Abstract
Background
Patient navigation is a promising intervention to address cancer disparities but requires a multisite controlled trial to assess its effectiveness.
Methods
The Patient Navigation Research Program compared patient navigation with usual care on time to diagnosis or treatment for participants with breast, cervical, colorectal, or prostate screening abnormalities and/or cancers between 2007 and 2010. Patient navigators developed individualized strategies to address barriers to care, with the focus on preventing delays in care. To assess timeliness of diagnostic resolution, we conducted a meta-analysis of center- and cancer-specific adjusted hazard ratios (aHRs) comparing patient navigation vs usual care. To assess initiation of cancer therapy, we calculated a single aHR, pooling data across all centers and cancer types. We conducted a metaregression to evaluate variability across centers. All statistical tests were two-sided.
Results
The 10521 participants with abnormal screening tests and 2105 with a cancer or precancer diagnosis were predominantly from racial/ethnic minority groups (73%) and publically insured (40%) or uninsured (31%). There was no benefit during the first 90 days of care, but a benefit of navigation was seen from 91 to 365 days for both diagnostic resolution (aHR = 1.51; 95% confidence interval [CI] = 1.23 to 1.84; P < .001)) and treatment initiation (aHR = 1.43; 95% CI = 1.10 to 1.86; P < .007). Metaregression revealed that navigation had its greatest benefits within centers with the greatest delays in follow-up under usual care.
Conclusions
Patient navigation demonstrated a moderate benefit in improving timely cancer care. These results support adoption of patient navigation in settings that serve populations at risk of being lost to follow-up.
Patient navigation refers to support and guidance offered to persons with abnormal cancer screening results or cancer, with the goal of improving access and coordination of timely care (1). The primary purposes of navigation are to identify and remove barriers to care. Patient navigation was conceived to address health disparities and assist those at risk for delays in care among racial and ethnic minority and lower-income populations. Although patient navigation is rapidly becoming a standard of care (2,3), and literature reviews (4,5) suggest that patient navigation improves timeliness of care, many of the studies have been small, conducted at a single institution, lacked concurrent control arms, or had dissimilar outcome metrics. Strategies and operational definitions of navigation that currently exist in the literature vary considerably, with little consensus on the roles and scope of patient navigation, which also limits the ability to assess the impact of this intervention (1,6,7).
The Patient Navigation Research Program (PNRP) is the first multicenter clinical trial to examine the benefits of patient navigation. Using community-based participatory research methods and addressing care to a diverse group of communities, PNRP targeted four common cancers (breast, cervical, colorectal, and prostate) with available screening tests and evidence of disparate outcomes in underserved populations. We present the findings of the two primary outcomes of the trial: 1) time from abnormal screening to diagnostic resolution and 2) time to initiation of treatment after a diagnosis of cancer or precancerous lesion.
Methods
Overall Study Design
We report here on the combined analyses of nine of the 10 PNRP centers. Each center designed and implemented the intervention within the context of the community setting in which it operated, most using community-based participatory research principles. Data sharing agreements with local communities at the 10th center precluded inclusion into the combined dataset (8). Two centers conducted an individually randomized clinical trial (9–11), two centers conducted a group-randomized trial (12–14), and five centers used quasi-experimental designs with nonrandom assignment into the intervention and controls arms at the group level (15–20). This individualization of study design based on community input required modifications from methods traditionally used for analyses of multicenter trials. For the abnormal cancer screening resolution analysis, eight of the nine centers had sufficient sample size and power to conduct a center-specific analysis (11,13,14,16–18); therefore we developed an a priori plan for a prospective meta-analysis (21). For analysis of the initiation of cancer treatment, none of the individual centers was powered to conduct separate analyses; therefore, we conducted a pooled analysis combining data across the nine centers and all cancer types. The institutional review board of each respective institution approved the research (ClinicalTrials.gov identifiers: NCT00613275, NCT00496678, NCT00375024, NCT01569672).
Participant Eligibility
Participants aged 18 years and older were included if they had an abnormal breast, cervical, colorectal, or prostate cancer screening result (22). Participants with invasive or preinvasive lesions with guidelines recommending treatment (23–26) were eligible for the cancer treatment initiation analysis. In addition to invasive cancers, we included breast ductal carcinoma in situ, cervical carcinoma in situ, cervical intraepithelial lesions grade II and III, and colorectal carcinoma in situ. Participants for the cancer treatment initiation analysis included both those with the abnormal screening and those recruited after their cancer diagnosis. Exclusion criteria included prior history of cancer, prior patient navigation support, cognitive conditions that would exclude participation in navigation, and pregnancy.
Study Centers
The study was conducted at nine centers recruiting participants from between one and 21 care sites. The majority of the sites were community health centers, in addition to several outpatient practice settings within and outside of safety-net hospitals. Most sites cared for primarily patients who were low income, uninsured or publically insured, and from racial and ethnic minority populations.
