Abstract
Antiporters are ubiquitous membrane proteins that catalyze obligatory exchange between two or more substrates across a membrane in opposite directions. Some utilize proton electrochemical gradients generated by primary pumps by coupling the downhill movement of one or more protons to the movement of a substrate. Since the direction of the proton gradient usually favors proton movement towards the cytoplasm their function results in removal of substrates other than protons from the cytoplasm, either into acidic intracellular compartments or out to the medium. H+-coupled antiporters play central roles in living organisms, e.g. storage of neurotransmitter and other small molecules, resistance to antibiotics, homeostasis of ionic content and more. Biochemical and structural data support a general mechanism for H+-coupled antiporters whereby the substrate and the protons cannot bind simultaneously to the protein. In several cases, it was shown that the binding sites overlap and therefore there is a direct competition between the protons and the substrate. In others, the “competition” seems to be indirect and it is most likely achieved by allosteric mechanisms. To ensure the feasibility of such a mechanism the pKa of one or more carboxyls in the protein must be tuned appropriately. In this review I discuss in detail the case of EmrE, a multidrug transporter from Escherichia coli and evaluate the information available for other H+-coupled antiporters.
Antiporters (also called exchangers) are ubiquitous membrane proteins that catalyze obligatory exchange between two or more substrates across a membrane in opposite directions. They are present in plasma membranes of bacteria, archaea, plant and animal cells and in intracellular organelles in the eukaryotic cells. Notable examples of this type of transporters are vesicular neurotransmitter transporters, multidrug transporters, Cl−/H+, Ca2+/H+ and Na+/H+ antiporters [1-7].
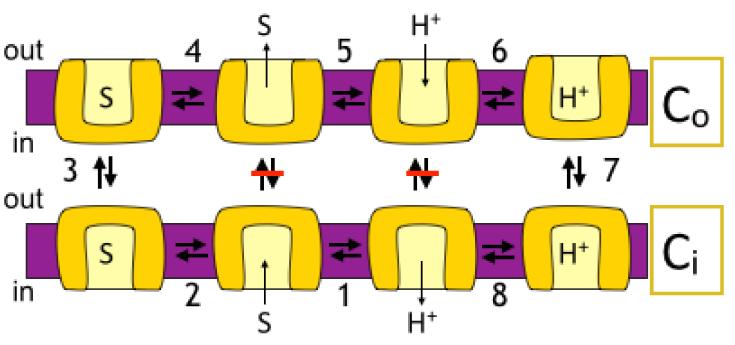
Many antiporters utilize proton electrochemical gradients generated by primary pumps by coupling the downhill movement of one or more protons to the movement of a substrate. For efficient coupling of H+ and substrate fluxes, H+-coupled antiporters, have been proposed to couple transport by utilizing a sequential binding and translocation mechanism, through which the substrate must be released prior to binding and translocation of the hydrogen ion (Fig.1). Such a mechanism anticipates two major conformations of the transporter, facing alternatively each side of the membrane (Co and Ci), which can interconvert only when one of the substrates is bound. Furthermore, simultaneous binding of both substrates is prohibited.
Figure 1. A simplified view of the catalytic cycle of an antiporter.
For the sake of simplicity the cartoon includes only the conformations where the protein faces the inside (Ci) or the outside (Co) with a proton (CiH and CoH) or substrate (CiS and COS) bound. Modified from figure 1 in [44]
What molecular determinants ensure such mutually exclusive occupancy of the binding domains and how does binding affect the associated conformational changes? In this review I describe what is known about the core of the coupling mechanism, i.e. the first part of the above question. I describe in detail our work on EmrE where a simple, direct competition between H+ and substrates for occupancy of a common binding site has been clearly demonstrated. I also discuss other mechanistic studies where competition has been demonstrated and new structures of antiporters that support a common binding site for H+ and substrates.
I. EmrE: a simple model for the coupling mechanism
I.A. One carboxyl per monomer is necessary and sufficient for coupling
EmrE is a small (110 residues) SMR transporter from Escherichia coli that functions as a dimer and extrudes one positively charged aromatic drug in exchange for two protons (one per subunit), thus rendering bacteria resistant to a variety of toxic compounds. Studies of this small, 110-residue multi-drug transporter from E. coli have provided information valuable for understanding coupling mechanisms in H+-coupled antiporters [7-13].
EmrE provides a unique experimental paradigm to study the coupling mechanism not only because of its size and stability. Most importantly, under proper conditions, the detergent solubilized protein binds substrate and releases protons in a manner that reflects with high fidelity its catalytic activity in the membrane. This property has enabled a detailed study of the molecular basis of coupling between protons and substrate [10-12, 14-17]. EmrE contains eight charged residues, seven of them located in the hydrophilic loops and only one membrane-embedded charged residue, Glu14, which is also conserved in hundreds of homologous proteins in bacteria and archaea [7, 18]. The first indications of its crucial role was supplied by experiments where replacement of Glu14 in EmrE or Smr from Staphylococcus aureus (equivalent Glu13) with Cys, Gln, His, Tyr or Asp had a profound effect on the phenotype [14, 19-21]. Further characterization of the mutants showed that the E14C and E14Q mutations yielded a protein completely devoid of activity. Even a conservative E14D mutation (to be discussed more in detail below) was impaired in its ability to couple substrate and proton fluxes [14]. Replacement of the two carboxyls in the loops with Cys residues generated an EmrE mutant with a single carboxyl per monomer at position 14. This single carboxyl mutant displays properties similar to those of the wild type protein, indicating that out of the three side-chain carboxyl residues of the protein, the only essential one is Glu14 [15].
