The MSI-High (MSI-H) phenotype is present in 15% of early stage CRC. DNA microsatellite instability (MSI) occurs when germ-line or sporadic mutations in mismatch repair (MMR) genes allows for replication errors or instability in repeat DNA sequences. MSI-H has been shown to confer a good prognosis in patients with localized disease. Given the prognostic effect of the MSI-H status, few patients present with metastatic disease. It is unclear whether MSI-H status confers a prognostic benefit or a predictive effect to therapy in this setting. In this study we use a multi-center approach to examine the survival outcomes and responses to chemotherapy for the largest reported cohort of MSI-H metastatic CRC. A total of 55 patients (33 from The Royal Melbourne Hospital and 22 from University of Texas MD Anderson Cancer Center) with metastatic CRC and known MSI-H status were analyzed.
Keywords: colorectal cancer, microsatellite instability-high, survival, BRAF V600E mutation, metastatectomy, chemotherapy
Abstract
Background
The microsatellite instability-high (MSI-H) phenotype, present in 15% of early colorectal cancer (CRC), confers good prognosis. MSI-H metastatic CRC is rare and its impact on outcomes is unknown. We describe survival outcomes and the impact of chemotherapy, metastatectomy, and BRAF V600E mutation status in the largest reported cohort of MSI-H metastatic colorectal cancer (CRC).
Patients and methods
A retrospective review of 55 MSI-H metastatic CRC patients from two institutions, Royal Melbourne Hospital (Australia) and The University of Texas MD Anderson Cancer Center (United States), was conducted. Statistical analyses utilized Kaplan–Meier method, Log-rank test, and Cox proportional hazards models.
Results
Median age was 67 years (20–90), 58% had poor differentiation, and 45% had stage IV disease at presentation. Median overall survival (OS) from metastatic disease was 15.4 months. Thirteen patients underwent R0/R1 metastatectomies, with median OS from metastatectomy 33.8 months. Thirty-one patients received first-line systemic chemotherapy for metastatic disease with median OS from the start of chemotherapy 11.5 months. No statistically significant difference in progression-free survival or OS was seen between fluoropyrimidine, oxaliplatin, or irinotecan based chemotherapy. BRAF V600E mutation was present in 14 of 47 patients (30%). BRAF V600E patients demonstrated significantly worse median OS; 10.1 versus 17.3 months, P = 0.03. In multivariate analyses, BRAF V600E mutants had worse OS (HR 4.04; P = 0.005), while patients undergoing metastatectomy (HR 0.11; P = <0.001) and patients who initially presented as stage IV disease had improved OS (HR 0.27; P = 0.003).
Conclusions
Patients with MSI-H metastatic CRC do not appear to have improved outcomes. BRAF V600E mutation is a poor prognostic factor in MSI-H metastatic CRC.
introduction
DNA microsatellite instability (MSI) occurs when germline or sporadic mutations in mismatch repair (MMR) genes allow for replication errors or instability in repeat DNA sequences. Germline mutations in the MLH1, MSH2, MSH6, and PMS2 genes, lead to an autosomal dominant hereditary syndrome named hereditary nonpolyposis colorectal cancer (HNPCC), or Lynch syndrome. Sporadic deficiency of the MMR system occurs with silencing of the MLH1 promoter via hypermethylation.
The MSI-high (MSI-H) phenotype is present in 15% of early-stage metastatic colorectal cancer (CRC). MSI-H tumors frequently present on the right side, are poorly differentiated with mucinous histological features, and marked peri- and intratumoral lymphocytic invasion [1]. MSI is more common among stage II (∼20%) than III (∼12%), and is even less frequent in stage IV CRC (∼4%) [2]. MSI-H has been shown to confer a good prognosis in patients with localized disease [3, 4].
