Focus on PAD patients with diabetes and chronic kidney disease
October 19th - 20th 2013 in Jeju, Korea
Co-chairs:
Day 1 – Dr. Moon Kyu Lee (Seoul, Korea) and
Dr. Hiroshi Shigematsu (Tokyo, Japan);
Day 2 – Dr. Bo Yang Suh (Daegu, Korea) and
Dr. Hiroshi Shigematsu (Tokyo, Japan)
The Asian PAD Workshop, the 5th in this series of medical education events, builds on the success of the previous Asian PAD Workshops (Jeju, 2009; Osaka, 2010; Gyeongju, 2011; Busan, 2012). This round of the workshop focused on the prevalence, diagnostic approaches and management of PAD, particularly those PAD patients with diabetes mellitus (DM) and chronic kidney disease (CKD). PAD has been progressively and sharply increasing in Asian countries and diabetes is one of the most critical factors in its onset. Unfortunately, the proportion of diabetic patients amongst PAD patients grows annually. The prevalence of the disease currently exceeds 40% in Japan, and other Asian countries have a similar prevalence. This increase of diabetic prevalence clearly contributes to the rise in the incidence of PAD. As it takes more than 10 years of diabetic history before the onset of PAD, we are faced with the spectra of increasing burden of PAD throughout Asia. In addition, renal dysfunction is one of the most critical comorbidity for the PAD patients with diabetes. Early PAD diagnosis also serves as a crucial “window” for other early stage diagnoses of possible cardiac and cerebral pathogenesis. The five distinguished speakers invited from Korea, Thailand, Indonesia and Japan provided an excellent opportunity to discuss the management and prevention of PAD with CKD induced by DM. In-depth discussions were held on various topics including early-stage diagnostic approaches and primary drug therapies such as beraprost sodium for PAD associated with diabetes and CKD. Raising awareness of PAD in order to encourage early diagnosis and intervention is mandatory to help prevent disease progression and achieve the best patient outcomes possible.
Day 1
Chairmen
Dr. Moon Kyu Lee
Professor, Endocrinology, Samsung Medical Center, Sungkyunkwan University School of medicine,
Seoul, Korea
Dr. Hiroshi, Shigematsu,
Professor of Vascular Surgery,
Director of Vascular Center, Sanno Medical Center, International University of Health and Welfare,
Tokyo, Japan
Progress of the Asian PAD Workshop
Dr. Hiroshi Shigematsu
In the 2011 focused update of the American College of Cardiology Foundation/American Heart Association Task Force (ACCF/AHA) guideline for the management of patients with PAD, it is recommended that the resting ankle brachial index (ABI) should be used to establish the lower extremity PAD diagnosis in patients with suspected lower extremity PAD, defined as individuals with one or more of the following: exertional leg symptoms, non-healing wounds, age ≥65 years or ≥50 years with a history of smoking or diabetes.1) ABI results should be uniformly reported with non-compressible values defined as >1.40, normal values 1.00 to 1.40, borderline 0.91 to 0.99 and abnormal ≤0.90. However, it is apparent that most borderline patients with an ABI of between 0.91 and 0.99, will be asymptomatic and will not visit medical doctor. The problem is therefore how to make a diagnosis in asymptomatic PAD patients.
To address this issue, we established a new organization in 2010 the Japanese Association for Cardiovascular Disease Prevention. A major initiative to make an early diagnosis of PAD was to establish a new nationwide trial to measure ABI for elderly people aged >65 years, diabetic patients and patients with smoking history. To date, around 20 or more institutes have joined this trial throughout Japan. Notably, we have measured the ABI of elderly people aged over 65 years, diabetic patients and patients with smoking history in order to make an early diagnosis of PAD on the same special national holiday, respect-for-the Aged Day. The results of the ABI measurement shows 3% of citizens have lower ABI below 0.9 and more than 6% of them belong to borderline, between 0.91 and 0.99, surprisingly. This movement is called “Prevent PAD - Green IVY movement“(see Fig. 1), and we aim to extend the Take ABI movement throughout Asian countries to help prevent PAD especially for patients with diabetes.
Fig. 1.
Tokyo tower was illuminated green on Aged Day in Japan
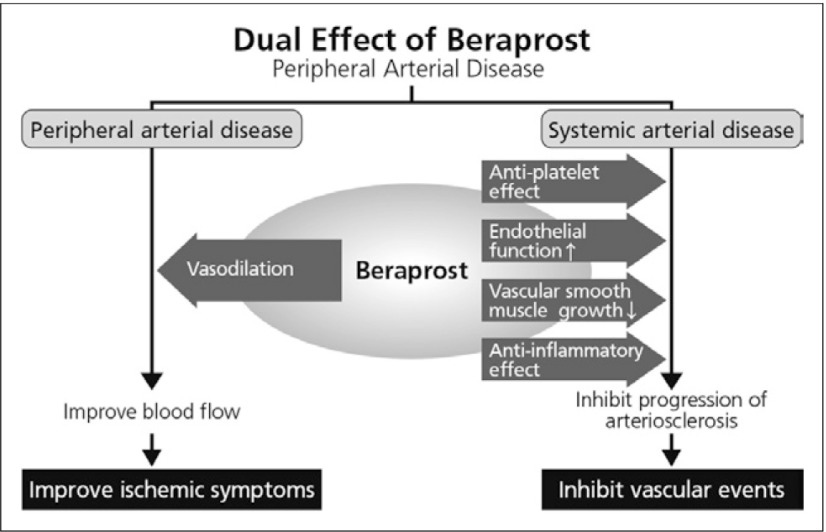
In the ACCF/AHA guideline recommendations, antiplatelet therapy is indicated to reduce the risk of MI, stroke, and vascular death in individuals with symptomatic atherosclerotic lower extremity PAD, with an evidence level A. Antiplatelet therapy can be useful to reduce the risk of MI, stroke, or vascular death in asymptomatic individuals with an ABI less than or equal to 0.90. But, at present, the usefulness of antiplatelet therapy to reduce the risk of MI, stroke, or vascular death in asymptomatic individuals with borderline abnormal ABI, defined as 0.91 to 0.99, is not well established. This therefore represents a new issue to be solved. It is useful to note that beraprost sodium is expected to have dual effects on improving ischemic symptoms due to PAD and on preventing CV events due to polyvascular disease (Fig. 2).
Fig. 2.
Dual effect of beraprost in PAD
Reference
- 1).2011 WRITING GROUP MEMBERS; 2005 WRITING COMMITTEE MEMBERS; ACCF/AHA TASK FORCE MEMBERS 2011 ACCF/AHA Focused Update of the Guideline for the Management of patients with peripheral artery disease (Updating the 2005 Guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2011; 124: 2020-45. [DOI] [PubMed] [Google Scholar]
Management of PAD patients with CKD in Thailand
Dr. Atiporn Ingsathit
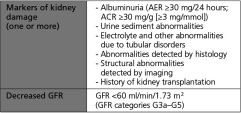
Chronic kidney disease is the primary cause of end-stage renal disease (ESRD) in the United States.1) A patient is diagnosed with CKD if either of the following are present for >3 months (based on the analyses of 45 study cohorts):
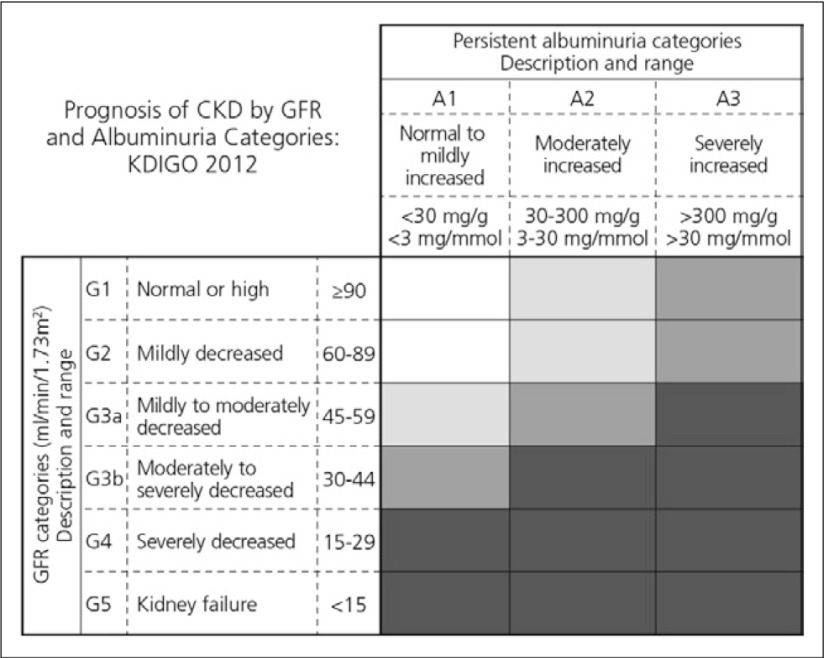
The definition and classification for CKD was proposed by the National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF-KDOQI) in 2002 and endorsed by Kidney Disease: Improving Global Outcomes (KDIGO) in 2004. KDIGO initiated a collaborative meta-analysis and sponsored a Controversies Conference in October 2009 to examine the relationship of estimated glomerular filtration rate (GFR) and albuminuria to mortality and kidney outcomes. Prognosis is based on clinical diagnosis, stage, and other key factors relevant to specific outcomes (Fig. 1). KDIGO has also convened a workgroup to develop a global clinical practice guideline for the definition, classification, and prognosis of CKD.2)
Fig. 1.
Prognosis of CKD by GFR and albuminuria categories (KIDGO 2012)2)
Prevalence of CKD
The population-based Thai Screening and Early Evaluation of Kidney Disease (SEEK) study revealed CKD prevalence in the Thai population to be much higher than previously known and published. As shown in Table 1, early stages of CKD appeared to be as common as later stages. However, albuminuria measurement was not confirmed and adjusting for persistent positive rates resulted in a prevalence of 14.4%. Awareness of CKD was also relatively low in the Thai population.3)
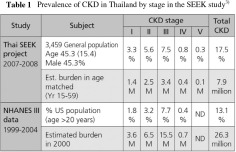
Keith et al. (2004) showed that the rate of renal replacement therapy over a 5-year observation period was 1.1%, 1.3%, and 19.9%, respectively, for the National Kidney Foundation Kidney Disease Outcomes Quality Initiative (K/DOQI) stages 2, 3, and 4, but that mortality rates were 19.5%, 24.3%, and 45.7%. Death was far more common than dialysis at all stages. Congestive heart failure, coronary artery disease, diabetes, and anaemia were also more prevalent in the patients who died but hypertension prevalence was similar across all stages.1)
Diagnosis and management strategies
PAD is the presence of significant narrowing of arteries distal to the arch of the aorta, most often due to atherosclerosis. Critical limb ischemia (CLI) is characterized by ischaemic pain at rest, ulcers or gangrene that can be directly attributed to arterial occlusive disease.4) According to the Fontaine’s classification, patients are defined as having CLI if they have chronic ischaemic pain at rest, ulcers or gangrene that can be directly attributed to arterial occlusive disease.
Ankle brachial index (ABI)
The ABI is calculated by dividing the higher ankle systolic blood pressure and the higher brachial systolic blood pressure. Diabetic patients undergoing dialysis have heavily calcified vessels, and so the arteries are frequently non-compressible. This results in an artificially elevated ankle pressure, which can cause misdiagnosis and underestimation of the severity of the disease.
An ABI of 1.00-1.30 is normal. However, a value of 0.91-0.99 is indicative of borderline PAD, whereas 0.41-0.90 and 0.00-0.40 is indicative of mild-to-moderate and severe PAD, respectively.5) According to the ACCF/AHA 2011 guidelines for PAD Recommendation for ABI, patients with suspected lower extremity PAD is defined as individuals with one or more followings:6)
Exertional leg symptoms
Non-healing wound
Age 65 years and older
Age 50 years and older with history of smoking or diabetes
Unfortunately, the diagnosis of PAD is often overlooked. Patients with subclavian artery stenosis can lead to lower central SBP and thus a high ABI. A Toe Brachial Index (TBI) is performed when the ABI is abnormally high due to plaque and calcification of the arteries in the leg; this is caused by atherosclerosis and is most often found in diabetic patients. Comparing the benefits and limitations of ABI and TBI as diagnostic tools (Table 2), TBI appears to offer certain benefits over ABI.
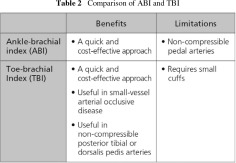
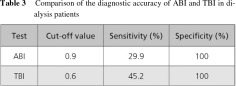
The diagnostic accuracy of ABI and TBI in dialysis patients is similar, although TBI is thought to be more sensitive (Table 3).7)
Prevalence of PAD in CKD patients
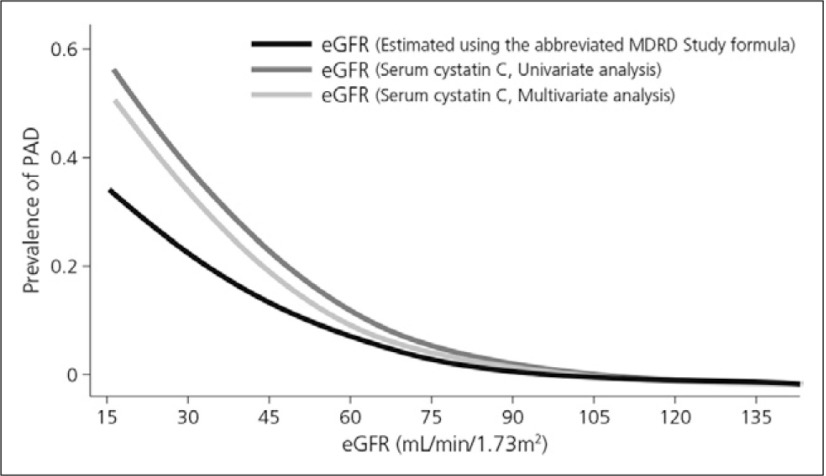
CKD is strongly and independently associated with the occurrence of PAD. For example, a cross-sectional study in 3,089 patients by Selvin et al. (2009) showed that the GFR estimated using cystatin C was more strongly associated with PAD compared with the estimated GFR (eGFR) using serum creatinine before and after multivariable adjustment (Fig. 2).8)
Fig. 2.