I.B. Time-sharing of the binding site
The pH dependence of substrate binding and the properties of an Asp replacement at this position reveal the essential and dual role of Glu14 in catalysis: it plays a role in binding of both substrate and H+, only one of which can bind at any one time. The availability of Tetraphenyl-phosphonium (TPP+), a substrate that is transported and binds with high affinity, was essential for the success of these studies. This compound, originally used for the measurement of membrane potentials in mitochondria and bacteria, bears a permanent positive charge surrounded by four phenyl rings that lower the charge density and provide enough hydrophobicity to allow passive permeation across membranes [22, 23]. As it turns out, it is also a substrate of EmrE [20] and many other multidrug transporters [4].
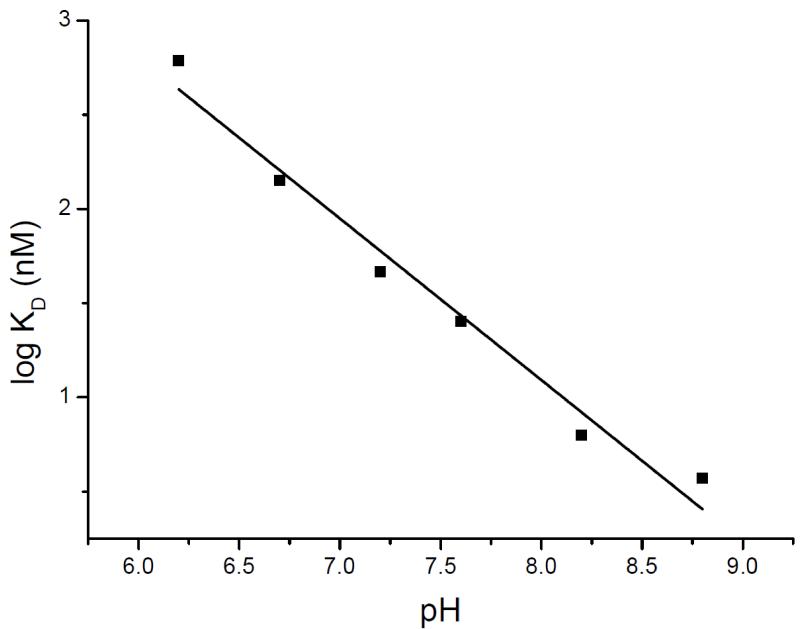
A demonstration of classical competition between TPP+ and H+ was obtained by measurement of TPP+ binding at various pH values: the KD decreases from about 600 nM at pH 6.2 to about 3 nM at pH 8.8 [9] (Figure 2). The number of binding sites (one per EmrE dimer) is constant at all the pHs tested [9, 21, 24]. These findings suggest that a residue in the protein must change its protonation state to allow substrate binding. As mentioned above, a mutant with the single Glu at position 14 is fully functional, displays the same affinity to TPP+ and the same pH dependency [15] consistent with the notion that deprotonation of this residue is necessary and sufficient for TPP+ binding [9, 21]. If so, the pH profile revealed by these measurements provides a rough estimate of the pKA of the Glu14 carboxyl in the range of 7-8 [9, 21].
Figure 2. pH dependence of the equilibrium KD of EmrE.
Purified protein was immobilized on Ni-NTA beads and bound to increasing [3H]TPP+ concentrations at various pH values as described [9]. Adapted from [9].
