Abstract
Background
Targeting smokers at higher lung cancer risk can improve efficiency and reduce false-positive detection in lung cancer screening. We evaluated whether time to first cigarette after waking (TTFC), a single-item measure of nicotine dependency, could improve stratification of lung cancer risk beyond standard smoking metrics (intensity, duration, and pack-years).
Methods
In 3249 ever-smokers (n = 1812 case subjects; n = 1437 control subjects) from a population-based case–control study in Italy, we examined the association between TTFC and lung cancer using logistic regression and estimated lung cancer incidence by levels of TTFC, and intensity, duration, and pack-years using absolute risk regression. Significance tests were two-sided.
Results
Compared with smokers with TTFC greater than 60 minutes, the lung cancer odds ratios for TTFC of 31 to 60 minutes, 6 to 30 minutes, and 5 or fewer minutes (by increasing dependency) were 2.57 (95% confidence interval [CI] = 2.03 to 3.26), 2.27 (95% CI = 1.79 to 2.88), and 3.50 (95% CI = 2.64 to 4.64), respectively (P trend < .0001). The average lung cancer incidence rates for smokers of 1 to 10, 11 to 20, 21 to 30 and more than 30 cigarettes per day were consistently higher among smokers with TTFC of 60 or fewer minutes vs more than 60 minutes (64.1 vs 11.7; 125.6 vs 28.6; 130.1 vs 40.7; and 260.8 vs 108.9 per 100000 person-years, respectively). The slopes of increase in lung cancer rates with smoking duration and pack-years were statistically significantly greater among smokers with higher dependency (P interaction < .001).
Conclusions
Lung cancer risk increases with shorter TTFC; this simple nicotine dependency measure increases lung cancer risk stratification beyond standard smoking measures. Assessing TTFC may improve lung cancer risk prediction and could be useful in lung cancer screening and smoking cessation programs.
Lung cancer risk varies substantially among smokers (1). Smoking intensity (cigarettes per day), duration, and the cumulative exposure (pack-years) are standard smoking metrics for the evaluation and prediction of lung cancer risk (2). However, smoking intensity only partially explains the wide range of heterogeneity in the biological measures of nicotine exposure, as measured by blood and urinary cotinine (3). Time to first cigarette (TTFC) after waking in the morning, a single-item measure of nicotine dependence, strongly predicts the biological uptake of nicotine (3) and smoking cessation success (4) independently of smoking intensity. TTFC also has a dose-dependent relationship with the tobacco-specific carcinogen urinary NNAL (4-methylnitrosamino-1-3-pyridyl-1-butanol); the NNAL concentration of smokers taking their first cigarette within 5 minutes after waking was twice as high as that of smokers with an equal number of daily cigarettes but who smoke their first cigarette an hour or more after waking (5). Thus, TTFC may capture another smoking dimension relevant to cancer risk and provide additional lung cancer risk stratification among smokers beyond the standard measures of smoking consumption (duration, intensity, and pack-years), thus improving the performance of lung cancer screening (6).
A previous report from a hospital-based case–control study found an association between TTFC and lung cancer risk (7), but this study did not sufficiently adjust for other smoking variables to reliably determine whether TTFC differentiates lung cancer risk beyond the standard smoking metrics. Our study evaluated the association between TTFC and lung cancer with comprehensive adjustment for smoking behavior factors. In addition, we thoroughly examined whether TTFC might improve risk stratification beyond standard smoking phenotypes in a large, population-based, case–control study in Lombardy, Italy, the Environment and Genetics in Lung Cancer Etiology (EAGLE) study (8). The association between TTFC and lung cancer was also assessed using prospectively collected data from the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial (9) and by synthesizing the up-to-date evidence.
Methods
Ethics Statement
EAGLE was approved by the institutional review board of each participating hospital and university in Italy and the National Cancer Institute, National Institutes of Health, in Bethesda, Maryland. The PLCO protocol was approved by the institutional review board of each screening center. Informed consent was received from each participant for both studies.
Study Design and Data Collection of EAGLE
EAGLE is a population-based, case–control study described in detail previously (8) that includes 2101 incident lung cancer case subjects and 2120 control subjects aged 35 to 79 years from five Italian cities (Milan, Monza, Brescia, Pavia, and Varese) and surrounding areas in the Lombardy region. The incident primary lung cancer case subjects were diagnosed between April 2002 and February 2005 from 13 hospitals covering 80% of the case subjects in the catchment area and were confirmed by pathology reports (95%) or clinical history and imaging (5%). Control subjects were randomly selected from the population database of the catchment area and were frequency matched with case subjects on residence, sex, and age. Participation rates for eligible subjects were 86.6% for case subjects and 72.4% for control subjects.
EAGLE collected questionnaire data on major lung cancer risk factors and detailed smoking information (8). For ever smokers, we used the six-item Fagerström Test for Nicotine Dependence (10). The question evaluating TTFC, the first item of the Fagerström Test for Nicotine Dependence, was, “How soon after you wake up do/did you smoke your first cigarette?” with four response categories (≤5, 6–30, 31–60, and >60 minutes).
