Abstract
Objective: To investigate the ability of source image of time-of-flight magnetic resonance angiography (TOF-MRA) in the detection of fibrous cap rupture of atherosclerotic carotid plaques.
Materials and Methods: From the database of radiological information in our hospital, 35 patients who underwent carotid MR imaging and subsequent carotid endoarterectomy within 2 weeks were included in this retrospective study. MR imaging included thin-slice time-of-flight MR angiography, black-blood T1- and T2-weighted imaging. Sensitivity, specificity and accuracy were calculated for the detection of fibrous cap rupture with source image of TOF-MRA. The Cohen k coefficient was also calculated to quantify the degree of concordance of source image of TOF-MRA with histopathological data.
Results: Sensitivity, specificity and accuracy in the detection of fibrous cap rupture were 90% (95%CI: 81–98), 69% (95%CI: 56–82) and 79% (95%CI: 71–87) with a k value of 0.59. The false positives (n = 15) were caused by partial-volume averaging between fibrous cap and lumen at the shoulder of carotid plaque. The false negatives (n = 5) were underestimated as partial thinning of fibrous cap.
Conclusion: Source image of TOF-MRA can be useful in the detection of fibrous cap rupture with high sensitivity, but further technical improvement should be necessary to overcome shortcomings causing image degradation.
Keywords: atherosclerotic plaque, carotid artery, MR imaging, fibrous cap, time of flight MR angiography
Introduction
The most common source of emboli in cerebral ischemia such as transient ischemic attacks and embolic stroke originates from atherosclerotic carotid plaque.1–3) It is important to detect unstable plaque for the purpose of preventing future cardiovascular events. There have been reported to be several key factors in advanced plaque that are likely to lead to cerebral ischemic disease: condition of the fibrous cap, size of necrotic core and hemorrhage, and extent of inflammatory activity within atherosclerotic carotid plaque.4–6) Progressive thinning of fibrous cap of carotid plaque can lead to plaque fissuring and ulceration. Acute thrombosis might occur and cause an occlusion of the arterial lumen prompted by the release of thrombogenic materials from the plaque.7)
Plaque rupture has been shown to occur regardless of the degree of luminal stenosis, and it is difficult to predict ischemic events with only the degree of narrowing of the carotid artery lumen.8,9) Thick fibrous caps are more likely to withstand pulsatile mechanical forces. In contrast, thin fibrous caps are vulnerable to fissuring or ulceration.10,11) Fibrous cap rupture is considered to be a predisposing factor to cause ischemic cerebral disease. Soft plaque components such as intraplaque hemorrhage and necrotic lipid core could worsen the degree of symptoms by massive ingredient outflows.7)
High-resolution magnetic resonance (MR) imaging has emerged as a potential technique for atherosclerotic plaque imaging.3,6,7,12–14) MR imaging provides tremendous information in evaluating plaque surface morphology such as ulceration and fibrous cap disruption as well as in measuring total plaque volume and identifying plaque components.3–7,10,13,15–18)
The purpose of this article is to investigate the ability of source image of time-of-flight MR angiography (TOF-MRA) in the detection of fibrous cap rupture of atherosclerotic carotid plaques.
Materials and Methods
Patient population
From the database of radiological information in our hospital, 44 symptomatic patients who underwent MR imaging of carotid arteries and subsequent carotid endoarterectomy within 2 weeks between June 2006 and April 2010 were nominated in this retrospective study. Excluded were 9 patients because of severe damage of the surgical specimen (n = 4) and motion artifact in TOF-MRA (n = 5). Thus, this retrospective study included 35 patients. The written informed consent from the patients was waived owing to the retrospective design of this study.
MR imaging
MR imaging was performed on a 1.5 Tesla super-conductive magnet system (Gyroscan ACS-Intera; Philips Medical systems, Best, the Netherlands). Each patient was positioned supine on the patient table. A pair of commercially available 8-cm circular surface coil was placed on each side of a patient’s neck centered over carotid bifurcation. After initial T1-weighted localizing images were obtained in sagittal, coronal and transaxial direction, MR angiography of carotid arteries was performed with fat-suppressed three-dimensional (3D) TOF sequence (TR/TE/FA: 40/1.8/20) in the transaxial direction. Other parameters included a 512 × 512 matrix, 1.4 mm section thickness, 0.7 mm section overlap, and 150 mm field of view yielding a voxel size of 0.29 × 0.29 × 0.7 mm3. A number of data sampling was two and the scan time was about 3 minutes 50 seconds.
