioned to full enteral nutrition. These data suggest that children with cirrhosis from PNALD can respond to FO therapy and display resolution of cholestasis, without progression to ESLD and the need for early liver transplantation. Utilization of FO therapy in this population may prevent liver failure and allow practitioners to bridge these children from PN-dependence to full enteral feeding as the small bowel grows and adapts.
In some children, advancement to full enteral feeding may require years of intestinal rehabilitation and small bowel transplantation is discussed as an option to achieve independence from PN. Children with cirrhosis due to PNALD undergoing small bowel transplant evaluation may be considered for combined liver-small bowel or multivisceral grafts, depending on the extent of their liver disease 41. The practice of considering children with cirrhosis due to PNALD for a liver graft, despite resolution of cholestasis and without signs of ESLD, stems from concern regarding the progression of hepatic fibrosis and cirrhosis, particularly in children who may continue to require PN. However, there are limited data to support this practice. Soden et al. reported experience with two children with intestinal failure and PNALD. The children had DB levels of 5.7 and 9.5 md/dL and both had bridging fibrosis at initial biopsy examination, while receiving parenteral SO. FO therapy was not initiated for 3 months in one infant and for 6 months in the other infant, with DB levels rising in the interim from 5.7 to 12.8 and 9.5 to 14.2 mg/dL. Repeat biopsies were obtained 3 months after initiation of FO in one infant and after 11 months in the other infant, with both biopsies demonstrating progression of fibrosis 22. Given the small sample size and lack of biopsies at the time of FO initiation, it is difficult to attribute the progression of fibrosis to the period after FO therapy. Both children experienced a delay in therapy compared to the protocols used widely today, where children are switched from SO to FO when DB levels sustain at greater than 2 mg/dL.
A more contemporary study by Mercer et al. reported outcomes in six children with intestinal failure and PNALD, focusing on children who had multiple liver biopsies. DB levels ranged from 2.9 to 6.2 mg/dL at the time of FO initiation. Of the six children, 2 children had no progression of fibrosis, 3 had progression of fibrosis, and one demonstrated regression of fibrosis over a follow-up period of 27 to 88 weeks from initial biopsy 23. Again, sample size was small and the findings subject to selection bias toward children with more severe liver disease requiring multiple liver biopsies, presumably to aid in management decisions. Despite these histologic findings, all 6 children had preserved liver function, no signs of portal hypertension, and most were making progress with advancement of enteral feeds 23.
These two case series are the only available studies evaluating the progression of liver disease in children after FO therapy and their limitations make it difficult to draw generalizable conclusions. However, both studies support the observation that normalization of cholestasis may not be a reliable marker of the extent of underlying hepatic fibrosis. Fitzgibbons et al. reported similar findings in a report of 83 liver biopsies in 66 children with PN-dependence due to intestinal failure. Fibrosis, of any amount, was observed in 89% of children, with 55% of these biopsies obtained in children without biochemical evidence of cholestasis. Cirrhosis was observed in 9.6% children, with 37% showing no biochemical evidence of cholestasis 42.
For these reasons, the present study focused on non-cholestatic markers of liver disease progression to better describe the status of cirrhosis from PNALD in children after resolution of cholestasis with FO therapy. The PELD score was evaluated as it is in widespread use as a prognostic factor for liver disease and tool for liver graft allocation in children awaiting transplantation. Like the score utilized for adults, the PELD score takes cholestasis and hepatic synthetic function into consideration, but also accounts for the increased mortality associated with age less than 1 year and the presence of growth failure in children with ESLD 43. In the present study, PELD scores normalized from a mean of 16 ± 4.6 at initiation of FO to −1.2 ± 4.6, 12 months after resolution of cholestasis. Even in the population of children who remained PN-dependent, the PELD score was never beyond the normal range up to 54 months after resolution of cholestasis. Similar trends were observed for the APRI, AST, ALT, and albumin in this high-risk cohort. None of the children required medications for the management of ascites, varices, or encephalopathy throughout the 1- to 9-year follow-up period. These data suggest that cirrhosis from PNALD after resolution of cholestasis with FO therapy may be more stable than previously thought, without clinical evidence of disease progression.