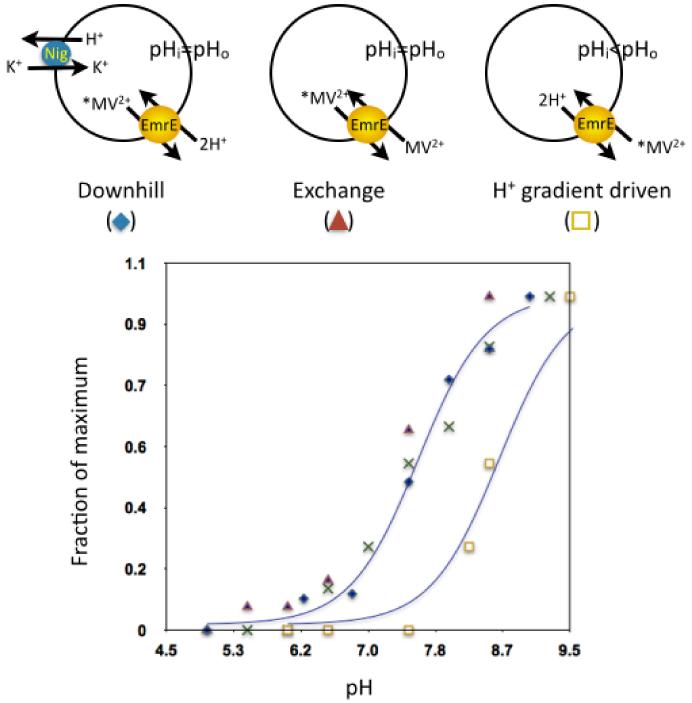
The measurements of the binding reaction provided invaluable information but since they were performed with a detergent solubilized protein they required further validation. This was provided by a series of experiments performed with purified EmrE reconstituted in proteoliposomes in which we followed transport of radiolabeled Methyl Viologen (MV2+), another substrate of EmrE [14]. MV2+ is a relatively hydrophilic substrate with a higher KM and yields excellent signal to noise data. Transport was assayed in three different modes: uphill, driven by a proton gradient, downhill and exchange (Fig. 3). Downhill transport of substrate involves all the steps described in figure 1 but is driven by the substrate electrochemical gradient and does not necessitate a gradient of H+ ions, i.e. the pH inside and outside the proteoliposome is the same. Moreover, generation of a pH gradient by the counter transported proton was prevented by addition of the ionophore nigericin. When the rate of MV2+ efflux was measured at various pH values it was found that the increase in the rate with an increase in pH was practically identical to that of the binding reaction measured in detergent (Fig. 3 and see also [14]). In exchange experiments, a saturating concentration of unlabeled substrate was included in the outside solution and the transporter thus interconverts between the inward- and outward-facing conformations in the “substrate-loaded” mode (steps 2-4 in figure 1). Even though exchange does not involve net proton movement the pH dependence of the reaction was similar to that of the binding reaction reflecting the fact that the rate of substrate binding is affected by pH [14]. When uphill transport was measured, a pH gradient of 2 units (acid inside) was imposed to drive the accumulation. In these experiments the rate increased with increasing external pH but there was a shift of approximately 1 pH unit to the alkaline side (Fig. 3 and see also [14]). It would appear as if Glu14 is now “sensing” the average between the internal and external pH of the proteoliposome. The mechanistic significance of this “averaging” necessitates further research.
Figure 3. Partial steps of the cycle.
Rates of substrate binding are dependent on pH because it binds only the unprotonated form of the transporter (bottom panel, x-x). Downhill transport of substrate includes all the steps in the cycle described in figure 1 (top left). The pH on both sides of the membrane is kept the same by the ionophore Nigericin and the reaction is driven by the substrate electrochemical gradient. The pH dependence of the reaction is shown at the bottom (◆-◆). The exchange reaction between radiolabeled (*MV2+) and unlabeled (MV2+) Methyl Viologen (top middle) does not include the proton translocation steps (6-8 in figure 1) but since rates of substrate binding are affected by pH also the exchange reaction shows pH dependence similar to that of the binding reaction and the downhill transport (▲-▲). The dependence of H+-driven uptake (■-■) is shifted about 1 pH unit to the alkaline side. In these experiments, at each external pH the internal pH is about 2 pH units lower. A likely interpretation of this shift is that Glu14 is exposed alternately to the internal and external pH of the proteoliposome and “senses” the average pH between the inside and the outside. Adapted from [14].
These results validate the experiments performed with the detergent solubilized transporter and support the dual role proposed for Glu14.
I. C. Direct measurements of proton release
The assumption that a proton must be released to allow for substrate binding was experimentally challenged and supported by equilibrium measurements where substrate-induced proton release was observed in the detergent solubilized protein for the first time in an antiporter [10]. These measurements provided further support for the pKA estimates for Glu14 and Asp14 and allowed determination of a stoichiometry of 2H+ released per substrate bound [10].
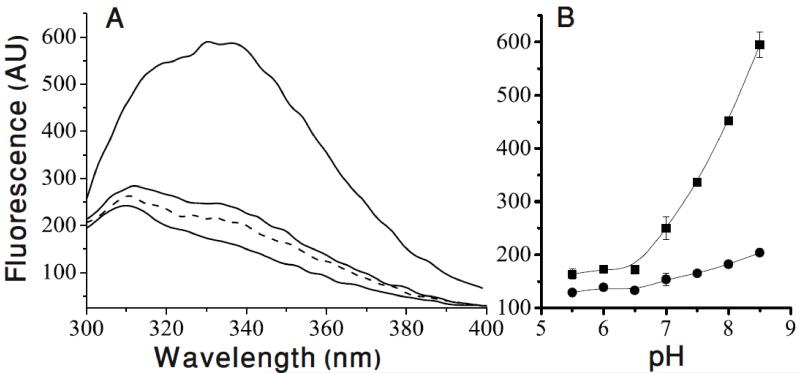
In addition to the equilibrium measurements the use of transient kinetics allowed us to break down this multistep process to its individual steps [9]. A powerful tool was provided by the study of the fluorescent properties of EmrE. The fluorescence of Trp63 in EmrE reflects the occupancy of the binding site in the protein: the highest fluorescence is observed when the protein is fully deprotonated, the protonated protein displays a lower fluorescence and the substrate bound protein displays the lowest (Fig.4). Interestingly, the environment to which Trp63 is exposed as reflected by the wavelength of its fluorescence peak does not change significantly at the three different states suggesting that Trp63 does not go through large conformational changes that expose it to different environments. A possible reason for the different fluorescence levels could be due to quenching or energy transfer to the substrate or to other amino acids not yet identified.