Because TTFC information is only relevant among smokers, we restricted our main analysis to 1812 case subjects and 1437 control subjects who were smokers (85.5% and 68.4% of total case subjects and control subjects, respectively). To compare lung cancer risk with never smokers, we also included never smokers (1939 case subjects and 2088 control subjects in total) in related analyses.
Study Design and Data Collection of PLCO
The PLCO study (9) was designed to evaluate the effects of prostate, lung, colorectal, and ovarian cancer screening on mortality and other endpoints. Briefly, 74000 men and 74000 women aged 55 to 74 years without a history of the PLCO cancers were enrolled at 10 clinical centers from 1993 to 2001 and randomly assigned into the screened or control arm. TTFC information was collected among current smokers by a supplementary questionnaire distributed in 2006. The relevant question was, “How soon after you wake up do you usually smoke your first cigarette of the day?” The four response categories were the same as those used in EAGLE. Approximately 104000 people responded to the supplementary questionnaire, and 6821 current smokers answered the TTFC question. After excluding 96 participants with an earlier lung cancer diagnosis, 6725 participants remained eligible for this study. The follow-up started at the date of supplementary questionnaire administration and ended at the date of lung cancer diagnosis; death; withdrawal from the study; loss to follow-up; on December 31, 2009; or the study end date, whichever occurred first. During the follow-up period of up to 4.5 years, 181 case subjects with lung cancer were diagnosed among current smokers with available TTFC data. Given the limited number of lung cancer case subjects, the PLCO cohort was only used to assess the association between TTFC and lung cancer, not to evaluate the lung cancer risk stratification by TTFC and other smoking variables.
Statistical Analyses
We examined associations of TTFC with covariables among EAGLE control subjects using the χ2 test for categorical variables and analyses of variance for continuous variables. For continuous smoking variables, we calculated Spearman correlation coefficients with TTFC.
We assessed the association between TTFC and lung cancer risk in EAGLE using logistic regression, adjusting for age, sex, study area, and lung cancer risk factors, including smoking intensity, smoking duration, pack-years, current/former smoking status, years since quitting, age at smoking initiation, body mass index, lung cancer family history, chronic obstructive pulmonary disease, education, and occupations associated with increased lung cancer risk (11). Missing categories were created for lung cancer family history and chronic obstructive pulmonary disease to retain samples missing these two covariables in the analyses. We estimated the odds ratio (OR) and its 95% confidence interval (CI) for each TTFC category (>60, 31–60, 6–30, and ≤5 minutes) with reference to TTFC of more than 60 minutes. We tested for a trend in association using an ordinal TTFC. Subgroup analyses were performed by sex, current/former smokers, smoking intensity (≤10, 11–20, >20 cigarettes per day), histology, and stage. We tested for the statistical significance of the pair-wise interactions of TTFC with sex, current/former smoking, and smoking intensity. To compare the lung cancer odds ratio of each TTFC category with never smokers, we included all EAGLE samples in the logistic regression using never smokers as the reference, adjusting for the same nonsmoking lung cancer risk factors as above.
We replicated the association between TTFC and lung cancer in PLCO, using a Cox proportional hazards model with days from questionnaire administration to lung cancer as the time scale. We adjusted for smoking duration, intensity, sex, lung cancer family history, education, and ethnicity. The proportional hazards assumption was tested by including interaction terms between TTFC categories and follow-up time (P > .05).
To summarize current evidence on TTFC and lung cancer risk and increase the generalizability of our findings (12,13), we systematically searched the literature through PubMed/MEDLINE as of June 7, 2013 (Supplementary Methods, available online). Epidemiological studies reporting data on TTFC and lung cancer were considered eligible and were included in a meta-analysis along with EAGLE and PLCO. Given the expected between-study heterogeneity in the synthesis of observational studies (14), we estimated the summary effect for each TTFC category using random effects (15).
To evaluate whether TTFC can improve lung cancer risk stratification beyond standard smoking phenotypes, we estimated incident rates of lung cancer per 100000 person-years in EAGLE samples for each joint category of TTFC (>60 and ≤60 minutes) and smoking intensity, pack-years, or duration, as well as in never smokers, using absolute risk regression in a binomial linear model (16). This program used the sampling fraction of the EAGLE study to recover the original cohort. To evaluate whether the linear effect of duration and pack-years on lung cancer incidence differed by TTFC, we included in the binomial linear model TTFC (dichotomized), duration or pack-years (coded ordinal), and the interaction terms to obtain P values for interaction (among smokers only). We assumed no secular change in incidence rate over the 34-month period of case ascertainment in the EAGLE study. Because sex has been previously shown to modify smoking–lung cancer association in EAGLE participants (17), we performed sensitivity analyses that stratified the regression by sex to avoid potential bias because of sex difference. We also estimated the odds ratio of each joint category of TTFC (four categories) and quartiles of pack-years, with reference to never smokers, using logistic regression, adjusting for nonsmoking lung cancer risk factors.
We used R to calculate the absolute lung cancer incidence rate (16), SAS 9.1 (SAS Institute, Cary, NC) for descriptive analyses and logistic regression, and Stata 11.0 (StataCorp, College Station, TX) for meta-analyses. All tests of statistical significance were two sided, with P value of .05 as the cut point.