Intervention
Navigators used the care management model (27) to identify barriers to recommended care, develop strategies to address these barriers, and track participants through the steps in their medical evaluation. Their focus was on timely diagnostic resolution and therapy initiation. Navigation was initiated after a clinician informed the participant of the abnormal test result. Most programs were imbedded within clinical care systems with close interface with the clinical practice, and most included opportunities for face-to-face interaction between participants and navigators, as well as telephone and mail contact. In addition to patient contact, navigators worked with families, health-care providers, and social service agencies to identify resources to address barriers to care. Navigators documented their activities in a standardized, structured template that captured the barriers identified and the activities performed to address the barriers for each encounter with the patient. Examples of navigation services included arranging financial support, scheduling and arranging for transportation to scheduled appointments, coordinating care among providers, arranging for interpreter services, and linking to community resources.
Each center hired navigators with a minimum of a high school diploma and used the same protocol for the intervention. Navigators participated in annual national trainings and webinars in order to standardize the intervention (28) and were assessed for national core competencies twice annually using a standardized checklist. Centers determined the specific job description and supplemented the national training with ongoing, local training to provide navigators with local context and resource information.
Data Collection
Definitions of all variables were developed by the PNRP investigators and adhered to by all centers. Coding questions were reviewed weekly by investigators to maintain rigorous data entry standards. Clinical variables were abstracted from the participants’ medical records, including type of screening abnormality, type and stage of cancer, dates and types of clinical services, and clinical outcomes. Each center coded race/ethnicity uniformly for all participants either from self-report or medical records. Race/ethnicity was collapsed into a single categorical variable: white, black, Hispanic, and other (Asian, Native American, unknown). Primary language was coded as English or other. Health insurance coverage at the time of study entry was hierarchically categorized into private, public, or no insurance coverage.
Statistical Analysis Plan
Time to Diagnostic Resolution.
The first outcome of interest was whether and when diagnostic resolution of the abnormal cancer screening result was achieved, defined as time from date of initial abnormal cancer screening test result to date when the definitive diagnostic test or evaluation was completed. We analyzed this outcome using two methods determined a priori (21): First, for each study center and cancer screening type, we calculated an unadjusted rate of achieving diagnostic resolution within 1 year, comparing the intervention with the control arm. The second method used a prospective meta-analysis and provided a single adjusted effect estimate (the adjusted hazard rate ratio [aHR]) across all centers. An adjusted hazard rate ratio was calculated for time to diagnostic resolution for each cancer type within each center. Models were adjusted for participant race/ethnicity, insurance status, language, marital status, and age, except for centers C, F, G, and H, which did not include marital status, and centers C and H, which did not include language, because of missing data. To account for potential intraclass correlations within the sites of care for centers with group-randomized trial or quasi-experimental designs, we used either a clustering variable in a proportional hazards regression (29) (centers A, B, C, F, G, and H) or a shared frailty model (30) (center E).
In developing the proportional hazards models, almost half of the models from centers had a violation of the proportional hazards assumptions, indicating that the effect of navigation varied across time. Because most of these changes occurred at approximately 90 days within the 365 days of observation, we addressed these violations by calculating a separate adjusted hazard rate ratio for 0 to 90 days and for 91 to 365 days, per methods previously described (31,32). Effect size (aHR) estimates were calculated for each time period for each center–cancer combination. Separate meta-analyses were conducted for each time period with a random effects model using the method described by DerSimonion and Laird (33) and a test for heterogeneity across the centers (34,35). Because of the heterogeneity noted, we conducted a meta-regression with two center-level variables, baseline rates of diagnostic resolution in the usual care arm at each center for each cancer type, and method of subject assignment to intervention at the center (random vs nonrandom designs). An influence analysis was conducted by comparing the recalculated effect estimate to the confidence interval to the initial estimate after removing one center at a time to determine whether the results were unduly influenced by a single center. All analyses were conducted using Stata version 10.0 (36).
Time to Treatment Initiation.
The second outcome was time to initiation of treatment for participants with invasive cancer or precancerous lesions. We pooled data across all centers for a single analysis. This method does not account for intraclass correlation at the site within center level. However, 27% of subjects were recruited from the sites conducting individual randomized clinical trial designs, and 50% of subjects were recruited from a single site within a center or sites with five or fewer cases, thereby reducing the potential impact of intraclass correlation. Guidelines developed by the Centers for Disease Control recommend that more than 90% of women initiate therapy within 90 days (37), and a number of analyses have suggested reduced cancer survival with delays beyond 60 days (37–41). Therefore, we compared the unadjusted proportion of participants who initiated treatment within 60, 90, and 365 days between the intervention and control arms. We calculated an adjusted hazard rate ratio adjusting for race/ethnicity, insurance status, age, language, marital status, and cancer type. Because of violations in proportional hazards, we calculated separate adjusted hazard rate ratios for 0 to 90 days and 91 to 365 days. Influence analyses recalculated the adjusted hazard rate ratio, removing the data from each center to assess whether the effect size was unduly influenced by one center. All statistical tests were two-sided.