Figure 4. Tryptophan fluorescence spectra of EmrE-single Trp63 at different pH values with/without substrate.
A- Emission spectra (excitation at 280 nm) of 2 μM EmrE-Single Trp 63. The lowest solid line is at pH 5.5 the middle is at pH 7.0 and the highest is at pH 8.5. The dashed line represents protein at pH 8.5 after addition of 50 μM of the substrate TPP+. The shoulder at around 310 nm in the spectra of the single Trp63 mutant (that bears 7 Tyr residues) is not observed upon excitation at 295 nm and was assigned to Tyr fluorescence.
B- Summary of the pH dependence of the fluorescence intensity at the emission peak at 338 nm of free protein (squares) and substrate bound protein (circles). Adapted from [9].
I.D. Fine tuning of the pKA of the essential carboxyl is necessary for efficient coupling
The findings described above allowed us to study the transient kinetics of substrate binding and proton release using spectroscopic techniques: the fluorescence of either the wild type protein or a mutant with a single Trp at position 63 was used to measure binding rates [9]. The maximal rates of substrate binding of about 2 × 107M−1s−1 are relatively close to typical values in diffusion limited processes, implying that, in detergent, binding does not necessitate major conformational changes of the protein. The kon values for binding to the single Trp63 mutant in the pH range measured are essentially identical to the kon values for binding to the wild type protein. These findings further validate the use of the mutant to study the mechanism of catalysis. Moreover, the results suggest that Trp63 is responsible for most of the fluorescence changes in the wild type protein. The changes in fluorescence of Trp63 are extremely large and under optimal conditions most of the fluorescence is quenched either by protons or by substrate (Fig. 4).
Taken as a whole, the results support a binding mechanism where binding of either protons or substrate requires dissociation of the other. The suggested reaction path implies that first, E-2H+S dissociate to E+S+2H+, and it rapidly leads to the formation of ES+2H+. The fit to a model that assumes, as a first approximation, no binding to either the singly or doubly protonated protein and that both protons have a single or very similar pKa is in agreement with the following experimental findings (summarized in ref. [9]): I. The stoichiometry of proton release as determined from steady state measurements is 2H+/functional unit (dimer) [10]. This finding is supported also by the stoichiometry of the whole transport process: 2H+/substrate [25]. II. In steady state measurements the maximal substrate-induced release of protons from a practically fully protonated protein is at around 7 and therefore we conclude that the pKa must be well above 7 [10]. This contention is supported also by the pH dependence of the Trp63 fluorescence [9]. III. Although EmrE releases two protons upon substrate binding, the substrate induced proton release is monophasic, suggesting that both protons display identical or very similar pKa values.
The pre-equilibrium pKa values calculated from the data discussed above are ~7.3 for Glu14 and 5.8 for Asp14. The pKa values from this fit are lower than the equilibrium pKa values (8.3-8.5 and 6.7, respectively) [10] and this could be due to different effects on rates and on steady states, to a lower accuracy of the steady state measurements or to the need to obtain a model that better describes the reaction.
Interestingly, kon values of substrates with much lower affinities (TPMP+ and Ethidium) are of the same order of magnitude as those measured with TPP+ [9]. The fact that the association constant is similar for substrates with completely different affinity implies that dissociation determines the large affinity differences between the various substrates.
A possible reason for the modified pKa of Glu14 in the EmrE dimer could be the proximity of the two carboxyls, one from each monomer, in the binding cavity. This possibility was ruled out in a study of hetero-dimers with a single carboxyl in position 14 generated by monomer swapping or by genetic fusion [26, 27]. Such heterodimers differ in their affinity to the substrates and the stoichiometry between substrate and proton fluxes but the pH dependence of substrate binding (i.e. the pKa) is practically indistinguishable from that of the wild type (S. Steiner-Mordoch and S. Schuldiner, unpublished results). Another probable explanation for a modified pKa may stem from experimental data that imply a role for at least three aromatic residues (Trp63, Tyr40 and Tyr60) in each EmrE monomer [28, 29]. In addition to increasing the pKa of the carboxyls, the low dielectric constant of the protein interior created by hydrophobic and aromatic residues would strongly favor the electrostatic interaction between the negatively charged glutamate and a positively charged drug.