Results
Shorter TTFC (ie, stronger dependency) was statistically significantly associated with a greater number of pack-years and cigarettes per day, a longer smoking duration, less time since quitting among former smokers, increased depth of inhalation, and younger age at smoking initiation (Table 1). A substantial fraction of smokers took their first cigarette within 60 minutes of waking in both EAGLE and PLCO. In EAGLE, the percentages of smokers with a TTFC greater than 60 minutes, from 31 to 60 minutes, from 6 to 30 minutes, and 5 or fewer minutes were 16%, 24%, 32%, and 28% among lung cancer case subjects and 51%, 18%, 21%, and 10% among control subjects (Supplementary Table 1, available online). In PLCO, the corresponding percentages of baseline current smokers for each of the TTFC categories were 23%, 19%, 42%, and 16%, respectively (data not shown).
Table 1.
Association between covariables and time to first cigarette in the Environment and Genetics in Lung Cancer Etiology study control subjects (n = 1437)*
| Characteristics | No.† | TTFC, min (increasing dependency with shorter minutes) | ||||
|---|---|---|---|---|---|---|
| >60 | 31–60 | 6–30 | ≤5 | P‡ | ||
| Total | 1437 | 51.0 | 18.2 | 20.5 | 10.3 | |
| Age, y | 1437 | 65.9 (8.8) | 65.4 (7.9) | 64.6 (8.2) | 65.6 (8.6) | .20 |
| Smoking status | <.0001 | |||||
| Former | 909 | 58.9 | 15.7 | 16.0 | 9.5 | |
| Current | 527 | 37.4 | 22.4 | 28.5 | 11.8 | |
| Pack-year, packs per day × y | 1435 | 16.4 (15.7) | 32.7 (20.2) | 38.1 (18.6) | 50.3 (27.2) | <.0001 |
| Cigarettes per day | 1435 | 10.9 (7.9) | 18.6 (9.6) | 20.4 (7.7) | 27.3 (12.1) | <.0001 |
| Smoke duration, y | 1435 | 27.5 (15.7) | 35.3 (13.7) | 37.5 (12.1) | 37.4 (12.5) | <.0001 |
| Years since quitting, y | 909 | 23.5 (13.5) | 19 (11.0) | 16.4 (10.7) | 17.7 (10.4) | <.0001 |
| Age at smoking initiation, y | 1437 | 20.1 (6.8) | 18.7 (5.0) | 17.8 (3.8) | 17.5 (4.8) | <.0001 |
| Depth of inhale | <.0001 | |||||
| Not inhale | 111 | 85.6 | 7.2 | 2.7 | 4.5 | |
| Slightly | 183 | 63.9 | 15.9 | 15.9 | 4.4 | |
| Moderately | 571 | 57.6 | 17.2 | 19.3 | 6.0 | |
| Deeply | 568 | 33.5 | 22.0 | 26.8 | 17.8 | |
| Body mass index, kg/m2 | 1437 | 25.9 (3.8) | 26 (3.6) | 26.2 (3.9) | 27.1 (5.9) | <.01 |
| Sex | <.01 | |||||
| Male | 1220 | 49.3 | 18.9 | 21.1 | 10.8 | |
| Female | 217 | 60.8 | 14.3 | 17.5 | 7.4 | |
| Study area | <.01 | |||||
| Brescia | 168 | 52.4 | 10.7 | 22.6 | 14.3 | |
| Milan | 977 | 51.2 | 20.7 | 18.2 | 9.9 | |
| Monza | 80 | 43.8 | 13.8 | 31.3 | 11.3 | |
| Pavia | 92 | 50.0 | 13.0 | 27.2 | 9.8 | |
| Varese | 120 | 53.3 | 15.0 | 24.2 | 7.5 | |
| Occupations with higher lung cancer risk | <.01 | |||||
| No | 1063 | 53.4 | 17.1 | 20.2 | 9.2 | |
| Yes | 374 | 44.1 | 21.1 | 21.4 | 13.4 | |
| Lung cancer family history | .03 | |||||
| No | 1142 | 52.9 | 17.6 | 19.9 | 9.6 | |
| Yes | 132 | 43.2 | 19.7 | 23.5 | 13.6 | |
| COPD | <.0001 | |||||
| No | 1265 | 52.5 | 18.2 | 20.2 | 9.2 | |
| Yes | 148 | 40.5 | 16.2 | 21.6 | 21.6 | |
| Education | <.0001 | |||||
| None | 58 | 43.1 | 15.5 | 20.7 | 20.7 | |
| Elementary | 389 | 45.0 | 21.6 | 22.4 | 11.1 | |
| Middle | 424 | 47.9 | 18.4 | 21.0 | 12.7 | |
| High | 389 | 57.6 | 15.7 | 19.8 | 6.9 | |
| University | 177 | 59.9 | 16.4 | 16.9 | 6.8 | |
| ETS exposure | 0.32 | |||||
| No | 87 | 59.8 | 17.2 | 13.8 | 9.2 | |
| Yes | 1341 | 50.5 | 18.3 | 20.9 | 10.3 | |
| ETS exposure at home in childhood | 0.001 | |||||
| No | 455 | 57.1 | 17.1 | 17.6 | 8.1 | |
| Yes | 973 | 48.1 | 18.8 | 21.9 | 11.2 | |
| ETS exposure at home in adulthood | 0.31 | |||||
| No | 765 | 52.4 | 18.0 | 18.8 | 10.7 | |
| Yes | 612 | 48.9 | 18.6 | 22.5 | 10.0 | |
| ETS exposure in the work place | 0.16 | |||||
| No | 404 | 52.5 | 15.8 | 22.3 | 9.4 | |
| Yes | 1024 | 50.5 | 19.2 | 19.8 | 10.4 | |
* For continuous variables we present mean and standard deviation; otherwise we present row percentages. COPD = chronic obstructive pulmonary disease; ETS = environmental tobacco smoke; TTFC = time to first cigarette.