Association between eGFR levels and prevalence of PAD8)
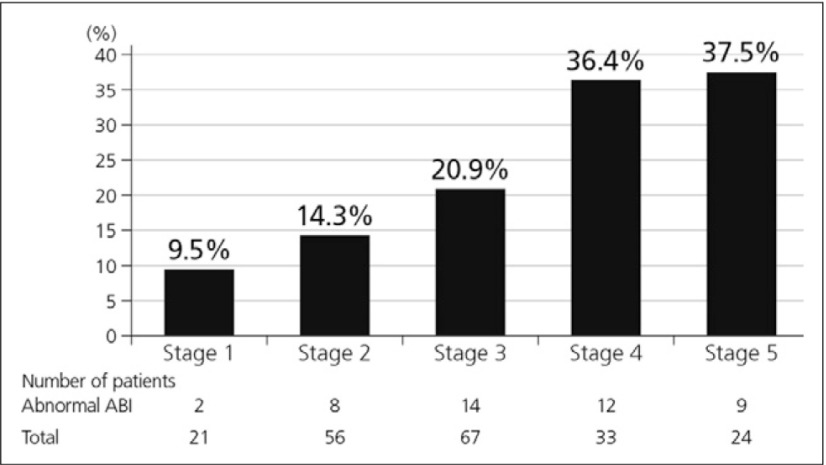
Furthermore, the prevalence of asymptomatic PAD increases with more advanced stage of CKD and diabetes and coronary artery disease are higher in patients with abnormal ABI (but with no statistical significance).9) Abnormal ABI was frequently seen in the patients with more advanced CKD and the mean ABI was lower in patients with more advanced CKD stage. The mean ABI of stage 4 and 5 CKD patients was lower than that of stage 1 and 2 (p<0.05) (Fig. 3).9)
Fig. 3.
Incremental correlation between prevalence of abnormal ABI and stage of CKD9)
Prevalence of PAD in dialysis patients
The value of ABI as a powerful and independent predictor for all-cause and cardiovascular mortality among haemodialysis patients was further established in a cohort of 1,010 Japanese patients undergoing chronic haemodialysis.10) The relative risk of a history of DM, cardiovascular, and cerebrovascular disease was significantly higher in patients with lower ABPI than those with ABPI ≥1.1 to <1.3. Even those with modest reductions in the ABI (≥0.9 to <1.1) appeared to be at increased risk for all-cause and cardiovascular mortality. Patients having abnormally high ABPI (≥1.3) also had poor prognosis.10)
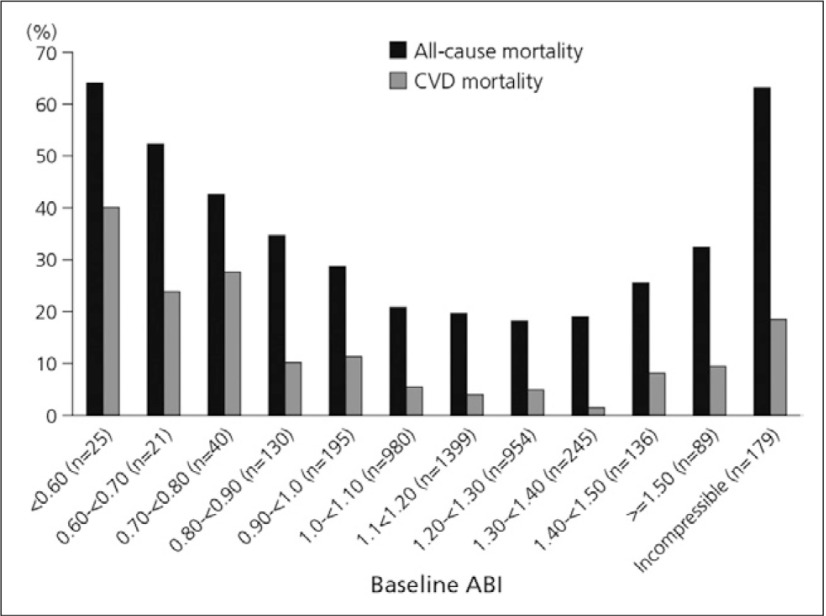
Overall, each decrement of 0.1 in the ABI is associated with a 10.2% increase in the relative risk of ischaemic stroke, nonfatal myocardial infarction or vascular death. However, the association between high ABI and mortality was found to be similar to that of low ABI and mortality, highlighting a U-shaped association between PAD and mortality risk (Fig. 4). These data suggest that the upper limit of normal ABI should not exceed 1.40.11)
Fig. 4.
Relationship of high and low ABI to all-cause and cardiovascular disease mortality in the Strong Heart Study11)
For patients with CKD and PAD, KDIGO 2012 recommends that adults with CKD be regularly examined for signs of PAD and be considered for therapy. KIDGO also suggest that adults with CKD and diabetes are offered regular podiatric assessment.12)
Management options for PAD
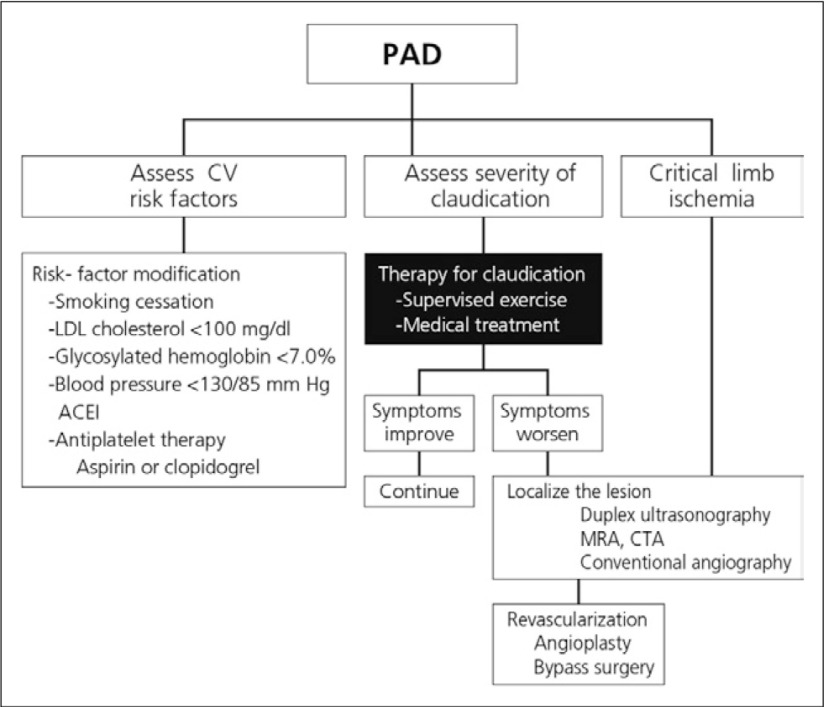
Exercise therapy for claudication includes the practice of exercise-rest-exercise, with patients advised to titrate to a speed that elicits claudication within 5 min follow by the brief period of rest. This should be done in 35 min sessions 3-5 times per week.13) Other lifestyle modifications and medical interventions for PAD are suggested in Fig. 5. It is noteworthy that both cilostazol and beraprost sodium are associated with beneficial PAD and cardiovascular outcomes.
Fig. 5.
Suggested patient management recommendations6)
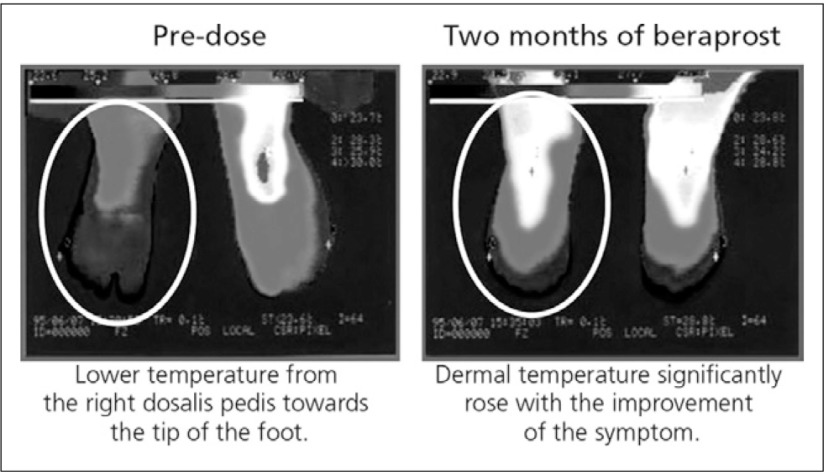
Beraprost is a stable, orally active PGI2 analogue with vasodilatory, antiplatelet, antiproliferative and cytoprotective effects.14) Beraprost acts by binding to prostacyclin membrane receptors, ultimately inhibiting the release of calcium from intracellular storage sites. This relaxes the smooth muscle cells and cause vasodilation. In early clinical studies, beraprost was demonstrated to be generally well tolerated and efficacious in the treatment of patients with PAD. Patients receiving beraprost exhibited reduction of ulcer size, reported improvement of granulation appearance of the tissue and showed improvement of pain at rest and sensation of cold in the extremities.14) This is illustrated in Fig. 6, where changes of dermal temperature in patients with concurrent Type 2 DM beraprost also elevated dermal temperature.
Fig. 6.
PAD patient with concurrent Type 2 DM on insulin therapy (duration of diabetes: 24 years, 65-year-old male) before and after beraprost 120μg/day15)
To conclude, PAD is a common but frequently overlooked condition and major risk factor for CVD. The prevalence of PAD is increased in CKD patients and cardiovascular disease is the major cause of death among CKD and PAD patients. The overall management strategy should focus on both the limb and CVD outcomes. Important differences in pharmacological actions, indications and contraindications can be used to help select the most appropriate agent for each individual patient in order to optimize outcomes.
Discussion points:
Many factors contribute to an increased risk of PAD in CKD patients, e.g. traditional factors such as diabetes and hypertension and less common factors such as disorders of metabolism and chronic inflammatory conditions that lead to atherosclerosis.
Every CKD patient should be screened (via physical examination including TBI where possible/feasible or ABI otherwise) for PAD even if they are asymptomatic. Skin perfusion pressure (SPP) can also be used.
The multiple pharmacological effects of beraprost make it a good treatment choice; patient comorbidities should also be taken into account when selecting treatment.
References
- 1).Keith DS, Nichols GA, Gullion CM, et al. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med 2004; 164: 659-63 [DOI] [PubMed] [Google Scholar]
- 2).International Society of Nephrology KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease, Chapter 1: Definition, and classification of CKD. Kidney International Supplements 2013; 3: 19-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Ingsathit A, Thakkinstian A, Chaiprasert A, et al. Prevalence and risk factors of chronic kidney disease in the Thai adult population: Thai SEEK study. Nephrol Dial Transplant 2010; 25: 1567-75 [DOI] [PubMed] [Google Scholar]
- 4).Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 2006; 113: e463-654 [DOI] [PubMed] [Google Scholar]
- 5).Hiatt WR. Medical treatment of peripheral arterial disease and claudication. N Engl J Med 2001; 344: 1608-21 [DOI] [PubMed] [Google Scholar]
- 6).2011 WRITING GROUP MEMBERS; 2005 WRITING COMMITTEE MEMBERS; ACCF/AHA TASK FORCE MEMBERS 2011 ACCF/AHA Focused Update of the Guideline for the Management of patients with peripheral artery disease (Updating the 2005 Guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2011; 124: 2020-45 [DOI] [PubMed] [Google Scholar]
- 7).Okamoto K, Oka M, Maesato K, et al. Non-invasive diagnostic modality for peripheral arterial occlusive disease in hemodialysis patients. J Jpn Coll Angiol 2006; 46: 829-835 [Google Scholar]
- 8).Selvin E, Köttgen A, Coresh J. Kidney function estimated from serum creatinine and cystatin C and peripheral arterial disease in NHANES 1999-2002. Eur Heart J 2009; 30: 1918-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Ungprasert P, et al. Correlation between peripheral arterial disease and stage of chronic kidney disease. J Med Assoc Thai 2011; 94Suppl 1: S46-50 [PubMed] [Google Scholar]
- 10).Ono K, Tsuchida A, Kawai H, et al. Ankle-brachial blood pressure index predicts all-cause and cardiovascular mortality in hemodialysis patients. J Am Soc Nephrol 2003; 14: 1591-8 [DOI] [PubMed] [Google Scholar]
- 11).Resnick HE, Lindsay RS, McDermott MM, et al. Relationship of high and low ankle brachial index to all-cause and cardiovascular disease mortality: the Strong Heart Study. Circulation 2004; 109: 733-9 [DOI] [PubMed] [Google Scholar]
- 12).Stevens PE; Levin A; Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med 2013; 158: 825-30 [DOI] [PubMed] [Google Scholar]
- 13).Nehler MR, Hiatt WR. Exercise therapy for claudication. Ann Vasc Surg 1999; 13: 109-14 [DOI] [PubMed] [Google Scholar]
- 14).Melian EB, Goa KL. Beraprost: a review of its pharmacology and therapeutic efficacy in the treatment of peripheral arterial disease and pulmonary arterial hypertension. Drugs 2002; 62: 107-33 [DOI] [PubMed] [Google Scholar]
- 15).Ishida K, et al. Gendai-Iryo 1997; 29(Additional IV): 2987-92 [Google Scholar]
Clinical experience of antiplatelet agents in PAD patients with CKD - a focus on Thailand
Dr. Dusit Lumlertgul
The prevalence of PAD in Thai patients with CKD is around 16.2%. Of these, 8.1% suffer from stage 3 and stage 4 CKD.1) Data from the Thai SEEK (Screening and Early Evaluation of Kidney disease) study (shown in Page 201 Table 1) show a high incidence of CKD, 17.5% of the general population compared with 13.1% in the US.1) Given this high incidence, a thorough consideration of the optimal current management strategies is essential.
