In a country hyperendemic for chronic hepatitis B virus infection, the authors found that the incidence of recent hepatitis D virus infection is increasing among HIV-infected patients over the 20-year study period, which is associated with syphilis and hepatitis flares.
Keywords: seroconversion, seroincidence, case-control study, syphilis, sexually transmitted diseases
Abstract
Background. Superinfection with hepatitis D virus (HDV) may increase the risk for hepatitis flares and chronic hepatic complications in patients with chronic hepatitis B virus (HBV) infection. This retrospective observational study aimed to examine the incidence of and factors associated with recent HDV superinfection among individuals coinfected with human immunodeficiency virus (HIV) and HBV.
Method. Anti-HDV immunoglobulin G (IgG) was sequentially determined in 375 HIV/HBV-coinfected patients to estimate the HDV incidence between 1992 and 2012. Plasma HDV and HBV loads and HBV surface antigen (HBsAg) levels were determined for the HDV seroconverters. A nested case-control study was conducted to identify the associated factors with HDV seroconversion. Phylogenetic analysis was performed using HDV sequences amplified from HDV seroconverters and HDV-seropositive patients at baseline.
Results. During 1762.4 person-years of follow-up [PYFU], 16 patients seroconverted for HDV, with an overall incidence rate of 9.07 per 1000 PYFU, which increased from 0 in 1992–2001, to 3.91 in 2002–2006, to 13.26 per 1000 PYFU in 2007–2012 (P < .05). Recent HDV infection was associated with elevated aminotransferase and bilirubin levels and elevated rapid plasma reagin titers. Of the 12 patients with HDV viremia, 2 were infected with genotype 2 and 10 with genotype 4. HBsAg levels remained elevated despite a significant decline of plasma HBV DNA load with combination antiretroviral therapy that contained lamivudine and/or tenofovir.
Conclusions. Our findings show that the incidence of recent HDV infection in HIV/HBV-coinfected patients increased significantly from 1992–2001 to 2007–2011, and was associated with hepatitis flares and syphilis.
Hepatitis D virus (HDV) is a defective RNA virus that requires the presence of the hepatitis B virus (HBV) surface antigen (HBsAg) to infect the hepatocytes [1]. The prevalence of HDV infection varies widely with different geographic regions studied [2, 3], depending on the prevalence of HBV infection in the general population and the risk factors for HBV transmission. It has been estimated that approximately 5% of HBV carriers are coinfected with HDV, leading to an estimate of 15 million persons infected with HDV worldwide [2, 3].
The majority of HDV infections are acquired through parenteral and sexual routes [2, 3], both of which are also important routes for human immunodeficiency virus (HIV) transmission. Compared with patients who are at risk for sexually transmitted infections, patients who are injection drug users (IDUs) have a significantly higher prevalence of HDV infection, suggesting that HDV is more efficiently transmitted by injections of contaminated blood or diluent than by sexual intercourse [4]. HDV infection has been considered to be the most severe form of viral hepatitis [2, 5, 6], and treatment options for HDV infection are limited [2, 7]. HDV coinfection increases the risk for hepatitis flares and chronic hepatic complications [2]; furthermore, patients with HBV/HDV coinfection have a significantly increased risk for hepatocellular carcinoma compared with patients with HBV monoinfection and the general population [8].
HDV infection can occur simultaneously with acute HBV infection in patients without preexisting HBV infection (coinfection), or HDV infection may occur in those patients with chronic HBV infection (superinfection) [2, 5, 6]. Superinfection with HDV in patients with chronic HBV infection is more likely to cause chronic HDV infection, which may lead to episodes of hepatitis exacerbations, rapid progression of chronic liver disease, hepatic failure, and deaths.
With the advent of combination antiretroviral therapy (cART) in 1996, the survival of HIV-infected patients has significantly improved, and many HIV/HBV-coinfected patients now live on to develop the late complications (including death) related to chronic hepatotropic virus infections [9]. Notably, among such hepatotropic viruses is hepatitis C virus (HCV), which is increasingly reported among men who have sex with men (MSM) in many developed countries because of the shared routes of transmission for HIV and HCV [10, 11]. However whether the same is true for HDV remains largely unknown, as HDV is less well studied. In this study, we aimed to investigate the incidence of and factors associated with recent HDV infection among HIV/HBV-coinfected patients in Taiwan, where the prevalence of chronic HBV infection is estimated to be 18%–20% among adults who were born before implementation of the nationwide HBV vaccination program in 1984 [12].
MATERIALS AND METHODS
Study Setting and Population
The retrospective cohort study was conducted at the National Taiwan University Hospital in Taiwan between 1992 and 2012. The cART that was introduced in 1997 was provided according to the national HIV treatment guidelines. Tenofovir was not available as the first-line agent until mid-2011. Although entecavir and adefovir were available in clinical use, HIV-infected patients who were already on lamivudine-containing cART rarely received entecavir or interferon simultaneously for chronic HBV infection. Monitoring of CD4 lymphocyte count, plasma HIV RNA load, and biochemistry was performed every 3–6 months within the first year of initiation of cART and every 6 months and on an as-needed basis thereafter.
HIV/HBV-coinfected patients who were aged ≥18 years, and had 2 or more follow-up visits at the hospital with an interval >3 months were enrolled for assessment of HDV seroepidemiology. The present study included all HIV/HBV-coinfected patients with negative anti-HDV immunoglobulin G (IgG) at baseline who had at least 1 subsequent follow-up of HBV DNA load to assess the virologic response to lamivudine with or without tenofovir. Anti-HDV antibody testing was performed in the last available blood samples, and all of the archived blood samples of the patients who tested positive for anti-HDV IgG were tested retrospectively to estimate the timing and incidence of recent HDV seroconversion. A standardized case record form was used to collect information on demographics; serologies of HBV, HCV, and syphilis; cART; aminotransferase levels; CD4 count; and plasma HIV RNA load. The research ethics committee of the hospital approved the study and the patients gave written informed consent (registration number 200705072M).
