Abstract
Aortoiliac arterial steno-occlusions in young or middle-aged patients are relatively rare and have been reported in the literature as small aorta syndrome (SAS) or hypoplastic aortoiliac syndrome. We report the case of a 48-year-old Japanese woman with intermittent claudication caused by SAS. We performed left iliofemoral bypass grafting with a Dacron graft via a retroperitoneal approach. Bypass grafts, endarterectomy, and sympathectomy have been used for surgical management. Given that post-procedural event rates are higher for SAS than for other common atherosclerotic diseases, patients with SAS should be closely followed up after surgery.
Keywords: small aorta syndrome, hypoplastic aortoiliac syndrome, intermittent claudication
Introduction
Aortoiliac arterial steno-occlusions in young or middle- aged patients are relatively rare and have been reported in the literature as small aorta syndrome (SAS)1,2) or hypoplastic aortoiliac syndrome (HAIS).3,4) In Western countries, approximately 5%–16% of aortoiliac occlusive diseases are SAS;5,6) however, SAS is very rare in Asia.7) We report the case of a Japanese woman with intermittent claudication caused by occlusion of the left external iliac artery. We performed iliofemoral artery bypass grafting via a retroperitoneal approach.
Case Report
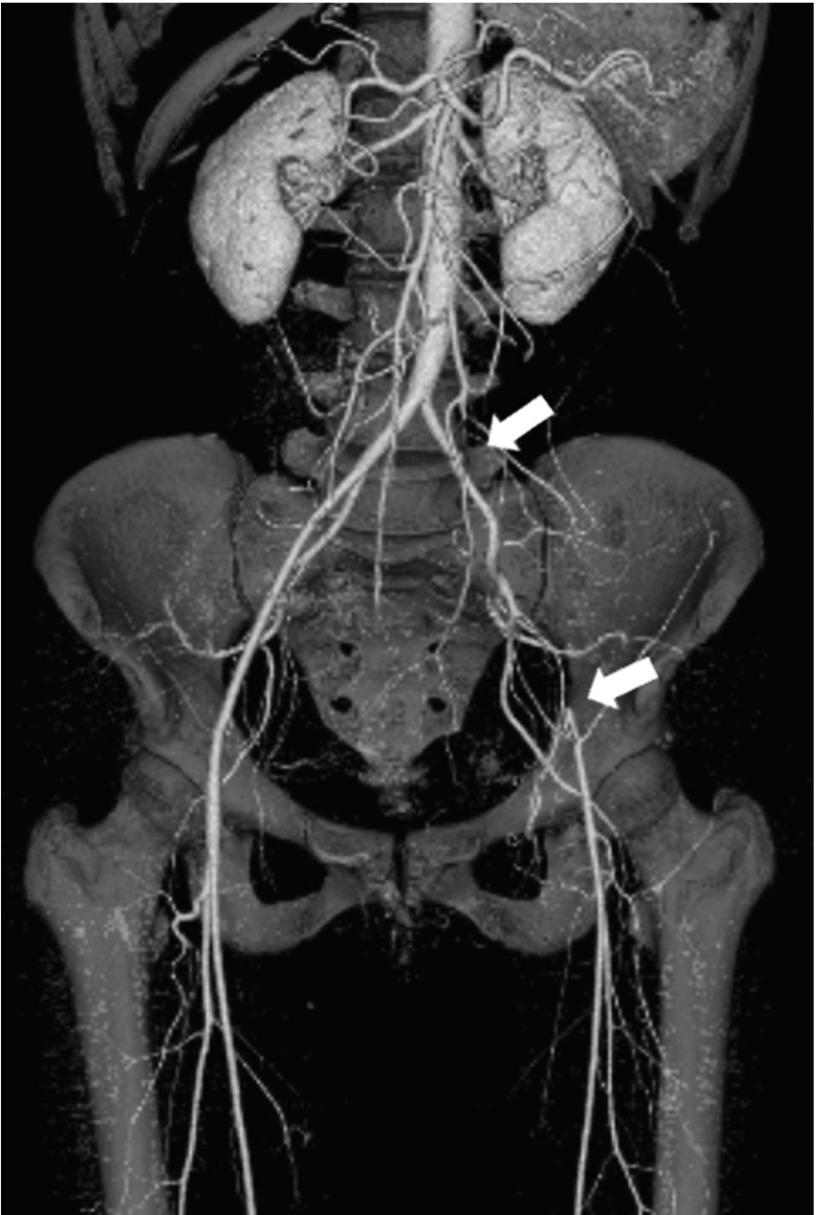
A 48-year-old woman was referred to our department with intermittent claudication in the left lower extremity since 1 year previously. The claudication distance in her left thigh and calf had shortened to 200 m, and ankle brachial pressure index (ABI) was 1.04 for the right leg and 0.59 for the left leg. Contrast-enhanced three-dimensional computed tomography (3D-CT) angiography revealed almost no atherosclerotic changes such as stenosis, kinking, or calcification from the abdominal aorta to both femoral arteries but revealed occlusion of the left external iliac artery (Fig. 1). Furthermore, the level of aortic bifurcation was high with a narrow angle. The diameter of the terminal aorta was 11.1 mm, left common iliac artery was 6.2 mm, and that of the left common femoral artery was 4.7 mm. Based on these findings, we diagnosed SAS.