Actions and relationship between PGI2 and TXA2
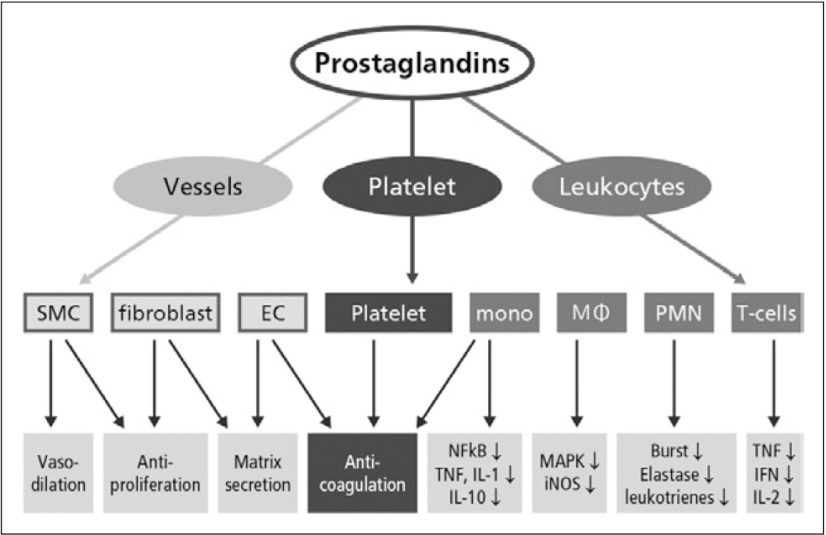
A variety of vascular cells, platelets and leukocytes have been identified as sensitive target cells of PGs (Fig. 1). On the cellular level, the objectives of PG therapy are to:2)
Fig. 1.
Target cells of PGs2)
Reduce the increased vascular wall stress by relaxation of vascular SMCs
Prevent or reverse the structural reorganization of pulmonary vessels (anti-remodeling)
Inhibit primary or secondary thrombus-mediated obliteration via inhibition of platelet aggregation
Suppress inflammatory activity near the vessels
EC, endothelial cells; mono, monocytes; MA, macrophages; PMN, polymorphonuclear neutrophils; NFkB, nuclear factor-kB; TNF-α, tumor necrosis factor α; IL, interleukin; MAPK, mitogen-activated protein kinases; iNOS, inducible NO synthase; burst, respiratory burst (generation of reactive oxygen species); elastase, elastase secretion; IFN, interferon-γ.
Aspirin inhibits the production of both TXA2 as well as PGI2. As detailed in Table 2, TXA2 accelerates platelet aggregation which leads to vasoconstriction while PGI2 inhibits platelet aggregation, promoting vasodilation. Platelet production of TXA2 in response to a variety of stimuli (including collagen, thrombin, and ADP) results in the amplification of the platelet aggregation response and in vasoconstriction. Conversely, vascular endothelial cell production of PGI2 results in inhibition of platelet aggregation and induces vasodilation. Aspirin induced inhibition of TXA2 and PGI2 has opposing effects on hemostasis; however, the available data suggest that the potentially prothrombotic effects of PGI2 inhibition are not clinically relevant and that the antithrombotic effects of TXA2 inhibition predominate. This may, in part, be a result of the ability of vascular endothelial cells to regenerate new COX and thus recover normal function, whereas COX inhibition in platelets is irreversible owing to the limited mRNA pool and protein synthesis in the nucleus cells.3)
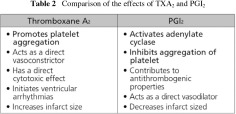
Effectiveness of beraprost in PAD
The antiplatelet and vasodilating properties of beraprost make it an effective symptomatic treatment in patients with claudication. The overall therapeutic effect is to improve the decreased blood flow of the lower limbs.4)
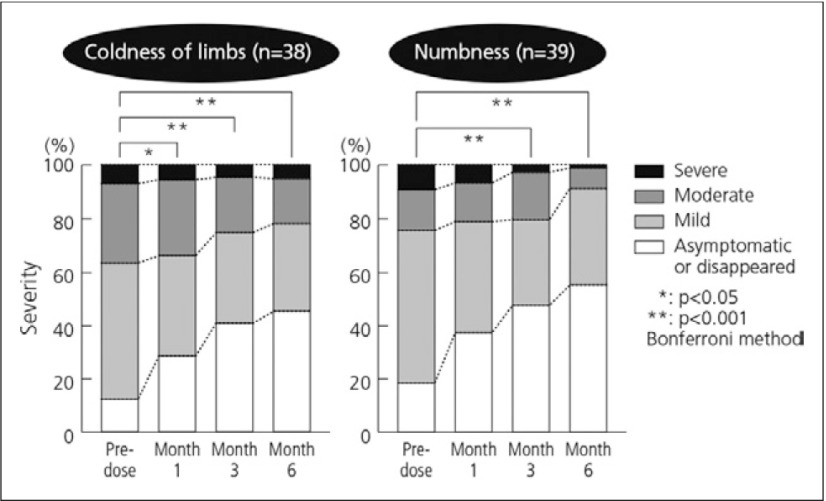
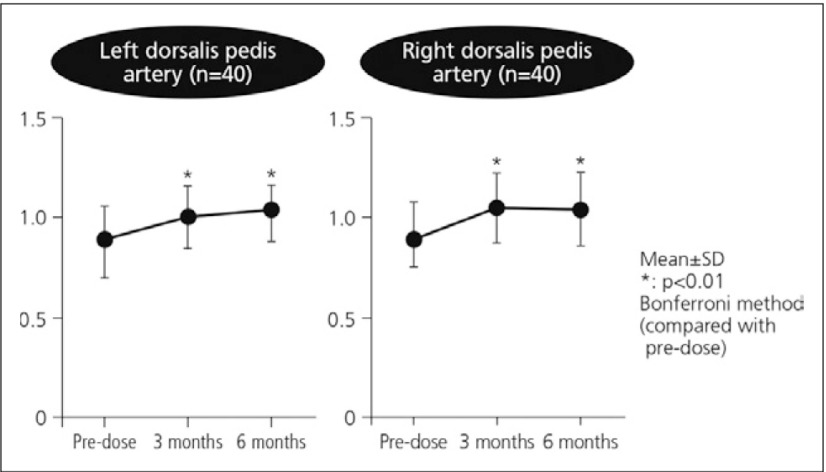
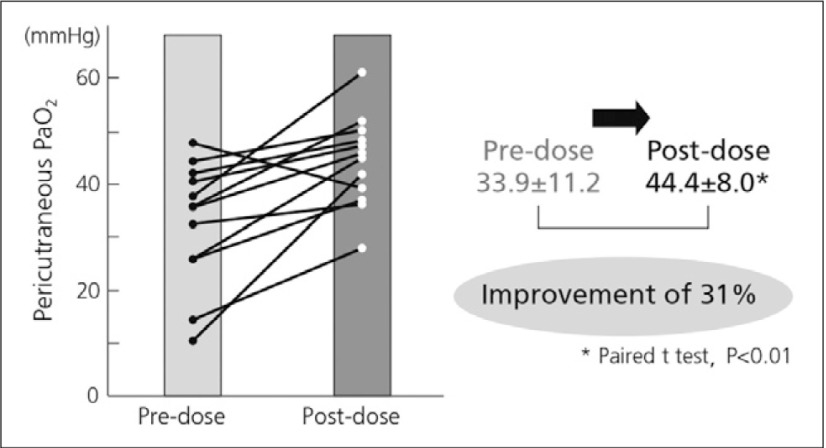
The clinical benefits of beraprost have been demonstrated in numerous clinical trials and clinical and nonclinical studies. For example, significant improvements were observed in a clinical study of the efficacy of beraprost in PAD in the coldness of limbs after one month and numbness after three months (Fig. 2) in diabetic patients with arteriosclerosis obliterans, demonstrating the enhanced improvement in the severity over time following beraprost treatment. After 3 months of beraprost treatment, ABIs of the left and right dorsalis pedis arteries showed significant improvement (Fig. 3).5) Beraprost also improved pericutaneous PaO2 by 31% in diabetic patients examined while lifting an upper limb upwards (Fig. 4).6)
Fig. 2.
Effects of beraprost on PAD symptoms: coldness of limbs and numbness5)
Fig. 3.
Effects of beraprost on blood flow in lower limbs: change of ABI5)
Fig. 4.
Improvement of pericutaneous PaO2 with beraprost6)
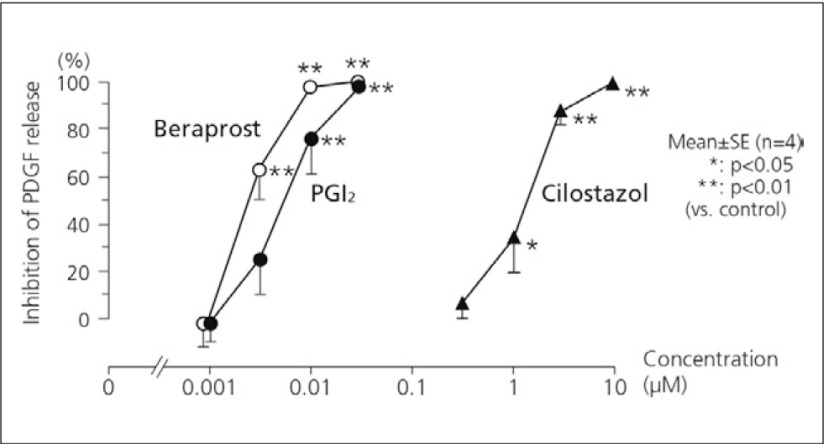
The comparative vasodilative action of beraprost was examined in dogs by measuring the blood flow of the femoral artery following intra-arterial administration of beraprost, PGI2, ticlopidine or cilostazol. Although each of the drugs showed a dose-dependent increase in blood flow, the order of the effect was PGI2 > beraprost > cilostazol > ticlopidine.7) Beraprost also inhibited the release of PDGF dose-dependently in human platelets in vitro. The effect was comparable or slightly more potent compared with PGI2 and stronger than cilostazol (Fig. 5).8)
Fig. 5.
Inhibition of the release of PDGF in vitro8)
A 3-year prospective observational cohort study was conducted to investigate the status of PAD drug treatment in Japan and the effects of drug treatment in PAD patients. Beraprost, cilostazol and aspirin were found to be the most frequently used treatments for PAD. Among patients who had not undergone vascular reconstruction before enrollment, there was a significant improvement in ABI after treatment with beraprost.9)
Therapeutic apheresis
Methods of therapeutic apheresis eliminate moderately aggregating macromolecules like fibrinogen, as well as strongly aggregating substances like alpha2-macroglobulin from blood. In microcirculation disorders, therapeutic apheresis seems to have two different effects. The first, achieved after only a few sessions, is acute, consisting of drastic reduction of blood viscosity and obtained with the use of low-density lipoprotein (LDL) apheresis, rheopheresis, or fibrinogen apheresis. It is likely that the acute effect of apheresis mainly influences the functional components of the vascular damage, and so the derived rheological benefit might last only for a short period. In contrast, a chronic effect, by acting on the morphological alterations of the vascular walls, requires the apheresis treatment to be prolonged for a longer period or even cycles of treatment to be programmed.10)
In a study investigating continuous changes of tissue blood flow in the lower limbs and head during LDL-apheresis (LDLA) treatment by a non-invasive method (the Non-Invasive COntinuous Monitoring Method [NICOMM] system), tissue blood flow in both the head and lower limbs showed significant enhancement with treatment. Tissue blood flow in the lower limbs showed a significantly larger improvement than observed in the head.11)
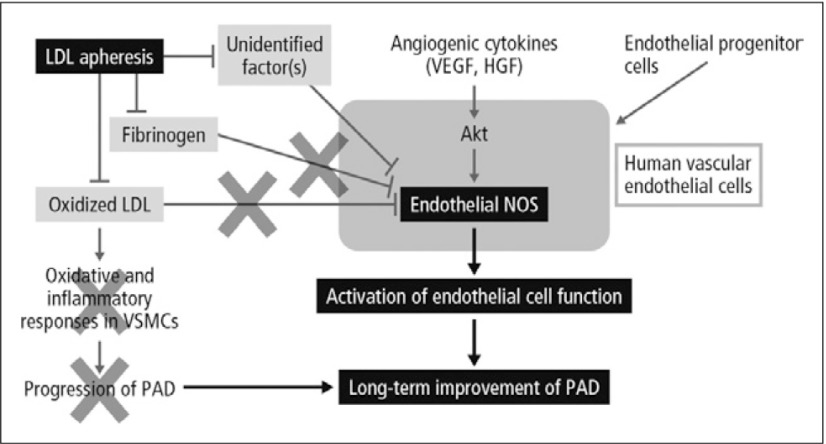
Suppression of oxidative stress, improvement of endothelial functions and alteration in the action of vasoactive compounds may occur with the improvement of the rheological property of blood as a result of aggressive removal of atherogenic factors including LDL, possibly resulting in the suppression of development of atherosclerosis. Repeated LDLA prevents the progression of atherosclerotic diseases and probably improves the long-term prognosis of patients with PAD.12) The proposed action of LDLA in this setting is outlined in Fig. 6.13) In clinical studies, approximately 50% reduction in incidence of major coronary events was achieved by LDLA. Similarly, in the studies with a angiographic parameter, coronary atherosclerosis was suppressed or regressed by LDLA.14)
Fig. 6.
Proposed action of LDLA13)
LDLA is currently indicated in Japan in patients with clinical signs of poor peripheral circulation, such as cold, discolored or ulcerated extremities, or intermittent claudication consistent with a Fontaine classification of Grade II or more, and with LDL-C >140 mg/dL or total cholesterol >220 mg/dL in spite of drug treatment.13)
In a study conducted in Thailand, Lumlertgul et al. (2013) applied Double Filtration Plasmapheresis (DFPP) to the treatment of two different categories from 100 cases of non-kidney disease that had been collected over a 5 year period (2007-2011). PAD was shown to benefit from significantly decreasing fibrinogen and IgM, which resulted in clinical tissue oxygenation.15)
To conclude, DFPP (rheopheresis) is advocated in arterial diseases or severe microvascular complications in diabetic vascular diseases, and hyperviscosity syndrome caused by IgM, fibrinogen, lipid, and α2-macroglobulin. Renal deterioration in diabetic nephropathy can be delayed by DFPP plus anticoagulants. In one study, DFPP was not associated with the significant adverse effect of severe bleeding or other serious complications. Overall, we should aggressively treat PAD and its complications before the patient loses a limb or their life.