The biochemical effects of parenteral FO on fibrosis progression and cirrhosis from PNALD have not been studied. However, studies of chronic Hepatitis C, alcoholism, heavy metal toxicity, and non-alcoholic steatohepatitis, have demonstrated that fibrosis and cirrhosis are in fact reversible processes and that removal of the source of injury may be sufficient to arrest or reverse fibrogenesis within the liver 44. The role of parenteral SO as a major source of hepatic injury in the development of PNALD is now well established. Specifically, SO contains plant-based steroids, or phytosterols, that are normally absorbed in limited quantities from the gut, but achieve high levels when given intravenously 45. Children receiving parenteral SO demonstrate high levels of serum phytosterols, with higher levels correlating with more severe cholestasis 9–12. Furthermore, piglets given parenteral phytosterols in isolation of other components of PN develop cholestasis similar to that seen in PNALD 46. The mechanism by which phytosterols may promote cholestasis and trigger hepatic inflammation may be through antagonism of the Farsenoid X Receptor (FXR), thereby preventing inhibition of 7-alpha hydroxylase’s role in bile acid synthesis 47.
The absence of phytosterols in parenteral FO may be the basis for its reduced toxicity in the liver. Furthermore, the anti-inflammatory and anti-oxidant effects of the omega-3 fatty acids and tochopherols (Vitamin E) abundant in FO may also have hepatoprotective effects 13,48. Considering these biochemical mechanisms, substitution of SO with FO may stabilize fibrogenesis by removing the source of hepatic injury. Given enough time, it is possible that children with cirrhosis from PNALD will display regression of fibrosis.
The results of this study suggest that children with cirrhosis from PNALD who experience resolution of cholestasis with FO therapy have preserved liver function and do not require liver transplantation in the long-term. This is of critical importance for practitioners considering the inclusion of liver grafts when planning for small bowel transplantation, because outcomes are worse for combined liver-small bowel transplantation than for isolated small bowel transplantation. Dopazo et al. recently reported a prospective analysis of 51 children with intestinal failure who underwent transplantation, 39 of whom received combined liver and small bowel grafts. Mortality at 6 months after transplantation was 19%, all in the combined liver-small bowel transplantation group 49. Similarly, Grant et al. reported increased mortality one year after combined liver/small bowel transplantation than isolated small bowel (77% versus 60%, p <0.001) 50. Sparing the native liver whenever possible is therefore a priority and approaches to prevent the need for liver transplantation are of paramount importance.
While the presented data suggest that liver transplantation may not be necessary in children with cirrhosis from PNALD, there are important limitations to this study that should be considered. Outcomes are based entirely on non-invasive markers using serum studies, measures of growth, need for medication, and validated risk-assessment scores. Biopsies were only used in the diagnosis of cirrhosis and were rarely performed thereafter, in accordance with the American Association for the Study of Liver Disease’s recommendations for indications for liver biopsy 51. In addition, this is a single-institution study subject to biases related to the specific management protocols used at BCH. However, BCH has the longest and largest experience with the use of FO therapy for the treatment of PNALD and has openly shared its protocol for the FDA’s Investigational New Drug application with all inquiring institutions. Lastly, the majority of the analysis was performed on data from 12 months after the resolution of biochemical cholestasis. However, data are described for children who have remained PN-dependent for up to 9 years for the small portion of patients who started FO therapy when it was introduced in 2004. The conclusions can only be interpreted within this time frame and longer follow-up is needed to confirm that these children continue to do well.
With the advent of FO therapy, children with cirrhosis from PNALD are now entering childhood, many with persistent PN-dependence. The presented work suggests that children who experience resolution of cholestasis with FO therapy have preserved liver function in the long-term and may not require liver transplantation. Instead, these children should be followed closely with continued monitoring of growth and serum markers of hepatic function and injury, with biopsies reserved for major management decisions. Normalization of hyperbilirubinemia, while encouraging, may not be a reliable marker of the extent of underlying hepatic fibrosis. Therefore, practitioners may gain additional insight by calculating and trending PELD scores to identify patients with progressive disease. Continued long-term follow-up of children with cirrhosis from PNALD after treatment with FO is necessary and will assist in refining the management of PNALD for children today.