Many studies have attempted to address the effect of chemotherapy in the MSI-H population with mixed results. In the adjuvant setting, it appears that MSI-H tumors are chemoresistant to 5-fluorouracil (5-FU), the cornerstone of CRC treatment [5]. One hypothesis is that an intact MMR system normally recognizes incorporated DNA adducts and mismatches to halt cell growth [6]. This does not occur with a defective MMR system. The effect of additional agents such as oxaliplatin and irinotecan in the adjuvant setting has been less defined [7, 8]. Some have postulated there may be a differential response to therapy based on sporadic versus germline MSI-H status, where germline MSI-H patients appear more chemosensitive [9].
Given the prognostic effect of MSI-H status, few patients present with metastatic disease. It is unclear whether MSI-H status confers a prognostic benefit or a predictive effect in this setting. A deeper understanding of the disease biology, mutational profile, and intricacies of treatment response in this rare subset is warranted. In this study, we use a multicenter approach to examine the survival outcomes and responses to chemotherapy for the largest reported cohort of MSI-H metastatic CRC.
materials and methods
patient selection
At the University of Texas MD Anderson Cancer Center (UTMDACC), a total of 554 CRC patients who underwent testing for MSI were identified from August 2002 to August 2010. At the Royal Melbourne Hospital (RMH), a previously described cohort of 316 patients was used to identify patients with metastatic CRC and MSI-H status. [10] A total of 55 patients (33 from RMH and 22 from UTMDACC) with metastatic CRC and known MSI-H status were analyzed. There was no effect of institution on overall survival (OS) (log-rank test, P = 0.22).
data collection
Medical records were reviewed on all 55 patients identified. Demographic data, tumor characteristics, treatment types, treatment responses, and survival rates were collected. Response evaluation was based on the treating physician's assessment. The analysis was approved by the UTMDACC and RMH Institutional Review Boards.
molecular testing
MSI analysis was carried out using the five National Cancer Institute recommended microsatellite markers [11]. MSI-H was defined as the presence of two or more (or >30%) loci showing instability. MSI-low (MSI-L) was defined as the presence of one (or <30%) loci showing instability, and MSI stable (MSS) as no loci of instability. Patients with MSI-L or MSS tumors were grouped together and excluded. BRAF V600E mutational testing by a mutation-specific real-time polymerase chain reaction assay was carried out on a subset of cases (N = 47).
Immunohistochemistry was carried out on the UTMDACC cohort to detect nuclear loss of DNA MMR gene products MLH1 (G168-15, 1:25; BD Biosciences Pharmingen, San Diego, CA), MSH2 (FE11, 1:100; Calbiochem, La Jolla, CA), MSH6 (44, 1:300; BD Biosciences Pharmingen), or PMS2 (Alb-4, 1:125; BD Biosciences Pharmingen). In patients with MLH1 loss, a methylation-specific polymerase chain reaction MLH1 promoter methylation assay was conducted.
statistical methods
The progression-free and OS distributions were estimated using the Kaplan–Meier method and differences in survival were evaluated by the log-rank test. Point estimates of median survival and the associated Brookmeyer and Crowley 95% confidence interval (CI) estimates are reported when appropriate. Univariate Cox's proportional hazards models were used to assess the effect of continuous variables on survival and multivariate Cox models were used to evaluate the effect of individual factors while simultaneously adjusting for additional co-variates. Bivariate associations of categorical variables were investigated using Pearson's χ2 test or Fisher's exact test as appropriate. All statistical analyses were carried out using SAS 9.2 (SAS Institute, Inc., Cary, NC) and statistical significance was defined as P < 0.05.
results
patient characteristics
Patient characteristics are displayed in Table 1. A total of 55 patients with metastatic MSI-H CRC were identified between the two sites. Median age was 67 years (range: 20–90 years), 42% were male, 58% had poor differentiation, and 64% were right-sided. The median time to metastases for the 30 (55%) patients who presented with localized disease was 9 months. BRAF mutational status was available in 47 patients of whom 14 harbored the BRAF V600E mutation. BRAF mutants were more common in those initially diagnosed with stage IV rather than I–III disease, although this was not statistically significant (40.9% versus 20%; P = 0.20). Within the UTMDACC cohort, five patients had BRAF V600E mutation, of whom four (80%) demonstrated MLH1 loss due to methylation of the MLH1 promoter.