In the characterization of carotid plaque, 3 transverse images with a section thickness of 3 mm were obtained using black-blood TSE sequences with a double inversion recovery technique.19) The parameters used are: fat-suppressed (FS) T1-(TR/TE: 750-1000/12), FS T2-weighted TSE (TR/TE: 1500-2000/ 80) images with ECG-triggering. Other imaging parameters were a 256 × 256 matrix, 3 mm section thickness, 150 mm field of view, yielding a voxel size of 0.6 × 0.6 × 3 mm3.
Histological evaluation
For each patient, a plaque resected on surgery served as the reference standard. A whole piece of carotid plaque was marked laterally and ventrally in the longitudinal direction and then fixed with formalin. It was then divided into 2-mm transverse contiguous slices, processed, embedded in paraffin, and those slices were subjected to hematoxylin-eosin, Masson-trichrome and Elastica-von-Gieson staining. The histologic slices were examined microscopically. Rupture of fibrous cap was determined by using Masson-trichrome and Elastica-von-Gieson staining. Major components such as lipid core, intraplaque hemorrhgae, fibrous tissue and calcification were also identified by the pathologists.
Assessment of plaque
Three imaging sections of TOF MRA source images matched with each section of FST1- and FST2-black blood images were selected in each patient and analyzed by two radiologists who jointly assessed all the sequences and expressed a consensus opinion on fibrous cap rupture as well as major plaque components.
Assuming that the fibrous cap is arranged in layers, it appears as a hypointense band of varying thickness in the TOF-MRA sequences. Fibrous cap covering carotid plaque was classified as thick, thin, disruption and unassessable. Lack of the hypointense band indicates that the cap has ruptured.
TOF-MRA imaging sections were then compared with the corresponding histological sections.
Data analysis
Histopathology was the standard of reference in the diagnosis of fibrous cap rupture. Sensitivity, specificity and accuracy were calculated for the detection of fibrous cap rupture with source image of TOF-MRA. The Cohen k coefficient was also calculated to quantify the degree of concordance of source image of TOF-MRA with the histopathological data. A k value >0.75 indicates good concordance; k between 0.40 and 0.75 indicates moderate concordance.20)
Results
Among the 105 sections of TOF-MRA source images in all the 35 patients, 8 sections proved not to be assessable because of complete occlusion (n = 3) and inappropriate slice direction (n = 5). Thus, 97 sections were assessed and compared with histopathology findings.
Out of the 97 sections, source image of TOF-MRA showed true positive and negative findings of disruption of fibrous cap in 44 and 33 sections, respectively (Figs. 1–3). The number of sections with false positive and negative results was 15 and 5, respectively. Sensitivity, specificity and accuracy in the detection of fibrous cap rupture were 90% (95%CI: 81–98), 69% (95%CI: 56–82) and 79% (95%CI: 71–87) with a k value of 0.59 (Table 1).