† Sample size may not sum up to 1437 because of missing values or restricting to former smokers (years since quitting).
‡ P values were calculated by χ2 test (categorical/dichotomized variables) or analyses of variance (continuous variables). All statistical tests were two-sided.
In EAGLE, lung cancer risk was higher in smokers with shorter TTFC (Table 2). After adjusting for smoking intensity, duration, pack-years, current/former smoking status, years since quitting, age at smoking initiation, and other lung cancer risk factors (details in Methods and Table 2 notes), the odds ratios compared with TTFC of greater than 60 minutes were 2.57 (95% CI = 2.03 to 3.26), 2.27 (95% CI = 1.79 to 2.88), and 3.50 (95% CI = 2.64 to 4.64), respectively, for TTFC of 31 to 60 minutes, 6 to 30 minutes, and 5 or fewer minutes (P trend < .0001). The association was apparent in each subgroup by sex, current/former smoking status, and smoking intensity (1–10, 11–20, >20 cigarettes per day) (Table 2). It was stronger in current smokers (P interaction = .03 compared with former smokers) and lighter smokers (P interaction = .02 compared with heavier smokers) but not statistically significantly different by sex. The association was also observed across all major lung histologies and clinical stages, with the strongest association found among squamous cell carcinoma and the weakest among adenocarcinoma (Supplementary Table 2, available online). The odds ratio relative to never smokers were 2.46 (95% CI = 1.91 to 3.20), 10.24 (95% CI = 7.84 to 13.37), 11.31 (95% CI = 8.73 to 14.64) and 19.66 (95% CI = 14.73 to 26.24) for smokers with TTFC of greater than 60, 31 to 60, 6 to 30, and 5 or fewer minutes, respectively (data not shown).
Table 2.
Association between time to first cigarette and lung cancer among smokers in the Environment and Genetics in Lung Cancer Etiology study*
| Group/Subgroup | Odds ratio (95% confidence interval) | ||||||
|---|---|---|---|---|---|---|---|
| Case subjects/ control subjects | TTFC >60 min | TTFC 31–60 min | TTFC 6–30 min | TTFC ≤5 min | P trend | P interaction | |
| All smokers | 1807/1434 | 1.00 | 2.57 (2.03 to 3.26) | 2.27 (1.79 to 2.88) | 3.50 (2.64 to 4.64) | <.001 | |
| Sex | |||||||
| Male | 1504/1217 | 1.00 | 2.71 (2.09 to 3.52) | 2.28 (1.75 to 2.96) | 3.68 (2.71 to 4.99) | <.001 | .78 |
| Female | 303/217 | 1.00 | 2.01 (1.06 to 3.82) | 2.48 (1.37 to 4.5) | 2.86 (1.28 to 6.43) | .002 | |
| Smoking status | |||||||
| Current | 971/525 | 1.00 | 3.13 (2.16 to 4.53) | 2.73 (1.9 to 3.92) | 4.20 (2.72 to 6.49) | <.001 | .03 |
| Former | 836/909 | 1.00 | 2.50 (1.81 to 3.46) | 2.15 (1.54 to 3) | 3.28 (2.24 to 4.81) | <.001 | |
| Cigarettes per day | |||||||
| ≤10 | 232/515 | 1.00 | 3.43 (2.00 to 5.87) | 3.03 (1.55 to 5.91) | 7.44 (2.04 to 27.20) | <.001 | .02 |
| 11–20 | 946/660 | 1.00 | 2.69 (1.96 to 3.68) | 2.06 (1.51 to 2.8) | 3.52 (2.38 to 5.19) | <.001 | |
| >20 | 629/259 | 1.00 | 1.56 (0.83 to 2.94) | 1.75 (0.98 to 3.11) | 2.29 (1.28 to 4.1) | .005 | |
* We used logistic regression, adjusting for age, area, sex, smoking intensity (in categories ≤5, >5–≤10, >10–≤20, >20–≤30, >30 cigarettes per day), smoking duration (≤20, >20–≤30, >30–≤40, >40–≤50, >50 years), pack-years (≤10, >10–≤20, >20–≤30, >30–≤40, >40–≤50, >50), current/former smoking status, years since quitting (≤2, >2–≤5, >5–≤10, >10–≤20, >20–≤30, >30 years), age at smoking initiation, body mass index (continuous), lung cancer family history (in categories yes, no, missing), chronic obstructive pulmonary disease (in categories: yes, no, missing), education, and occupations associated with increased lung cancer risk. P trend was obtained by treating time to first cigarette (TTFC) as an ordinal variable; Pinteraction was obtained by adding the interaction term of TTFC (ordinal) and sex, current/former, and cigarettes per day (ordinal). Sex was removed in the male and female models, smoking status was removed in the current and former smoker models, years since quitting was removed in the current smoker model. All statistical tests were two-sided. Sample sizes are slightly less than 1812/1437 due to missing data in some continuous covariates.