Table 1.
Patient characteristics
| Characteristic | Number of patient | Percent (%) |
|---|---|---|
| Median age at diagnosis of metastatic disease (years) | 67 (range: 20–90) | |
| Gender | ||
| Male | 23 | 42 |
| Female | 32 | 58 |
| Stage at diagnosis | ||
| Stage I–III | 30 | 55 |
| Stage IV | 25 | 45 |
| Tumor site | ||
| Right | 35 | 64 |
| Left | 20 | 36 |
| Tumor grade | ||
| Well | 0 | 0 |
| Moderate | 19 | 35 |
| Poor | 32 | 58 |
| Unknown | 4 | 7 |
| BRAF status | ||
| BRAF WT | 33 | 60 |
| BRAF mutant | 14 | 25 |
| Unknown | 8 | 15 |
| Metastatectomy | ||
| R0 | 12 | 92 |
| R1 | 1 | 8 |
| Sites of metastatectomy | ||
| Liver | 7 | |
| Lymph nodesa | 3 | |
| Peritoneum | 4 | |
| Front-line chemotherapy regimen | ||
| Flouropyridime alone | 7 | |
| Oxaliplatin based | 14 | |
| Irinotecan based | 10 | |
aExternal iliac (1), supraclavicular (1), retroperitoneal (1).
entire cohort
A total of 42 deaths (76%) had occurred at the time of analysis, with median follow-up for all patients of 19.3 months (range 1.1–105.3 months). The median survival from diagnosis of metastatic disease was 15.4 months (95% CI 10.61% to 17.74%; supplementary Figure S1, available at Annals of Oncology online) and 20.2 months (95% CI 15.6–34.6) from the date of initial diagnosis. There was no correlation between stage at initial diagnosis (stage I–III versus IV) and OS from the date of metastatic disease (13.2 versus 16.2 months; P = 0.83; supplementary Figure S2, available at Annals of Oncology online). Patients with BRAF wild-type when compared with mutant tumors had an improved outcome (17.3 versus 10.1 months; P = 0.029; Figure 1A).
Figure 1.
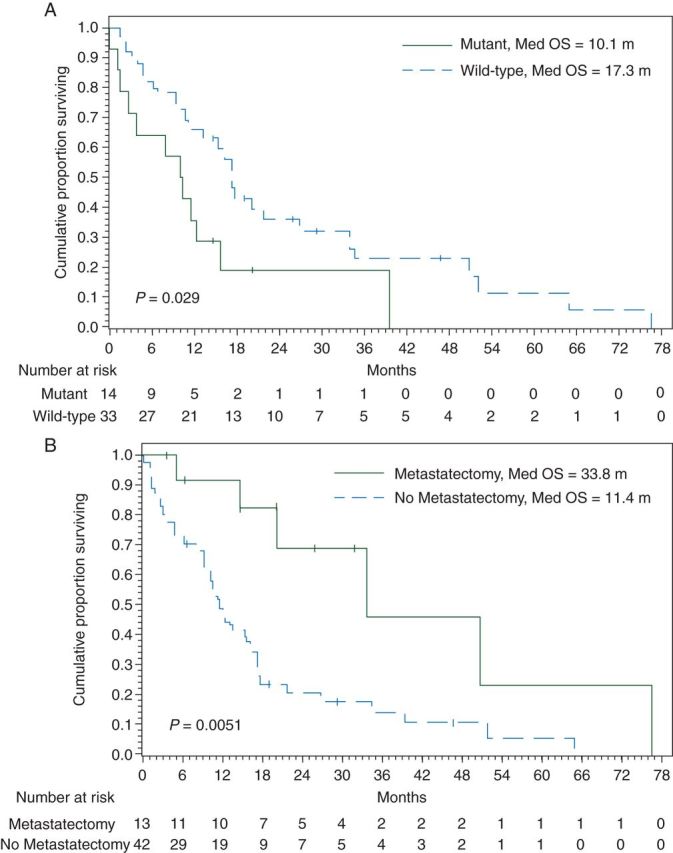
(A) Overall survival from the date of diagnosis of metastatic disease by BRAF. (B) Overall survival from the date of diagnosis of metastatic disease by metastatectomy.