Fig. 1.
Contrast-enhanced three-dimensional computed tomography angiography revealing no atherosclerotic change from the abdominal aorta to both femoral arteries but showing occlusion of the left external iliac artery. Furthermore, the level of aortic bifurcation is high with a narrow angle (White arrows indicate occlusion of the left external iliac artery).
The patient’s height was 166 cm and body weight was 59 kg (BMI 21.4). Her blood pressure was 126/72 mmHg on admission. She had a 28-year history of smoking (10 cigarettes/day) and had been treated for LDL-hypercholesterolemia with a statin for 9 months. Blood chemistry revealed a serum total cholesterol level of 246 mg/dL, low-density lipoprotein (LDL) cholesterol level of 171 mg/dL, and triglyceride level of 121 mg/dL.
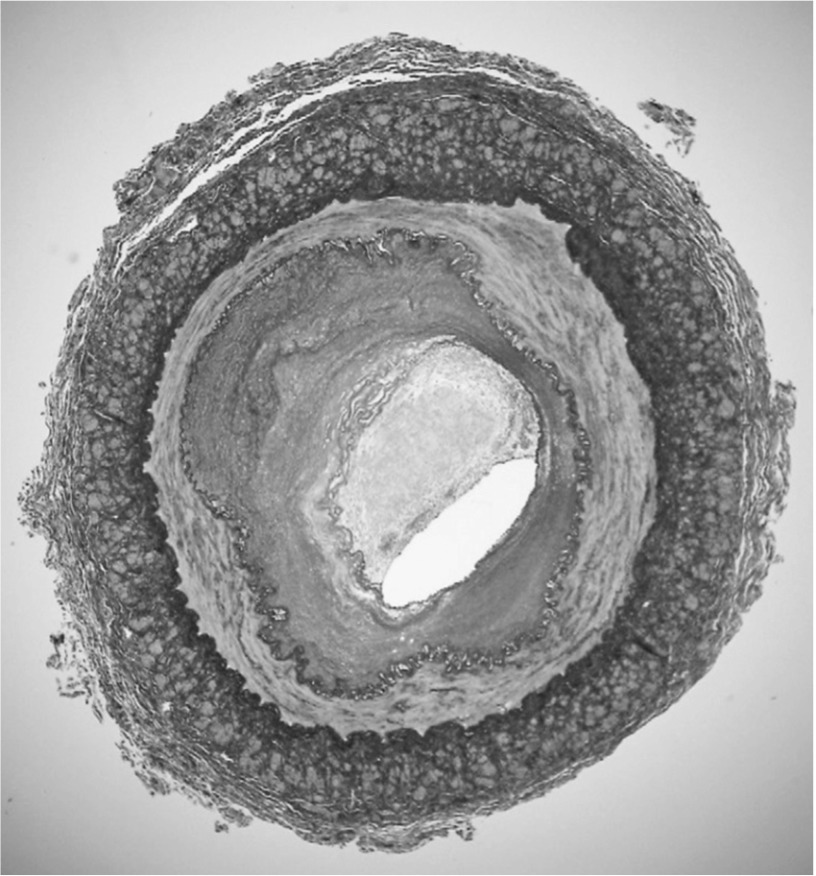
We performed left iliofemoral bypass grafting with a 6-mm Dacron graft via a retroperitoneal approach. The anastomosis of left common iliac artery and Dacron graft was performed with a 10-mm side-to-end anastomosis. The left common femoral artery was exposed, and anastomosis was performed using a Dacron graft in the left common femoral artery with a 10-mm end-to-side anastomosis. Furthermore, the proximal and distal edges of the external iliac artery (EIA) were ligated, and we harvested the EIA. The diameter of the artery from the left common iliac artery to the common femoral artery was small, and no atherosclerotic change was noted. The harvested EIA was occluded and had a diameter of 4 mm. Hematoxylin-eosin staining and elastica van Gieson staining showed the following: a thrombus-occluded internal vessel, thickened intima, disrupted and/or diminished elastic fibers of the media, and longitudinally arranged adventitial smooth muscle cells (Fig. 2). After surgery, the patient was administered clopidogrel (75 mg/day) and aspirin (100 mg/day) and has remained free of claudication since then. The ABI of the left limb 1 month after surgery was 0.96, and 3D-CT angiography at 6 months revealed graft patency.