Discussion points:
Apparent differences between Western and Asian populations in the prevalence of PAD in patients with CKD are most likely not consistent or significant; any differences in incidence most likely reflects differences in classification.
There may be gender-based difference in prostaglandin activity that relate to testosterone levels - these could potentially lead to gender differences in efficacy for therapeutic agents but this remains to be determined.
The immediate use of beraprost is important in patients with critical limb ischemia - after two or three weeks, leg pain symptoms are usually reduced.
References
- 1).Ingsathit A, Thakkinstian A, Chaiprasert A, et al. Prevalence and risk factors of chronic kidney disease in the Thai adult population: Thai SEEK study. Nephrol Dial Transplant 2010; 25: 1567-75 [DOI] [PubMed] [Google Scholar]
- 2).Olschewski H, Olschewski A, Rose F, et al. Physiologic basis for the treatment of pulmonary hypertension. J Lab Clin Med 2001; 138: 287-97 [DOI] [PubMed] [Google Scholar]
- 3).Awtry EH, Loscalzo J. Aspirin. Circulation 2000; 101: 1206-18 [DOI] [PubMed] [Google Scholar]
- 4).Melian EB, Goa KL. Beraprost: a review of its pharmacology and therapeutic efficacy in the treatment of peripheral arterial disease and pulmonary arterial hypertension. Drugs 2002; 62: 107-33 [DOI] [PubMed] [Google Scholar]
- 5).Toyota T, Oikawa S; Beraprost Sodium Study Group. Effects of beraprost sodium (Dorner) in patients with diabetes mellitus complicated by chronic arterial obstruction. Angiology 2002; 53: 7-13 [DOI] [PubMed] [Google Scholar]
- 6).Kosugi K, et al. Prog Med 2004; 24: 797-801 [Google Scholar]
- 7).Ishikawa J, et al. Gendai-Iryo 1992; 24 (Special ed.): 153 [Google Scholar]
- 8).Nishio S. Gendai-Iryo 1992; 24 (Special ed.): 109 [Google Scholar]
- 9).Shigematsu H, Nishibe T, Obitsu Y, et al. Three-year cardiovascular events and disease progress in patients with peripheral arterial disease: results from the Japan Medication Therapy for Peripheral Arterial Disease (J-METHOD). Int Angiol 2010; 292 Suppl: 2-13 [PubMed] [Google Scholar]
- 10).Ramunni A, Brescia P, De Fino G, et al. Therapeutic apheresis in peripheral and retinal circulatory disorders. Clin Res Cardiol Suppl 2012; 7Suppl 1: 41-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Ebihara I, Sato T, Hirayama K, et al. Blood flow analysis of the head and lower limbs by the laser Doppler blood flowmeter during LDL apheresis. Ther Apher Dial 2007; 11: 325-30 [DOI] [PubMed] [Google Scholar]
- 12).Sato M, Amano I. Changes in oxidative stress and microcirculation by low-density lipoprotein apheresis. Ther Apher Dial 2003; 7: 419-24 [DOI] [PubMed] [Google Scholar]
- 13).Tamura K, Tsurumi-Ikeya Y, Wakui H, et al. Therapeutic potential of low-density lipoprotein apheresis in the management of peripheral artery disease in patients with chronic kidney disease. Ther Apher Dial 2013; 17: 185-92 [DOI] [PubMed] [Google Scholar]
- 14).Tasaki H. Low-density lipoprotein apheresis in the prevention of recurrent coronary heart disease: a review. Ther Apher Dial 2003; 7: 408-12 [DOI] [PubMed] [Google Scholar]
- 15).Lumlertgul D, Suteeka Y, Tumpong S, et al. Double filtration plasmapheresis in different diseases in Thailand. Ther Apher Dial 2013; 17: 99-116. [DOI] [PubMed] [Google Scholar]
Vascular calcification in chronic kidney disease
Dr. Ketut Suwitra
Arterial media calcification in ESRD
Patients with CKD, especially those with chronic dialysis have more frequent vascular calcification than the general population which will increase morbidity and mortality. Arterial intimal calcification (AIC) is a typical risk factor associated with atherosclerotic disease and is usually observed in older patients with a clinical history of atherosclerosis before starting haemodialysis. Arterial medial calcification (AMC) is closely associated with the duration of haemodialysis and calcium-phosphate disorders, including the dose of calcium prescribed as phosphate binder. It is observed in young and middle-aged patients without the conventional atherosclerotic risk factors.1)
The number of dialysis patients in the US has been increasing consistently. Experts from the US Renal Data System (USRDS) have projected that between 1998 to 2010 the number of patients with long-term ESRD will increase at an average rate of 6.4% per year, and that the prevalence of ESRD will increase from approximately 372,000 in 2000 to 651,000 by 2010.2)
Applying KDOQI guidelines for the definition of CKD, Coresh et al. (2003) estimated the prevalence of CKD in the Third National Health and Nutrition Examination Survey (NHANES III). Results showed that stage 3 CKD affects 4.3% of the population (7.6 million), a much larger group of individuals than those now on dialysis. As the population ages, the number of people at risk for CKD is therefore thought to increase.3)
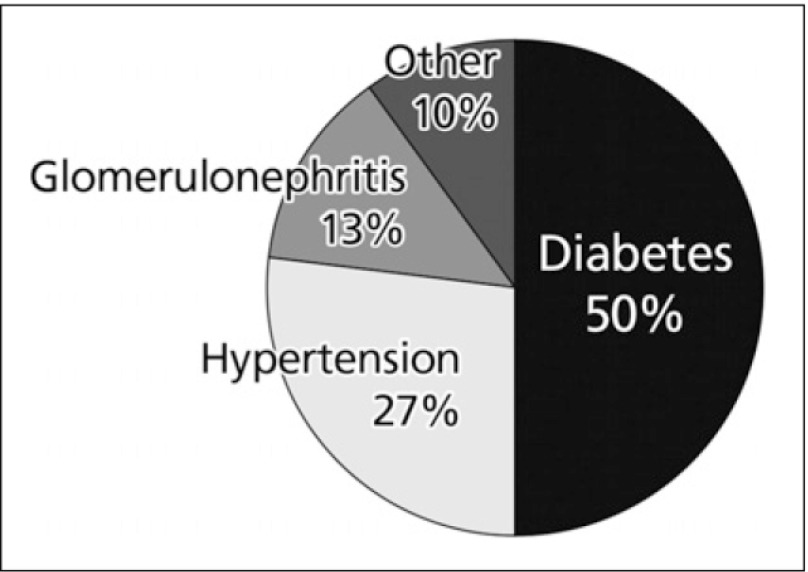
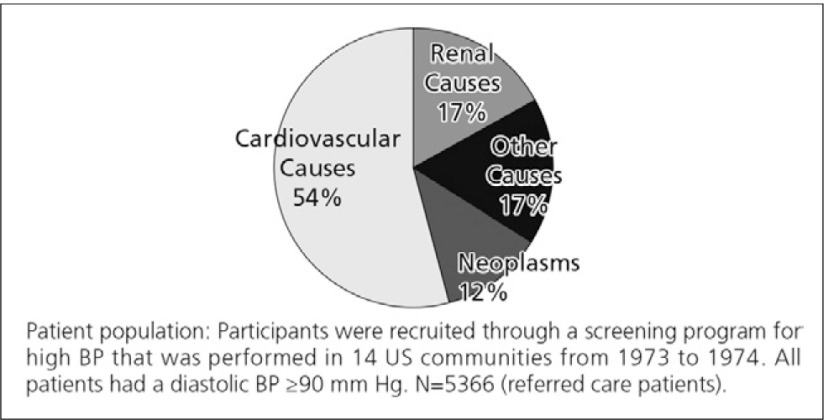
As shown in Fig. 1, diabetes is the major cause of ESRD. Hypertension is the second most common cause. However, a substantial proportion of diabetics will have hypertension (blood pressure >140/90 mmHg) as an important contributing factor to their loss of renal function.4) Shulman et al. (1989) followed up 10,940 CKD patients for 5 years in a community-based trial for the treatment hypertension, the Hypertension Detection and Follow-up Program. The leading cause of death was found to be cardiovascular disease (54.3%).5)
Fig. 1.
Causes of ESRD4)
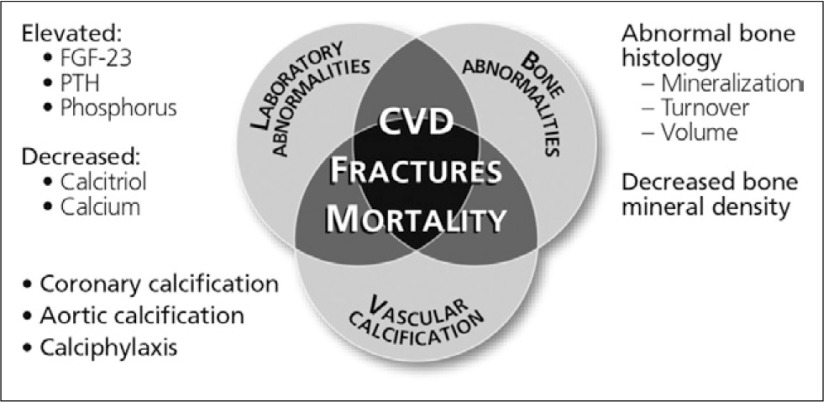
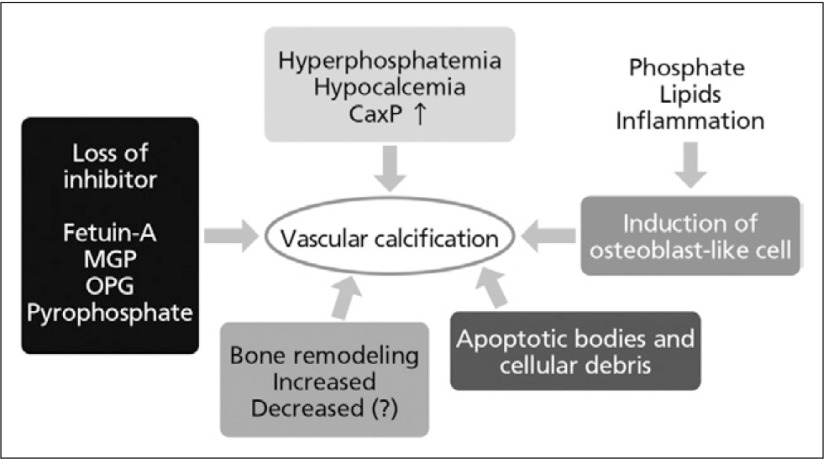
In 2009, KDIGO categorised vascular and soft tissue calcification in CKD on Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) beside abnormality metabolism of calcium, phosphorus, parathyroid hormone, vitamin D and abnormality of bone turnover, mineralisation, volume, linear growth or strength. Figure 2 illustrates how the consequences of CKD-MBD interact to produce poor outcomes for ESRD patients,6, 7) while Fig. 3 outlines mechanisms of vascular calcification in CKD.
Fig. 2.
Fig. 3.
Mechanisms of vascular calcification in CKD6,7) MGP, matrix GLA protein; OPG, osteoprotegerin
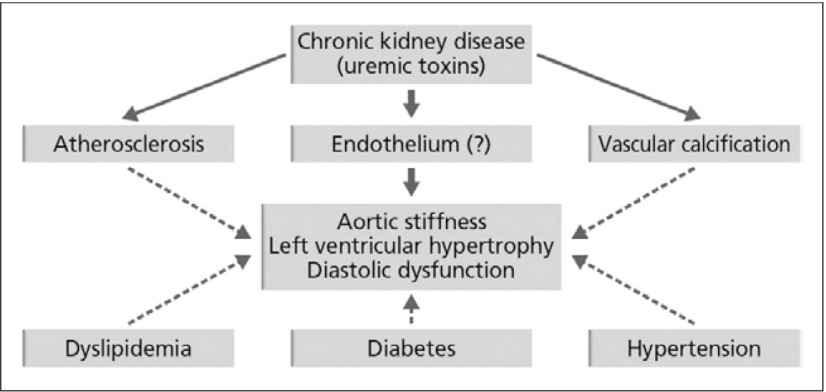
Researchers in the 1970s proposed that atherosclerosis was the main cause of cardiovascular disease in patients with CKD and that its progression. As showed in Fig. 4 that, cardiovascular event still the main cause of death in CKD patients, so vascular calcification of course has a contribution for that matter. Based on observations of patients on long-term dialysis, vascular calcification was accelerated by the uremic state. Subsequent reports, however, favor the involvement of other mechanisms, such as arteriosclerosis, vascular calcification, ‘myocyte/capillary mismatch’ and congestive cardiomyopathy.The prevalence of atherosclerosis increases with age and is aggravated, but not specifically induced, by CKD. Other risk factors for atherosclerosis in CKD are detailedin Table 1. The cardiovascular events associated with atherosclerosis are more often fatal in patients with CKD than in individuals without CKD. The authors put forward a hypothetical role of mild to moderate CKD in the occurrence of cardiovascular dysfunction, which is shown in Fig. 5.8)
Fig. 4.
Major cause of death5)

Fig. 5.