metastatectomy cohort
A margin-negative or microscopically margin-positive resection of all metastatic disease was carried out in 13 (23.6%) of the 55 patients. There was a statistically significant difference between the median OS from the date of metastatic disease in those that underwent metastatectomy (R0/1) than those that did not (33.8 versus 11.4 months; P = 0.005; Figure 1B). Of the 13 patients who underwent metastatectomy, relapse-free survival from the date of metastatic disease was 13.6 months. OS was not significantly different based on stage at diagnosis (stage I–III versus IV) in metastatectomy patients (38.7 versus 33.8 months; P = 0.393).
chemotherapy cohort
Thirty-one patients received front-line systemic chemotherapy: single-agent fluoropyrimidine (5-FU) in 7, oxaliplatin-based in 14, and irinotecan-based in 10. The rate of response to 5-FU, irinotecan-based and oxaliplatin-based chemotherapy were 14%, 40%, and 43%, respectively (P = 0.37 for 5-FU versus Other). The median survival from date of first-line chemotherapy was 11.5 months (95% CI 8.0–21.8; supplementary Figure S3, available at Annals of Oncology online). OS was not significantly different between types of chemotherapy 5-FU versus Other (10.02 versus 11.63 months; P = 0.803, Figure 2A). Progression-free survival was not significantly different between types of chemotherapy 5-FU versus Other (2.2 versus 5.4 months; P = 0.66, Figure 2B).
Figure 2.
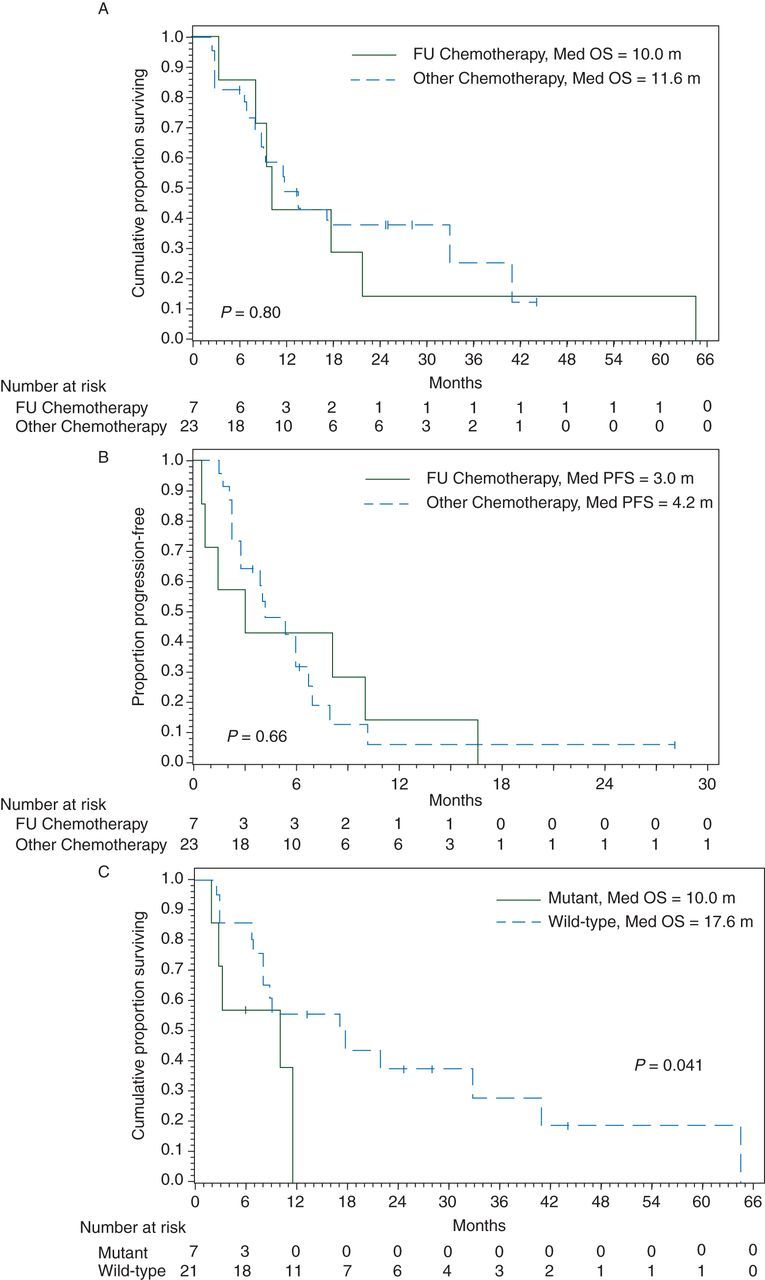
(A) Overall survival from date of first-line chemotherapy by type of chemotherapy. (B) Progression-free survival from the date of first-line chemotherapy by type of chemotherapy. (C) Overall survival from the date of first-line chemotherapy start by BRAF.
In the chemotherapy cohort, of the 28 patients with known BRAF status, 7 had a BRAF V600E mutation. The median OS among BRAF mutant patients from start of chemotherapy was statistically worse than the wild-type patients (10 versus 17.6 months, P = 0.041; Figure 2C).
