Abstract
Hepatobiliary tuberculosis is a rare manifestation of Mycobacterium tuberculosis infection and is usually secondary to tuberculosis of the lungs or gastrointestinal tract. Diagnosis is difficult pre-operatively in local (focal and tubular) forms because of its rarity and presentation in the form of non-specific symptoms and signs and lack of any defined criteria on imaging studies. Histopathological examination is necessary for definite diagnosis but in cases where there is suspicion of hepatobiliary tuberculosis, with PCR assay diagnosis it is possible pre-operatively. Recommended treatment is with conventional antituberculous drugs and surgical intervention in tuberculous abscess or granulomas. The disease is usually associated with good prognosis under complete antituberculous treatment. The author encountered 4 cases of hepatobiliary tuberculosis over 5 years. The aim of this article is to present current knowledge of hepatobiliary tuberculosis and to comprehensively review all the available literature.
Keywords: Hepatobiliary tuberculosis, difficult diagnosis, histopathology, antituberculous drugs
Introduction
The involvement of liver with various infectious agents may be caused by primary infections or may represent a component of multisystem disorders, and the clinical presentation often mimics other conditions. Hepatic tuberculosis (TB) is an uncommon manifestation of one of the most common infections, caused by Mycobacterium tuberculosis. The term hepatobiliary TB refers to either isolated hepatic, biliary, or hepatobiliary involvement with other organ system involvement. The manifestations of tuberculous liver involvement are protean, and terms in the literature used to describe hepatobiliary system involvement with TB include tuberculous pseudotumor, tuberculous cholangitis, tuberculous liver abscess, tuberculous hepatitis, and tuberculous cholangitis [1-5].
Hepatic TB can be classified into the following: 1) Local, with focal involvement of liver in the form of the tuberculous primary complex with caseation of the associated hepatic hilar lymph nodes. These involved lymph nodes may become the source of spread causing early systemic generalization. 2) Miliary TB, a part of generalized disease, results in an attack on the liver by clustered miliary tubercles. It is the most common form of liver TB and is said to occur in 50-80% of all patients dying of pulmonary TB [6]. 3) Tuberculomas, or granulomatous disease, can develop through enlargement and subsequent confluence of the miliary foci or tubercles as well as through nodular development of tuberculous foci in the tertiary stage. They appear as nodules with a diameter of 1-4 cm. Embedded calcifications are typical features of tuberculomas.
We encountered 4 cases of hepatic TB in the last 5 years. Out of these 4 cases, hepatic TB was a part of generalized disease i.e. miliary TB in 2 patients, 1 patient had isolated gallbladder TB and 1 patient was found to have tuberculous liver abscess.
Epidemiological features
TB is a worldwide health problem with a high prevalence, approximately 95%, in developing countries [7]. During the late 1980s and early 1990s, numbers of reported cases of TB increased in developed countries and these increases were largely due to immigration from countries with a high prevalence of TB, drug abuse and infection with human immunodeficiency virus (HIV). Abdominal TB is uncommon, comprising 3.5% of extrapulmonary TB, and hepatic TB is considered very rare among abdominal TB patients.
Hepatic TB is encountered more frequently in Asian countries and in a study, it has been observed that hepatobiliary TB is seen commonly in the Philippines and among Filipino patients abroad and there is no explanation for this kind of occurrence but it has been suggested that Filipinos may have racial vulnerability to the tubercle bacilli. Some of the case reports in the world literature of localized hepatic TB, especially causing obstructive jaundice, involved Filipino patients [8-11]. In a retrospective study from Southern Taiwan, out of 1,251 patients with TB 10 were found to have hepatic TB including 4 cases of isolated hepatic TB [12]. A study from South Africa showed that hepatic TB accounted for only 1.2% of all cases of TB diagnosed at general hospitals [13]. In 2 of our cases, gallbladder TB and tuberculous hepatic abscess, TB was diagnosed on histopathological examination. None of the pre-operative investigations were suggestive of hepatic TB. Lack of familiarity and enough literature on this particular condition is apparently responsible for the diagnosis being made after surgery on histopathological examination. There are many case reports of hepatic TB mimicking other conditions with hepatic or non-hepatic manifestations. Hepatic involvement was found clinically in 50-80% of all patients dying of pulmonary TB and in up to 91% on autopsy [6].