Results
Each center focused on different cancers, eight of nine centers enrolled in breast cancer, four enrolled in cervical cancer, four enrolled in colorectal cancer, and two enrolled in prostate cancer. Table 1 presents the demographic data on the two outcomes, the sample size for each cancer type, and the enrollment at each of the centers. We enrolled 10521 participants with abnormal cancer screening results and 2105 with a diagnosis of cancer or of a precancerous lesion. Refusal rates by center ranged from less than 1% to 23%. Enrolled participants were diverse; 73% were from racial and ethnic minority groups, 40% were publically insured, and 31% were uninsured at the time of study enrollment. The imbalances in recruitment by race/ethnicity and insurance reflect the group-allocated designs. More than 85% of all participants were women because most participants had abnormal breast or cervical cancer screening results. The low enrollment in colorectal cancer reflected the shift at most institutions to colonoscopy, where concurrent diagnostic biopsy during the screening test eliminated the need for follow-up.
Table 1.
Demographic characteristics of participants enrolled in the Patient Navigation Research Program
| Variable | Outcome 1: diagnostic evaluation (n = 10521) | Outcome 2: cancer treatment (n = 2105) | ||
|---|---|---|---|---|
| Intervention | Control | Intervention | Control | |
| No. (%) | No. (%) | No. (%) | No. (%) | |
| Race /ethnicity | ||||
| White | 1224 (24) | 1370 (25) | 285 (28) | 376 (35) |
| Black | 1487 (29) | 1843 (34) | 385 (37) | 425 (40) |
| Hispanic | 2142 (42) | 1964 (36) | 338 (33) | 213 (20) |
| Other | 207 (4) | 185 (3) | 16 (2) | 39 (4) |
| Insurance | ||||
| Private | 1202 (24) | 1599 (29) | 342 (33) | 461 (43) |
| Public | 1969 (39) | 2290 (42) | 448 (43) | 492 (46) |
| Uninsured | 1837 (36) | 1548 (28) | 236 (23) | 119 (11) |
| Sex | ||||
| Female | 4665 (92) | 5006 (92) | 874 (85) | 920 (86) |
| Marital status | ||||
| Married | 1772 (35) | 1588 (29) | 383 (37) | 397 (37) |
| Age, y | ||||
| Mean ± standard deviation | 43.6±14.8 | 47.2±14.9 | 51.7±15.0 | 53.8±15.3 |
| Cancer type | ||||
| Breast | 3083 (61) | 3643 (67) | 605 (59) | 683 (64) |
| Cervical | 1455 (29) | 1226 (22) | 245 (24) | 207 (19) |
| Colorectal | 219 (4) | 278 (5) | 52 (5) | 58 (5) |
| Prostate | 306 (6) | 311 (6) | 130 (13) | 125 (12) |
| Sites | ||||
| A | 1496 (30) | 1543 (28) | 126 (12) | 74 (7) |
| B | 357 (7) | 542 (10) | 68 (7) | 100 (9) |
| C | 243 (5) | 245 (4) | 85 (8) | 86 (8) |
| D | 490 (10) | 508 (9) | 121 (12) | 114 (11) |
| E | 444 (9) | 346 (6) | 46 (4) | 24 (2) |
| F | 639 (13) | 408 (7) | 226 (22) | 145 (14) |
| G | 586 (12) | 683 (13) | 25 (2) | 47 (4) |
| H | 808 (16) | 1183 (22) | 167 (16) | 328 (31) |
| I | 0 | 0 | 168 (16) | 155 (14) |
| Total | 5063 (100) | 5458 (100) | 1032 (100) | 1073 (100) |
Diagnostic Resolution Trial
Figure 1 shows the unadjusted proportions of diagnostic resolution within 365 days, organized by cancer screening type and resolution rates in the control arms of the centers. For most centers, the navigated arm had a higher percentage of participants who reached a diagnostic resolution than the control arm, and this was similar for all four cancer types. The effect of navigation was greatest at centers where the control arms had the lowest rates, with differences of 20% at several centers. We observed a ceiling effect, where navigation had little or no impact when the control arms had 90% or greater resolution by 1 year.
Figure 1.
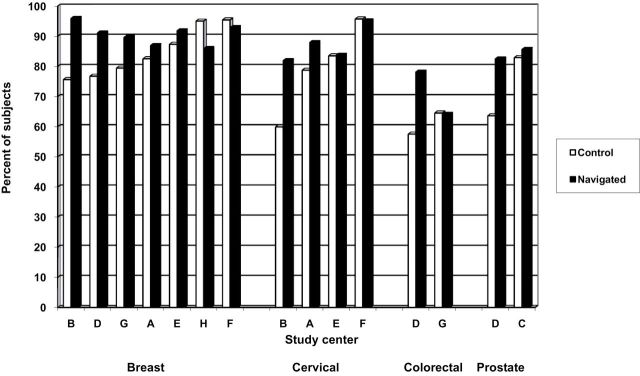
Unadjusted proportion of participants with abnormal cancer screening or symptoms who reach diagnostic resolution within 365 days in navigated and control arms by cancer screening type, study center: Patient Navigation Research Program.