Among PLCO smokers, lung cancer risk increased with shorter TTFC in a borderline statistically significant trend (P trend = .05), although the association was not statistically significant for each TTFC category. The hazard ratios among smokers with TTFC of 31 to 60, 6 to 30, and 5 or fewer minutes compared with TTFC of greater than 60 minutes were 1.00 (95% CI = 0.55 to 1.80), 1.50 (95% CI = 0.91 to 2.49) and 1.57 (95% CI = 0.86 to 2.85), respectively.
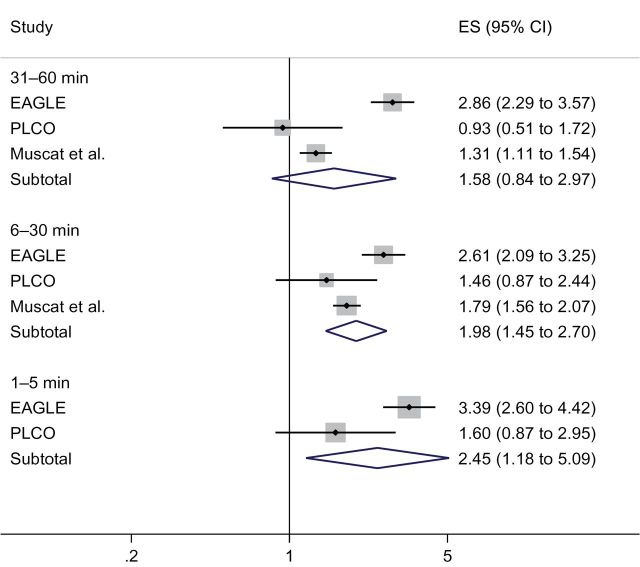
The article by Muscat et al., a hospital-based case–control study in New York, described the only eligible study retrieved by our systematic literature search (7) (see Supplementary Methods, available onine, for details). They found a consistently increasing risk of lung cancer with shorter TTFC. The effect size is weaker than EAGLE but stronger than PLCO: the odds ratios were 1.79 (95% CI = 1.56 to 2.07) for TTFC of 1 to 30 minutes and 1.31 (95% CI = 1.11 to 1.54) for TTFC of 31 to 60 minutes, compared with TTFC of greater than 60 minutes. The meta-analysis of the three studies confirmed the increased risk of lung cancer with shorter TTFC. The relative risk estimates were 1.58 (95% CI = 0.84 to 2.97), 1.98 (95% CI = 1.45 to 2.70), and 2.45 (95% CI = 1.18 to 5.09), respectively, for smokers with TTFC of 31 to 60, 6 to 30, and 5 or fewer minutes (Figure 1).
Figure 1.
Meta-analyses for the association between time to first cigarette (TTFC) and lung cancer. Lung cancer odds ratios and 95% confidence intervals (TTFC >60 minutes as reference) were estimated using random-effects meta-analysis. In the Environment and Genetics in Lung Cancer Etiology study (EAGLE) and Prostate, Lung, Colorectal, and Ovarian Cancer Screening trial (PLCO), we adjusted for the same covariates as in the Muscat et al. report (7) (age, sex, race, education, body mass index, and pack-years; in PLCO, we used duration and frequency instead of pack-years because of the high missing rate in pack-years). The Muscat et al. report (7) lumped TTFC of 1 to 5 minutes and 6 to 30 minutes together, which was categorized as a TTFC of 6 to 30 minutes group, based on the fact that the majority of smokers with TTFC of 1 to 30 minutes belong to TTFC of 6 to 30 minutes for both EAGLE (66%) and PLCO (72%). All statistical tests were two-sided. CI = confidence interval; ES = effect size.