Hepatobiliary TB is more common in males with a male to female ratio of 2:1 and there is no specific age group but according to one study the majority of patients fall within the age range of 11-50 years [14].
Pathogenesis
Tuberculous hepatic infections are transmitted prenatally, perinatally and postnatally. Prenatally and perinatally tuberculous hepatic infections are transmitted via the umbilical vein or the amniotic fluid and presence of maternal placenta tuberculosa is a precondition for both infections. Postnatally, tubercle bacilli reach the liver via hematogenous dissemination or hepatopetal lymph vessels. In hematogenous dissemination, the portal of entry in the case of miliary TB is through the hepatic artery whereas it is via the portal vein in the case of focal liver TB or tuberculous primary complex. Hematogenous dissemination is more common than dissemination via portal vein or lymphatic vessels. In miliary TB, the hematogenous spread, occurring as intermittent episodes, provokes a number of small tuberculous liver foci.
Pathology
Irrespective of the mode of entry, the liver responds by granuloma formation. Both caseating and non-caseating granulomas are seen. In miliary TB, multiple small tuberculous liver foci show central caseation and fibrinoid necrosis. In the periphery, a corona of epitheloid cells of variable diameter is found, in which Langhans’ giant cells are embedded. These granulomatous tubercles are surrounded by a loose rim of lymphocytes. Tuberculous granulomata are most frequently found in the periportal areas. Miliary tubercles or primary tuberculous complex in local TB coalesce to form tuberculomas. They appear as nodules with a diameter of 1-4 cm. Embedded calcifications are typical features of tuberculomas and they become encapsulated in the course of time. These nodules are sometimes the source of hematogenic spread.
Clinical presentation
The clinical manifestations of hepatobiliary TB are those of the extrahepatic disease (Table 1); hepatic involvement is usually asymptomatic. In most of the series right upper quadrant or non-specific abdominal pain appeared to be the most common symptom present in 65-87% of patients [14,15]. In a South African study in 1964, almost 50% of patients presented with upper abdominal pain [13]. One of the patients with isolated TB of gallbladder presented with features suggestive of acute cholecystitis and there was no suspicion of TB pre-operatively. Jaundice is an uncommon presentation, being present in 20-35% of patients [11,14,15]. The presence of jaundice suggests biliary involvement, and the biochemical profile may simulate extrahepatic biliary obstruction. Most authors agree that jaundice or biliary stasis is due either to porta hepatis nodes causing biliary compression or to pericholangitis or possibly by direct involvement of biliary epithelium or the rupture of a tuberculous granuloma into the bile ducts [16]. Intrahepatic bile duct obstruction may result from granulomatous involvement often as part of miliary TB. The entity of bile duct TB may manifest as bile duct dilatation with common hepatic duct strictures and such patients mostly have painless jaundice and weight loss that mimics pancreatic or cholangiocarcinoma. In jaundiced patients, associated abdominal pain was present only in 45% of patients [11]. Other than non-specific abdominal pain, common presenting symptoms in several series were fever of unknown origin, anorexia and weight loss, present in 55-90% of patients. The authors report that the patient with tuberculous liver abscess presented with non-specific symptoms and after investigations based on imaging studies, a diagnosis of hydatid cyst of liver was made pre-operatively. In several series fever was present in more than 50% of patients [11,13,15,17]. Gallbladder TB is reportedly increasing in incidence and may manifest as biliary colic or acute cholecystitis. Isolated pancreatic TB may manifest in a manner similar to that of a pancreatic neoplasm.
Table 1.
Presenting common symptoms and signs
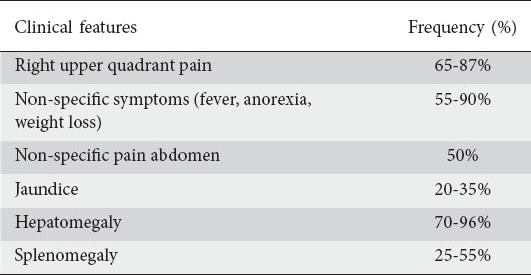
In most of the series, hepatomegaly is the commonest finding, being present in 70-96% of patients, and presented as an isolated liver tumor or abscess [11,15]. Liver was hard and nodular in about half the cases simulating cancer of the liver, so in most cases, the clinical presentation was that of a neoplasm and it was tender in 36% of cases simulating liver abscess. Splenomegaly was present in 25-55% of patients [11].