multivariate analysis
A Cox proportional hazards model was used to assess the effect of multiple factors on OS from the date of metastatic disease (Table 2). BRAF mutational status was associated with a worse OS (P = 0.005, HR 4.04, 95% CI 1.52–10.71), while presenting with stage IV disease (P = 0.003, HR 0.27, 95% CI 0.12–0.63) and undergoing a metastatectomy (P = 0.001, HR 0.11, 95% CI 0.03–0.38) were associated with improved OS.
Table 2.
Univariate and multivariate analysis for overall survival from the date of metastatic disease
| Variables | Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|
| Mediana | 95% CI | P-value | HR | 95% CI | P-value | |
| Stage | ||||||
| IV | 16.20 | 9.86–33.81 | 0.83 | 0.27 | 0.12–0.63 | 0.0025 |
| I–III | 13.21 | 7.95–21.78 | 1 | |||
| Grade | ||||||
| Poor differentiation | 11.07 | 5.09–17.45 | 0.49 | 1.58 | 0.64–3.85 | 0.32 |
| Well-mod differentiation | 17.31 | 13.21–26.74 | 1 | |||
| BRAF mutation status | ||||||
| Mutant | 10.10 | 1.41–15.64 | 0.029 | 4.04 | 1.52–10.71 | 0.0051 |
| Wild type | 17.31 | 11.07–26.74 | 1 | |||
| Gender | ||||||
| Male | 17.74 | 11.72–39.59 | 0.15 | 0.46 | 0.18–1.13 | 0.09 |
| Female | 13.21 | 6.31–17.31 | 1 | |||
| Metastatectomy | ||||||
| Yes | 33.81 | 14.55–76.58 | 0.0051 | 0.11 | 0.03–0.38 | 0.0006 |
| No | 11.43 | 9.23–16.20 | 1 | |||
| Systemic chemotherapy | ||||||
| Yes | 15.64 | 11.43–33.81 | 0.25 | 0.73 | 0.32–1.63 | 0.44 |
| No | 10.35 | 4.86–17.31 | 1 | |||
| Age at initial diagnosis (years) | ||||||
| ≥65 | 11.43 | 6.31–20.24 | 0.18 | 1.97 | 0.84–4.66 | 0.12 |
| <65 | 15.64 | 11.07–50.79 | 1 | |||
aMedian survival in months.
discussion
In this study, we expanded on the current body of literature with the largest multi-institutional cohort of MSI-H metastatic CRC. Our report is also unique in that we analyzed the influence of BRAF status and metastatectomy in this population.