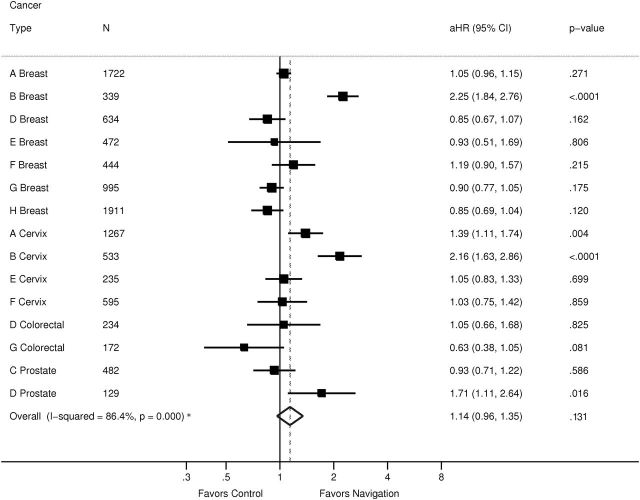
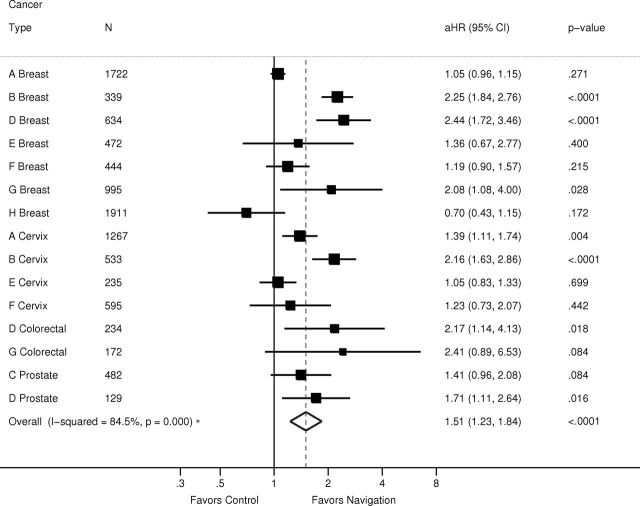
Figures 2 and 3 use forest plots by cancer type to report the meta-analysis of the adjusted hazard rate ratios of time to resolution of abnormal cancer screening results for each of the two time periods, where adjusted hazard rate ratios greater than one indicate a benefit in the navigated arm. The adjusted hazard rate ratio from the metanalysis was 1.14 (95% confidence interval [CI] of 0.96 to 1.35; P =.14) from 0 to 90 days and 1.51 (95% CI = 1.23 to 1.84; P < .001) from 91 to 365 days. Influence analyses always produced an effect size within the confidence interval of the original calculation.
Figure 2.
Meta-analysis of impact of patient navigation on diagnostic resolution after cancer screening abnormality from 0 to 90 days: Patient Navigation Research Program. I 2 addresses the heterogeneity of the model and is not the overall effect of the intervention. The solid vertical line denotes 1, or no effect. The squares denote the adjusted hazard ratio for each center and cancer type, with the horizontal line indicating the 95% confidence interval. The dotted vertical line denotes the adjusted hazard ratio for the meta-analysis. The diamond indicates the 95% confidence interval of the adjusted hazard ratio. The letters (A–H) in the first column denote the study center. aHR = adjusted hazard ratio; CI = confidence interval.
Figure 3.
Meta-analysis of impact of patient navigation on diagnostic resolution after cancer screening abnormality from 91 to 365 days: Patient Navigation Research Program. I 2 addresses the heterogeneity of the model and is not the overall effect of the intervention. The solid vertical line denotes 1, or no effect. The squares denote the adjusted hazard ratio for each center and cancer type, with the horizontal line indicating the 95% confidence interval. The dotted vertical line denotes the adjusted hazard ratio for the meta-analysis. The diamond indicates the 95% confidence interval of the adjusted hazard ratio. The letters (A–H) in the first column denote the study center.
Because there was substantial heterogeneity among the effect size estimates (overall I 2 for heterogeneity = 84.5 %; P < .001), a metaregression was performed on the 91 to 365 day period, the period demonstrating a benefit of navigation. Two study center–level variables examined were patient assignment (random vs nonrandom) to intervention arm and diagnostic resolution rate of control subjects (a continuous variable). The results indicated that patient assignment was not related to effect size estimates (P > .79), whereas resolution rate of control subjects was statistically significantly related to the adjusted hazard rate ratio (P < .01), confirming that navigation had a larger effect when the time to diagnostic resolution was delayed in the usual care arm.