Sequelae and complications
Tuberculous nodules may penetrate bile ducts and cause tuberculous cholangitis with bile duct stricture [16]. Presence of tubercle bacilli and caseous necrosis in these tuberculous nodules may lead to formation of hepatic cavities and thus tuberculous abscesses or gravitation abscesses or pseudotumoral liver TB and differential diagnosis from these conditions is very difficult [11,18,19]. Development of portal hypertension caused by the compression of the portal vein by tuberculous lymph nodes, with resultant variceal hemorrhage is an uncommon manifestation of hepatobiliary TB. Tuberculous pseudocirrhosis may result because of scarring of multiple tubercles or small disseminated diffuse foci during the healing phase but no major hepatic dysfunction results from the cicatrization of the healing process. Secondary hepatic amyloidosis, developing in the course of chronic lung TB, has also been documented. A restriction of hepatic function in chronic TB has been described in several studies [20-22]. Massive miliary spread to the liver may cause acute liver failure as well as septic shock with multiorgan failure [23-25].
Diagnosis
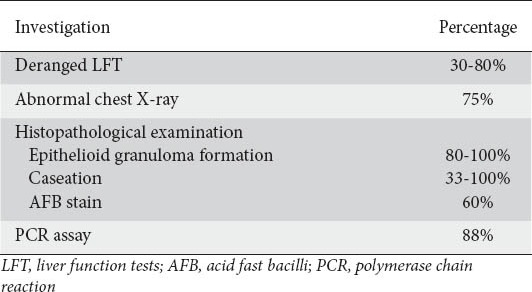
Biochemical clues to the presence of hepatic TB are non-specific. Liver function tests including aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, gamma-glutamyltranspeptidase, total protein and albumin-globulin ratio, although found to be elevated in 30-80% of patients, were non-specific and were not diagnostic of hepatoboliary TB [11]. A disproportionately increased serum alkaline phosphatase level is a consistent finding suggestive of an infiltrative hepatic process.
Approximately 75% of patients with hepatic TB were found to have abnormal chest x-rays demonstrating pulmonary TB [17]. Calcification in the hepatic region on plain x-ray of the abdomen may occasionally be seen in local hepatic TB. In tuberculous liver abscess and pseudotumoral liver TB, ultrasonography shows hypoechoic leisons and complex masses, either solitary or multiple, without a distinct wall and it was difficult to differentiate them from carcinoma on ultrasound. Computed tomography (CT) and magnetic resonance imaging (MRI) are helpful in diagnosing tuberculoma or tuberculous liver abscess. On CT scan, liver tuberculoma appears as a non-enhancing, central, low-density lesion due to caseation necrosis with a slightly enhancing peripheral rim corresponding to surrounding granulation tissue but these have also been seen in necrotic tumor such as hepatocellular and metastatic carcinoma [26,27]. Liver calcifications can also be demonstrated by CT scan. CT-guided aspiration and biopsy can confirm the diagnosis. MRI offers no added advantage in diagnosing hepatobiliary TB.