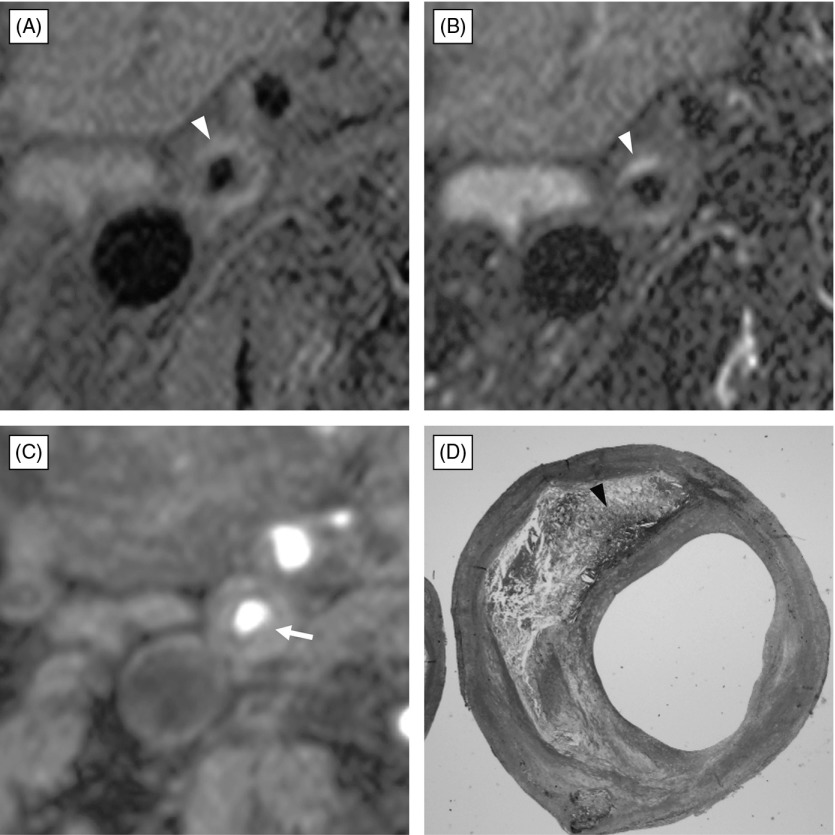
Fig. 1.
Atherosclerotic plaque composed of fibrous tissue and small amount of intraplaque hemorrhage with no fibrous cap rupture. (A) black-blood fat-suppressed T1-weighted image, (B) black-blood fat-suppressed T2-weighted image, (C) source image of time-of-flight magnetic resonance (TOF MR) angiogram, (D) histological section with Masson-Trichrome staining. Black-blood fat-suppressed T1-weighted image (A) and T2-weighted image (B) demonstrates carotid plaque composed of a large isointensity portion and small crescent-shape high intensity (arrowhead) relative to the submandibular gland. Source image of TOF MR angiography (C) shows moderate stenosis of the right carotid artery with thin hypointensity band of fibrous cap covering the plaque completely. Note partial thinning (arrow) on the fibrous plaque. Histological section with Masson-Trichrome staining (D) demonstrates fibrous cap covering the fibrous plaque with intraplaque hemorrhage (arrowhead), and no disruption is found.
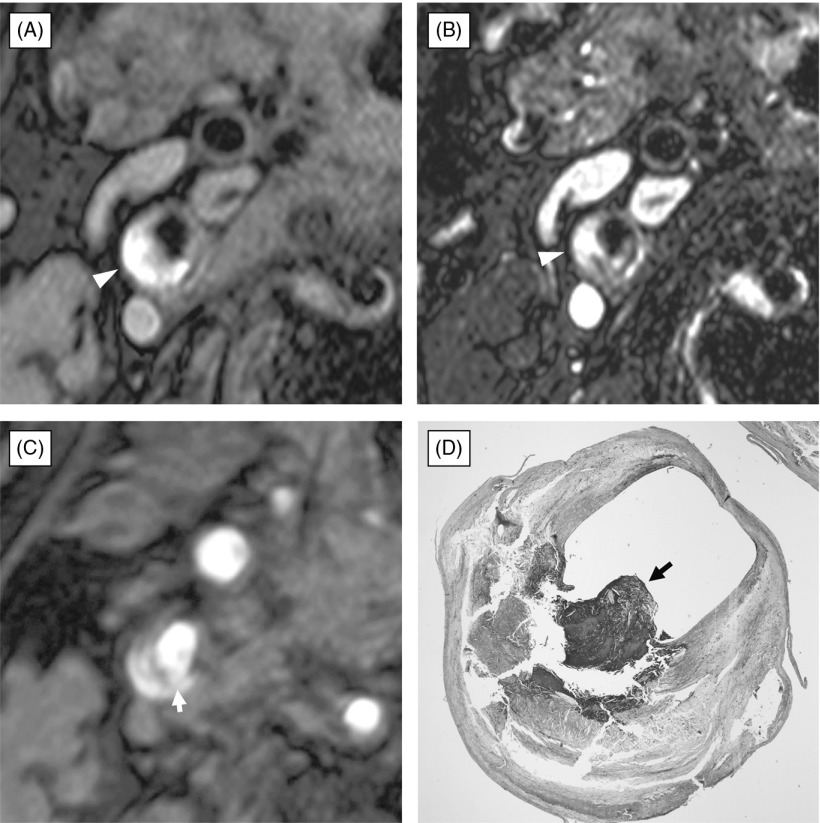
Fig. 3.