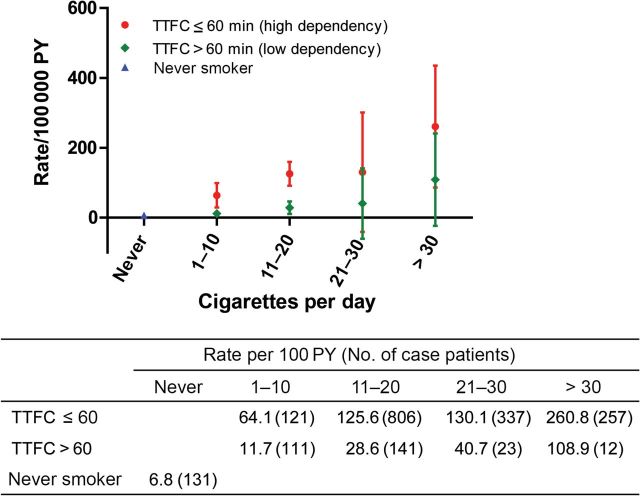
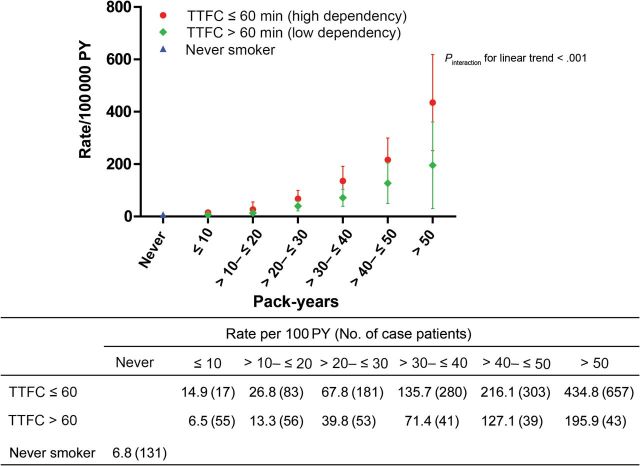
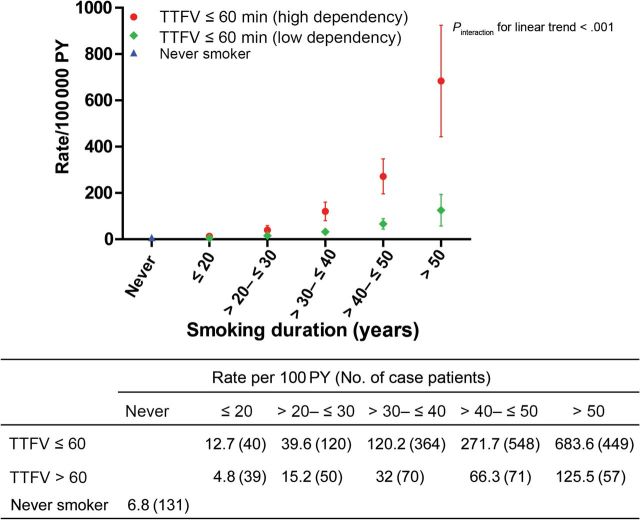
TTFC increased lung cancer risk stratification in EAGLE, beyond smoking intensity, smoking duration, or pack-years (Figures 2–4; Supplementary Table 3, available online). The average lung cancer rates for smokers of 1 to 10, 11 to 20, 21 to 30, and more than 30 cigarettes per day were consistently higher among smokers with TTFC or 60 minutes or less than greater than 60 minutes (64.1 vs 11.7; 125.6 vs 28.6; 130.1 vs 40.7; and 260.8 vs 108.9 per 100000 person-years, respectively), in spite of some overlapped 95% confidence intervals among heavier smokers (>20 cigarettes per day) (Figure 2). Lung cancer rates among those smoking 1 to 10 cigarettes per day who began smoking within an hour were as high or higher than those smoking 20 cigarettes per day who waited an hour or more before starting to smoke. The slopes of the linear increase in lung cancer rate with duration and pack-years were markedly greater (63 more case subjects per 100000 person-years per 10 years smoking duration and 34 more case subjects per 100000 person-years per 10 pack-years) in the higher dependency group (TTFC ≤60 minutes; P interaction < .001) (Figures 3 and 4). This leads to the faster lung cancer rate increase with duration and pack-years among higher-dependency smokers. For example, among high-dependency smokers of 21 to 30 pack-years, the lung cancer rate per 100000 person-years was 68 (95% CI = 36 to 100), similar to the rate of low-dependency smokers with 31 to 40 pack-years (lung cancer rate per 100000 person-years = 71; 95% CI = 39 to 104). Similar patterns were observed when we stratified by sex, with lung cancer incidence rates being higher among men than women (Supplementary Figures 1–3, available online).
Figure 2.
Lung cancer incidence in each category of time to first cigarette (TTFC) and smoking intensity in the Environment and Genetics in Lung Cancer Etiology study (EAGLE). The figure shows the point estimate and 95% confidence interval of the absolute lung cancer rate per 100000 person-years (PY). The table under the figure shows the value of the point estimate (number of lung cancer case subjects). Incidence rates were estimated using a binomial linear model with eight joint categories of TTFC and smoking intensity as well as the never smokers category. All statistical tests were two-sided.
Figure 4.
Lung cancer incidences in each category of time to first cigarette (TTFC) and pack-years in the Environment and Genetics in Lung Cancer Etiology study (EAGLE). The figure shows the point estimate and 95% confidence interval of the absolute lung cancer rate per 100000 person-years (PY). The table under the figure shows the value of the point estimate (number of lung cancer case subjects). Incidence rates were estimated using a binomial linear model with 12 joint categories of TTFC and pack-years as well as the never smokers category. To evaluate whether the linear trend of lung cancer incidence with increased pack-years differed by TTFC statistically, we tested the interaction of TTFC and pack-years (treated as an ordinal variable) among smokers only. All statistical tests were two-sided.
Figure 3.