Nested Case-Control Study
To better understand the factors associated with HDV seroconversion, a nested case-control study with 2 controls for each case was conducted. The case patients were recent HDV seroconverters, whereas the control patients were those who had similar follow-up duration to the case patients but without HDV seroconversion throughout the follow-up period. Cases and controls were further matched for age (±5 years), sex, risk for HIV transmission, baseline CD4 count (±20 cells/µL), and plasma HIV RNA load (±0.5 log10 copies/mL). Because our cohort consisted of a small proportion of IDUs and only 1 of 34 IDUs seroconverted for HDV during the observation period (data not shown), we excluded IDUs from the case-control study.
Definitions
Recent HDV seroconversion was defined as the first positive anti-HDV antibody detected within 1 year of the last negative anti-HDV. The date of HDV seroconversion was arbitrarily assigned as the midpoint between the date of the last negative and that of the first positive anti-HDV result. The overall incidence rate of HDV seroconversion in 1992–2012 was estimated, and 3 study periods were defined to assess the trends of recent HDV infection: 1992–2001, 2002–2006, and 2007–2012. Within the 6 months of HDV seroconversion or within the 6 months of the last anti-HDV testing, patients with new rapid plasma reagin (RPR) seroreactivity or a 4-fold increase in RPR titers were diagnosed as having recent syphilis. The end date of the study was the last date the patients had follow-up visits at the hospital, death, or 31 December 2012, whichever occurred first.
Combination ART was defined as the combination of at least 3 antiretroviral agents that contained 2 nucleoside reverse transcriptase inhibitors (NRTIs) plus protease inhibitor, 1 non-NRTI, or integrase inhibitor; or 3 NRTIs.
Laboratory Investigations
HBsAg and anti-HDV antibody were determined using the HBsAg enzyme-linked immunosorbent assay (ELISA) kit (Abbott Laboratories, Abbott Park, Illinois) and ANTI-HDV ELISA kit (Dia.Pro Diagnostic Bioprobes, Srl, Milan, Italy), respectively. Anti-HCV antibody was determined by anti-HCV ELISA kit (Ax SYM HCV III, Abbott Laboratories, North Chicago, Illinois). Syphilis was diagnosed by a positive RPR titer (BD Macro-VueTM RPR Card tests) and Treponema pallidum hemagglutination test (FTI-SERODIA-TPPA, Fujirebio, Taoyuan, Taiwan).
Plasma HBV load was quantified by the Cobas AmpliPrep/Cobas TaqMan HBV Test version 2.0 (Roche Diagnostics Corporation). HDV load was determined using SYBR green real-time polymerase chain reaction (PCR) assay with a lower detection limit of 80 copies/mL. The primer pairs used were HDV-856 (5′-AGG TGG AGA TGC CAT GCC GAC-3′) and HDV-1275 (5′-GGA YCA CCG AAG AAG GAA GGC C-3′). For phylogenetic analysis, HDV delta-gene fragments (nt 856–1275 relative to HDV reference strain JA-M27) were PCR amplified and sequenced using an automatic sequencer (3100 Avent Genetic Analyzer, ABI). GenBank accession numbers for sequences derived in this study were KF678406 through KF678433, wherein KF678406 to KF678417 were the sequences for HDV identified from the HDV seroconverters. Sequences were aligned with Clustal W listed in the MEGA (molecular evolutionary genetics analysis) analytical package (version 3.0) with minor manual adjustments. The phylogenetic trees were constructed by the neighbor-joining method based on the Kimura 2-parameter distance matrix listed in the MEGA software. Bootstrap values >700 of 1000 replicates were considered significant.
Statistical Analysis
All statistical analyses were performed using SPSS software version 16.0 (SPSS Inc, Chicago, Illinois). Categorical variables were compared using χ2 or Fisher exact test and noncategorical variables were compared using Student t test or Mann-Whitney U test. All tests were 2-tailed and a P value <.05 was considered to be statistically significant. The incidence rate of HDV seroconversion in each study period was calculated as the number of HDV seroconversion per 1000 person-years of follow-up (PYFU). Poisson regression was used to compare incidence rates of HDV seroconversion among the 3 study periods (1992–2001, 2002–2006, and 2007–2012).