Acknowledgments
Funding Sources:
This study was funded by the Boston Children’s Hospital Surgical Foundation, Boston MA, National Institute of Health grants T32 DK007754-14 (MIC), T32 HD007466-17 (EC), F32 HD071715-01 (SJC), and the Joshua Ryan Rappaport Fellowship (PN). The funders did not participate in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Financial Disclosure:
A license agreement for the use of Omegaven® has been signed by Boston Children’s Hospital and Fresenius Kabi and a patent has been submitted by Boston Children’s Hospital on behalf of Mark Puder and Kathleen Gura.
References
- 1.Freund HR. Abnormalities of liver function and hepatic damage associated with total parenteral nutrition. Nutrition. 1991;7:1–6. [PubMed] [Google Scholar]
- 2.Mullick FG, Moran CA, Ishak KG. Total parenteral nutrition: a histopathologic analysis of the liver changes in 20 children. Mod pathol. 1994;7:190–4. [PubMed] [Google Scholar]
- 3.Touloukian RJ, Seashore JH. Hepatic secretory obstruction with total parenteral nutrition in the infant. J Pediatr Surg. 1975;10:349–352. doi: 10.1016/0022-3468(75)90098-6. [DOI] [PubMed] [Google Scholar]
- 4.Cohen C, Olsen MM. Pediatric total parenteral nutrition. Liver histopathology. Arch of pathol & lab med. 1981;105:152–6. [PubMed] [Google Scholar]
- 5.Zambrano E, et al. Total parenteral nutrition induced liver pathology: an autopsy series of 24 newborn cases. Pediatr devel pathol. 2004;7:425–32. doi: 10.1007/s10024-001-0154-7. [DOI] [PubMed] [Google Scholar]
- 6.Kelly DA. Intestinal failure-associated liver disease: what do we know today? Gastroenterology. 2006;130:S70–7. doi: 10.1053/j.gastro.2005.10.066. [DOI] [PubMed] [Google Scholar]
- 7.Chan S, et al. Incidence, prognosis, and etiology of end-stage liver disease in patients receiving home total parenteral nutrition. Surgery. 1999;126:28–34. doi: 10.1067/msy.1999.98925. [DOI] [PubMed] [Google Scholar]
- 8.Squires RH, et al. Natural history of pediatric intestinal failure: initial report from the Pediatric Intestinal Failure Consortium. The J of pediatr. 2012;161:723–8. e2. doi: 10.1016/j.jpeds.2012.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clayton PT, Whitfield P, Iyer K. The role of phytosterols in the pathogenesis of liver complications of pediatric parenteral nutrition. Nutrition. 1998;14:158–64. doi: 10.1016/s0899-9007(97)00233-5. [DOI] [PubMed] [Google Scholar]
- 10.Ellegård L, Sunesson A, Bosaeus I. High serum phytosterol levels in short bowel patients on parenteral nutrition support. Clin nutr. 2005;24:415–20. doi: 10.1016/j.clnu.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 11.Kurvinen A, et al. Effects of long-term parenteral nutrition on serum lipids, plant sterols, cholesterol metabolism, and liver histology in pediatric intestinal failure. J pediatr gastroenterol and nutr. 2011;53:440–6. doi: 10.1097/MPG.0b013e3182212130. [DOI] [PubMed] [Google Scholar]
- 12.Llop JM, et al. Phytosterolemia in parenteral nutrition patients: implications for liver disease development. Nutrition. 2008;24:1145–52. doi: 10.1016/j.nut.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 13.Calder PC. Immunomodulation by omega-3 fatty acids. Prostagland, leukotr, and essent fatty acids. 2007;77:327–35. doi: 10.1016/j.plefa.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 14.Chen WY, et al. Beneficial effect of docosahexaenoic acid on cholestatic liver injury in rats. J nutrit biochem. 2012;23:252–64. doi: 10.1016/j.jnutbio.2010.11.022. [DOI] [PubMed] [Google Scholar]
- 15.Sanchez SE, et al. The effect of lipid restriction on the prevention of parenteral nutrition-associated cholestasis in surgical infants. J pediatr surg. 2013;48:573–8. doi: 10.1016/j.jpedsurg.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nehra D, et al. Provision of a Soy-Based Intravenous Lipid Emulsion at 1 g/kg/d Does Not Prevent Cholestasis in Neonates. JPEN. 2012;37:498–505. doi: 10.1177/0148607112453072. [DOI] [PubMed] [Google Scholar]