Hypothetical role of mild to moderate CKD in the occurrence of cardiovascular dysfunction8)
Controlling the development of vascular calcification in CKD is essential, principally because it is associated with increased cardiovascular pathology. However, the precise mechanism of vascular calcification remains to be completely elucidated.9)
In histological specimens from the inferior epigastric artery of dialysis patients, expression of the osteoblast differentiation factor core binding factor alpha-1 (Cbfa1) and several bone-associated proteins (osteopontin, bone sialoprotein, alkaline phosphatase, type I collagen) have been found in both the intima and medial layers when calcification was present. In cultured vascular smooth muscle cells, the addition of pooled serum from dialysis patients accelerated mineralisation and increased expression of Cbfa1, osteopontin, and alkaline phosphatase to a similar magnitude as does beta-glycerophosphate alone. However, a lack of inhibitors of calcification may also be important. Dialysis patients with low levels of serum fetuin-A, a circulating inhibitor of mineralisation, have increased coronary artery calcification and fetuin-A can inhibit mineralization of vascular smooth muscle cells in vitro. These data support that elevated levels of phosphorus and/or other potential uremic toxins may play an important role by transforming vascular smooth muscle cells into osteoblast-like cells, which can produce a matrix of bone collagen and noncollagenous proteins. This nidus can then mineralize if the balance of pro-mineralizing factors outweighs inhibitory factors.10)
Diagnosis of CKD-MBD
The KDIGO working group recommend that screening for vascular calcification is done at the discretion of the treating physician, especially when knowledge of the presence of vascular calcification may impact therapeutic decision making. In CKD stages 3-5D, the suggestions indicate that:11)
-
It is reasonable to use alternatives to computed tomography-based imaging to detect the presence or absence of vascular calcification, including:
Lateral abdominal radiograph
Echocardiogram
Patients with known vascular/valvular calcification can be considered at highest cardiovascular risk
In the initial CKD stage, the recommendation is to monitor serum levels of:11)
Phosphorus
Calcium
PTH
Alkaline phosphatase
In CKD stages 3-5D, frequency of monitoring serum calcium, phosphorus, and PTH should be based:11)
On the presence and magnitude of abnormalities
The rate of progression of CKD
In children, the suggestion is to begin monitoring in CKD stage 2.
In patients with CKD stages 3-5, it is recommended to maintain serum phosphorus and calcium in the normal range. Phosphate binders are suggested in the treatment of hyperphosphataemia. For choice of phosphate binder, it is reasonable to take into account:11)
CKD stage
Presence of other components of CKD-MBD
Concomitant therapies
Side-effect profile (not graded)
In patients with CKD stages 3-5 not on dialysis, the optimal PTH level is unknown. In patients with levels of intact PTH (iPTH) above the upper normal limit of the assay, the suggestion is to first evaluate for hyperphosphataemia, hypocalcaemia and vitamin D deficiency. It is reasonable to correct these abnormalities with any or all of the following; reducing dietary phosphate intake and administering phosphate binders, calcium supplements, and/or native vitamin D. The suggestion is to treat with calcitriol or vitamin D analogs if serum PTH is progressively rising and remains persistently above the upper limit of normal for the assay despite correction of modifiable factors.11)
Kidney function and the risk of PAD
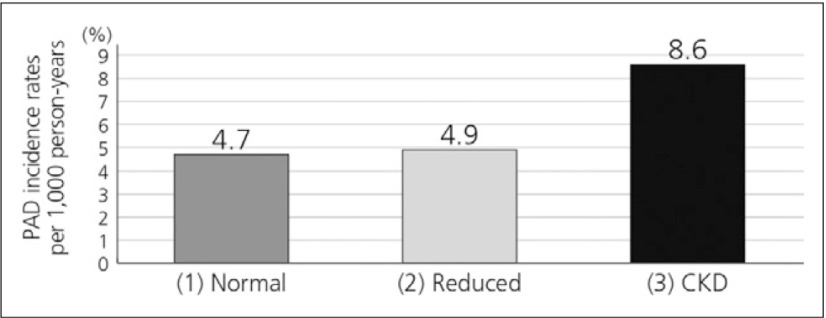
The Atherosclerosis Risk in Communities (ARIC) study, which enrolled 14,280 middle-aged adults, showed an increase in risk for incident PAD in participants with CKD (Fig. 6), with a multivariable adjusted RR of 1.56 (95% CI 1.13 to 2.14).12)
Fig. 6.
Risk of PAD in CKD patients in the ARIC study12)
Cystatin C, a novel marker of kidney function, may have clinical utility when combined with serum creatinine in evaluation of individuals who may have PAD. A clinical study in 3,089 patients suggested that GFR estimated using cystatin C was more strongly associated with PAD compared with eGFR using serum creatinine before and after multivariable adjustment. Further, after adjustment for cystatin C, kidney function based on serum creatinine was no longer significantly associated with PAD. However, cystatin C remained significantly associated with PAD even after adjustment for GFR estimated by serum creatinine.13)
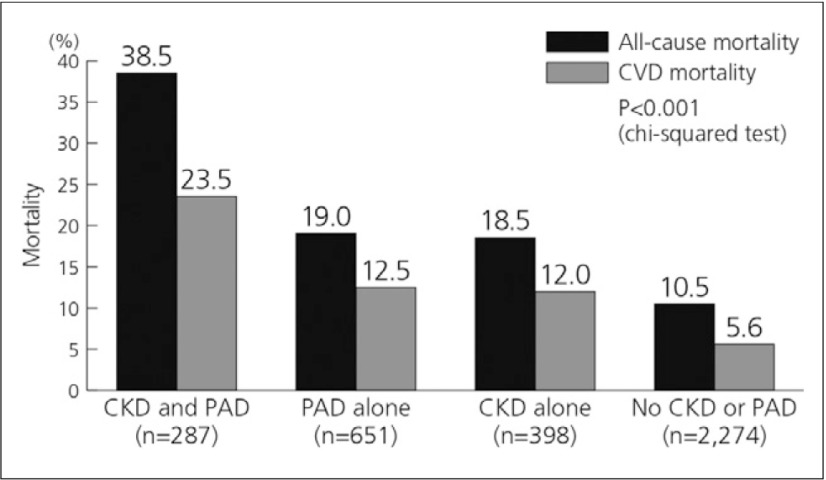
A further study investigated the association between CKD and PAD. The prevalence of PAD in patients with and without CKD was 41.9% and 22.3%, respectively. The survival rate for the CKD and PAD group was significantly lower than that for any single disease, for both all-cause and CVD mortality (Fig. 7).14)
Fig. 7.
Mortality rate in CKD/PAD patients14)
Effects of beraprost on vascular endothelium in patients with PAD and renal dysfunction
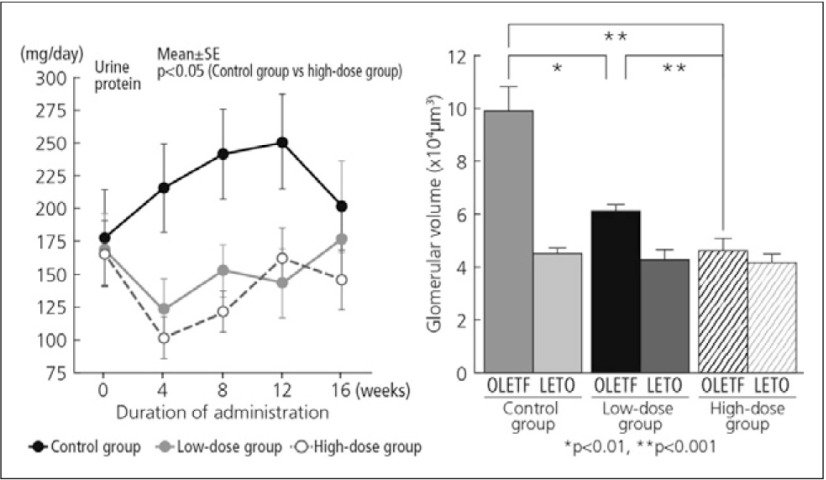
In a preclinical study, beraprost had a therapeutic effect on diabetic nephropathy in the OLETF (Otsuka Long-Evans Tokushima Fatty) rat, suggesting a potential application in the treatment of human diabetic nephropathy. The values for urine protein excretion and serum blood urea nitrogen in beraprost-treated rats were significantly lower than those in untreated rats (Fig. 7). Index of glomerulosclerosis and glomerular volume were also significantly reduced compared with untreated rats (Fig. 8).15) The administration of beraprost to PAD patients undergoing maintenance dialysis was associated with a decrease in plasma endothelin-1 concentration, a marker of vascular endothelial cell damage.16)
Fig. 8.
Urine protein excretion and glomerular volume in OLETF rats following beraprost treatment15)
To conclude, death in >50% of patients with CKD is a result of cardiovascular complications. Vascular calcification has been clearly defined as a risk factor and highly prevalent in CKD especially in end stage of the disease. While the pathophysiology of vascular calcification in CKD still unclear, a disturbance in calcium and phosphate metabolism is apparently dominant. The frequency and severity of atherosclerotic events is higher in patients with CKD and studies have shown that beraprost has an important therapeutic role in this setting.
References
- 1).London GM, Guérin AP, Marchais SJ, et al. Arterial media calcification in end-stage renal disease: impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant 2003; 18: 1731-40 [DOI] [PubMed] [Google Scholar]
- 2).Xue JL, Ma JZ, Louis TA, et al. Forecast of the number of patients with end-stage renal disease in the United States to the year 2010. J Am Soc Nephrol 2001; 12: 2753-8 [DOI] [PubMed] [Google Scholar]
- 3).Coresh J, Astor BC, Greene T, et al. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis 2003; 41: 1-12 [DOI] [PubMed] [Google Scholar]
- 4).United States Renal Data System (USRDS) 2000 Annual Data Report. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases – Division of Kidney, Urologic and Hematologic Diseases. USRDS Coordinating Center operated by the Minneapolis Medical Research Foundation. URL: www.usrds.org
- 5).Shulman NB, Ford CE, Hall WD, et al. Prognostic value of serum creatinine and effect of treatment of hypertension on renal function. Results from the hypertension detection and follow-up program. The Hypertension Detection and Follow-up Program Cooperative Group. Hypertension 1989; 135 Suppl: I80-93 [DOI] [PubMed] [Google Scholar]
- 6).KDIGO® Overview slide presentation at: http://www.kdigo.org/speaker_center.php. Accessed September 11, 2008
- 7).Moe S, Drüeke T, Cunningham J, et al. Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2006; 69: 1945-53 [DOI] [PubMed] [Google Scholar]
- 8).Drüeke TB, Massy ZA. Atherosclerosis in CKD: differences from the general population. Nat Rev Nephrol 2010; 6: 723-35 [DOI] [PubMed] [Google Scholar]
- 9).Huybers S, Bindels RJ. Vascular calcification in chronic kidney disease: new developments in drug therapy. Kidney Int 2007; 72: 663-5 [DOI] [PubMed] [Google Scholar]
- 10).Moe SM, Chen NX. Pathophysiology of vascular calcification in chronic kidney disease. Circ Res 2004; 95: 560-7 [DOI] [PubMed] [Google Scholar]
- 11).Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl 2009; (113): S1-130 [DOI] [PubMed] [Google Scholar]
- 12).Wattanakit K, Folsom AR, Selvin E, et al. Kidney function and risk of peripheral arterial disease: results from the Atherosclerosis Risk in Communities (ARIC) Study. J Am Soc Nephrol 2007; 18: 629-36 [DOI] [PubMed] [Google Scholar]
- 13).Selvin E, Köttgen A, Coresh J. Kidney function estimated from serum creatinine and cystatin C and peripheral arterial disease in NHANES 1999-2002. Eur Heart J 2009; 30: 1918-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Luo Y, Li X, Li J, et al. Peripheral arterial disease, chronic kidney disease, and mortality: the Chinese Ankle Brachial Index Cohort Study. Vasc Med 2010; 15: 107-12 [DOI] [PubMed] [Google Scholar]
- 15).Watanabe M, Nakashima H, Mochizuki S, et al. Amelioration of diabetic nephropathy in OLETF rats by prostaglandin I(2) analog, beraprost sodium. Am J Nephrol 2009; 30: 1-11 [DOI] [PubMed] [Google Scholar]
- 16).Kuriyama T, Tomonari H. Effect of prostacyclin in dialysis patients with ASO. Jap Coll Angiol 1997; 37: 455-9. [Google Scholar]
Association between albuminuria, atherosclerosis and PAD in Korean type 2 diabetic patients - detecting high risk PAD patients
Dr. Dong Sun Kim
Risk factors of albuminuria are the same as those associated with vascular disease. Many analyses have also identified albuminuria as a potent risk factor for the development of progressive kidney disease. As the conditions associated with endothelial damage predispose individuals to albuminuria, PAD might also be related to albuminuria. However, since albuminuria and renal insufficiency are closely related, the association between albuminuria and PAD could be confounded by renal function.
The endothelial cell lining of the arterial vasculature modulates the relationship between the cellular elements of the blood and the vascular wall, mediating the normal balance between thrombosis and fibrinolysis. Abnormalities of endothelial function can change the arterial system susceptible to atherosclerosis and its associated adverse outcomes.1)
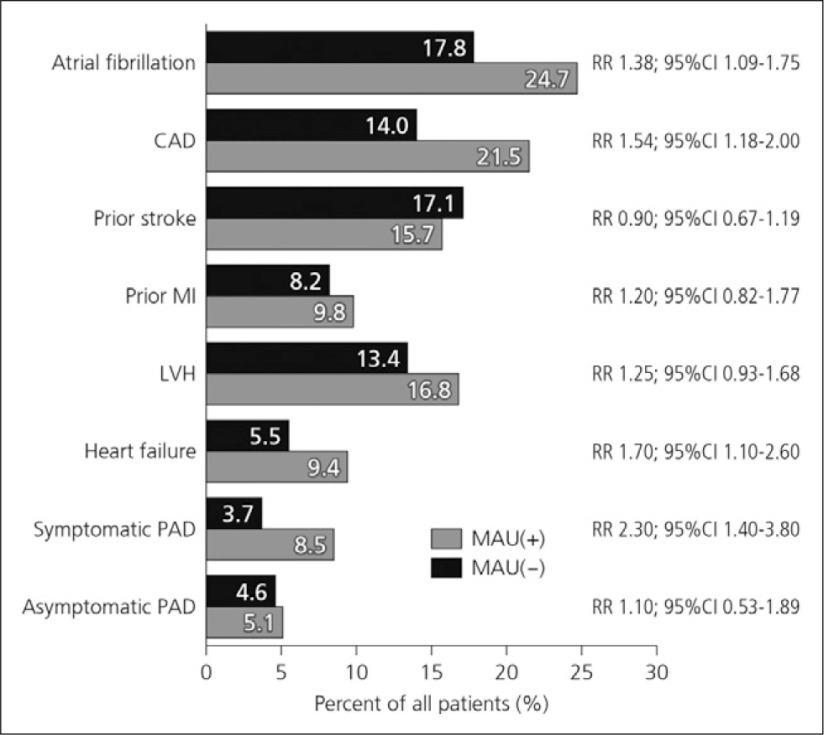
Patients with microalbuminuria were found to have higher rates of atrial fibrillation, coronary artery disease, heart failure and symptomatic PAD compared to patients without microalbuminuria (Fig. 1).2)
Fig. 1.