RESULTS
HDV Seroincidence in the 3 Study Periods
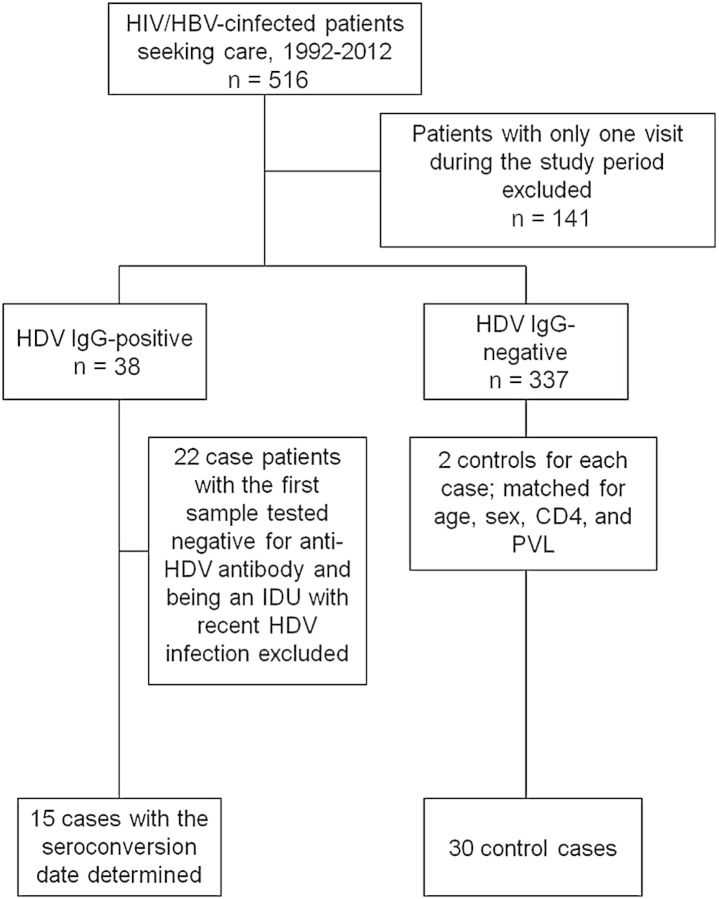
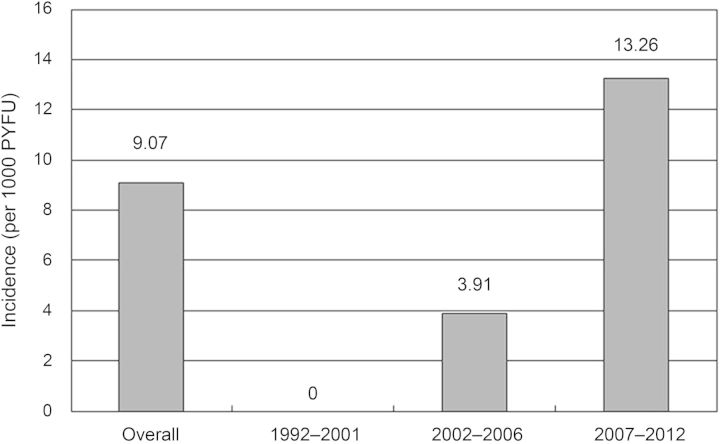
During the 20-year study period, 516 HIV/HBV-coinfected patients sought HIV care at the hospital and 375 patients (72.7%) with at least 2 blood samples for quantification of plasma HBV load were enrolled for determinations of anti-HDV IgG. The study flow is shown in Figure 1. Compared with patients who were excluded from analysis, those included were more likely to be male (96.8% vs 92.9%, P = .05) and MSM (72.5% vs 50.4%, P < .0001) (data not shown). Overall, HDV seropositivity was noted in 38 of 375 (10.1%) HIV/HBV-coinfected patients (Table 1), including 22 who were HDV seropositive at baseline (prevalent HDV infections) and 16 who seroconverted for HDV during the 1762.4 PYFU (incident HDV infections), accounting for an overall HDV seroincidence rate of 9.07 per 1000 PYFU (95% confidence interval [CI], 5.73–14.43 per 1000 PYFU). In the study period between 1992 and 2001, none of 55 patients during the 186.1 PYFU seroconverted for HDV (incidence rate, 0); between 2002 and 2006, 2 of 163 patients within 509.7 PYFU seroconverted (3.1 per 1000 PYFU); and between 2007 and 2012, 14 of 364 within 1066.6 PYFU seroconverted (13.26 per 1000 PYFU) (Figure 2). The incidence rate of recent syphilis increased from 3.22 per 100 PYFU (95% CI, 1.23–5.22 per 100 PYFU) in 1992–2006 to 6.07 per 100 PYFU (95% CI, 4.99–7.15 per 100 PYFU) in 2007–2012 (P = .05) (data not shown).
Figure 1.
Study flow of the incidence of recent hepatitis D virus infection among patients with hepatitis B virus and human immunodeficiency virus coinfection. Abbreviations: HBV, hepatitis B virus; HDV, hepatitis D virus; HIV, human immunodeficiency virus; IDU, injection drug user; IgG, immunoglobulin G; PVL, plasma HIV RNA load.
Table 1.