In our study, we show a median OS of 15.4 months from the date of diagnosis of metastatic disease and 20.2 months from date of initial diagnosis. These findings appear similar to previous retrospective studies of metastatic MSI-H CRC where median OS ranges from 9 to 33 months [2, 12–15]. Although our study does not contain a MSS cohort for prognostic comparison, our findings of a median OS of 15.4 months appears similar to a recently published large retrospective multicenter study that reported a median OS of 18 months for stage IV CRC [12, 16].
The survival advantage for stage I–III CRC is dependent on the fact that MSI tumors experience lower rates of tumor recurrence than do MSS tumors [9, 17]. Whether this finding infers a similar benefit for the resection of oligometastatic disease has not been evaluated. The median survival following successful metastatectomy in our cohort was 33.8 months, which appears similar in comparison with the expected outcomes following metastatectomy. In a recent meta-analysis of CRC liver hepatectomies, the median OS was 42 months [18]. Although the small sample size limits definitive conclusions, our data do not support an improved outcome following metastatectomy of oligometastatic disease in patients with MSI-H CRC.
While MSI status is a proven prognostic marker in the treatment of localized CRC, its role as a predictive marker for chemotherapy is not certain due to conflicting evidence. Currently, the European Society for Medical Oncology does not consider MSI to be a predictive marker [19]. Both Sargent et al. and Bertognoli et al. demonstrate the lack of efficacy of 5-fluorouracil-based regimens in the adjuvant setting in locally advanced MSI-H CRC [5, 8]. Though limited by small sample size our data did not demonstrate improved outcomes in the metastatic setting with any chemotherapy combination, though numerically there were less responses in the 5-FU group compared with oxaliplatin and irinotecan based combinations. This is comparable with reports looking at oxaliplatin-based (CAPOX or FOLFOX/FUFOX) [15, 20, 21] and irinotecan-based regimens [22] therapies that have not shown improved outcomes in the MSI-H cohort. These studies, however, are also limited by sample size, the largest of which had 23 MSI-H patients. Similarly, in a meta-analysis that included 964 metastatic CRC patients, of which 91 were MSI-high, MSI-high status did not predict for a differential chemotherapy benefit [23].
Two studies have reported improved outcomes with 5-FU-based chemotherapy. In a retrospective study, Brueckl et al. demonstrated an improved outcome for the use of 5-FU in MSI-H in comparison with MSS patients; however, only seven patients with MSI-H were included [14]. In a non-randomized prospective study where 5-FU chemotherapy use was determined by each individual patient, Liang et al. demonstrated an improved median OS for the 35 MSI-H patients and the 134 MSS patients that received chemotherapy (24 versus 13 months, P < 0.001, respectively) [13].
MSI is strongly associated with mutations in BRAF, limited to sporadic MSI-H tumors [24], and is used clinically to differentiate these from HNPCC cases. The presence of BRAF V600E mutations has been correlated with markedly worse outcome in both localized and metastatic patients [10, 25]. Though less studied, it does not appear that the presence of BRAF V600E correlates with a worse prognosis in MSI-H metastatic CRC [10, 26]. In the current study, BRAF V600E was a strong negative prognostic marker of survival in metastatic CRC with median OS of 10.1 months compared with 17.3 months for BRAF wild-type (P = 0.029). A similar result was seen in patients who were treated with chemotherapy (10.0 versus 17.6 months; log-rank test P = 0.041). BRAF V600E mutation status was more frequent in stage IV patients, 41%, than stage I–III patients, 20%.