Lung cancer incidences in each category of time to first cigarette (TTFC) and smoking duration in the Environment and Genetics in Lung Cancer Etiology study (EAGLE). The figure shows the point estimate and 95% confidence interval of the absolute lung cancer rate per 100000 person-years (PY). The table under the figure shows the value of the point estimate (number of lung cancer case subjects). Incidence rates were estimated using a binomial linear model with 10 joint categories of TTFC and duration as well as the never smokers category. To evaluate whether the linear trend of lung cancer incidence with increased duration differed by TTFC statistically, we tested the interaction of TTFC and duration (treated as an ordinal variable) among smokers only. All statistical tests were two-sided.
Discussion
In our study, lung cancer risks are substantially higher among those smoking cigarettes immediately after waking up, reflecting higher nicotine dependence. Lung cancer risks in smokers with shorter TTFC remain elevated, even after accounting for comprehensive smoking measurements including intensity, smoking duration, pack-years, current/former smoking status, years since quitting, and age at smoking initiation, emphasizing the independent effect of TTFC. We further demonstrate that TTFC differentiates lung cancer risk beyond smoking intensity, duration, and pack-years. TTFC increases stratification of lung cancer risk within each cigarettes-per-day category. Lung cancer risk increases more rapidly with the increase in smoking duration and pack-years among more highly dependent smokers.
The association between TTFC and lung cancer is observed in men and women, former and current smokers, heavy and light smokers, and in all histologies of lung cancer; it was notably stronger in current smokers and light smokers. A US prospective cohort study and a meta-analysis that included data from an earlier report provide evidence supporting our primary findings from a population-based, Italian case–control study (7). The joint effects of TTFC and smoking intensity, duration, and pack-years suggest that TTFC captures a large amount of heterogeneity of lung cancer risk beyond standard smoking variables.
The increased risk of lung cancer with shorter TTFC in EAGLE is consistent with findings in PLCO reported here and in a previous publication by Muscat et al. (7). Evidence for an association was observed in each study, but the estimates of effect are higher in EAGLE. The weaker PLCO finding was based on just 181 eligible lung cancer case subjects (10-fold fewer than in EAGLE); therefore, chance variation could be a source of heterogeneity between studies. EAGLE’s questionnaire, specifically designed to study smoking and lung cancer, collected more precise smoking information than PLCO; the effect size therefore will be less attenuated by nondifferential misclassification. In addition, geographical location, sex, and age might contribute to heterogeneity. Exclusion of smokers with higher dependency, who were more likely to be diagnosed with lung cancer before TTFC collection (5 to 13 years after baseline) and therefore were excluded from the analytical cohort, may have attenuated the hazard ratios found in PLCO. Whether other differences among these studies contributed to our observed heterogeneity is unclear; for example, although EAGLE consists of 100% whites, PLCO and the Muscat report included 84% and 89% whites, respectively. Restricting PLCO to whites had no effect on our risk estimates. Alternatively, higher odds ratios in the case–control studies, relative to the cohort study, could reflect bias if case subjects differentially reported a shorter TTFC than control subjects; the impact of this bias is likely to be small because TTFC is not generally recognized as a lung cancer risk factor. Nevertheless, future studies are needed to investigate these associations to precisely estimate the magnitude of these associations and to confirm the findings in other populations.
Several limitations of our work, beyond possible heterogeneity, should be noted. Self-reported TTFC from a single questionnaire in EAGLE and PLCO imperfectly represents long-term TTFC. Given the small number of case subjects in the PLCO cohort, PLCO was primarily used to check the pattern consistency of the TTFC and lung cancer association.
The higher lung cancer incidence among smokers of shorter TTFC in each category of smoking intensity, as well as the faster lung cancer rate increase with duration and pack-years among shorter TTFC, is consistent with the evidence that shorter TTFC was associated with higher blood levels of NNAL, a tobacco-specific carcinogen, after adjusting for smoking intensity (5). Mechanistically, smokers who reduce smoking intensity maintain a high level of carcinogen absorption (dose) (18), possibly through adapting their smoking behaviors to sustain nicotine levels in spite of lower cigarette consumption. Concordantly, in EAGLE, shorter TTFC smokers tend to inhale more deeply (Spearman r = 0.36; P < .001) (Supplementary Table 4, available online). Additionally, differences in TTFC could also reflect genetically mediated differences in the pharmacodynamics and pharmacokinetics of tobacco smoke metabolism (19), which in turn, influence tobacco toxicity and lung cancer risk.
Our findings have clinical, public health, and research implications. TTFC, a single-item measure of smoking dependency, appears to capture additional dimensions of smoking that contribute to lung cancer risk. The demonstration that a simple smoking question can explain a substantial variation of lung cancer risk after considering smoking intensity, duration, and pack-years has the potential to increase the accuracy of lung cancer prediction models. Improved risk stratification may also improve the performance of spiral computerized tomography screening programs (20). Routine collection of TTFC along with standard smoking measurements may also help smokers and clinicians better gauge lung cancer risk and thereby motivate smokers to quit and may improve clinicians’ recommendations to patients for effective smoking cessation therapies. Our finding reinforces the importance of smoking cessation even among light smokers (21). Further, TTFC may similarly improve risk stratification for other important smoking-related health outcomes, including cardiovascular disease and chronic obstructive pulmonary disease. Finally, understanding potential determinants of short TTFC [such as the environmental tobacco smoking exposure in childhood (Table 1) and genetic factors] may help smoking cessation and risk reduction.