II. Challenging the classical view of antiporters
The behavior of a mutant where the essential Glu14 was replaced with Asp raises a general question regarding antiporter mechanisms. In a classical scheme of an antiporter cycle, it is assumed that the transition of the “unloaded” transporter between the conformations facing the opposite sides of a membrane is not allowed (Fig.1). Should such a transition be permitted the coupling between the two fluxes would be compromised. Noteworthy, the single conservative mutation of Glu14 to Asp generates a protein with very interesting properties: as a result of the lower pKA of the Asp carboxyl, it binds TPP+ with high affinity but the pH dependence of this reaction is shifted to the acidic values; it does not confer to cells a significant resistance to EmrE substrates; it does not catalyze proton driven accumulation of substrate but it can transport it downhill [9, 14, 21]. In other words, the E14D mutant functions now as a uniporter suggesting that, in the mutant, the transition of the “unloaded” transporter is permitted. It is possible that the mutation has an effect on the transporter that modifies the likelihood of this step to occur. Another more interesting possibility is that the transition is always allowed, even in the wild type EmrE. The reason that such a transition is not detectable in the wild type is that the concentration of the unprotonated form of transporter is extremely low and therefore the net flux would be nil. As demonstrated by the studies described above, the unprotonated form is extremely short lived in the wild type protein: it either binds protons or substrate rapidly upon its generation. The conformational change would be many times slower than the binding of protons or substrate and it would not happen frequently enough to uncouple. The lower pKA of the E14D mutant means that most of the protein will be unprotonated during interconversion between Co and Ci, uncoupling transport from proton movements.
III. Competition in other H+ coupled antiporters
As described in detail above, in the case of EmrE, the coupling mechanism is based on a direct competition of the substrate and the H+ for the same or overlapping binding site where Glu14 plays the central role. Is there evidence for a similar mechanism for the larger modern antiporters? The answer is most likely a complex one.
NhaA provides another well-documented case of competition between the two substrates [30]. NhaA, is a Na+/H+ antiporter from E. coli that belongs to the CPA family of monovalent cation/proton antiporters [31]. It plays a central role in sodium and proton homeostasis by exchanging two H+ with one Na+ ion [32]. The activity of NhaA increases with increasing pH similar to what was described above for EmrE [33]. Electrophysiological measurement of the activity of NhaA was tested over a wide pH range from pH 5.0 to 9.5. Forward and reverse transport directions were investigated at zero membrane potential and the pH dependence was found to be associated with a systematic increase of the KM for Na+ at acidic pH [30]. This classical competition seems to be at a common binding site, as suggested by biochemical and structural evidence that support a mechanism whereby two Asp residues, Asp163 and Asp164, provide the site for both H+ and Na+ [31, 34]. This conclusion is further supported by equilibrium molecular dynamics simulations of outward-facing NapA, an NhaA homologue from T. thermophiles, in a model membrane bilayer. Simulations were carried out with both Asp 157 and Asp 156 (the residues corresponding to Ap163 and 164 in NhaA) deprotonated, as they are likely to be in the crystal structure, and also as combinations of their neutral and charged forms. With both aspartates charged, Na+ ions spontaneously entered the negatively charged extracellular cavity to bind to Asp 157. Na+ ions were concentrated at Asp 157 and multiple distinct binding and unbinding events could be observed, which is qualitatively consistent with weak binding. By contrast, Na+ ion binding was not observed when Asp 157 was protonated and was markedly reduced when Asp 156 was neutral. In molecular dynamics simulations of inward-facing NhaA, Na+ binds to the equivalent aspartate (Asp 164), which is positioned at the base of the cytoplasmic cavity [35].
Recent structures of antiporters support a mechanism where there is direct competition for a binding site shared by H+ and the counter transported substrate. Thus, the Vcx1 protein from Saccharomyces cerevisiae and CAX_Af from Archaeoglobus fulgidus are two proteins from the CaCa family that display Ca2+/H+ antiporter activity. Their crystal structures suggested that Ca2+ and H+ binding to the cation-binding site are mutually exclusive [5, 36]. Also in the case of DinF, a H+-coupled MATE transporter from Bacillus halodurans, the structure unveils a membrane-embedded substrate-binding chamber that suggests a common binding site for H+ and substrate [37].
On the other hand, at least in two cases, it has been proposed that the counter-transported substrates bind at different sites without compromising the competitive nature of their binding. Since we are now talking about different sites on the protein, the term competition is used here in a free way. Thus, in the case of MdfA, a proton/drug antiporter from E. coli that belongs to the MFS family, genetic and biochemical evidence support the contention that distinct binding sites may exist for substrates and protons [38]. PfMATE, a H+/drug antiporter from Pyrococcus furiosus that belongs to the MATE family, was recently crystallized [39] and, also in this case, the authors suggest that the binding sites for H+ and substrates do not overlap.
An extreme example of “long-distance” competition is provided by the AcrAB-TolC complex from E. coli, a large tripartite complex that belongs to the RND family and provides the major intrinsic resistance of these cells. It actively removes a large variety of drugs from the periplasm by a mechanism that utilizes the proton electrochemical gradient across the cytoplasmic membrane. In the case of the AcrAB-TolC complex, there is a complete spatial separation of the sites: the proton site is in the membrane domain while the substrate site is in the periplasmic portion of this large complex [40]. The conformational changes occurring upon protonation and deprotonation of carboxylic residues in the membrane domain are coupled to conformational changes in distant domains where the substrate transport takes place [40].
A notable, and maybe not unique, exception to the mechanism depicted in Fig. 1 is presented by the ClC Cl−/H+ antiporter [41]. In general, antiporters bind their substrates alternately in the two different states and shuttle them across the membrane in ping-pong like conformational changes according to the alternating access model. In contrast, it has been proposed that ClC is able to bind both its substrates simultaneously, and, importantly, proton movement along its pathway seems to be possible only when the central anion binding site is occupied by Cl− [41].