The main limitation inherent to our study is the retrospective nature and small sample size of patients with metastatic MSI-H CRC. The small sample size limits the interpretation of subgroup analyses. This study was conducted over an extended time period and thus, tumor response assessment could only be based on the treating physician's assessment. The choice of therapy for each patient was dependent upon the treating physician and the rationale for such decisions could not be determined. Despite these limitations, this study does contain the largest reported cohort of metastatic MSI-H CRC and represents the only study to attempt to evaluate the impact of metastatectomy in this cohort.
Compared with historical controls, patients with MSI-H metastatic CRC do not appear to have improved outcomes following R0/R1 metastatectomy. This study did not find any support for MSI-H status predicting for any differential chemotherapy benefit in metastatic patients. BRAF V600E mutation is a poor prognostic factor in metastatic MSI-H. Further studies including this unique subset of CRC patients should be conducted to further delineate the prognostic and predictive impact of MSI.
disclosure
The authors have declared no conflicts of interest.
Supplementary Material
references
- 1.Kim H, Jen J, Vogelstein B, et al. Clinical and pathological characteristics of sporadic colorectal carcinomas with DNA replication errors in microsatellite sequences. Am J Pathol. 1994;145:148–156. [PMC free article] [PubMed] [Google Scholar]
- 2.Koopman M, Kortman GA, Mekenkamp L, et al. Deficient mismatch repair system in patients with sporadic advanced colorectal cancer. Br J Cancer. 2009;100:266–273. doi: 10.1038/sj.bjc.6604867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lothe RA, Peltomaki P, Meling GI, et al. Genomic instability in colorectal cancer: relationship to clinicopathological variables and family history. Cancer Res. 1993;53:5849–5852. [PubMed] [Google Scholar]
- 4.Thibodeau SN, Bren G, Schaid D. Microsatellite instability in cancer of the proximal colon. Science. 1993;260:816–819. doi: 10.1126/science.8484122. [DOI] [PubMed] [Google Scholar]
- 5.Sargent DJ, Marsoni S, Monges G, et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol. 2010;28:3219–3226. doi: 10.1200/JCO.2009.27.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carethers JM, Chauhan DP, Fink D, et al. Mismatch repair proficiency and in vitro response to 5-fluorouracil. Gastroenterology. 1999;117:123–131. doi: 10.1016/s0016-5085(99)70558-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gavin PG, Colangelo LH, Fumagalli D, et al. Mutation profiling and microsatellite instability in stage II and III colon cancer: an assessment of their prognostic and oxaliplatin predictive value. Clin Cancer Res. 2012;18:6531–6541. doi: 10.1158/1078-0432.CCR-12-0605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bertagnolli MM, Niedzwiecki D, Compton CC, et al. Microsatellite instability predicts improved response to adjuvant therapy with irinotecan, fluorouracil, and leucovorin in stage III colon cancer: Cancer and Leukemia Group B Protocol 89803. J Clin Oncol. 2009;27:1814–1821. doi: 10.1200/JCO.2008.18.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sinicrope FA, Foster NR, Thibodeau SN, et al. DNA mismatch repair status and colon cancer recurrence and survival in clinical trials of 5-fluorouracil-based adjuvant therapy. J Natl Cancer Inst. 2011;103:863–875. doi: 10.1093/jnci/djr153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tran B, Kopetz S, Tie J, et al. Impact of BRAF mutation and microsatellite instability on the pattern of metastatic spread and prognosis in metastatic colorectal cancer. Cancer. 2011;117:4623–4632. doi: 10.1002/cncr.26086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boland CR, Thibodeau SN, Hamilton SR, et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58:5248–5257. [PubMed] [Google Scholar]