The final diagnosis of hepatic TB, local as well as diffuse, rests on histopathologic evidence of caseating granuloma or demonstration of acid fast bacilli (AFB) on smear or culture of biopsy specimen (Table 2). The aim should always be to demonstrate the presence of tubercle bacilli in the liver tissue, either directly or by culture. The granulomas are generally 1-2 mm in size but large tuberculomas up to 12 cm have also been reported. The granulomas are composed of epithelioid cells surrounded by lymphocytes, with or without Langhans’ type multinucleated giant cells. Epithelioid granuloma formation in hepatic TB can be demonstrated in 80-100% of cases. Granuloma formation has also been reported in brucellosis, coccidioidomycosis and Hodgkin’s disease, but occurrence of these conditions is high in the western hemisphere [28] while TB is the commonest cause of granuloma formation in India [29]. Caseation, a hallmark finding of TB granulomas, is present in 33-100% of liver biopsy specimens from various series. Alvarez et al [30] reported caseation necrosis in 67%, Essop et al [13] in 83%, Maharaj et al [17] in 51%, and Korn et al [31] in 30% of the cases in their series. AFB stains are positive in about 60%, in contrast to cultures often being negative. The demonstration of AFB has generally been low in pulmonary TB and higher in miliary disease, but positive cultures have been rare in the literature. Demonstration of AFB is more common in tubercular abscess as compared to solid tuberculomas because AFB is abundant in liquified caseous material. In 2 of our cases, isolated gallbladder and isolated tuberculous liver abscess, diagnosis of hepatobiliary TB was made only after histopathological confirmation. In isolated gallbladder TB, pre-operative diagnosis was acute calculous cholecystitis, while in isolated hepatic tuberculous abscess, two differential diagnoses were kept-hydatid cyst and pyogenic abscess. The diagnosis of hepatic tuberculous abscess on histopathological examination was a complete surprise in this case and both the patients were then properly investigated to look for primary or any other focus in the body but no other focus was found. AFB was not found in three series from India despite the presence of caseation necrosis and epithelioid granulomas.
Table 2.
Presenting lab investigations
PCR assays are diagnostic in most patients with TB granulomas in the liver. Alcantara-Payawal [18] reported a 100% success rate for those with definitive diagnosis of TB (those with caseating granuloma) and a 78% success rate for those with presumptive diagnosis of hepatobiliary TB, with an overall PCR assay positivity of 88%. The author did not perform PCR assay on 2 of the patients as there was no suspicion of TB, clinically or radiologically.
Laparoscopy has been used widely in suspected cases of abdominal and hepatobiliary TB. In 1983, Alvarez et al [11] advocated that laparoscopy combined with direct vision liver biopsy may be the preferred mode of investigation for hepatobiliary TB as this modality was successful in the diagnosis of liver tuberculoma in all the cases and described the laparoscopic appearance of hepatobiliary TB as cheesy white, irregular nodules of varying sizes, some of which resembled tumor masses. If laboratory tests and imaging techniques have proved unsuccessful, it is only possible to obtain a reliable diagnosis and differentiation of the multiple manifestations of liver TB by means of laparoscopy and targeted biopsy. In case of bile duct TB, biliary cytologic findings from endoscopic cholangiography may yield the diagnosis. Biliary tract involvement in hepatobiliary TB in the majority of cases was due to enlarged tuberculous lymph nodes. In 2006, Alvarez et al [30] described ERCP findings in 26 patients with hepatobiliary TB presenting with obstructive jaundice. Experience with therapeutic biliary stenting has been variable, and in unsuccessful cases percutaneous biliary drainage decompresses the obstruction.
Treatment
The treatment of hepatic TB is the same as any other extrapulmonary tuberculous lesion. Conventional antituberculous therapy involving the use of at least four drugs remains the cornerstone of treatment. Treatment consists of a fourfold combination: isoniazid (5 mg/kg BW/day), rifampicin (10 mg/kg BW/day), pyrazinamide (30 mg/kg BW/day), ethambutol (20 mg/kg BW/day) generally for 2-4 months, subsequently isoniazid and rifampicin for 6-12 months. Although therapy traditionally lasts for 6 months, multidrug-resistant organisms and hepatotoxicity of the agents require alternative regimens. Drug induced liver injury secondary to antituberculous drugs, especially isoniazid, is idiosyncratic and potentially lethal. Drug induced hepatotoxicity is not mentioned in most reports of hepatic TB and this is probably due to the small number of patients with hepatic TB. Careful monitoring for drug induced hepatitis may obviate fatality. The American Thoracic Society recommended more vigilant serum ALT monitoring in patients who consume alcohol, take concomitant hepatotoxic drugs, have HIV, have an abnormal baseline ALT, or pre-existing liver disease or viral hepatitis, have a prior history of isoniazid hepatitis, are pregnant, or are within 3 months postpartum [32]. Alvarez et al [12] has shown good clinical response with the use of standard antituberculous drugs in 67% of cases with disappearance of abdominal pain, fever, increase in appetite, weight gain, and reduction in the size of the liver. Essop et al [13] have shown that when INH and rifampicin are used together, mortality rates are significantly lower than those with the use of non-rifampicin containing regimens (0% vs 17-50%).