Fig. 2.
Microscopic examination with elastica van Gieson staining: (1) thrombus-occluded internal vessel occlusion; (2) thickened intima; (3) disrupted and/or diminished elastic fiber of the media; (4) longitudinally arranged adventitial smooth muscle cells.
Discussion
SAS was first described by Quain in 1847 in women with aortoiliac hypoplasia. SAS has been reported in the literature from Western countries; however, similar reports from Asia are rare.7) Approximately 5%–16% of patients with aortoiliac occlusion are diagnosed with SAS.6,8)
Arteries of patients with aortoiliac occlusive disease (AIOD) have atherosclerotic changes such as stenosis, kinking, or calcification. However, arteries of patients with SAS have few atherosclerotic changes. Although SAS is diagnosed by the anatomic findings of the aorta or iliac arteries, its definition is arbitrary. Several of the following definitions are available in the literature: (1) aortic lumen diameter <12 mm just below the renal artery1,9) or <10.3 mm just above the aortic bifurcation;1) (2) aortic bifurcation level as high as the third to fourth lumbar vertebra with a narrow angle (20°–30°);3) (3) straight-shaped iliac artery;3) and (4) corresponding femoral artery only approximately 5 mm in diameter.1)
The etiology of SAS remains unclear; however, the literature suggests that it is associated with developmental defects,4,10–12) inflammation,13) infection,10,14) radiation,4) and atherosclerosis-induced shrinkage.4) Most patients with SAS have one or more risk factors for atherosclerosis, such as smoking, hypertension, and hypercholesterolemia.4,15) In this case, the patient had two risk factors: smoking and LDL-hypercholesterolemia. As SAS generally presents in the fourth or fifth decade of life, whether its pathogenesis is congenital or acquired remains controversial.6,12) However, several authors have suggested that SAS may result from a combination of congenital hypoplasia and aggressive atherosclerotic disease.4,6,12,16) To some extent, SAS has characteristics similar to those of Leriche syndrome, Takayasu arteritis, and coarctation of the descending thoracic and abdominal aorta.4,11) Certain risk factors distinguish SAS from these conditions, such as age <55 years, smoking history, female sex, and stenotic or occlusive lesions from the infrarenal aorta to the femoral artery.4)
SAS symptoms arise from stenotic or occlusive lesions. Intermittent claudication is common in patients with iliac arterial stenosis or occlusion, and hypertension is a common and severe symptom in patients with renal arterial stenosis.12) However, since stenosis of the renal artery is more common in other AIODs than in SAS, intermittent claudication is the most common symptom of SAS, as shown here in our patient.
Surgical revascularization has been described in four series comprising 60 patients. Bypass grafts, endarterectomy, and sympathectomy have been used for surgical management; however, aortofemoral bypass is the most commonly used grafting technique.4) The review of a study that included nine patients with hypoplastic infrarenal aorta who underwent surgical revascularization with a follow-up duration of 19 months revealed a repeated revascularization or claudication rate of 33%.17) Magnoni et al. suggested that the small diameter of the aorta usually requires anterior patch angioplasty at the time of grafting or thromboendarterectomy and that the primary patency rate at a mean of 4 years is 80%.18) Furthermore, Walton et al. reported 28 aortoiliac percutaneous interventions of HAIS and revealed that the post-procedural event rate was 28.6% over a mean follow-up period of 31 months. Therefore, they concluded that endovascular therapy for SAS with subtotal occlusion of the iliac arteries is a viable alternative to surgical revascularization.4) In any case, patients with SAS should be closely followed up after surgery since the long-term patency of revascularization is lower than that in patients with AIOD. These patients should take anti-platelet drugs and be monitored claudication, ABI, and CT angiography more often than patients with AIOD.