Vascular risk in patients with microalbuminuria2)
Microalbuminuria is therefore an early sign of intra-renal vascular dysfunction, and reflects generalised atherosclerosis. An improvement in endothelial function is reflected in a decrease of albuminuria. Since the conditions associated with endothelial damage predispose individuals to microalbuminuria, it is natural that PAD might also be related to albuminuria.
Cardiovascular mortality in patients with microalbuminuria and PAD
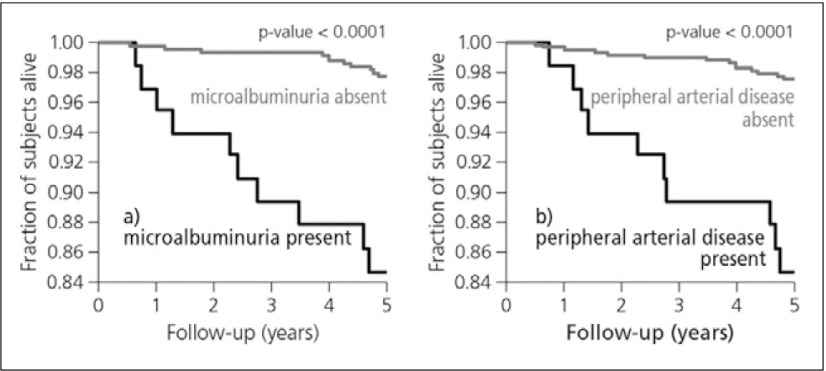
Data from a population-based cohort, followed prospectively for 5 years (n=631), showed both microalbuminuria and PAD to be associated with an increased risk of cardiovascular mortality (Fig. 2). The risk of cardiovascular mortality increased around 13-fold when both existed. The associations of microalbuminuria and PAD with all-cause mortality are somewhat weaker. They are more pronounced in the presence of hypertension than in its absence. Data from this study suggest that microalbuminuria affects mortality risk through a mechanism different from generalized atherosclerosis.3)
Fig. 2.
Cardiovascular survival according to the absence vs the presence of microalbuminuria (b) and PAD (b)3)
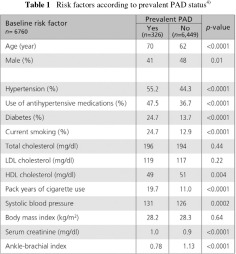
Most epidemiologic studies have shown an increased risk of PAD in patients with CKD. However, the role of albuminuria as a risk factor for PAD has mostly been evaluated small studies. The Multi-Ethnic Study of Atherosclerosis (MESA), for example, is a population-based investigation of the prevalence, correlates, and progression of subclinical CVD. This study cohort comprised 6,814 subjects. Compared to subjects without PAD, those who had PAD were more likely to be older, male and have higher prevalence of hypertension, diabetes, and smoking as well as a greater mean values for systolic blood pressure, serum creatinine and a lower mean HDL cholesterol level (Table 1). The study suggested that albuminuria may be an important risk factor for PAD in the general population and in high-risk populations of diabetic subjects or hypertensive subjects.4)
Interesting results from the National Health and Nutrition Examination Surveys (NHANES) in US have recently been published. The objective of this study was to examine the association of PAD with low GFR and albuminuria in a large general population. Of the 7068 subjects, in non-diabetics, PAD occurred more frequently in those with albuminuria (13.7%) than in those without albuminuria (4.6%). However, in the diabetics, the difference was not statistically significant (12.3% vs 14.9%; p=0.43). Among non-diabetic subjects, PAD was 2.77 times more likely in the presence of albuminuria. Even after controlling for the confounding effect of CKD, the OR of albuminuria for PAD was still significant (p=0.0003). For diabetic subjects, there was no association between albuminuria and PAD before adjustment and after adjustment. However, the association between CKD and PAD was statistically significant (OR: 2.3).5)
To see the impact of glucose control on the association between albuminuria and PAD, the diabetic patients were stratified into those with tight glucose control and poor control. In the tight controlled group (HbA1C <7), ACR was associated with PAD in univariate analysis (OR: 1.25; p=0.0015), but only tended to be associated in multivariate analysis (OR: 1.16; p=0.09). In the poorly controlled group(HbA1C ≥7), there was no association between ln(ACR) and PAD in the unadjusted and multivariate analysis. Considering albuminuria is especially common in those with poor glucose control, the severity of endothelial damage cannot be determined simply by the presence of albuminuria. Deterioration of renal function, instead of the presence of albuminuria, may reflect more accurately the severity of generalized atherosclerosis. Thus, renal function (i.e., GFR), rather than albuminuria, might be a better predictor of PAD in subjects with diabetes.5)
Overall, these NHANES data indicate that albuminuria, independent of renal function, is strongly associated with PAD in non-diabetic subjects. It also appears that as diabetes develops and HbA1c level increases, the predictive value of albuminuria gradually diminishes after adjustment for renal function.5)
Kidney function and risk of PAD
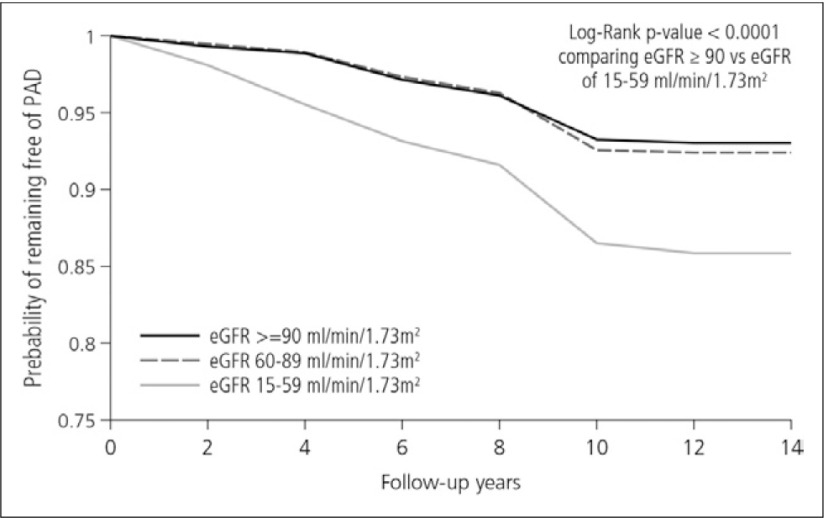
Kidney disease is also associated with an increased risk for cardiovascular disease, and kidney function correlates with a risk of PAD, as shown by findings from the ARIC study.6) A total of 14,280 middle-aged adults were categorized on the basis of estimated GFR ≥90, 60 to 89, and 15 to 59 ml/min per 1.73 m2) for normal kidney function, mildly decreased kidney function and stages 3 to 4 CKD, respectively. During a mean follow-up time of 13.1 years, 1,016 participants developed PAD. Figure 3 shows Kaplan-Meier curve for remaining free of PAD by level of estimated GFR. It is clear that individuals having low eGFR (15-59) show a high incidence rate of PAD.6) Patients with CKD are therefore at an increased risk for incident PAD.
Fig. 3.
Kaplan-Meier curve for remaining free of PAD by level of eGFR6)
Diabetes, inflammation and the risk for PAD
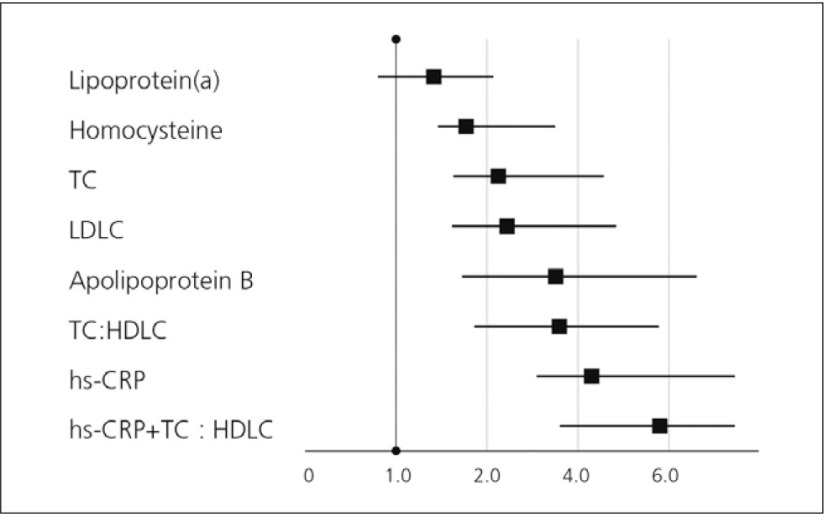
Inflammation has been established as both a risk marker and perhaps a risk factor for atherothrombotic diseases, including PAD. Elevated levels of CRP are strongly associated with the development of PAD. A high-sensitive C-reactive protein (hs-CRP) assay was developed that can detect slight but significant increases in CRP levels within the normal range. Hs-CRP is considered as an independent predictor of CV disease. In addition, levels of CRP are abnormally elevated in patients with diabetes. CRP was discovered 70 years ago. Historically, CRP has been used in the diagnosis and monitoring of active inflammation and infection. In response to tissue damage or infection, hepatocytes are stimulated by cytokines, especially IL6, IL1 and TNFα, to synthesize CRP. So, acute-phase CRP has been used as a marker of systemic inflammatory changes in patients with sepsis or connective tissue disease. Inflammation has been considered, at least in part, to lead to the development and progression of atherosclerosis. Hs-CRP is considered to be a consistent marker for evaluating the extent of CVD in clinical studies. When directly compared with many other novel risk factors including homocysteine, lipoprotein(a), and standard lipid measures, hs-CRP has proven to be the single strongest predictor of risk, as is shown by data from the Women’s Health Study (Fig. 4).7)
Fig. 4.
Relative risks of future CV events7)
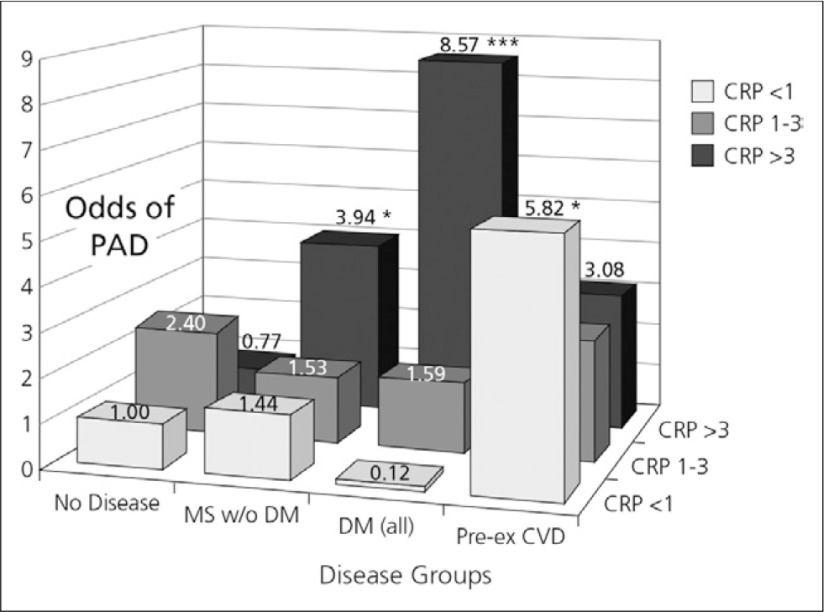
Data from NHANES also illustrate the risk of PAD by disease and CRP category (Fig. 5). Subjects with diabetes and an elevated CRP had the greatest likelihood of PAD (OR: 8.57; p<0.001). PAD is more likely to occur among those with DM and increased CRP, suggesting that enhanced inflammation may identify these subjects. The important findings of this study were that increased CRP levels are associated with a greater likelihood of PAD among those with MS, even if DM is absent. In those with metabolic syndrome alone, an elevated CRP also resulted in an increased likelihood of PAD (OR: 3.94; p=0.04).8)
Fig. 5.
Risk of PAD by disease and CRP category8)
CRP has been found to bind to endothelial cell receptors promoting apoptosis and has been shown to colocalize with oxidized LDL in atherosclerotic plaques. Therefore, in addition to being a marker of disease presence, elevation of CRP may also be a culprit in the causation or exacerbation of PAD.9) Summarizing the many studies that have investigated the role of CRP in peripheral vascular disease (PVD), it’s very clear high CRP levels are an independent risk factor for PAD development and severity. CRP may also predict the risk of restenosis after angioplasty.10)
Data from our clinic in patients with type 2 diabetes (n=254) reveal an association between albuminuria and hs-CRP with PAD.11) The clinical characteristics of the study population by the presence of albuminuria are shown in Table 2. It is apparent that PAD prevalence was much higher in the microalbuminuria and macroalbuminuria group compared to the no albuminuria group.