Clinical Characteristics of Hepatitis D Virus–Seropositive and –Seronegative Subjects
| Characteristic | Seropositive for HDV IgG | Seronegative for HDV IgG | P Value |
|---|---|---|---|
| No. of subjects | 38 | 337 | |
| Age, y, mean (SD) | 38 (7) | 40 (10) | .03 |
| Male sex, % (No.) | 94.7 (36) | 97.0 (327) | .45 |
| CD4, cells/µL, mean (SD) | 581 (293) | 479 (277) | .20 |
| CD4 count <200 cells/µL, % (No.) | 7.9 (3/38) | 14.7 (49/333) | .25 |
| Plasma HIV RNA, log10 copies/mL, mean (SD) | 2.00 (1.23) | 2.29 (1.19) | .25 |
| Plasma HIV RNA <400 copies/mL, % (No.) | 63.2 (24/38) | 75.0 (252/336) | .12 |
| Risk behavior, % (No.) | |||
| MSM | 50.0 (19) | 70.6 (238) | .02 |
| Heterosexual | 7.9 (3) | 13.7 (46) | .47 |
| IDU | 39.5 (15) | 5.6 (19) | <.001 |
| Others | 2.6 (1) | 10.0 (34) | .21 |
| Receipt of cART, % (No.) | 68.4 (26/38) | 87.7 (292/333) | .001 |
| Receipt of lamivudine, % (No.) | 68.4 (26/38) | 87.7 (292/333) | .001 |
| Receipt of TDF, % (No.) | 23.7 (9/38) | 31.7 (105/331) | .31 |
| Anti-HCV positive, % (No.) | 39.5 (15/38) | 10.6 (34/320) | <.001 |
| Plasma HBV DNA, log10 copies/mL, mean (SD) | 2.21 (1.22) | 2.71 (1.89) | .03 |
| Total bilirubin, mg/dL, mean (SD) | 1.43 (1.15) | 1.23 (1.10) | .08 |
| Bilirubin level ≥1.2 mg/dL, % (No.) | 47.6 (10/21) | 34.0 (53/156) | .22 |
| AST, U/L, mean (SD) | 51.8 (87.4) | 45.0 (138.7) | .24 |
| AST level ≥37 U/L, % (No.) | 60.0 (15/25) | 26.7 (60/225) | .001 |
| ALT, U/L, mean (SD) | 57.7 (98.6) | 52.4 (140.4) | .31 |
| ALT level ≥41 U/L, % (No.) | 64.5 (20/31) | 29.7 (66/222) | <.001 |
| ALP, U/L, mean (SD) | 173.3 (136.8) | 176.3 (184.2) | .89 |
| Recent syphilisa, % (No.) | 13.0 (3/23) | 5.6 (16/286) | .15 |
| RPR ≥32, % (No.) | 8.8 (3/34) | 4.8 (15/318) | .30 |
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; cART, combination antiretroviral therapy; HBV, hepatitis B virus; HCV, hepatitis C virus; HDV, hepatitis D virus; HIV, human immunodeficiency virus; IDU, injection drug use; IgG, immunoglobulin G; MSM, men who have sex with men; RPR, rapid plasma reagin; SD, standard deviation; TDF, tenofovir disoproxil fumarate.
a Recent syphilis was defined as having new RPR seroreactivity or a 4-fold increase in RPR titers within the 6 months of HDV seroconversion or within the 6 months of the last anti-HDV testing.
Figure 2.
Incidence rate of recent hepatitis D virus infection among patients with hepatitis B virus and human immunodeficiency virus coinfection in 3 study periods, 1992–2012. The incidence rate increased significantly from 0 per 1000 person-years of follow-up (PYFU) between 1992 and 2001 (zero cases for 186.1 PYFU), to 3.91 per 1000 PYFU between 2002 and 2006 (2 cases for 509.7 PYFU), to 13.26 per 1000 PYFU between 2007 and 2012 (14 cases for 1066.6 PYFU).
Case-Control Study
For the 15 case patients who had recent HDV seroconversion and were not IDUs, 30 matched controls were identified. The clinical characteristics of the case and control patients are shown in Table 2. At baseline, case and control patients had similar CD4 count, plasma HIV RNA and HBV loads, HCV seropositivity, and cART containing lamivudine and/or tenofovir. However, compared with controls, case patients were significantly more likely to have elevated levels of total bilirubin and aminotransferases and RPR titers ≥1:32 (26.7% vs 3.3%; Table 2). Recent syphilis was more common in case patients than in controls (23.1% vs 11.5%, P = .346), although statistical significance was not reached because of small case numbers.
Table 2.
Characteristics of 15 HIV/Hepatitis B Virus (HBV)–Coinfected Patients Who Seroconverted for Hepatitis D Virus and 30 Matched Controls Who Did Not Seroconvert During Follow-up
| Characteristic | HDV Seroconverters | Seronegative for HDV IgG | P Value |
|---|---|---|---|
| No. of subjects | 15 | 30 | |
| Age, y, mean (SD) | 37 (6) | 38 (6) | .672 |
| Male sex, % (No.) | 100 (15) | 100 (30) | >.999 |
| CD4, cells/µL, mean (SD) | 452 (220) | 452 (212) | .997 |
| CD4 count <200 cells/µL, % (No.) | 13.3 (2/15) | 13.3 (4/30) | >.999 |
| Plasma HIV RNA, log10 copies/mL, mean (SD) | 2.10 (1.15) | 2.04 (1.00) | .848 |
| Plasma HIV RNA <400 copies/mL, % (No.) | 86.7 (13/15) | 86.7 (26/30) | >.999 |
| Risk behavior, % (No.) | |||
| MSM | 86.7 (13) | 90 (27) | >.999 |
| Heterosexual | 13.3 (2) | 6.7 (2) | .853 |
| Other | 0 (0) | 3.3 (1) | >.999 |
| Receipt of cART, % (No.) | 100.0 (15/15) | 96.7 (29/30) | .475 |
| Receipt of lamivudine, % (No.) | 100.0 (15/15) | 96.7 (29/30) | .475 |
| Receipt of TDF, % (No.) | 40.0 (6/15) | 43.3 (13/30) | .831 |
| Anti-HCV positive, % (No.) | 0 (0/15) | 6.9 (2/29) | .298 |
| HBV DNA, log10 copies/mL, mean (SD) | 2.13 (0.76) | 2.25 (1.31) | .768 |
| Total bilirubin, mg/dL, mean (SD) | 1.94 (1.41) | 0.80 (0.56) | .035 |
| Bilirubin levels ≥1.2 mg/dL, % (No.) | 63.6 (7/11) | 25.0 (5/20) | .004 |
| AST, U/L, mean (SD) | 67.6 (54.9) | 25.9 (5.6) | <.001 |
| AST level ≥37 U/L, % (No.) | 76.9 (10/13) | 4.8 (1/21) | <.001 |
| ALT, U/L, mean (SD) | 96.0 (87.9) | 31.5 (13.6) | <.001 |
| ALT level ≥41 U/L, % (No.) | 76.9 (10/13) | 19.0 (4/21) | .001 |
| ALP, U/L, mean (SD) | 245.6 (107.7) | 160.5 (89.6) | .154 |
| Recent syphilis, % (No.) | 23.1 (3/13) | 11.5 (3/26) | .346 |
| RPR ≥32, % (No.) | 26.7 (4/15) | 3.33 (1/30) | .019 |
The case patients and controls were matched for age (±5 years), sex, risk for HIV transmission, baseline CD4 count (±20 cells/µL), and plasma HIV RNA load (±0.5 log10 copies/mL).