Although our measure of time to diagnostic resolution began at the date that the screening test was performed, patient navigation efforts did not begin immediately. Navigation was subject to delays in receiving the test report, in the initial contact by a clinician with the participant, and in contacting the participant for consent to enroll. The range by center of median times to initiation of navigation was longer with cervical (24–64 days), colorectal (19–48 days), and prostate (33–34 days) cancer screening tests than breast (7–33 days) cancer screening tests. Initiation of navigation after a diagnosis of cancer or of a precancerous lesion had fewer delays because 60% of these participants were already consented and enrolled at the time of their diagnosis.
Treatment Initiation Trial
We calculated the unadjusted proportions of participants who initiated treatment at specified time points. The navigated arm had a smaller proportion of participants who had initiated treatment at both 60 days (57% vs 62%) and 90 days (73% vs 75%) compared with the control arm; however, at 365 days, the findings had reversed, and navigated participants had a higher proportion (89%) who had initiated treatment compared with the control participants (87%). We calculated from the adjusted Cox regression analysis separate adjusted hazard rate ratios for 0 to 90 days and 91 to 365 days. The adjusted hazard rate ratio was 0.85 (95% CI = 0.71 to 1.01; P = .07) from 0 to 90 days and 1.43 (95% CI = 1.10 to 1.86; P < .007) from 91 to 365 days. Influence analyses removing one center and recalculating the adjusted hazard rate ratio always produced an effect size within the confidence interval of the original calculation.
Table 2 presents the adjusted hazard rate ratio at 0 to 90 days and 91 to 365 days for diagnostic resolution and treatment initiation. The findings for the two outcomes parallel one another, with no impact of patient navigation in the first 90 days of observation. For both outcomes, navigation showed a statistically significant benefit from 91 to 365 days (diagnostic metanalysis: aHR = 1.51, 95% CI = 1.23 to 1.84; P < .001; treatment: adjusted Cox regression aHR = 1.43, 95% CI = 1.10 to 1.86, P < .007).
Table 2.
Adjusted hazards ratios for diagnostic care and cancer care trials of the Patient Navigation Research Program*
| Outcome | Days 0–90 adjusted HR (95% CI) | Days 91–365 adjusted HR (95% CI) |
|---|---|---|
| Diagnostic phase | 1.14 (0.96 to 1.35) | 1.51 (1.23 to 1.84) |
| Treatment phase | 0.85 (0.7 to 1.01) | 1.43 (1.10 to 1.86) |
* CI = confidence interval; HR = hazard ratio.
Discussion
This is the first multisite study of patient navigation as an intervention to reduce disparities in cancer outcomes by addressing barriers to follow-up care and treatment for underserved and minority populations. The goal of this study was to investigate the efficacy of patient navigation in reducing delays in resolving abnormal cancer screening tests and initiating treatment of cancer among diverse populations and four cancer types. Results of this trial indicate a statistically significant, although modest, benefit of navigation on timely cancer care. Both diagnostic evaluation and cancer treatment were initiated earlier in the navigator arm compared with the control arm from 91 to 365 days of observation, but not in the first 90 days.
Our finding of no benefit of patient navigation in the first 90 days may reflect the time required to connect navigators with participants. We note, for example, that 13% of participants with abnormal breast cancer screening results were not able to be contacted by their navigator within 60 days. Our finding of no benefit in the first 90 days may also reflect the fact that some participants are able to overcome barriers without a navigator. We observed the greatest benefits among centers where control participants experienced longer delays; conversely, we observed little benefit among centers where those in usual care achieved 90% resolution at 1 year. These findings suggest that navigation is likely to show the greatest effects in centers and populations with the greatest delays in follow-up under usual care.
Previous studies have reported mixed results in regard to the benefit of navigation. Whereas some studies reported more timely care (42–47), others have not (46,48). In the PNRP, the impact of patient navigation was greatest among centers with low baseline resolution or treatment initiation rates in the control arm. This speaks to a need for patient navigation services in settings that possibly have few resources to assist underserved participants to complete timely diagnostic resolution and initiate cancer treatment. Other studies have found stronger effects of patient navigation interventions among populations with low adherence rates (49).
Some of this variation in prior studies is because of wide variation in what is considered patient navigation. A limitation of our study is the ability to assess the fidelity of implementation of the intervention across the sites and navigators. Although we were unable to assess all of the variability of navigator activities across the centers, we addressed this variability by having a standardized definition of navigation, navigator training and protocols, templates for assessing and recording barriers to care and navigator actions to address barriers, and a standardized competency assessment of navigation standards. Variation in implementation of the intervention may account for some of the heterogeneity of the effect sizes seen. Even when navigators were imbedded within clinical practices, we observed delays in initiating navigation, which likely limited their impact during the first 90 days of care. New data on the efficacy of prostate cancer screening has emerged since the design of the PNRP (50,51), with new guidelines advising against prostate cancer screening or for informed decision making, as opposed to population based screening (52–56). We did not have a priori rules for removing centers from the analysis; thus we kept the two prostate screening centers in our analysis. Our sensitivity analysis indicated that no one center affected the overall findings of our analysis. The generalizability of the findings for treatment initiation is limited by the loss of data for 11% of the participants diagnosed with cancer or with a precancerous lesion. We compared the participants with and without a known date for treatment initiation. This former group was more likely to be black and less likely to be Hispanic/Latino or white. No differences were found between these two groups on insurance coverage, age, primary language, or type of cancer. For many of the participants without information on start date of cancer treatment, chart review indicated that participants had received care at another institution, but without specific dates of care available.