In addition to chemotherapy, good clinical responses have also been reported in tuberculous liver abscess after percutaneous aspiration and drainage of the abscess plus antituberculous treatment [3,26,33]. Two of our cases with isolated gallbladder and hepatic tuberculous abscess after surgical intervention received standard antituberculous treatment while other 2 patients with miliary TB, received antituberculous drugs with no surgical intervention. Since the definite diagnosis is often difficult and occult malignancy is possible, surgical intervention with hepatectomy is an alternative option in treating nodular hepatic TB when necessary. In a study by Wei-Chen et al [12] in 2008, 2 patients with solitary tuberculous pseudotumor received left hepatectomy. In a study by Xing et al [33], 8 hepatic TB pseudotumor patients received segmentectomy or hepatectomy. Patients who present with obstructive jaundice, in addition to the use of anti tuberculous treatment, biliary decompression should also be done either by stent placement during ERCP, percutaneous transhepatic biliary drainage or by surgical decompression whenever feasible.
Cumulative mortality for hepatic TB ranges between 15% and 42%. Essop et al reported a mortality rate of 42% [13]. Hepatic failure is not a usual cause of death from hepatobiliary TB. Nearly 50% of deaths in the Philippines study were due to respiratory failure and another third from ruptured esophageal varices due to associated cirrhosis [11]. The factors associated with adverse prognosis are: military TB, concurrent steroid therapy, age less than 20, cachexia, HIV, associated cirrhosis and liver failure. The authors report that out of 4 patients, 1 patient with miliary TB died due to septic shock and respiratory failure while the other 3 patients recovered well with standard treatment.
Concluding remarks
Hepatobiliary TB is a rare extrapulmonary manifestation of one of the commonest infections caused by Mycobacterium tuberculosis. It occurs in various forms; local, miliary, and tuberculomas or granulomatous TB of liver. Diagnosis may not often be possible pre-operatively and is confirmed by histopathological examination in a local (focal and tubular) form. In cases of pre-operative suspicion, PCR assay is helpful in diagnosis. Recommended treatment is quadruple therapy with antituberculous drugs and prognosis is good.
Biography
Dr Ram Manohar Lohia Hospital (Dr RMLH and PGIMER), New Delhi, India
Footnotes
Conflict of Interest: None
References
- 1.Alvarez SZ. Hepatobiliary tuberculosis. J Gastroenterol Hepatol. 1998;13:833–839. doi: 10.1111/j.1440-1746.1998.tb00743.x. [DOI] [PubMed] [Google Scholar]
- 2.Chong VH. Hepatobiliary tuberculosis: a review of presentations and outcome. South Med J. 2008;101:356–361. doi: 10.1097/SMJ.0b013e318164ddbb. [DOI] [PubMed] [Google Scholar]
- 3.Goh KL, Pathmanathan R, Chang IW, Wong NW. Tuberculous liver abscess. J Trop Med. 1987;90:255–257. [PubMed] [Google Scholar]
- 4.Weinberg II, Cohen P, Malhotra R. Primary tuberculous liver abscess associated with human immunodeficiency virus. Tubercle. 1988;69:145–147. doi: 10.1016/0041-3879(88)90078-5. [DOI] [PubMed] [Google Scholar]
- 5.Spiegel CT, Tuozon CD. Tuberculous liver abscess. Tubercle. 1984;65:127–131. doi: 10.1016/0041-3879(84)90065-5. [DOI] [PubMed] [Google Scholar]
- 6.Morris E. Tuberculosis of the liver. Am Rev Tuberc. 1930;22:585–592. [Google Scholar]