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; cART, combination antiretroviral therapy; HBV, hepatitis B virus; HCV, hepatitis C virus; HDV, hepatitis D virus; HIV, human immunodeficiency virus; IgG, immunoglobulin G; MSM, men who have sex with men; RPR, rapid plasma reagin; SD, standard deviation; TDF, tenofovir disoproxil fumarate.
HDV Virology
Of the 38 patients who were seropositive for HDV at baseline (prevalent HDV infection) or during follow-up (incident HDV infection), 28 (73.7%) had HDV viremia, and 9 (32.1%) were infected with genotype 2 and 19 (67.9%) with genotype 4. Of the 16 patients who had HDV seroconversion during the follow-up, HDV fragments could be successfully amplified from 12 (75.0%); 2 patients (16.7%) were infected with genotype 2 and 10 patients (83.3%) with genotype 4 (Figure 3). The genotypes remained unchanged in the blood samples collected sequentially from those 12 patients with HDV seroconversion (data not shown). In the phylogenetic analysis of all HDV strains from HIV-infected or HIV-uninfected IDUs in our previous study (Supplementary Figure 1) [13] and from HIV-infected patients with prevalent and incident HDV infections who were not IDUs in this study (Figure 3), we did not identify clusters of HDV sequences.
Figure 3.
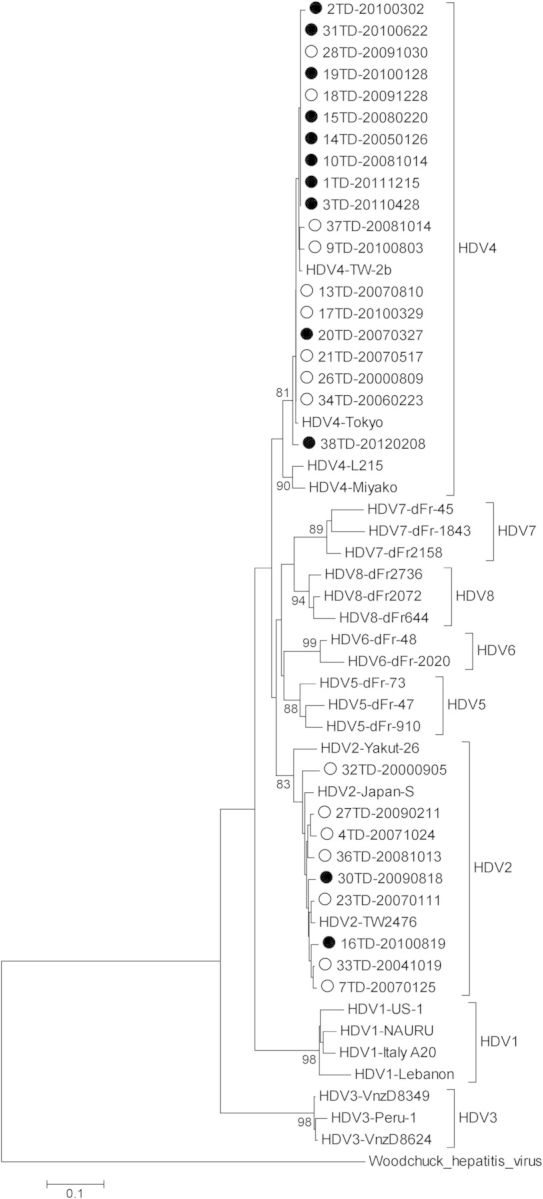
Phylogenetic analysis of hepatitis D virus (HDV) identified from patients with HDV seropositivity at baseline (prevalent HDV infection, open circle, n = 16) and those with HDV seroconversion (incident HDV infection, filled circle, n = 12) during the follow-up. The phylogenetic tree was constructed by the neighbor-joining method based on the Kimura 2-parameter distance matrix listed in MEGA software (version 3.0). The study and reference sequences were aligned using the Clustal W program with minor manual adjustment. The horizontal branch was drawn in accordance with their relative genetic distances. Bootstrap values >700 of 1000 replicates were considered significant and indicated at the nodes of the corresponding branches.