- 12.Golan T, Urban D, Berger R, et al. Changing prognosis of metastatic colorectal adenocarcinoma: Differential improvement by age and tumor location. Cancer. 2013;119:3084–3091. doi: 10.1002/cncr.28143. [DOI] [PubMed] [Google Scholar]
- 13.Liang JT, Huang KC, Lai HS, et al. High-frequency microsatellite instability predicts better chemosensitivity to high-dose 5-fluorouracil plus leucovorin chemotherapy for stage IV sporadic colorectal cancer after palliative bowel resection. Int J Cancer. 2002;101:519–525. doi: 10.1002/ijc.10643. [DOI] [PubMed] [Google Scholar]
- 14.Brueckl WM, Moesch C, Brabletz T, et al. Relationship between microsatellite instability, response and survival in palliative patients with colorectal cancer undergoing first-line chemotherapy. Anticancer Res. 2003;23:1773–1777. [PubMed] [Google Scholar]
- 15.des Guetz G, Mariani P, Cucherousset J, et al. Microsatellite instability and sensitivity to FOLFOX treatment in metastatic colorectal cancer. Anticancer Res. 2007;27:2715–2719. [PubMed] [Google Scholar]
- 16.Kopetz S, Chang GJ, Overman MJ, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol. 2009;27:3677–3683. doi: 10.1200/JCO.2008.20.5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tejpar S, Saridaki Z, Delorenzi M, et al. Microsatellite instability, prognosis and drug sensitivity of stage II and III colorectal cancer: more complexity to the puzzle. J Natl Cancer Inst. 2011;103:841–844. doi: 10.1093/jnci/djr170. [DOI] [PubMed] [Google Scholar]
- 18.Kanas GP, Taylor A, Primrose JN, et al. Survival after liver resection in metastatic colorectal cancer: review and meta-analysis of prognostic factors. Clin Epidemiol. 2012;4:283–301. doi: 10.2147/CLEP.S34285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmoll HJ, van Cutsem E, Stein A, et al. ESMO Consensus Guidelines for management of patients with colon and rectal cancer. A personalized approach to clinical decision making. Ann Oncol. 2012;23:2479–2516. doi: 10.1093/annonc/mds236. [DOI] [PubMed] [Google Scholar]
- 20.Kim ST, Lee J, Park SH, et al. The effect of DNA mismatch repair (MMR) status on oxaliplatin-based first-line chemotherapy as in recurrent or metastatic colon cancer. Med Oncol. 2010;27:1277–1285. doi: 10.1007/s12032-009-9374-x. [DOI] [PubMed] [Google Scholar]
- 21.Muller CI, Schulmann K, Reinacher-Schick A, et al. Predictive and prognostic value of microsatellite instability in patients with advanced colorectal cancer treated with a fluoropyrimidine and oxaliplatin containing first-line chemotherapy. A report of the AIO Colorectal Study Group. Int J Colorectal Dis. 2008;23:1033–1039. doi: 10.1007/s00384-008-0504-2. [DOI] [PubMed] [Google Scholar]
- 22.Kim JE, Hong YS, Ryu MH, et al. Association between deficient mismatch repair system and efficacy to irinotecan-containing chemotherapy in metastatic colon cancer. Cancer Sci. 2011;102:1706–1711. doi: 10.1111/j.1349-7006.2011.02009.x. [DOI] [PubMed] [Google Scholar]
- 23.Des Guetz G, Uzzan B, Nicolas P, et al. Microsatellite instability does not predict the efficacy of chemotherapy in metastatic colorectal cancer. A systematic review and meta-analysis. Anticancer Res. 2009;29:1615–1620. [PubMed] [Google Scholar]
- 24.Vilar E, Tabernero J. Molecular dissection of microsatellite instable colorectal cancer. Cancer Discov. 2013;3:502–511. doi: 10.1158/2159-8290.CD-12-0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Samowitz WS, Sweeney C, Herrick J, et al. Poor survival associated with the BRAF V600E mutation in microsatellite-stable colon cancers. Cancer Res. 2005;65:6063–6069. doi: 10.1158/0008-5472.CAN-05-0404. [DOI] [PubMed] [Google Scholar]
- 26.Lochhead P, Kuchiba A, Imamura Y, et al. Microsatellite instability and BRAF mutation testing in colorectal cancer prognostication. J Natl Cancer Inst. 2013;105:1151–1156. doi: 10.1093/jnci/djt173. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