Ulcerated atherosclerotic plaque composed of lipid core and intraplaque hemorrhage with rupture of fibrous cap. (A) black-blood fat-suppressed T1-weighted image, (B) black-blood fat-suppressed T2-weighted image, (C) source image of time-of-flight magnetic resonance (TOF MR) angiogram, (D) histological section with Masson-Trichrome staining. The atherosclerotic carotid plaque demonstrates a large portion of high signal intensity on black-blood fat-suppressed T1-weighted image (A) and mixed high and intermediate signal intensity on black-blood fat-suppressed T2-weighted image (B). Source image of TOF MRA (C) shows disruption of thick fibrous cap (arrow) covering the hyperintense carotid plaque. Histopathological examination with Masson-Trichrome staining (D) clearly reveals that a plaque composed of lipid core and intraplaque hemorrhage has small ulceration with disruption of fibrous cap (arrow). Note protruded lumen (arrow) into hyperintense plaque on black-blood fat-suppressed T2-weighted image (B).
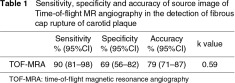
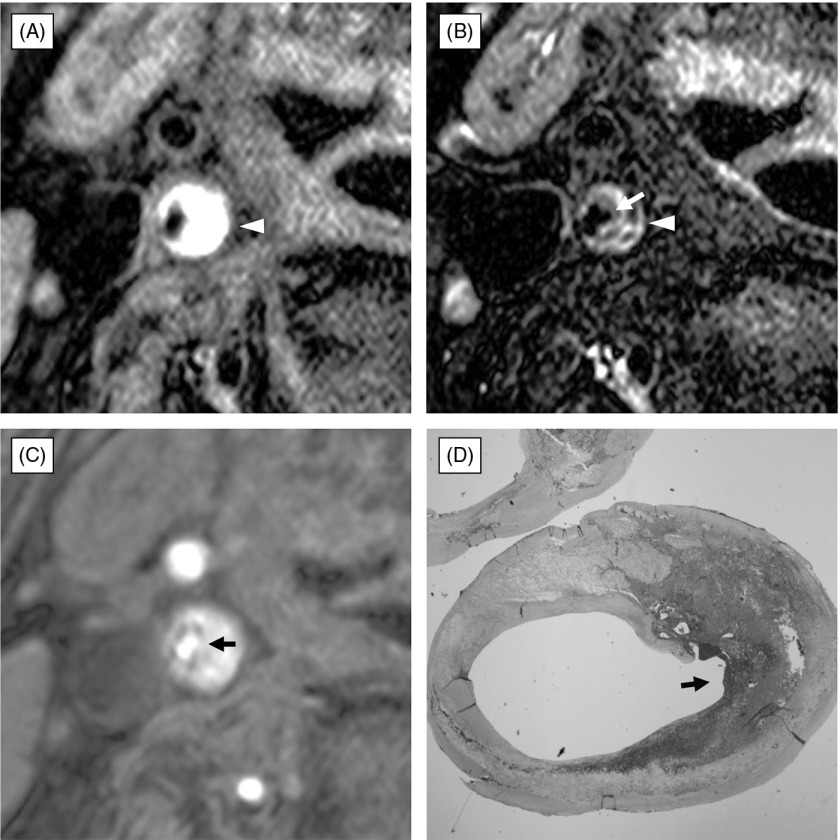
Fig. 2.