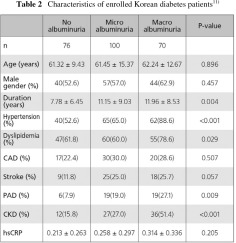
Clinical characteristics of the study population by the presence of PAD are shown in Table 3. Among the 254 diabetic subjects, those with PAD had a worse risk factor profile. Compared with subjects without PAD, those who had PAD were more likely to be older and male and have longer duration, higher prevalences of albuminuria and hypertension, as well as greater mean values for hsCRP.11)
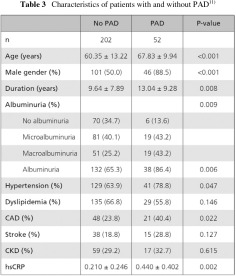
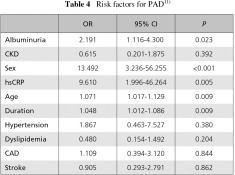
The occurrence of PAD was found to be 2.2 times more likely in the presence than in the absence of albuminuria. Odds ratio of hs-CRP was 9.6. An association between sex, age, and duration was also identified. However, the results of CKD, hypertension, dyslipidemia and a history of CAD were not statistically significant. This is likely due to the small number of PAD cases in this study (Table 4).11)
To summarize, non-diabetic and diabetic adults with albuminuria have a higher prevalence of PAD independent of current renal function. Individuals with CKD or albuminuria are at moderately increased risk for developing PAD. High CRP levels are an independent risk factor for PAD development and severity in patients with type 2 DM. As the early diagnosis of PAD is critical to prevent lower extremity revascularization procedures and amputations, measurement of microalbuminuria with hs-CRP is clinically useful in identifying diabetic subjects at risk for PAD.
Discussion points:
While screening for albuminuria is important for the detection of patients with PAD, there are no direct pathophysiological links between these conditions. Patients with albuminuria are more likely to experience more rapid deterioration of kidney function and cardiovascular status.
References
- 1).Caballero AE. Endothelial dysfunction, inflammation, and insulin resistance: a focus on subjects at risk for type 2 diabetes. Curr Diab Rep 2004; 4: 237-46 [DOI] [PubMed] [Google Scholar]
- 2).Sander D, Weimar C, Bramlage P, et al. Microalbuminuria indicates long-term vascular risk in patients after acute stroke undergoing in-patient rehabilitation. BMC Neurol 2012; 12: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Jager A, Kostense PJ, Ruhé HG, et al. Microalbuminuria and peripheral arterial disease are independent predictors of cardiovascular and all-cause mortality, especially among hypertensive subjects: five-year follow-up of the Hoorn Study. Arterioscler Thromb Vasc Biol 1999; 19: 617-24 [DOI] [PubMed] [Google Scholar]
- 4).Wattanakit K, Folsom AR, Criqui MH, et al. Albuminuria and peripheral arterial disease: results from the multi-ethnic study of atherosclerosis (MESA). Atherosclerosis 2008; 201: 212-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Wu CK, Yang CY, Tsai CT, et al. Association of low glomerular filtration rate and albuminuria with peripheral arterial disease: the National Health and Nutrition Examination Survey, 1999-2004. Atherosclerosis 2010; 209: 230-4 [DOI] [PubMed] [Google Scholar]
- 6).Wattanakit K, Folsom AR, Selvin E, et al. Kidney function and risk of peripheral arterial disease: results from the Atherosclerosis Risk in Communities (ARIC) Study. J Am Soc Nephrol 2007; 18: 629-36 [DOI] [PubMed] [Google Scholar]
- 7).Ridker PM, Hennekens CH, Buring JE, et al. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med 2000; 342: 836-43 [DOI] [PubMed] [Google Scholar]
- 8).Vu JD, Vu JB, Pio JR, et al. Impact of C-reactive protein on the likelihood of peripheral arterial disease in United States adults with the metabolic syndrome, diabetes mellitus, and preexisting cardiovascular disease. Am J Cardiol 2005; 96: 655-8 [DOI] [PubMed] [Google Scholar]
- 9).Vainas T, Stassen FR, de Graaf R, et al. C-reactive protein in peripheral arterial disease: relation to severity of the disease and to future cardiovascular events. J Vasc Surg 2005; 42: 243-51 [DOI] [PubMed] [Google Scholar]
- 10).Abdellaoui A, Al-Khaffaf H. C-reactive protein (CRP) as a marker in peripheral vascular disease. Eur J Vasc Endovasc Surg 2007; 34: 18-22 [DOI] [PubMed] [Google Scholar]
- 11).Jung Hwan Park et al. Diabetes & Metabolism Journal 2013; 37(Suppl 1): 168 [Google Scholar]
Day 2
Chairmen
Dr. Bo Yang Suh
Professor, Vascular Surgery,
Yeungnam University Hospital, Daegu, Korea
Dr. Hiroshi, Shigematsu,
Professor of Vascular Surgery,
Director of Vascular Center, Sanno Medical Center, International University of Health and Welfare,
Tokyo, Japan
Treatment strategies for PAD in CKD patients: its characteristics and importance of early detection - findings from the Japanese J-PADD study
Dr. Shuzo Kobayashi
CKD, particularly end-stage renal disease (ESRD), is an independent risk factor for PAD irrespective of the presence or absence of diabetes. When looking at the underlying cause of dialysis, diabetes is increasing while glomerulonephritis is apparently decreasing. Recently, however, the number of patients with diabetes has decreased due to an improvement in medical care and treatment prior to dialysis. In Japan, the 5-year survival rate of haemodialysis (HD) patients is 67% and many dialysis patients tend to live longer. But this is still poor compared to that of early gastric cancer with a 5-year survival rate of 71%. It is known that as renal function decreases, the prevalence of PAD increases. PAD accounts for significant morbidity and mortality among individuals with dialysis patients. Therefore, early diagnosis is very important to improve survival rate.
Prognosis after major amputation
The prognosis after major amputation is extremely poor. Dossa et al. (1994) found that ESRD has a profound negative impact on morbidity, mortality and survival rates after lower extremity amputation.1) In a retrospective study, survival in ESRD patients was 51.9% and 14.4% vs 75.4% and 42.2% in patients without renal failure at 1 and 5 years, respectively.2) A high incidence of progressive ischaemic gangrene (PIG) in patients undergoing maintenance dialysis (MD) has been observed, especially in those with DM. A study in Kuwait reported that PIG developed in 41.4/1000 person years of observation of diabetic patients who received MD, compared to 7.1/1000 in non-diabetic patients on MD and 0.14/1000 in diabetics without renal disease.3) Moreover, among patients selected to undergo surgical bypass for infrainguinal disease, a risk stratification model reliably identified a category of critical limb ischaemia (CLI) patients with a >50% chance of death or major amputation at 1 year.4)
Evaluation and treatment of cardiovascular complications in HD patients
In 2011, the Japanese society of dialysis therapy produced the guideline for the evaluation and treatment of cardiovascular complications in dialysis patients in which PAD is included, as follows:5)
HD is an independent risk factor of PAD with or without diabetes
In PAD patients, simultaneous evaluation of cardiovascular disease is recommended
HD patients often have below-knee calcification, but symptoms are poor - early detection is therefore important
ABI measurement is recommended at least once a year
It is important to fully evaluate the morbidity of ischaemia
Procedures for PAD diagnosis
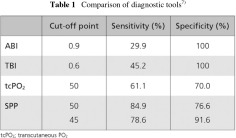
Skin perfusion pressure (SPP) measurement is an objective, noninvasive method that can be used to diagnose CLI with approximately 80% accuracy. In one study, the sensitivity of SPP <30 mmHg as a diagnostic test of CLI was 85%, and the specificity was 73%.6) In a comparison of the sensitivity and specificity of various diagnostic tools, SPP was the most useful for detecting PAD in HD patients, with an accuracy rate of 84.9% (Table 1). The sensitivity of the ABI was only 29.9%. For lesions located above the knee, TBI provided a sensitivity rate of 45.2% and specificity 100%.7)
Prevalence of PAD of HD patients using SPP
The normal cut-off value of SPP in HD patients is 40 mmHg. However, 50 mmHg is good for raising sensitivity. Using SPP, the prevalence of PAD in dialysis patients is considered to be 37.2%. In Japan, around 100,000 dialysis patients have PAD.8) Results from a Japanese survey in >4,000 dialysis patients showed that, despite a high-risk population, the proportion of patients on foot care was as low as 40%. Very few patients are diagnosed, examined and treated to a necessary and sufficient level. In particular, assessment and treatment for blood flow are not sufficient despite both being indispensable to PAD management. Unfortunately, PAD often goes undetected. In the US, for example, 29% of high risk patients in the primary care setting have an ABI of <0.9. Approximately half did not have a prior diagnosis of PAD and only 49% of their physicians were aware of the prior diagnosis.9)
Peripheral artery calcification associated with PAD severity
Ohtake et al. (2011) demonstrated that calcification of lower limb arteries is closely associated with the prevalence and severity of PAD in HD patients. Furthermore, arterial calcification of below-knee arteries and micro-inflammation represented as CRP were independent associating factors for low TBI, which was the independent associating factor for PAD and CLI in HD patients.10) Uremia causes chronic inflammation through an increases in TNF-α or IL-6. Recently, higher FGF23 levels have been found to be independently associated with higher levels of inflammatory markers in patients with CKD and with significantly greater odds of severe inflammation. FGF23 correlated directly with IL-6, CRP, TNF-α and fibrinogen.11)
A clinical study showed that monocyte-platelet conjugates play an important role in aortic stiffness and intima-media thickness (IMT) in HD patients. Flow cytometry demonstrated that leukocyte-aggregates (LA) were predominantly monocytes. Leukocyte aggregates were positively associated with plasma levels of fibrinogen, or serum high-sensitive CRP. Moreover, LA had highly significant associations with brachial-ankle pulse wave velocity and IMT.12) Dialysis patients with PAD are more likely to suffer from LA, than dialysis patients without PAD. The use of anti-platelet therapy is therefore recommended. Beraprost sodium (BPS), for example, decreased LA by 28%. Unfortunately, it has recently been reported that CKD patients have poor responsiveness to antiplatelet therapy.13)
Endothelial dysfunction, leading to platelet activation, is fundamental to atherosclerosis pathophysiology. In turn, platelet aggregation, smooth muscle contraction and proliferation disturbs peripheral microcirculation, leading to the development of ischemia.
Several treatment modalities are available. From an instruction of cessation of smoking, foot care, and foot wear, exercise, antiplatelet therapy, revascularization, debridement, LDL-apheresis, hyperoxgen therapy and rehabilitation. A multidisciplinary approach to treatment is important to optimize outcomes. These include:
Smoking cessation
Ongoing observation, evaluation and physical assessment of legs
Anti-platelet therapy
Evaluation of ischemia and infection
Suitable debridement and dressing technology are important
Don’t miss the point at which amputation is necessary
Mitigation of the role of amputation and prudent examination
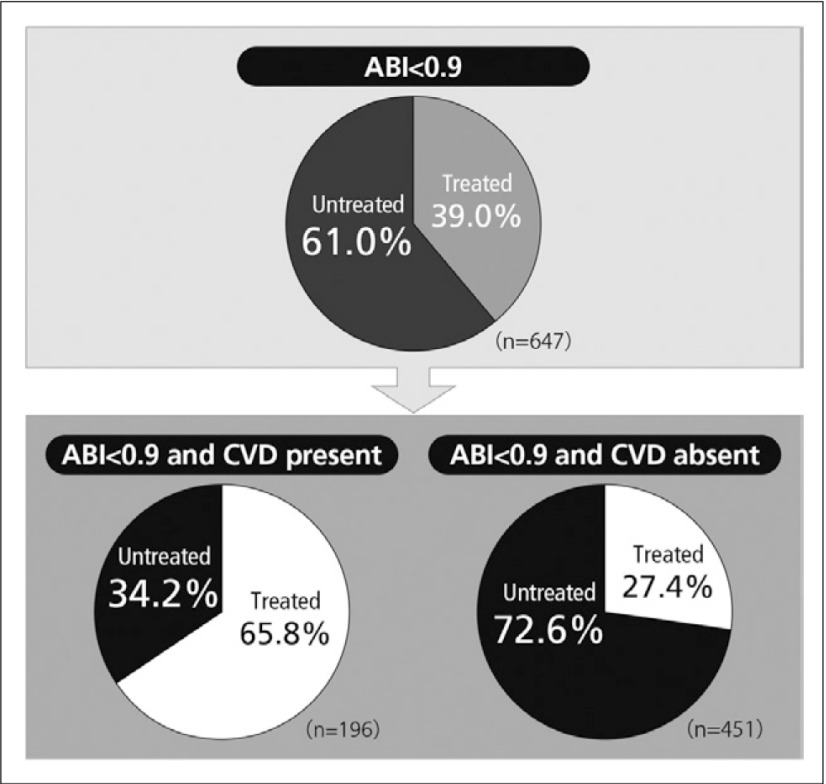
Despite the fact that PAD is a vascular disease, PAD patients are often not treated with antiplatelet drug therapy. When the ABI of 7,458 individuals were studied in a clinical trial in the USA, 5.9% had a suggestion of PAD and of these, 61% received no antiplatelet drug (Fig. 1). When analysed in relation to a complication by cardiovascular disease, the percentage of cases receiving antiplatelet drug therapy was higher in the complication positive other than PAD group, but the percentage of cases receiving no antiplatelet drug was more than 70% in the complication-free group, suggesting that PAD is ignored despite similar cardiovascular complications.14)
Fig. 1.
Percentage of antiplatelet drug-treated patients having ABI <0.914)
Cilostazol is known to improve walking distance as well as ischaemic symptoms. However, cilostazol is contraindicated for patients with heart failure and its use is therefore generally avoided where effective alternatives, such as beraprost, are available.