- 17.Gura KM, et al. Safety and efficacy of a fish-oil-based fat emulsion in the treatment of parenteral nutrition-associated liver disease. Pediatrics. 2008;121:e678–86. doi: 10.1542/peds.2007-2248. [DOI] [PubMed] [Google Scholar]
- 18.Puder M, et al. Parenteral fish oil improves outcomes in patients with parenteral nutrition-associated liver injury. Ann surg. 2009;250:395–402. doi: 10.1097/SLA.0b013e3181b36657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chung PHY, et al. Clinical experience in managing pediatric patients with ultra-short bowel syndrome using omega-3 fatty acid. Euro j pediatr surg. 2010;20:139–42. doi: 10.1055/s-0029-1238283. [DOI] [PubMed] [Google Scholar]
- 20.Sant’Anna AMGA, et al. Implementation of a multidisciplinary team approach and fish oil emulsion administration in the management of infants with short bowel syndrome and parenteral nutrition-associated liver disease. Canad j gastroenter. 2012;26:277–80. doi: 10.1155/2012/571829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Premkumar MH, et al. High Rates of Resolution of Cholestasis in Parenteral Nutrition-Associated Liver Disease with Fish Oil-Based Lipid Emulsion Monotherapy. J pediatr. 2012;162:793–798. doi: 10.1016/j.jpeds.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 22.Soden JS, et al. Failure of resolution of portal fibrosis during omega-3 fatty acid lipid emulsion therapy in two patients with irreversible intestinal failure. J pediatr. 2010;156:327–31. doi: 10.1016/j.jpeds.2009.08.033. [DOI] [PubMed] [Google Scholar]
- 23.Mercer DF, et al. Hepatic fibrosis persists and progresses despite biochemical improvement in children treated with intravenous fish oil emulsion. J pediatr gastroenterol nutr. 2013;56:364–9. doi: 10.1097/MPG.0b013e31827e208c. [DOI] [PubMed] [Google Scholar]
- 24.Moss RL, Das JB, Raffensperger JG. Total parenteral nutrition-associated cholestasis: Clinical and histopathologic correlation. J Pediatr Surg. 1993;28:1270–1275. doi: 10.1016/s0022-3468(05)80311-2. [DOI] [PubMed] [Google Scholar]
- 25.Rodgers BM, Hollenbeck JI, Donnelly WH, Talbert JL. Intrahepatic cholestasis with parenteral alimentation. Am J Surg. 1975;131:149–155. doi: 10.1016/0002-9610(76)90088-x. [DOI] [PubMed] [Google Scholar]
- 26.Dahms BB, Halpin TC. Serial liver biopsies in parenteral nutrition-associated cholestasis of early infancy. Gastroenterology. 1981;81:136–44. [PubMed] [Google Scholar]
- 27.Yang CFJ, et al. Persistent alanine aminotransferase elevations in children with parenteral nutrition-associated liver disease. J pediatr surg. 2009;44:1084–8. doi: 10.1016/j.jpedsurg.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mirtallo J, et al. Safe practices for parenteral nutrition. JPEN. 2004;28:S39–70. doi: 10.1177/0148607104028006s39. [DOI] [PubMed] [Google Scholar]
- 29.Garcia-Tsao G, et al. Now there are many (stages) where before there was one: In search of a pathophysiological classification of cirrhosis. Hepatology. 2010;51:1445–9. doi: 10.1002/hep.23478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goyal N, et al. Non-invasive evaluation of liver cirrhosis using ultrasound. Clinical radiology. 2009;64:1056–66. doi: 10.1016/j.crad.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 31.Sanchez MC, D’Agostino DE. Pediatric end-stage liver disease score in acute liver failure to assess poor prognosis. J pediatr gastroenterol nutr. 2012;54:193–6. doi: 10.1097/MPG.0b013e3182349a04. [DOI] [PubMed] [Google Scholar]
- 32.Mangus RS, et al. Use of the aspartate aminotransferase to platelet ratio index to follow liver fibrosis progression in infants with short gut. J pediatr surg. 2010;45:1266–73. doi: 10.1016/j.jpedsurg.2010.02.098. [DOI] [PubMed] [Google Scholar]