Rupture of thick fibrous cap covering atherosclerotic plaque composed of intraplaque hemorrhage. (A) black-blood fat-suppressed T1-weighted image, (B) black-blood fat-suppressed T2-weighted image, (C) source image of time-of-flight magnetic resonance (TOF MR) angiogram, (D) histological section with Masson-Trichrome staining. The atherosclerotic carotid plaque consists of a large portion of high signal intensity on black-blood fat-suppressed T1-weighted image (A) and mixed high and intermediate signal intensity on black-blood fat-suppressed T2-weighted image (B). Fibrous cap cannot be identified on the black-blood MR imaging. Source image of TOF MRA (C) demonstrates rupture of thick fibrous cap (arrow) lying on the hyperintense carotid plaque. Histopathological examination with Masson-Trichrome staining (D) clearly reveals disruption of fibrous cap (arrow) covering a large portion of intraplaque hemorrhage.
The false positives of source image of TOF-MRA (n = 15) were caused by partial-volume averaging between fibrous cap and lumen at the shoulder of carotid plaque. The false negatives (n = 5) were underestimated as partial thinning of fibrous cap.
Discussion
Fibrous cap was first reported as hypointense band or rim surrounding bright lumen on source image of three-dimensional (3D) TOF MR angiography by Hatsukami, et al.,21) and it was also demonstrated that high-resolution MRI with a 3D TOF protocol was capable of distinguishing an intact thick fibrous cap from an intact thin and/or ruptured fibrous cap.10,11,21) Hatsukami, et al.21) reported high sensitivity and specificity for the detection of fibrous cap rupture with source image of TOF-MRA. Our study also showed high sensitivity with a kappa value of 0.59, indicating moderate concordance between source image of TOF-MRA and histopathology findings in the evaluation of fibrous cap rupture.
However, there was considerable number of cases that could not be assessed for the condition of fibrous cap. As Pappin22) and Touze23) reported, a major problem can be low assessability of source image of TOF-MRA for the identification of fibrous cap. In our study, a major cause of failure in the identification of fibrous cap lying on the carotid plaque was image degradation due to motion artifact in obtaining TOF-MRA. Even respiratory and pulsatile movement could spoil image quality. Another is inappropriate slice direction resulting in partial volume averaging between hypointensity of fibrous cap and hyperintensity of lumen, which makes hypointensity band obscure. To overcome these disadvantages, TOF-MRA should be taken by using ECG-gating in multiangle transaxial directions against long axis of carotid arteries.
The specificity of 69% obtained in this study was relatively low, and most of false positives were found at the shoulder of carotid plaque. The section thickness of 1.4 mm used in this study might not be enough to avoid partial volume averaging between lumen and carotid plaque at the shoulder. The section thickness should be much thinner to minimize this unfavorable effect, though it may need longer acquisition time which lead to increase of motion artifact. False positives were also found with thin fibrous cap. The spatial resolution of this method was 290 um, which might give false positive results to thin fibrous cap.
Fibrous cap was reported to show hyperintensity in comparison to the underlying lipid-rich necrotic core on T2-weighted images by Trivedi, et al.10,24) However, intraplaque hemorrhage within lipid-rich necrotic core increases signal intensity and may obscure the boundary between fibrous cap and the underlying lipid-rich necrotic core on T2-weighted images.7) In our study, T2-weighted image could not identify the condition of fibrous cap, which seems that T2-weighted imaging may not be appropriate in the evaluation of fibrous cap rupture.
Plaque surface morphology on carotid angiography could be a sensitive marker of plaque instability.25) An appearance of ulceration, when found at carotid stenosis in a symptomatic patient, is a strong independent predictor of stroke.25,26) TOF-MR angiography can allow for accurate evaluation of plaque surface morphology at carotid stenosis by observing luminal stenosis from multi-direction view points. However, when carotid plaques including lipid core and/or intraplaque hemorrhage appears as hyperintensity on TOF-MRA, source image of TOF-MRA may be difficult to distinguish hyperintense plaque from protruded lumen. In such a case, black-blood T1- and T2-weighted imaging could be good to determine the presence or absence of plaque ulceration as shown in Fig. 3. This may suggest that simultaneous interpretation of source image of TOF-MRA with black-blood MR images can facilitate to evaluate the overall risk factors including plaque ulceration, fibrous cap rupture, underlying plaque components of lipid core and intraplaque hemorrhage, etc.7) Therefore, information concerning the condition of fibrous cap as well as the plaque composition should be included in the determination of treatment plan; carotid endarterectomy, carotid artery stenting, and medical treatment with lipid-lowering therapy.5,7,12,27–30)
In conclusion, the condition of fibrous cap can be assessed in source image of TOF-MRA with high sensitivity, but further technical improvement should be necessary in obtaining source image of TOF-MRA to overcome problems causing image degradation.