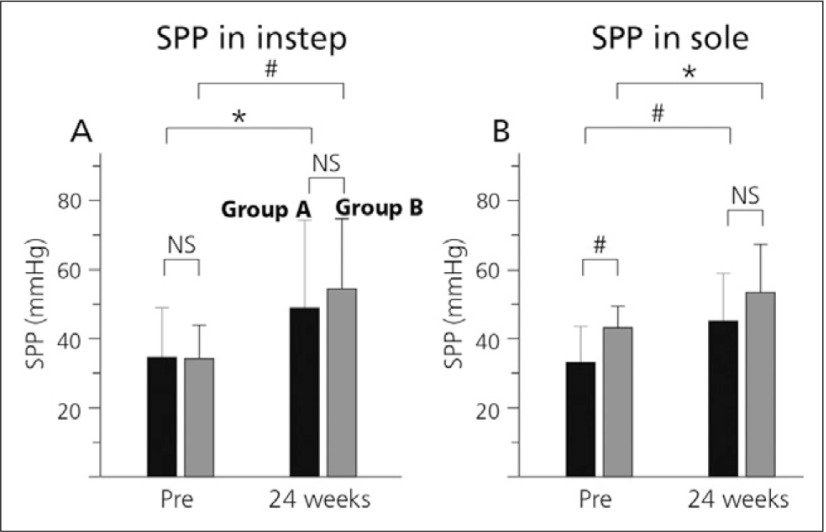
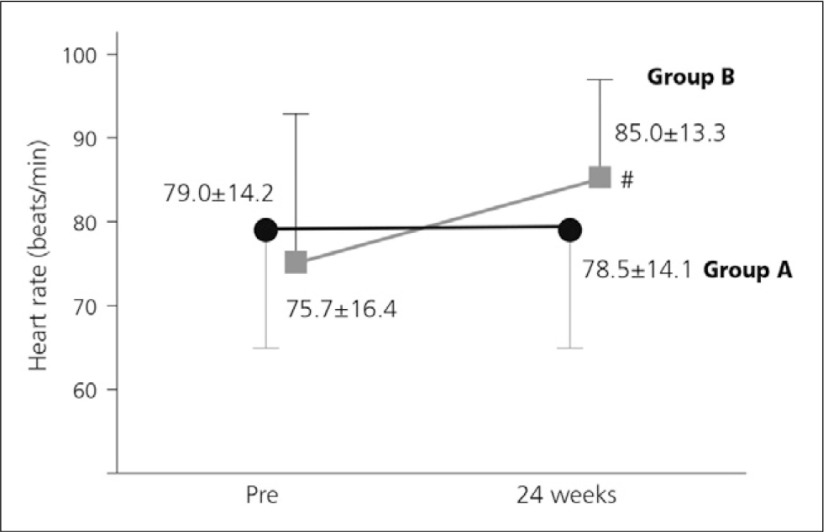
The Japan-Peripheral Arterial Disease study in patients with Dialysis (J-PADD) was a randomised, controlled, multicentre study comparing BPS (group A) and other anti-platelet therapies (antiplatelets, cilostazol or sarpogrelate, group B) in 72 HD patients with PAD. Patient SPP was found to increase significantly in both groups in instep and in sole (Fig. 2). Importantly, patients in the BPS group had no increase in heart rate (Fig. 3).15)
Fig. 2.
Changes in SPP.
(A) SPP in instep, (B) SPP in sole.
■ indicate group A (beraprost, n=35) and
■ indicate group B (cilostazol or sarpogrelate, n=33).15)
*p<0.05, #p<0.01 vs pretreatment values. NS, not significant
Fig. 3.
Effect of treatment on heart rate.
● indicate heart rate of group A (beraprost, n=35),
■ indicate heart rate of cilostazol-treated patients in group B (n=18).15) #p<0.01 vs pretreatment values
While both ABI and TBI did not show a significant change (Table 2), TBI, which reflects more distal circulatory changes, appeared to increase.15)
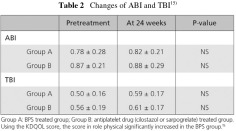
In both groups, neither cardiovascular events nor PAD events show any difference between two groups.15)
Renal anemia in dialysis patients
It has been reported that PGI2 has pleiotropic effects that are anti-inflammatory and anti-atherogenic. Findings from a clinical report suggest that beraprost may improve renal anemia in HD patients. After six months, the titer of haemoglobin had significantly increased in the BPS group compared to the baseline, and there was a significant difference between the BPS group and the control group.16)
Bypass grafting endovascular therapy
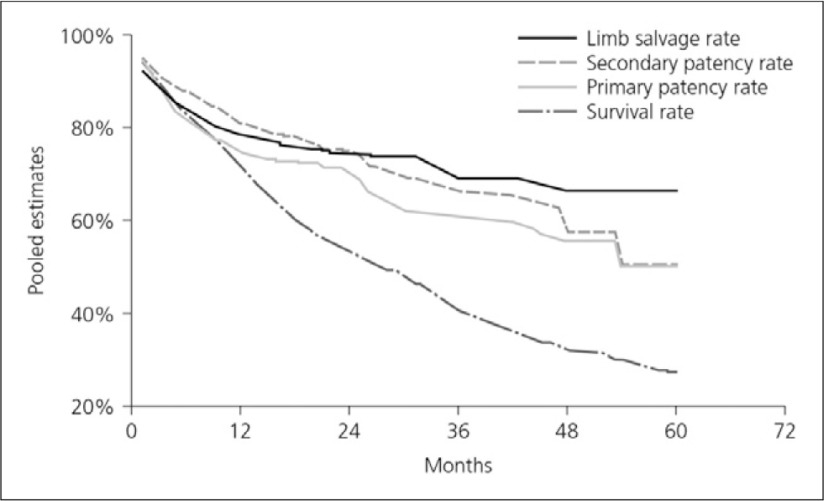
The results of revascularisation tend to be very poor. Vascular calcification and the lesion located in more distal sites make treatment difficult. A meta-analysis found that limb salvage can be achieved in most ESRD patients who undergo bypass surgery for critical ischemia, but survival is poor. To avoid early amputation despite a patent graft, bypass grafting should not be offered to patients with a great amount of tissue loss or extensive infection. The 2-year survival rate was 50% although the limb salvage rate was 75% (Fig. 4).17)
Fig. 4.
Pooled survival curves (meta-analysis)17)
LDL-apheresis (LDLA) treated scene
LDL-apheresis was originally used for familial hyperlipidemia, and in Japan extended to use for the treatment of patients with PAD and nephrotic syndrome due to steroid-resistant focal glomerular sclerosis (FGS). The reason why this treatment is applicable for these disorders is due to the fact that LDLA exerts its favorable effects beyond the lipid-lowering effect. The main underlying mechanisms, for example, in the case of LDLA application in patients with PAD are: (1) improvement of hemorheology, (2) improvement of endothelial dysfunction, (3) elevations of serum levels of NO and bradykinin, (4) increase in serum levels of vascular endothelial growth factor, and (5) reduction of adhesion molecules on monocytes.18)
Massive proteinuria is also an important challenge in nephrology. The possible mechanisms besides removal of toxic lipids are the reduction of the vasoconstrictive prostanoid and TXA2 and an improvement in macrophage function evidenced by a significant amelioration of interleukin-8 production by LPS-stimulated peripheral blood mononuclear cells. It is intriguing to note that in terms of pharmacodynamics, LDLA improves steroid and cyclosporine uptake into lymphocytes.18)
Moreover, in patients with peripheral arterial occlusive disease, serum levels in VEGF statistically increased after 10 sessions of LDLA, and further increased up 3 months after a completion of this intervention. After 10-times therapy, IGF-I significantly decreased (p<0.05), but increased over the basal value 3 months after this therapy. Plasma fibrinogen statistically decreased and remained low for 3 months. The favorable effects of LDLA may be ascribed to up-regulation of VEGF and IGF-I associated with decreased fibrinogen levels.19)
Maggot debridement therapy (MDT) is effective for treating intractable wounds. Results from a clinical study suggest that the formation of healthy granulation tissue observed during MDT results from the increased hepatocyte growth factor (HGF), which is clearly an important factor involved in cutaneous wound healing.20)
Several kinds of treatment strategies are available. Antiplatelet agents should be continuously used from an early stage even without overt symptoms until revascularization or debridement
Even if the patients are required to receive an amputation, healthcare professionals should not hesitate in putting their patients forward for amputation if it is going to help their lives. In addition, as with cancer patients, that mental care is also important. A multi-disciplinary team approach is important, and for limb salvage and to save a patient’s life, early diagnosis is essential. To diagnose the patient, SPP and angiography should be used for examination. In terms of treatment, antiplatelet agents continue to play an important role.
Interestingly, amongst its other effects, beraprost has been demonstrated to also improve insulin resistance such that it contributes to an overall systemic vascular protective action beyond antiplatelet effects. Beraprost sodium is also reported to attenuate inflammation. Treatment with beraprost improved glucose tolerance and insulin action without body weight change in mice. Beraprost is therefore thought to improve glucose intolerance through suppression of inflammatory cytokines in white adipose tissue.21) These and other properties suggest that this agent has an important role to play in the management of PAD patients with CKD and/or DM.
Discussion points:
For asymptomatic patients at high risk for PAD, we would typically assess coronary and peripheral artery function prior to initiation of therapy, which will most likely be an antiplatelet agent. J-PADD study suggests that beraprost was effective in increasing the levels of SPP without influencing the heart rate, and that it improves the QOL in HD patients with PAD. Beraprost sodium is suitable and would be preferable to aspirin as this agent does not have any further pleiotropic effects beyond antiplatelet action. As beraprost also helps to protect endothelial and renal function, it offers the potential for more diverse clinical benefits and improved outcomes.
Regarding the use of dietary phosphate restriction, it is important that this does not also result in malnutrition and so the advice to the patient is to not restrict intake. In this case, we need to use phosphate binders or hemodialysis to reduce serum phosphate. Calcium-containing phosphate binders should absolutely be avoided. Patient pill burden is also an issue to be considered in optimal management.
References
- 1).Dossa CD, Shepard AD, Amos AM, et al. Results of lower extremity amputations in patients with end-stage renal disease. J Vasc Surg 1994; 20: 14-9 [DOI] [PubMed] [Google Scholar]
- 2).Aulivola B, Hile CN, Hamdan AD, et al. Major lower extremity amputation: outcome of a modern series. Arch Surg 2004; 139: 395-9; discussion 399 [DOI] [PubMed] [Google Scholar]
- 3).el-Reshaid K, Madda JP, al-Duwairi Q, et al. Progressive ischemic gangrene in dialysis patients: a clinicopathological correlation. Ren Fail 1995; 17: 437-47 [DOI] [PubMed] [Google Scholar]
- 4).Schanzer A, Mega J, Meadows J, et al. Risk stratification in critical limb ischemia: derivation and validation of a model to predict amputation-free survival using multicenter surgical outcomes data. J Vasc Surg 2008; 48: 1464-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Hirakata H, Nitta K, Inaba M, et al. Japanese Society for Dialysis Therapy guidelines for management of cardiovascular diseases in patients on chronic hemodialysis. Ther Apher Dial 2012; 16: 387-435 [DOI] [PubMed] [Google Scholar]
- 6).Castronuovo JJ, Adera HM, Smiell JM, et al. Skin perfusion pressure measurement is valuable in the diagnosis of critical limb ischemia. J Vasc Surg 1997; 26: 629-37 [DOI] [PubMed] [Google Scholar]
- 7).Okamoto K, Oka M, Maesato K, et al. Peripheral arterial occlusive disease is more prevalent in patients with hemodialysis: comparison with the findings of multidetector-row computed tomography. Am J Kidney Dis 2006; 48: 269-76 [DOI] [PubMed] [Google Scholar]
- 8).Okamoto K, Oka M, Maesato K, et al. J Jpn Coll Angiol 2006; 46: 829-35 [Google Scholar]
- 9).Hirsch AT, Criqui MH, Treat-Jacobson D, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA 2001; 286: 1317-24 [DOI] [PubMed] [Google Scholar]
- 10).Ohtake T, Oka M, Ikee R, et al. Impact of lower limbs' arterial calcification on the prevalence and severity of PAD in patients on hemodialysis. J Vasc Surg 2011; 53: 676-83 [DOI] [PubMed] [Google Scholar]
- 11).Munoz Mendoza J, Isakova T, Ricardo AC, et al. Fibroblast growth factor 23 and Inflammation in CKD. Clin J Am Soc Nephrol 2012; 7: 1155-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Kobayashi S, Miyamoto M, Kurumatani H, et al. Increased leukocyte aggregates are associated with atherosclerosis in patients with hemodialysis. Hemodial Int 2009; 13: 286-92 [DOI] [PubMed] [Google Scholar]
- 13).Gremmel T, Müller M, Steiner S, et al. Chronic kidney disease is associated with increased platelet activation and poor response to antiplatelet therapy. Nephrol Dial Transplant 2013; 28: 2116-22 [DOI] [PubMed] [Google Scholar]
- 14).Pande RL, Perlstein TS, Beckman JA, et al. Secondary prevention and mortality in peripheral artery disease: National Health and Nutrition Examination Study, 1999 to 2004. Circulation 2011; 124: 17-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Ohtake T, et al. Ther Apher Dial. 2013.
- 16).Moriya H, Ishioka K, Honda K, et al. Beraprost sodium, an orally active prostaglandin I(2) analog, improves renal anemia in hemodialysis patients with peripheral arterial disease. Ther Apher Dial 2010; 14: 472-6 [DOI] [PubMed] [Google Scholar]
- 17).Albers M, Romiti M, De Luccia N, et al. An updated meta-analysis of infrainguinal arterial reconstruction in patients with end-stage renal disease. J Vasc Surg 2007; 45: 536-42 [DOI] [PubMed] [Google Scholar]
- 18).Kobayashi S. Applications of LDL-apheresis in nephrology. Clin Exp Nephrol 2008; 12: 9-15 [DOI] [PubMed] [Google Scholar]
- 19).Kobayashi S, Moriya H, Negishi K, et al. LDL-apheresis up-regulates VEGF and IGF-I in patients with ischemic limb. J Clin Apher 2003; 18: 115-9 [DOI] [PubMed] [Google Scholar]
- 20).Honda K, Okamoto K, Mochida Y, et al. A novel mechanism in maggot debridement therapy: protease in excretion/secretion promotes hepatocyte growth factor production. Am J Physiol, Cell Physiol 2011; 301: C1423-30 [DOI] [PubMed] [Google Scholar]
- 21).Inoue E, Ichiki T, Takeda K, et al. Beraprost sodium, a stable prostacyclin analogue, improves insulin resistance in high-fat diet-induced obese mice. J Endocrinol 2012; 213: 285-91. [DOI] [PubMed] [Google Scholar]