Plasma HBV Viral Load and HBsAg Titers
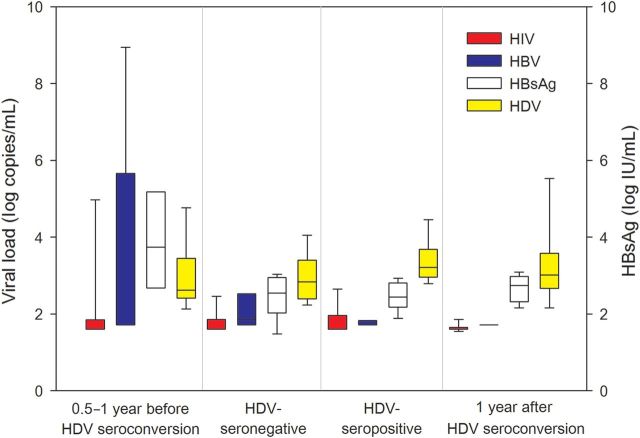
The sequential changes of plasma HIV, HBV, and HDV loads and HBsAg titers of the HDV seroconverters before and after HDV seroconversion are shown in Figure 4. Among the 16 seroconverters, 3 were excluded from analysis: 1 who died within 6 months of HDV seroconversion; 1 IDU who did not receive cART during the study period; and 1 who received cART after HDV seroconversion. All the remaining 13 patients had been diagnosed with HIV/HBV coinfection and were given cART containing lamivudine at enrollment. Before HDV seroconversion, a significant decline of median plasma HBV DNA load from 6.84 to 1.85 log10 copies/mL (P = .009) and a significant but a less dramatic decline of HBsAg titers from 3.16 to 2.54 log10 IU/mL (P = .013) were observed in patients following initiation of cART. HDV seroconversions occurred 0.5–4 years after initiation of cART. No significant changes of plasma HIV, HBV, and HDV loads and HBsAg titers in the HDV seroconverters were observed between the last seronegative and the first seropositive time points. The median plasma HBV and HDV load and HBsAg titer when HDV seroconversion was detected was 1.71 log10 copies/mL (range, 1.71–3.66 log10 copies/mL), 3.21 log10 copies/mL (range, 2.76–4.64 log10 copies/mL), and 2.39 log10 IU/mL (range, 1.71–2.94 log10 IU/mL), respectively (Figure 4). One year after HDV seroconversion, a decrease of plasma HDV load (median, 3.01 log10 copies/mL; P = .81) and an increase of HBsAg titer (2.74 log10 IU/mL; P = .22) were observed in the patients, whereas the plasma HBV loads remained stably suppressed (1.71 log10 copies/mL; P = .05).
Figure 4.
Trends of plasma human immunodeficiency virus (HIV) RNA load, hepatitis B virus (HBV) DNA load, hepatitis D virus (HDV) RNA load, and HBV surface antigen (HBsAg) titers in the HDV seroconverters before and after HDV seroconversion. The 13 patients had been diagnosed with HIV/HBV coinfection and were given combination antiretroviral therapy containing lamivudine at enrollment (0.5–1 year before HDV seroconversion). The case number tested for HDV RNA at 0.5–1 year before HDV seroconversion and HDV seronegativity is 12 and 13, respectively. Quantification of HBsAg was determined using the chemiluminescent microparticle immunoassay, the Architect QT, according to the manufacturer's recommendation (Abbott Laboratories, Abbott Park, Illinois).
DISCUSSION
In this longitudinal follow-up study conducted in a country that used to be hyperendemic for chronic HBV infection [12, 14], we found that the incidence rate of recent HDV infection among HIV/HBV-coinfected patients who were not IDUs increased between 1992 and 2012; furthermore, HDV seroconversion among these patients was associated with hepatitis flares and recent syphilis.
The prevalence of HBV infection has been on the decrease in the developed world where HBV vaccination programs are implemented [15, 16]. However, HDV seroprevalence has not shown a similar decline in these developed countries, which may be related to ongoing risk behaviors that facilitate HDV transmission among persons at risk and immigration of persons from regions endemic for HBV and HDV infections [17, 18]. In Taiwan, the prevalence of chronic HBV infection remains in the range of 15%–20% among adults who were born before the implementation of the nationwide neonatal HBV vaccination program in 1984 [12, 14]. Despite the high prevalence of chronic HBV infection, HDV seroprevalence was 9.3% and 2.3% in HIV-infected and HIV-uninfected patients who were not IDUs, respectively, in our previous survey [13], which is similar to the rates reported in developed countries with much lower HBV seroprevalence [19].
Similar to HCV, HDV can be acquired through sexual routes [20–23]. The findings of the increasing trends of HDV seroconversion and the association of HDV seroconversion with syphilis in this study echo the concurrent finding of recent HCV infections in Taiwan and many other developed countries [10, 11]. In Taiwan, the incidence of recent HCV seroconversion (overall incidence rate of 7.03 per 1000 PY) increased from 0 in 1994–2000, to 2.29 in 2001–2005, to 10.13 per 1000 PY in 2006–2010 (P < .05) [11]. The association between the increasing trends of recent syphilis and both trends of HCV and HDV seroconversion suggests that HDV or HCV transmission may be facilitated by syphilis that may present with ulceration in cases of primary or secondary syphilis, or that syphilis is a surrogate marker for risky sexual behaviors that may increase HDV or HCV transmission.
Long-term treatment with potent anti-HBV therapy such as entecavir and tenofovir can maintain prolonged suppression of HBV replication, which has been shown to result in regression of cirrhosis of the liver and reduction of liver-related and all-cause mortality in HIV-uninfected patients [24, 25]. Therapeutic options for HDV infection are limited, however, and current potent anti-HBV agents such as entecavir with or without interferon fail to eradicate HDV [26, 27]. Despite long-term suppression of HBV replication using potent nucleoside/nucleotide analogues, HBsAg titers decay very gradually, so much so that the probability of HBsAg clearance is remote during the patient's lifetime [28], thus providing a wide window of opportunity for HDV superinfection, as demonstrated in our study.