This study had several strengths, including a large and diverse population of participants geographically, demographically, and by cancer type. The study benefited from the use of community-based participatory methods to reach populations often not included in clinical trials. The resulting different research methodologies required a prospective meta-analysis for diagnostic resolution and a pooled analysis for treatment initiation. This heterogeneity in research design and treatment implementation highlights the reality of community-based participatory research. There is a need for further research to examine which activities of navigation are of greatest benefit and the role of lay vs clinically trained navigators.
In conclusion, the PNRP demonstrates the effectiveness of patient navigation in settings where resources are low or there is a history of poor follow-up rates and among patients at risk of failure to comply with follow-up or treatment recommendations after an abnormal cancer screening test. The impact of navigation to achieve better cancer care for all populations with the overall goal of reducing incidence, morbidity, and mortality from cancer will become more important as the Affordable Care Act is implemented.
Funding
This work was supported by the National Cancer Institute , National Institutes of Health (U01 CA116892, U01 CA117281, U01CA116903, 01CA116937, U01CA116924, U01CA116885, U01CA116875, and U01CA116925); the American Cancer Society (#SIRSG-05-253-01 and CRP-12-219-01-CPRB); and the Avon Foundation.
Employees of the National Cancer Institute participated in the design of the study and in review and approval of the manuscript. The funding sources had no role in the conduct of the study, in the collection, analysis, and interpretation of the data. The American Cancer Society and the Avon Foundation had no role in the study design, preparation, review or approval of the manuscripts. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the Center to Reduce Cancer Health Disparities, National Cancer Institute. DMM completed his work on this study before assuming his role at the National Institutes of Health.
We acknowledge the contributions of the following members of the Patient Navigation Research Program: Patient Navigation Research Program Investigators: Mollie Howerton, Ken Chu, Emmanuel Taylor, and Mary Ann Van Dyun (National Cancer Institute, Center to Reduce Cancer Health Disparities); Paul Young (NOVA Research Company); Heather A. Young, Heather J. Hoffman (George Washington University Cancer Institute); Cathy Meade and Kristen J. Wells (H. Lee Moffitt Cancer Center and Research Institute); Douglas Post and Mira Katz (Ohio State University); Samantha Hendren (University of Rochester); and Kevin Hall, Anand Karnard, and Amelie Ramirez (University of Texas Health Science Center at San Antonio Cancer Therapy and Research Center).
Preliminary findings were presented at the American Association of Cancer Research, Health Disparities National Meeting, Washington DC, September 2011.
References
- 1. Jean-Pierre P, Hendren S, Fiscella L, et al. Understanding the processes of patient navigation to reduce disparities in cancer care: perspectives of trained navigators from the field. J Cancer Educ. 2011;26(1):111–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. American College of Surgeons. National Accrediation Program for Breast Centers Breast Center Components Chicago: American College of Surgeons; 2010. http://accreditedbreastcenters.org/standards/standards.html Accessed November 21, 2010 [Google Scholar]
- 3. National Comprehensive Cancer Network. The case manager or patient navigator: providing support for cancer patients during treatment and beyond National Comprehensive Cancer Network. http://www.nccn.com/living-with-cancer/understanding-treatment/152-case-managers-for-cancer-patients.html Accessed April 16, 2014
- 4. Wells KJ, Battaglia TA, Dudley DJ, et al. Patient navigation: state of the art or is it science? Cancer. 2008;113(8):1999–2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Robinson-White S, Conroy B, Slavish KH, Rosenzweig M. Patient navigation in breast cancer: a systematic review. Cancer Nurs. 2010;33(2):127–140 [DOI] [PubMed] [Google Scholar]
- 6. Clark JA, Parker VA, Battaglia TA, Freund KM. Patterns of task and network actions performed by navigators to facilitate cancer care. Health Care Manage Rev. 2014;39(2):90–101. [DOI] [PubMed] [Google Scholar]
- 7. Parker VA, Clark JA, Leyson J, et al. Patient navigation: development of a protocol for describing what navigators do. Health Serv Res. 2010;45(2):514–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Warren-Mears V, Dankovchik J, Patil M, Fu R. Impact of patient navigation on cancer diagnostic resolution among Northwest Tribal communities. J Cancer Educ. 2013;28(1):109–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Friedman LM, Furberg CD, DeMets DL. Fundamentals of Clinical Trials. 4th ed. New York: Springer; 2010 [Google Scholar]