- 33.Zeger SL, Liang KY. An overview of methods for the analysis of longitudinal data. Stat med. 1992;11:1825–39. doi: 10.1002/sim.4780111406. [DOI] [PubMed] [Google Scholar]
- 34.Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988;44:1049–60. [PubMed] [Google Scholar]
- 35.Murray KF, Carithers RL. AASLD practice guidelines: Evaluation of the patient for liver transplantation. Hepatology. 2005;41:1407–32. doi: 10.1002/hep.20704. [DOI] [PubMed] [Google Scholar]
- 36.Loaeza-del-Castillo A, et al. AST to platelet ratio index (APRI) for the noninvasive evaluation of liver fibrosis. Ann hepatol. 2008;7:350–7. [PubMed] [Google Scholar]
- 37.O’Connor M, et al. Utility of liver function tests including aminotransferase-to-platelet ratio index in monitoring liver dysfunction in short-gut infants of varying ages and intestinal lengths. J pediatr surg. 2011;46:1057–63. doi: 10.1016/j.jpedsurg.2011.03.029. [DOI] [PubMed] [Google Scholar]
- 38.Seyama Y, Kokudo N. Assessment of liver function for safe hepatic resection. Hepatol res. 2009;39:107–16. doi: 10.1111/j.1872-034X.2008.00441.x. [DOI] [PubMed] [Google Scholar]
- 39.Parsons HG, George MA, Innis SM. Growth assessment in clinical practice: whose growth curve? Curr gastroenterol rep. 2011;13:286–92. doi: 10.1007/s11894-011-0187-7. [DOI] [PubMed] [Google Scholar]
- 40.Kaufman SS, et al. Predicting liver failure in parenteral nutrition-dependent short bowel syndrome of infancy. J pediatr. 2010;156:580–5. e1. doi: 10.1016/j.jpeds.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 41.Nayyar N, et al. Pediatric small bowel transplantation. Sem pediatr surg. 2010;19:68–77. doi: 10.1053/j.sempedsurg.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 42.Fitzgibbons SC, et al. Relationship between biopsy-proven parenteralnutrition-associated liver fibrosis and biochemical cholestasis in children with short bowel syndrome. J pediatr surg. 2010;45:95–9. doi: 10.1016/j.jpedsurg.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McDiarmid SV, Anand R, Lindblad AS. Development of a pediatric end-stage liver disease score to predict poor outcome in children awaiting liver transplantation. Transplantation. 2002;74:173–81. doi: 10.1097/00007890-200207270-00006. [DOI] [PubMed] [Google Scholar]
- 44.Bataller R, Brenner DA. Liver fibrosis. J clin invest. 2005;115:209–18. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salen G, Ahrens EH, Grundy SM. Metabolism of beta-sitosterol in man. J clin invest. 1970;49:952–67. doi: 10.1172/JCI106315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iyer KR, Spitz L, Clayton P. BAPS prize lecture: New insight into mechanisms of parenteral nutrition-associated cholestasis: role of plant sterols. British Association of Paediatric Surgeons. J pediatr surg. 1998;33:1–6. doi: 10.1016/s0022-3468(98)90349-9. [DOI] [PubMed] [Google Scholar]
- 47.Carter BA, et al. Stigmasterol, a soy lipid-derived phytosterol, is an antagonist of the bile acid nuclear receptor FXR. Pediatr res. 2007;62:301–6. doi: 10.1203/PDR.0b013e3181256492. [DOI] [PubMed] [Google Scholar]
- 48.Chung MY, et al. Dietary α- and γ-tocopherol supplementation attenuates lipopolysaccharide-induced oxidative stress and inflammatory-related responses in an obese mouse model of nonalcoholic steatohepatitis. J nutr biochem. 2010;21:1200–6. doi: 10.1016/j.jnutbio.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 49.Dopazo C, et al. Combined liver-intestine grafts compared with isolated intestinal transplantation in children: a single-center experience. Transplantation. 2012;94:859–65. doi: 10.1097/TP.0b013e318265c508. [DOI] [PubMed] [Google Scholar]
- 50.Grant D, et al. 2003 report of the intestine transplant registry: a new era has dawned. Ann surg. 2005;241:607–13. doi: 10.1097/01.sla.0000157265.85388.a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rockey DC, et al. AASLD Position Paper: Liver Biopsy. Hepatology. 2009;49:1017–1044. doi: 10.1002/hep.22742. [DOI] [PubMed] [Google Scholar]