The findings of our study are important in terms of long-term successful management of HIV-infected patients in the era of cART containing tenofovir when AIDS-related morbidity and mortality have significantly declined [29], A recent study from Japan demonstrated that use of tenofovir with or without lamivudine significantly decreased the risk for HBV acquisition by 90% [30]; however, complacency with the effectiveness of cART containing tenofovir or lamivudine with resultant inconsistent adoption of safe sex practices may allow breakthrough of HBV, HCV, and other sexually transmitted infections to occur, which may pave the way for HDV coinfection or superinfection [31]. Moreover, in HIV/HBV-coinfected patients with hepatitis flares or possibly even new syphilis, it is important to consider anti-HDV antibody and HDV RNA testing in high-risk patient populations.
The strengths of the study are the relatively large number of HIV/HBV-coinfected patients in a country with easy access to HIV and HBV care and the concomitant detection of plasma HBV and HDV loads, and HBsAg levels of sequentially collected blood samples to examine dynamic trends while patients are on cART. However, there are also several limitations to our study. First, the case number of HDV seroconversion remains small, which makes the identification of significant associated factors and clusters of recent HDV infections challenging. Second, we may have underestimated or overestimated the HDV seroincidence because not all HIV/HBV-coinfected patients had sequential blood samples for anti-HDV antibody testing. Third, we were not able to estimate the timing of HDV seroconversion precisely because blood sampling was performed at 3- to 6-month intervals when the patients returned for monitoring of plasma HIV RNA loads and CD4 counts. Fourth, although we identified the association between HDV seroconversion and higher RPR titers, we did not have information on sexual behaviors, a history of bleeding or injury during the sexual encounters, or substance abuse, which may preclude identification of the behaviors that facilitate HDV transmission. Fifth, 4 patients had HDV viremia when they were seronegative for anti-HDV antibody (Figure 4), and the HDV seroconversion occurred 1–8 months after HDV viremia. Therefore, the delayed seroconversion might lead to our underestimation of HDV incidence, although this did not affect the trend of increasing HDV incidence in the 3 study periods. Last, the numbers of IDUs (who used to have the highest HDV seroprevalence) and heterosexuals are small in our study, which may limit the generalizability of our findings.
In conclusion, the overall seroincidence of recent HDV infections in HIV/HBV-coinfected patients was 9.07 per 1000 PYFU in Taiwan, which increased significantly from 1992–2001 to 2007–2011. Recent HDV seroconversion was associated with hepatitis flares and syphilis despite the use of cART containing lamivudine or tenofovir. Vigilance should be maintained against acquisition of sexually transmitted infections that include hepatotropic viruses in the cART era.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank Aristine Cheng, National Taiwan University Hospital Hsin-Chu Branch, for review and English editing of the manuscript.
Disclaimer. The funding source had no role in the study design, conduct of the study, data collection and analysis, preparation of the manuscript, or decision to submit for publication.
Financial support. This work was sponsored by the Centers for Disease Control, Taiwan (grant number DOH102-DC-1401).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Rizzetto M, Hoyer B, Canese MG, Shih JW, Purcell RH, Gerin JL. delta Agent: association of delta antigen with hepatitis B surface antigen and RNA in serum of delta-infected chimpanzees. Proc Natl Acad Sci U S A. 1980;77:6124–8. doi: 10.1073/pnas.77.10.6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hughes SA, Wedemeyer H, Harrison PM. Hepatitis delta virus. Lancet. 2011;378:73–85. doi: 10.1016/S0140-6736(10)61931-9. [DOI] [PubMed] [Google Scholar]
- 3.Rizzetto M, Ciancio A. Epidemiology of hepatitis D. Semin Liver Dis. 2012;32:211–9. doi: 10.1055/s-0032-1323626. [DOI] [PubMed] [Google Scholar]
- 4.Calle Serrano B, Manns MP, Wedemeyer H. Hepatitis delta and HIV infection. Semin Liver Dis. 2012;32:120–9. doi: 10.1055/s-0032-1316467. [DOI] [PubMed] [Google Scholar]
- 5.Rizzetto M. Hepatitis D: thirty years after. J Hepatol. 2009;50:1043–50. doi: 10.1016/j.jhep.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Wedemeyer H, Manns MP. Epidemiology, pathogenesis and management of hepatitis D: update and challenges ahead. Nat Rev Gastroenterol Hepatol. 2010;7:31–40. doi: 10.1038/nrgastro.2009.205. [DOI] [PubMed] [Google Scholar]
- 7.Heidrich B, Manns MP, Wedemeyer H. Treatment options for hepatitis delta virus infection. Curr Infect Dis Rep. 2013;15:31–8. doi: 10.1007/s11908-012-0307-z. [DOI] [PubMed] [Google Scholar]
- 8.Ji J, Sundquist K, Sundquist J. A population-based study of hepatitis D virus as potential risk factor for hepatocellular carcinoma. J Natl Cancer Inst. 2012;104:790–2. doi: 10.1093/jnci/djs168. [DOI] [PubMed] [Google Scholar]
- 9.Thio CL, Seaberg EC, Skolasky R, Jr, et al. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS) Lancet. 2002;360:1921–6. doi: 10.1016/s0140-6736(02)11913-1. [DOI] [PubMed] [Google Scholar]