- 10. Fiscella K, Whitley E, Hendren S, et al. Patient navigation for breast and colorectal cancer treatment: a randomized trial. Cancer Epidemiol Biomarkers Prev. 2012;21(10):1673–1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Raich PC, Whitley EM, Thorland W, et al. Patient navigation improves cancer diagnostic resolution: an individually randomized clinical trial in an underserved population. Cancer Epidemiol Biomarkers Prev. 2012;21(10):1629–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Murray D. Design and Analysis of Group-Randomized Trials. New York: Oxford University Press; 1998 [Google Scholar]
- 13. Paskett ED, Katz ML, Post DM, et al. The Ohio Patient Navigation Research Program: does the American Cancer Society patient navigation model improve time to resolution in patients with abnormal screening tests? Cancer Epidemiol Biomarkers Prev. 2012;21(10):1620–1628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wells KJ, Lee JH, Calcano ER, et al. A cluster randomized trial evaluating the efficacy of patient navigation in improving quality of diagnostic care for patients with breast or colorectal cancer abnormalities. Cancer Epidemiol Biomarkers Prev. 2012;21(10):1664–1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shadish WR, Cook TD, Campbell DT. Experimental and Quasi-Experimental Designs for Generalized Causal Inference. Boston, MA: Houghton Mifflin Company; 2002 [Google Scholar]
- 16. Battaglia TA, Bak SM, Heeren T, et al. Boston Patient Navigation Research Program: the impact of navigation on time to diagnostic resolution after abnormal cancer screening. Cancer Epidemiol Biomarkers Prev. 2012;21(10):1645–1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hoffman HJ, LaVerda NL, Young HA, et al. Patient navigation significantly reduces delays in breast cancer diagnosis in the District of Columbia. Cancer Epidemiol Biomarkers Prev. 2012;21(10):1655–1663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dudley DJ, Drake J, Quinlan J, et al. Beneficial effects of a combined navigator/promotora approach for Hispanic women diagnosed with breast abnormalities. Cancer Epidemiol Biomarkers Prev. 2012;21(10):1639–1644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Markossian TW, Darnell JS, Calhoun EA. Follow-up and timeliness after an abnormal cancer screening among underserved, urban women in a patient navigation program. Cancer Epidemiol Biomarkers Prev. 2012;21(10):1691–1700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Simon MA, Nonzee NJ, McKoy JM, et al. Navigating veterans with an abnormal prostate cancer screening test: a quasi-experimental study. BMC Health Serv Res. 2013;13:314. 10.1186/1472-6963-13-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Roetzheim RG, Freund KM, Corle DK, et al. Analysis of combined data from heterogeneous study designs: an applied example from the patient navigation research program. Clin Trials. 2012;9(2):176–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Freund KM, Battaglia TA, Calhoun E, et al. National Cancer Institute Patient Navigation Research Program: methods, protocol, and measures. Cancer. 2008;113(12):3391–3399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology—Colorectal Cancer http://www.nccn.org/professionals/physician_gls/f_guidelines.asp Accessed April 16, 2014
- 24. National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology—Cervical Cancer http://www.nccn.org/professionals/physician_gls/f_guidelines.asp Accessed April 16, 2014
- 25. National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology— Breast Cancer http://www.nccn.org/professionals/physician_gls/f_guidelines.asp Accessed April 16, 2014
- 26. National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology—Prostate Cancer http://www.nccn.org/professionals/physician_gls/f_guidelines.asp Accessed April 16, 2014
- 27. Longest BB, Young GJ. Coordination and communication. In: Shortell SM, ed. Health Care Management: Organizational Design and Behavior. 4th ed. Albany, NY: Delmar Publishers; 2000:237–275 [Google Scholar]
- 28. Calhoun EA, Whitley EM, Esparza A, et al. A national patient navigator training program. Health Promot Pract. 2010;11(2):205–215 [DOI] [PubMed] [Google Scholar]
- 29. Clayton D, Cuzick J. Multivariate generalizations of the proportional hazards model. J R Stat Soc Ser A Stat Soc. 1985(148):82–117 [Google Scholar]
- 30. Vaupel JW, Manton KG, Stallard E. The impact of heterogeneity in individual frailty on the dynamics of mortality. Demography. 1979;16(3):439–454 [PubMed] [Google Scholar]
- 31. Klein JP, Rizzo JD, Zhang MJ, Keiding N. Statistical methods for the analysis and presentation of the results of bone marrow transplants. Part 2: regression modeling. Bone Marrow Transplant. 2001;28(11):1001–1011 [DOI] [PubMed] [Google Scholar]