Although the specific histological features of SAS have rarely been reported, some studies have suggested from the clinical perspective that a combination of atherosclerotic changes induced by smoking and/or hypercholesterolemia and congenital hypoplasia may cause aortoiliac occlusion in SAS.4,6,12,16) In this case, histological analysis of the harvested external iliac artery with elastica van Gieson stain showed a partial atherosclerotic change observed as intimal thickening and a non-atherosclerotic change observed as the fragmentation and extinction of elastic fiber of the media and longitudinal arrangement of smooth muscle cells of the adventitia. These features of the medial elastic fibers are similar to those of aortic aneurysm; on the other hand, features of adventitial smooth muscle cells are apparently different from those of normal arteries, typical aortic aneurysms, and atherosclerosis. These findings suggest that SAS is mostly due to a congenital arterial malformation rather than atherosclerotic changes.
Conclusions
SAS is less common in Asia than in Western countries. Its standard therapy consists of surgical management. Given that post-procedural event rates are higher for SAS than for other common atherosclerotic diseases, patients with SAS should be closely followed up after surgery.
Disclosure Statement
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this manuscript and received no financial support for its research, authorship, and/or publication.
References
- Cronenwett JL, Davis JT, Gooch JB, et al. Aortoiliac occlusive disease in women. Surgery 1980; 88: 775-84 [PubMed] [Google Scholar]
- Ingegnoli F, Soldi A, Meani L, et al. Primary antiphospholipid syndrome associated with small aorta syndrome: a case report. Lupus 2006; 15: 236-9 [DOI] [PubMed] [Google Scholar]
- Jernigan WR, Fallat ME, Hatfield DR. Hypoplastic aortoiliac syndrome: an entity peculiar to women. Surgery 1983; 94: 752-7 [PubMed] [Google Scholar]
- Walton BL, Dougherty K, Mortazavi A, et al. Percutaneous intervention for the treatment of hypoplastic aortoiliac syndrome. Catheter Cardiovasc Interv 2003; 60: 329-34 [DOI] [PubMed] [Google Scholar]
- Rutherford RB. Vascular Surgery. 6th ed.Philadelphia: Saunders, 2005: 1120-1 [Google Scholar]
- DeLaurentis DA, Friedman P, Wolferth CC, et al. Atherosclerosis and the hypoplastic aortoiliac system. Surgery 1978; 83: 27-37 [PubMed] [Google Scholar]
- Ito M, Mishima Y. Small aorta syndrome. Surg Today 1993; 23: 256-9 [DOI] [PubMed] [Google Scholar]
- Palmaz JC, Carson SN, Hunter G, et al. Male hypoplastic infrarenal aorta and premature atherosclerosis. Surgery 1983; 94: 91-4 [PubMed] [Google Scholar]
- Burke PM, Herrmann JB, Cutler BS. Optimal grafting methods for the small abdominal aorta. J Cardiovasc Surg (Torino) 1987; 28: 420-6 [PubMed] [Google Scholar]
- Rossi MA. Infrarenal aortic coarctation and diffuse hypoplasia of the aortoiliac-femoral system. Acta Cardiol 1997; 52: 373-9 [PubMed] [Google Scholar]
- Celik T, Kursaklioglu H, Iyisoy A, et al. Hypoplasia of the descending thoracic and abdominal aorta: a case report and review of literature. J Thorac Imaging 2006; 21: 296-9 [DOI] [PubMed] [Google Scholar]
- Alpagut U, Ugurlucan M, Sayin OA, et al. Infrarenal aortic coarctation. Wien Med Wochenschr 2010; 160: 372-5 [DOI] [PubMed] [Google Scholar]
- Hada Y, Ikeda H, Oku J, et al. Diffuse hypoplasia of the aorta as a possible cause of cardiac hypertrophy. Clin Cardiol 1985; 8: 359-62 [DOI] [PubMed] [Google Scholar]
- Siassi B, Klyman G, Emmanouilides GC. Hypoplasia of the abdominal aorta associated with the rubella syndrome. Am J Dis Child 1970; 120: 476-9 [DOI] [PubMed] [Google Scholar]
- Bucci F, Fiengo L, Hamati S, et al. Abdominal aortic occlusion of young adults. Interact Cardiovasc Thorac Surg 2012; 14: 99-101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poskitt KR, Perkin GD, Greenhalgh RM. A relationship between claudication of the cauda equina and the small aorta syndrome. J Neurol Neurosurg Psychiatr 1985; 48: 75-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caes F, Cham B, Van den Brande P, et al. Small artery syndrome in women. Surg Gynecol Obstet 1985; 161: 165-70 [PubMed] [Google Scholar]
- Magnoni F, Pisano E, Cirelli M, et al. Abdominal aortic hypoplasia: clinical and technical considerations. Cardiovasc Surg 1994; 2: 760-2 [PubMed] [Google Scholar]