IV. Tuning of the pKA is determined by the functional needs
Noteworthy, in addition to EmrE, in all the Escherichia coli antiporters described above, NhaA, MdfA and AcrAB-TolC complex, the dependence of the transport reaction on pH is very similar with no activity at acidic pH and an increase to a maximum at the alkaline pHs and an approximate middle point at around 7.5-7.8. Using a variety of approaches, the pKA of the carboxylic residues considered essential for coupling has been estimated to be at around 7.5 [30, 34, 38, 40], a value well within the range of the intracellular pH of E. coli cells [42]. Thus, it seems that regardless of their specific structures or mechanisms the transporters have evolved so that they are exquisitely tuned to function at the very constant cytoplasmic pH maintained by E. coli cells [42]. If the pKa were too low, such as in the E14D mutant, it would generate a protein that at physiological pH has already released the previously bound protons, binds substrate but cannot couple the substrate flux to the proton gradient [10, 14]. If the pKa were too high, substrate binding would be inhibited and it would, therefore, allow for very little activity at around the intracellular pH of E. coli [42].
Interestingly, such a pH dependence of the antiporters’ activity may provide the explanation on the reported involvement of these antiporters in bacteria and archaea in regulation of the internal pH at alkaline pH values, as already suggested for MdfA and MdtM, two H+-coupled multidrug antiporters from E. coli [38, 43]. Thus, when the intracellular pH increases, so will the rates of the H+-coupled multidrug transporters and, as a result, increased rates of H+ influx. In the cases above quoted, it has been suggested that the countertransported substrate is K+.
V. Conclusions
Biochemical and structural data support a general mechanism for H+-coupled antiporters whereby the substrate and the protons cannot bind simultaneously to the protein. In several cases, it was shown that the binding sites overlap and, therefore, there is a direct competition between the protons and the substrate. In others, the “competition” seems to be indirect and it is most likely achieved by some allosteric mechanism. To ensure the viability of such a mechanism the pKa of one or more carboxyls in the protein must be tuned appropriately and as shown for three E. coli proteins this ensures activity at the very constant cytoplasmic pH maintained by this organism.
The basic coupling mechanism for H+-coupled antiporters described here is probably the simplest and the result of early events in evolution. Modifications of the proposed mechanism may have evolved in specific cases to fulfill dedicated and/or regulated functions. The mirror image of this mechanism seems to have been adopted by H+-coupled symporters where binding of the proton and the substrate is essential for the transport reaction.
Highlights.
Antiporters catalyze obligatory exchange between substrates across a membrane.
In H+-coupled antiporters substrate and protons cannot bind simultaneously.
When binding sites for protons and substrate overlap there is direct competition.
Accurate tuning of the pKa of one or more carboxyls provides the chore of the mechanism.
Acknowledgements
Shimon Schuldiner is Mathilda Marks-Kennedy Professor of Biochemistry at the Hebrew University of Jerusalem. Work in our laboratory is supported by National Institutes of Health Grant NS16708 and Grant 97/12 from the Israel Science Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Donowitz M, Ming Tse C, Fuster D. SLC9/NHE gene family, a plasma membrane and organellar family of Na+/H+ exchangers. Mol Aspects Med. 2013;34:236–51. doi: 10.1016/j.mam.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Accardi A, Picollo A. CLC channels and transporters: proteins with borderline personalities. Biochim Biophys Acta. 2010;1798:1457–64. doi: 10.1016/j.bbamem.2010.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lawal HO, Krantz DE. SLC18: Vesicular neurotransmitter transporters for monoamines and acetylcholine. Mol Aspects Med. 2013;34:360–72. doi: 10.1016/j.mam.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Fluman N, Bibi E. Bacterial multidrug transport through the lens of the major facilitator superfamily. Biochim Biophys Acta. 2009;1794:738–47. doi: 10.1016/j.bbapap.2008.11.020. [DOI] [PubMed] [Google Scholar]