- 32. Weesie J. Survival analysis with time-varying covariates. Stata Technical Bulletin. 1998;7(41):25–43 [Google Scholar]
- 33. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188 [DOI] [PubMed] [Google Scholar]
- 34. Sharp S, Sterne J. New syntax and output for the meta-analysis command. Stata Technical Bulletin. 1998;7(42):6–8 [Google Scholar]
- 35. Sharp, Sterne J. Meta-analysis. Stata Technical Bulletin. 1997;7(38):9–14 [Google Scholar]
- 36. StataCorp LP. Stata Statisical Software [computer program]. College Station, TX: StataCorp LP; 2007 [Google Scholar]
- 37. Richardson LC, Royalty J, Howe W, et al. Timeliness of breast cancer diagnosis and initiation of treatment in the National Breast and Cervical Cancer Early Detection Program, 1996–2005. Am J Public Health. 2010;100(9):1769–1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Richards MA, Westcombe AM, Love SB, Littlejohns P, Ramirez AJ. Influence of delay on survival in patients with breast cancer: a systematic review. Lancet. 1999;353(9159):1119–1126 [DOI] [PubMed] [Google Scholar]
- 39. Ganry O, Peng J, Dubreuil A. Influence of abnormal screens on delays and prognostic indicators of screen-detected breast carcinoma. J Med Screen. 2004;11(1):28–31 [DOI] [PubMed] [Google Scholar]
- 40. Olivotto IA, Gomi A, Bancej C, et al. Influence of delay to diagnosis on prognostic indicators of screen-detected breast carcinoma. Cancer. 2002;94(8):2143–2150 [DOI] [PubMed] [Google Scholar]
- 41. McLaughlin JM, Anderson RT, Ferketich AK, et al. Effect on survival of longer intervals between confirmed diagnosis and treatment initiation among low-income women with breast cancer. J Clin Oncol. 2012;30(36):4493–4500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Palmieri FM, DePeri ER, Mincey BA, et al. Comprehensive diagnostic program for medically underserved women with abnormal breast screening evaluations in an urban population. Mayo Clin Proc. 2009;84(4):317–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lasser KE, Murillo J, Lisboa S, et al. Colorectal cancer screening among ethnically diverse, low-income patients: a randomized controlled trial. Arch Intern Med. 2011;171(10):906–912 [DOI] [PubMed] [Google Scholar]
- 44. Phillips CE, Rothstein JD, Beaver K, et al. Patient navigation to increase mammography screening among inner city women. J Gen Intern Med. 2011;26(2):123–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ma GX, Shive S, Tan Y, et al. Community-based colorectal cancer intervention in underserved Korean Americans. Cancer Epidemiol. 2009;33(5):381–386 [DOI] [PubMed] [Google Scholar]
- 46. Ell K, Vourlekis B, Lee PJ, Xie B. Patient navigation and case management following an abnormal mammogram: a randomized clinical trial. Prev Med. 2007;44(1):26–33 [DOI] [PubMed] [Google Scholar]
- 47. Ell K, Vourlekis B, Xie B, et al. Cancer treatment adherence among low-income women with breast or gynecologic cancer: a randomized controlled trial of patient navigation. Cancer. 2009;115(19):4606–4615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Clark CR, Baril N, Kunicki M, et al. Addressing social determinants of health to improve access to early breast cancer detection: results of the Boston REACH 2010 Breast and Cervical Cancer Coalition Women’s Health Demonstration Project. J Womens Health (Larchmt). 2009;18(5):677–690 [DOI] [PubMed] [Google Scholar]
- 49. Freeman HP, Muth BJ, Kerner JF. Expanding access to cancer screening and clinical follow-up among the medically underserved. Cancer Pract. 1995;3(1):19–30 [PubMed] [Google Scholar]
- 50. Andriole GL, Crawford ED, Grubb RL, 3rd, et al. Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: mortality results after 13 years of follow-up. J Natl Cancer Inst. 2012;104(2):125–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Schroder FH, Hugosson J, Roobol MJ, et al. Prostate-cancer mortality at 11 years of follow-up. N Engl J Med. 2012;366(11):981–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Heidenreich A, Bastian PJ, Bellmunt J, et al. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol. 2014;65(1):124–137 [DOI] [PubMed] [Google Scholar]
- 53. US Preventive Services Task Force. Screening for Prostate Cancer http://www.uspreventiveservicestaskforce.org/prostatecancerscreening.htm Accessed April 16, 2014
- 54. Chou R, Croswell JM, Dana T, et al. Screening for prostate cancer: a review of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2011;155(11):762–771 [DOI] [PubMed] [Google Scholar]
- 55. American Cancer Society. Recommendations for prostate cancer early detection http://www.cancer.org/cancer/prostatecancer/moreinformation/prostatecancerearlydetection/prostate-cancer-early-detection-acs-recommendations Accessed April 16, 2014
- 56. Qaseem A, Barry MJ, Denberg TD, et al. Screening for prostate cancer: a guidance statement from the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med. 2013;158(10):761–769 [DOI] [PubMed] [Google Scholar]