Acknowledgement
We thank our secretaries Ms. Hiroko Suyama and Ms. Rui Tamura for preparing this paper and figures, and also thank radiological technologists of MR division for technical support.
Disclosure Statement
The authors have no conflict of interest.
References
- North American Symptomatic Carotid Endarterectomy Trial Methods, patient characteristics, and progress. Stroke 1991; 22: 711-20 [DOI] [PubMed] [Google Scholar]
- Golledge J, Greenhalgh RM, Davies AH. The symptomatic carotid plaque. Stroke 2000; 31: 774-81 [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Nagayama M, Suga T, et al. Characterization of atherosclerotic plaque of carotid arteries with histopathological correlation: vascular wall MR imaging vs. color Doppler ultrasonography (US). J Magn Reson Imaging 2008; 28: 478-85 [DOI] [PubMed] [Google Scholar]
- Murphy RE, Moody AR, Morgan PS, et al. Prevalence of complicated carotid atheroma as detected by magnetic resonance direct thrombus imaging in patients with suspected carotid artery stenosis and previous acute cerebral ischemia. Circulation 2003; 107: 3053-8 [DOI] [PubMed] [Google Scholar]
- Ouhlous M, Flach HZ, de Weert TT, et al. Carotid plaque composition and cerebral infarction: MR imaging study. AJNR Am J Neuroradiol 2005; 26: 1044-9 [PMC free article] [PubMed] [Google Scholar]
- Yuan C, Zhang SX, Polissar NL, et al. Identification of fibrous cap rupture with magnetic resonance imaging is highly associated with recent transient ischemic attack or stroke. Circulation 2002; 105: 181-5 [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Nagayama M. MR plaque imaging of the carotid artery. Neuroradiology 2010; 52: 253-74 [DOI] [PubMed] [Google Scholar]
- Sadat U, Weerakkody RA, Bowden DJ, et al. Utility of high resolution MR imaging to assess carotid plaque morphology: a comparison of acute symptomatic, recently symptomatic and asymptomatic patients with carotid artery disease. Atherosclerosis 2009; 207: 434-9 [DOI] [PubMed] [Google Scholar]
- Wasserman BA, Wityk RJ, Trout HH, et al. Low-grade carotid stenosis: looking beyond the lumen with MRI. Stroke 2005; 36: 2504-13 [DOI] [PubMed] [Google Scholar]
- Cai J, Hatsukami TS, Ferguson MS, et al. In vivo quantitative measurement of intact fibrous cap and lipid-rich necrotic core size in atherosclerotic carotid plaque: comparison of high-resolution, contrast-enhanced magnetic resonance imaging and histology. Circulation 2005; 112: 3437-44 [DOI] [PubMed] [Google Scholar]
- Yuan C, Mitsumori LM, Beach KW, et al. Carotid atherosclerotic plaque: noninvasive MR characterization and identification of vulnerable lesions. Radiology 2001; 221: 285-99 [DOI] [PubMed] [Google Scholar]
- Corti R, Fayad ZA, Fuster V, et al. Effects of lipid-lowering by simvastatin on human atherosclerotic lesions: a longitudinal study by high-resolution, noninvasive magnetic resonance imaging. Circulation 2001; 104: 249-52 [DOI] [PubMed] [Google Scholar]
- Yuan C, Mitsumori LM, Ferguson MS, et al. In vivo accuracy of multispectral magnetic resonance imaging for identifying lipid-rich necrotic cores and intraplaque hemorrhage in advanced human carotid plaques. Circulation 2001; 104: 2051-6 [DOI] [PubMed] [Google Scholar]
- Yuan C, Tsuruda JS, Beach KN, et al. Techniques for high-resolution MR imaging of atherosclerotic plaque. J Magn Reson Imaging 1994; 4: 43-9 [DOI] [PubMed] [Google Scholar]