- 10.van de Laar T, Pybus O, Bruisten S, et al. Evidence of a large, international network of HCV transmission in HIV-positive men who have sex with men. Gastroenterology. 2009;136:1609–17. doi: 10.1053/j.gastro.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun HY, Chang SY, Yang ZY, et al. Recent hepatitis C virus infections in HIV-infected patients in Taiwan: incidence and risk factors. J Clin Microbiol. 2012;50:781–7. doi: 10.1128/JCM.06014-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun HY, Ko WC, Tsai JJ, et al. Seroprevalence of chronic hepatitis B virus infection among Taiwanese human immunodeficiency virus type 1-positive persons in the era of nationwide hepatitis B vaccination. Am J Gastroenterol. 2009;104:877–84. doi: 10.1038/ajg.2008.159. [DOI] [PubMed] [Google Scholar]
- 13.Chang SY, Yang CL, Ko WS, et al. Molecular epidemiology of hepatitis D virus infection among injecting drug users with and without human immunodeficiency virus infection in Taiwan. J Clin Microbiol. 2011;49:1083–9. doi: 10.1128/JCM.01154-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen CH, Yang PM, Huang GT, Lee HS, Sung JL, Sheu JC. Estimation of seroprevalence of hepatitis B virus and hepatitis C virus in Taiwan from a large-scale survey of free hepatitis screening participants. J Formos Med Assoc. 2007;106:148–55. doi: 10.1016/S0929-6646(09)60231-X. [DOI] [PubMed] [Google Scholar]
- 15.Ni YH, Chang MH, Wu JF, Hsu HY, Chen HL, Chen DS. Minimization of hepatitis B infection by a 25-year universal vaccination program. J Hepatol. 2012;57:730–5. doi: 10.1016/j.jhep.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 16.McMahon BJ, Bruden DL, Petersen KM, et al. Antibody levels and protection after hepatitis B vaccination: results of a 15-year follow-up. Ann Intern Med. 2005;142:333–41. doi: 10.7326/0003-4819-142-5-200503010-00008. [DOI] [PubMed] [Google Scholar]
- 17.William Tong CY, Asher R, Toby M, et al. A re-assessment of the epidemiology and patient characteristics of hepatitis D virus infection in inner city London. J Infect. 2013;66:521–7. doi: 10.1016/j.jinf.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 18.Gish RG, Yi DH, Kane S, et al. Coinfection with hepatitis B and D: epidemiology, prevalence and disease in patients in Northern California. J Gastroenterol Hepatol. 2013;28:1521–5. doi: 10.1111/jgh.12217. [DOI] [PubMed] [Google Scholar]
- 19.Soriano V, Grint D, d'Arminio Monforte A, et al. Hepatitis delta in HIV-infected individuals in Europe. AIDS. 2011;25:1987–92. doi: 10.1097/QAD.0b013e32834babb3. [DOI] [PubMed] [Google Scholar]
- 20.Liaw YF, Chiu KW, Chu CM, Sheen IS, Huang MJ. Heterosexual transmission of hepatitis delta virus in the general population of an area endemic for hepatitis B virus infection: a prospective study. J Infect Dis. 1990;162:1170–2. doi: 10.1093/infdis/162.5.1170. [DOI] [PubMed] [Google Scholar]
- 21.Wu JC, Lee SD, Govindarajan S, et al. Sexual transmission of hepatitis D virus infection in Taiwan. Hepatology. 1990;11:1057–61. doi: 10.1002/hep.1840110623. [DOI] [PubMed] [Google Scholar]
- 22.Mele A, Franco E, Caprilli F, et al. Hepatitis B and delta virus infection among heterosexuals, homosexuals and bisexual men. Eur J Epidemiol. 1988;4:488–91. doi: 10.1007/BF00146404. [DOI] [PubMed] [Google Scholar]
- 23.Rosenblum L, Darrow W, Witte J, et al. Sexual practices in the transmission of hepatitis B virus and prevalence of hepatitis delta virus infection in female prostitutes in the United States. JAMA. 1992;267:2477–81. [PubMed] [Google Scholar]
- 24.Marcellin P, Gane E, Buti M, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381:468–75. doi: 10.1016/S0140-6736(12)61425-1. [DOI] [PubMed] [Google Scholar]
- 25.Wong GL, Chan HL, Mak CW, et al. Entecavir treatment reduces hepatic events and deaths in chronic hepatitis B patients with liver cirrhosis. Hepatology. 2013;58:1537–47. doi: 10.1002/hep.26301. [DOI] [PubMed] [Google Scholar]
- 26.Wedemeyer H, Yurdaydin C, Dalekos GN, et al. Peginterferon plus adefovir versus either drug alone for hepatitis delta. N Engl J Med. 2011;364:322–31. doi: 10.1056/NEJMoa0912696. [DOI] [PubMed] [Google Scholar]
- 27.Kabacam G, Onder FO, Yakut M, et al. Entecavir treatment of chronic hepatitis D. Clin Infect Dis. 2012;55:645–50. doi: 10.1093/cid/cis459. [DOI] [PubMed] [Google Scholar]
- 28.Chevaliez S, Hezode C, Bahrami S, Grare M, Pawlotsky JM. Long-term hepatitis B surface antigen (HBsAg) kinetics during nucleoside/nucleotide analogue therapy: finite treatment duration unlikely. J Hepatol. 2013;58:676–83. doi: 10.1016/j.jhep.2012.11.039. [DOI] [PubMed] [Google Scholar]
- 29.Sterne JA, May M, Costagliola D, et al. Timing of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients: a collaborative analysis of 18 HIV cohort studies. Lancet. 2009;373:1352–63. doi: 10.1016/S0140-6736(09)60612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gatanaga H, Hayashida T, Tanuma J, Oka S. Prophylactic effect of antiretroviral therapy on hepatitis B virus infection. Clin Infect Dis. 2013;56:1812–9. doi: 10.1093/cid/cit145. [DOI] [PubMed] [Google Scholar]
- 31.Sheng WH, Chuang YC, Sun HY, et al. Prophylactic effect of lamivudine-based antiretroviral therapy on incident hepatitis B virus infection among HIV-infected patients. Clin Infect Dis. 2013;57:1504–6. doi: 10.1093/cid/cit511. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.