- [5].Waight AB, Pedersen BP, Schlessinger A, Bonomi M, Chau BH. Roe-Zurz Z, et al. Structural basis for alternating access of a eukaryotic calcium/proton exchanger. Nature. 2013;499:107–10. doi: 10.1038/nature12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Schuldiner S, Shirvan A, Linial M. Vesicular neurotransmitter transporters: from bacteria to humans. Physiol Rev. 1995;75:369–92. doi: 10.1152/physrev.1995.75.2.369. [DOI] [PubMed] [Google Scholar]
- [7].Schuldiner S. EmrE, a model for studying evolution and mechanism of ion-coupled transporters. Biochim Biophys Acta. 2009;1794:748–62. doi: 10.1016/j.bbapap.2008.12.018. [DOI] [PubMed] [Google Scholar]
- [8].Schuldiner S. When biochemistry meets structural biology: the cautionary tale of EmrE. Trends BiochemSci. 2007;32:252–8. doi: 10.1016/j.tibs.2007.04.002. [DOI] [PubMed] [Google Scholar]
- [9].Adam Y, Tayer N, Rotem D, Schreiber G, Schuldiner S. The fast release of sticky protons: Kinetics of substrate binding and proton release in a multidrug transporter. Proc Natl Acad Sci U S A. 2007;104:17989–94. doi: 10.1073/pnas.0704425104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Soskine M, Adam Y, Schuldiner S. Direct evidence for substrate induced proton release in detergent solubilized EmrE, a multidrug transporter. J. Biol. Chem. 2004;279:9951–5. doi: 10.1074/jbc.M312853200. [DOI] [PubMed] [Google Scholar]
- [11].Yerushalmi H, Schuldiner S. A model for coupling of H+ and substrate fluxes based on “time-sharing” of a common binding site. Biochemistry. 2000;39:14711–9. doi: 10.1021/bi001892i. [DOI] [PubMed] [Google Scholar]
- [12].Yerushalmi H, Schuldiner S. A common binding site for substrates and protons in EmrE, an ion-coupled multidrug transporter. FEBS LETTERS. 2000;476:93–7. doi: 10.1016/s0014-5793(00)01677-x. [DOI] [PubMed] [Google Scholar]
- [13].Schuldiner S. What Can a Living Fossil Tell Us About Evolution and Mechanism of Ion-Coupled Transporters: The Story of Small Multidrug Transporters. In: Krämer R, Ziegler C, editors. Membrane Transport Mechanism. Springer; Berlin Heidelberg: 2014. pp. 233–48. [Google Scholar]
- [14].Yerushalmi H, Schuldiner S. An Essential Glutamyl Residue in EmrE, a Multidrug Antiporter from Escherichia coli. J. Biol. Chem. 2000;275:5264–9. doi: 10.1074/jbc.275.8.5264. [DOI] [PubMed] [Google Scholar]
- [15].Yerushalmi H, Mordoch SS, Schuldiner S. A single carboxyl mutant of the multidrug transporter EmrE is fully functional. J. Biol. Chem. 2001;276:12744–8. doi: 10.1074/jbc.M010979200. [DOI] [PubMed] [Google Scholar]
- [16].Gutman N, Steiner-Mordoch S, Schuldiner S. An amino acid cluster around the essential Glu-14 is part of the substrate and proton binding domain of EmrE, a multidrug transporter from Escherichia coli. J. Biol. Chem. 2003;278:16082–7. doi: 10.1074/jbc.M213120200. [DOI] [PubMed] [Google Scholar]
- [17].Weinglass AB, Soskine M, Vazquez-Ibar JL, Whitelegge JP, Faull KF, Kaback HR, et al. Exploring the role of a unique carboxyl residue in EmrE by mass spectrometry. J. Biol. Chem. 2005;280:7487–92. doi: 10.1074/jbc.M413555200. [DOI] [PubMed] [Google Scholar]
- [18].Bay DC, Rommens KL, Turner RJ. Small multidrug resistance proteins: A multidrug transporter family that continues to grow. Biochim Biophys Acta. 2008;1778:1814–38. doi: 10.1016/j.bbamem.2007.08.015. [DOI] [PubMed] [Google Scholar]
- [19].Grinius L, Goldberg E. Bacterial Multidrug Resistance is Due to a Single Membrane Protein Which Functions as a Drug Pump. J. Biol. Chem. 1994;269:29998–30004. [PubMed] [Google Scholar]
- [20].Yerushalmi H, Lebendiker M, Schuldiner S. EmrE, an Escherichia coli 12-kDa multidrug transporter, exchanges toxic cations and H+ and is soluble in organic solvents. J. Biol. Chem. 1995;270:6856–63. doi: 10.1074/jbc.270.12.6856. [DOI] [PubMed] [Google Scholar]
- [21].Muth TR, Schuldiner S. A membrane-embedded glutamate is required for ligand binding to the multidrug transporter EmrE. EMBO J. 2000;19:234–40. doi: 10.1093/emboj/19.2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bakeeva LE, Grinius LL, Jasaitis AA, Kuliene VV, Levitsky DO, Liberman EA, et al. Conversion of biomembrane-produced energy into electric form. II. Intact mitochondria. Biochim Biophys Acta. 1970;216:13–21. doi: 10.1016/0005-2728(70)90154-4. [DOI] [PubMed] [Google Scholar]
- [23].Schuldiner S, Kaback HR. Membrane Potential and Active Transport in Membrane Vesicles from Escherichia coli. Biochemistry. 1975;14:5451–61. doi: 10.1021/bi00696a011. [DOI] [PubMed] [Google Scholar]
- [24].Miller D, Charalambous K, Rotem D, Schuldiner S, Curnow P, Booth PJ. In vitro unfolding and refolding of the small multidrug transporter EmrE. J Mol Biol. 2009;393:815–32. doi: 10.1016/j.jmb.2009.08.039. [DOI] [PubMed] [Google Scholar]