- Cappendijk VC, Kessels AG, Heeneman S, et al. Comparison of lipid-rich necrotic core size in symptomatic and asymptomatic carotid atherosclerotic plaque: initial results. J Magn Reson Imaging 2008; 27: 1356-61 [DOI] [PubMed] [Google Scholar]
- Chu B, Kampschulte A, Ferguson MS, et al. Hemorrhage in the atherosclerotic carotid plaque: a high-resolution MRI study. Stroke 2004; 35: 1079-84 [DOI] [PubMed] [Google Scholar]
- Keenan NG, Grasso A, Locca D, et al. Comparison of 2D and multislab 3D magnetic resonance techniques for measuring carotid wall volumes. J Magn Reson Imaging 2008; 28: 1476-82 [DOI] [PubMed] [Google Scholar]
- Saam T, Ferguson MS, Yarnykh VL, et al. Quantitative evaluation of carotid plaque composition by in vivo MRI. Arterioscler Thromb Vasc Biol 2005; 25: 234-9 [DOI] [PubMed] [Google Scholar]
- Fayad ZA, Fuster V. Characterization of atherosclerotic plaques by magnetic resonance imaging. Ann N Y Acad Sci 2000; 902: 173-86 [DOI] [PubMed] [Google Scholar]
- Medina LS. Study design and analysis in neuroradiology: a practical approach. AJNR Am J Neuroradiol 1999; 20: 1584-96 [PMC free article] [PubMed] [Google Scholar]
- Hatsukami TS, Ross R, Polissar NL, et al. Visualization of fibrous cap thickness and rupture in human atherosclerotic carotid plaque in vivo with high-resolution magnetic resonance imaging. Circulation 2000; 102: 959-64 [DOI] [PubMed] [Google Scholar]
- Puppini G, Furlan F, Cirota N, et al. Characterisation of carotid atherosclerotic plaque: comparison between magnetic resonance imaging and histology. Radiol Med 2006; 111: 921-30 [DOI] [PubMed] [Google Scholar]
- Touzé E, Toussaint JF, Coste J, et al. Reproducibility of high-resolution MRI for the identification and the quantification of carotid atherosclerotic plaque components: consequences for prognosis studies and therapeutic trials. Stroke 2007; 38: 1812-9 [DOI] [PubMed] [Google Scholar]
- Trivedi RA, Mallawarachi C, U-King-Im JM, et al. Identifying inflamed carotid plaques using in vivo USPIO-enhanced MR imaging to label plaque macrophages. Arterioscler Thromb Vasc Biol 2006; 26: 1601-6 [DOI] [PubMed] [Google Scholar]
- Lovett JK, Gallagher PJ, Hands LJ, et al. Histological correlates of carotid plaque surface morphology on lumen contrast imaging. Circulation 2004; 110: 2190-7 [DOI] [PubMed] [Google Scholar]
- Eliasziw M, Streifler JY, Fox AJ, et al. Significance of plaque ulceration in symptomatic patients with high-grade carotid stenosis. North American Symptomatic Carotid Endarterectomy Trial. Stroke 1994; 25: 304-8 [DOI] [PubMed] [Google Scholar]
- Drazen JM, Jarcho JA, Morrissey S, et al. Cholesterol lowering and ezetimibe. N Engl J Med 2008; 358: 1507-8 [DOI] [PubMed] [Google Scholar]
- Mani V, Muntner P, Gidding SS, et al. Cardiovascular magnetic resonance parameters of atherosclerotic plaque burden improve discrimination of prior major adverse cardiovascular events. J Cardiovasc Magn Reson 2009; 11: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perona F, Castellazzi G, Valvassori L, et al. Safety of unprotected carotid artery stent placement in symptomatic and asymptomatic patients: a retrospective analysis of 30-day combined adverse outcomes. Radiology 2009; 250: 178-83 [DOI] [PubMed] [Google Scholar]
- Saam T, Yuan C, Chu B, et al. Predictors of carotid atherosclerotic plaque progression as measured by noninvasive magnetic resonance imaging. Atherosclerosis 2007; 194: e34-42 [DOI] [PMC free article] [PubMed] [Google Scholar]