In summary, this study establishes TTFC as a novel and substantial determinant of lung cancer risk. TTFC explains differences in lung cancer risk among smokers after accounting for smoking intensity, duration, and pack-years. The ease of obtaining TTFC suggests opportunities for using measures of tobacco dependency in the clinic and in research. Finally, although this study supports the role of TTFC in lung cancer risk stratification, future results from prospective and screening studies are needed to determine whether TTFC will improve risk prediction models and aid in the identification of high-risk subjects. Such work should be performed in diverse populations with distinct geographic origins and ethnicities.
Funding
This work was supported by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics at the National Cancer Institute, and AWB was supported by the National Institute on Drug Abuse (DA020830 and DA033813).
We are grateful to the EAGLE participants and the large number of EAGLE collaborators (listed in http://dceg.cancer.gov/eagle). We thank the National Cancer Institute for providing the human material collected by the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. We thank Jianhong Chen for assistance in rendering Figures 2–4.
References
- 1. Bach PB, Kattan MW, Thornquist MD, et al. Variations in lung cancer risk among smokers. J Natl Cancer Inst. 2003;95(6):470–478 [DOI] [PubMed] [Google Scholar]
- 2. Tammemagi CM, Pinsky PF, Caporaso NE, et al. Lung cancer risk prediction: Prostate, Lung, Colorectal And Ovarian Cancer Screening Trial models and validation. J Natl Cancer Inst. 2011;103(13):1058–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Muscat JE, Stellman SD, Caraballo RS, et al. Time to first cigarette after waking predicts cotinine levels. Cancer Epidemiol Biomarkers Prev. 2009;18(12):3415–3420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baker TB, Piper ME, McCarthy DE, et al. Time to first cigarette in the morning as an index of ability to quit smoking: implications for nicotine dependence. Nicotine Tob Res. 2007;9(Suppl 4):S555–S570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Branstetter SA, Muscat JE. Time to first cigarette and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (nnal) levels in adult smokers; National Health and Nutrition Examination Survey (NHANES), 2007–2010. Cancer Epidemiol Biomarkers Prev. 2013;22(4):615–622 [DOI] [PubMed] [Google Scholar]
- 6. Kovalchik SA, Tammemagi M, Berg CD, et al. Targeting of low-dose CT screening according to the risk of lung-cancer death. N Engl J Med. 2013;369(3):245–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Muscat JE, Ahn K, Richie JP, Jr, et al. Nicotine dependence phenotype and lung cancer risk. Cancer. 2011;117(23):5370–5376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Landi MT, Consonni D, Rotunno M, et al. Environment and Genetics in Lung Cancer Etiology (EAGLE) study: an integrative population-based case-control study of lung cancer. BMC Public Health. 2008;8:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Prorok PC, Andriole GL, Bresalier RS, et al. Design of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Control Clin Trials. 2000;21(6 Suppl):273S–309S [DOI] [PubMed] [Google Scholar]
- 10. Heatherton TF, Kozlowski LT, Frecker RC, et al. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127 [DOI] [PubMed] [Google Scholar]
- 11. Ahrens W, Merletti F. A standard tool for the analysis of occupational lung cancer in epidemiologic studies. Int J Occup Environ Health. 1998;4(4):236–240 [DOI] [PubMed] [Google Scholar]
- 12. Clark S, Horton R. Putting research into context—revisited. Lancet. 2010;376(9734):10–11 [DOI] [PubMed] [Google Scholar]
- 13. Murad MH, Montori VM. Synthesizing evidence: shifting the focus from individual studies to the body of evidence. JAMA. 2013;309(21): 2217–2218 [DOI] [PubMed] [Google Scholar]
- 14. Higgins JPT, Green S.eds. Cochrane Handbook for Systematic Reviews of Interventions. Chichester, UK: John Wiley & Sons; 2008 [Google Scholar]
- 15. DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. 2007;28(2):105–114 [DOI] [PubMed] [Google Scholar]
- 16. Kovalchik SA, Varadhan R, Fetterman B, et al. A general binomial regression model to estimate standardized risk differences from binary response data. Stat Med. 2013;32(5):808–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kovalchik SA, De Matteis S, Landi MT. et al. A regression model for risk difference estimation in population-based case-control studies clarifies gender differences in lung cancer risk of smokers and never smokers. BMC Med Res Methodol. 2013;13:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hatsukami DK, Le CT, Zhang Y, et al. Toxicant exposure in cigarette reducers versus light smokers. Cancer Epidemiol Biomarkers Prev. 2006;15(12):2355–2358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Strasser AA, Malaiyandi V, Hoffmann E, et al. An association of CYP2A6 genotype and smoking topography. Nicotine Tob Res. 2007;9(4):511–518 [DOI] [PubMed] [Google Scholar]
- 20. Tammemagi MC, Katki HA, Hocking WG, et al. Selection criteria for lung-cancer screening. N Engl J Med. 2013;368(8):728–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Coggins CR, Murrelle EL, Carchman RA, et al. Light and intermittent cigarette smokers: a review (1989–2009). Psychopharmacology (Berl). 2009;207(3):343–363 [DOI] [PubMed] [Google Scholar]