- [25].Rotem D, Schuldiner S. EmrE, a multidrug transporter from Escherichia coli, transports monovalent and divalent substrates with the same stoichiometry. J. Biol. Chem. 2004;279:48787–93. doi: 10.1074/jbc.M408187200. [DOI] [PubMed] [Google Scholar]
- [26].Steiner-Mordoch S, Soskine M, Solomon D, Rotem D, Gold A, Yechieli M, et al. Parallel topology of genetically fused EmrE homodimers. EMBO J. 2008;27:17–26. doi: 10.1038/sj.emboj.7601951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Rotem D, Salman N, Schuldiner S. In vitro monomer swapping in EmrE, a multidrug transporter from Escherichia coli, reveals that the oligomer is the functional unit. J. Biol. Chem. 2001;276:48243–9. doi: 10.1074/jbc.M108229200. [DOI] [PubMed] [Google Scholar]
- [28].Rotem D, Steiner-Mordoch S, Schuldiner S. Identification of tyrosine residues critical for the function of an ion-coupled multidrug transporter. J. Biol. Chem. 2006;281:18715–22. doi: 10.1074/jbc.M602088200. [DOI] [PubMed] [Google Scholar]
- [29].Elbaz Y, Tayer N, Steinfels E, Steiner-Mordoch S, Schuldiner S. Substrate-Induced Tryptophan Fluorescence Changes in EmrE, the Smallest Ion-Coupled Multidrug Transporter. Biochemistry. 2005;44:7369–77. doi: 10.1021/bi050356t. [DOI] [PubMed] [Google Scholar]
- [30].Mager T, Rimon A, Padan E, Fendler K. Transport mechanism and pH regulation of the Na+/H+ antiporter NhaA from Escherichia coli: An electrophysiological study. J. Biol. Chem. 2011;286:23570–81. doi: 10.1074/jbc.M111.230235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Padan E, Kozachkov L, Herz K, Rimon A. NhaA crystal structure: functional-structural insights. J Exp Biol. 2009;212:1593–603. doi: 10.1242/jeb.026708. [DOI] [PubMed] [Google Scholar]
- [32].Taglicht D, Padan E, Schuldiner S. Proton-sodium stoichiometry of NhaA, an electrogenic antiporter from Escherichia coli. J. Biol. Chem. 1993;268:5382–7. [PubMed] [Google Scholar]
- [33].Taglicht D, Padan E, Schuldiner S. Overproduction and purification of a functional Na+/H+ antiporter coded by nhaA (ant) from Escherichia coli. J. Biol. Chem. 1991;266:11289–94. [PubMed] [Google Scholar]
- [34].Arkin IT, Xu H, Jensen MO, Arbely E, Bennett ER, Bowers KJ, et al. Mechanism of Na+/H+ antiporting. Science. 2007;317:799–803. doi: 10.1126/science.1142824. [DOI] [PubMed] [Google Scholar]
- [35].Lee C, Kang HJ, von Ballmoos C, Newstead S, Uzdavinys P, Dotson DL, et al. A two-domain elevator mechanism for sodium/proton antiport. Nature. 2013;501:573–7. doi: 10.1038/nature12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Nishizawa T, Kita S, Maturana AD, Furuya N, Hirata K, Kasuya G, et al. Structural basis for the counter-transport mechanism of a H+/Ca2+ exchanger. Science. 2013;341:168–72. doi: 10.1126/science.1239002. [DOI] [PubMed] [Google Scholar]
- [37].Lu M, Radchenko M, Symersky J, Nie R, Guo Y. Structural insights into H-coupled multidrug extrusion by a MATE transporter. Nat Struct Mol Biol. 2013 doi: 10.1038/nsmb.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Fluman N, Ryan CM, Whitelegge JP, Bibi E. Dissection of mechanistic principles of a secondary multidrug efflux protein. Mol Cell. 2012;47:777–87. doi: 10.1016/j.molcel.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Tanaka Y, Hipolito CJ, Maturana AD, Ito K, Kuroda T, Higuchi T, et al. Structural basis for the drug extrusion mechanism by a MATE multidrug transporter. Nature. 2013;496:247–51. doi: 10.1038/nature12014. [DOI] [PubMed] [Google Scholar]
- [40].Seeger MA, von Ballmoos C, Verrey F, Pos KM. Crucial role of Asp408 in the proton translocation pathway of multidrug transporter AcrB: evidence from site-directed mutagenesis and carbodiimide labeling. Biochemistry. 2009;48:5801–12. doi: 10.1021/bi900446j. [DOI] [PubMed] [Google Scholar]
- [41].Miller C, Nguitragool W. A provisional transport mechanism for a chloride channel-type Cl-/H+ exchanger. Philos Trans R Soc Lond B Biol Sci. 2009;364:175–80. doi: 10.1098/rstb.2008.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Padan E, Zilberstein D, Schuldiner S. pH Homeostasis in Bacteria. Biochimica et Biophysica Acta. 1981;650:151–66. doi: 10.1016/0304-4157(81)90004-6. [DOI] [PubMed] [Google Scholar]
- [43].Holdsworth SR, Law CJ. Multidrug resistance protein MdtM adds to the repertoire of antiporters involved in alkaline pH homeostasis in Escherichia coli. BMC microbiology. 2013;13:113. doi: 10.1186/1471-2180-13-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Rudnick G. How do transporters couple solute movements? Mol Membr Biol. 2013;30:355–9. doi: 10.3109/09687688.2013.842658. [DOI] [PMC free article] [PubMed] [Google Scholar]