- 7.Raviglione MC, O’Brien RJ. Harrison's Principles of Internal Medicine. In: Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL, Loscalzo J, editors. 18th ed. McGraw Hill; 2012. p. 1340. [Google Scholar]
- 8.Bristowe JS. On the connection between abscess of the liver and gastrointestinal ulceration. Trans Pathol Soc. 1858;9:241. [Google Scholar]
- 9.Fan ST, Ng IOL, Choi TK, Lai ECS. Tuberculosis of the bile duct. A rare cause of biliary stricture. Am J Gastroenterol. 1989;84:413–414. [PubMed] [Google Scholar]
- 10.Gallinger S, Strasberg SM, Marcus HI, Brunton J. Local hepatic tuberculosis, the cause of a painful hepatic mass: case report and review of literature. Can J Surg. 1986;29:451–452. [PubMed] [Google Scholar]
- 11.Alvarez SZ, Carpio R. Hepatobiliary tuberculosis. Dig Dis Sci. 1983;28:193–200. doi: 10.1007/BF01295113. [DOI] [PubMed] [Google Scholar]
- 12.Tai WC, Kuo CM, Lee CH, et al. Liver tuberculosis in Southern Taiwan: 15-years clinical experience. J Intern Med Taiwan. 2008;19:410–417. [Google Scholar]
- 13.Essop AR, Posen JA, Hodkinson JH, Segal I. Tuberculous hepatitis: a clinical review of 96 cases. Quart J Med. 1984;53:465–77. [PubMed] [Google Scholar]
- 14.Oliva A, Duarte B, Jonasson O, Nadimpalli V. The nodular form of local hepatic tuberculosis. J Clin Gastroenterol. 1990;12:166. doi: 10.1097/00004836-199004000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Hersch C. Tuberculosis of the liver: a study of 200 cases. South Afr Med J. 1964;38:857–863. [PubMed] [Google Scholar]
- 16.Kok KY, Yapp SK. Tuberculosis of the bile duct: a rare cause of obstructive jaundice. J Clin Gastroenterol. 1999;29:161–164. doi: 10.1097/00004836-199909000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Maharaj B, Leary WP, Pudifin DJ. A prospective study of hepatic tuberculosis in 41 black patients. Quart J Med. 1987;63:517–522. [PubMed] [Google Scholar]
- 18.Alcantara-Payawal DE, Matsumura M, Shiratori Y, et al. Direct detection of Mycobacterium tuberculosis using polymerase chain reaction assay among patients with hepatic granuloma. J Hepatol. 1997;27:620–627. doi: 10.1016/s0168-8278(97)80078-5. [DOI] [PubMed] [Google Scholar]
- 19.Blangy S, Cornud F, Sibert A, et al. Hepatitis tuberculosis presenting as tumoral disease on ultrasonography. Gastrointest Radiol. 1988;13:52–54. doi: 10.1007/BF01889024. [DOI] [PubMed] [Google Scholar]
- 20.Lamache A, Bourel M, Chevrel ML, Richier JL. Foieettuberculose. Hop (Paris) 1961;37:803–813. [Google Scholar]
- 21.Pariente R, Etienne JP, Chretien J. Etude de 22 cas de tuberculose du foieanatomiquementdemontree (considerations etiologiques, cliniques, laparoscopiques et histologiques) Rev Tuberc Pneumol. 1963;27:1177–1192. [PubMed] [Google Scholar]
- 22.Thomas MR, Goldin RD. Tuberculosis presenting as jaundice. Brit J Clin Pract. 1990;44:161–163. [PubMed] [Google Scholar]
- 23.Asada Y, Hayashi T, Sumiyoshi A, et al. Miliary tuberculosis presenting as fever and jaundice with hepatic failure. Hum Pathol. 1991;22:92–94. doi: 10.1016/0046-8177(91)90068-z. [DOI] [PubMed] [Google Scholar]
- 24.Hussain W, Mutimer D, Harrison R, et al. Fulminant hepatic failure caused by tuberculosis. Gut. 1995;36:792–794. doi: 10.1136/gut.36.5.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mandak M, Kerbl U, Kleinert R, et al. Miliare tuberkulose der lebere als ursache eines septischen schocks mit multiorganversagen as this seems to be a rather long word. Wien Klin Wschr. 1999;106:111–114. [PubMed] [Google Scholar]
- 26.Reed DH, Nash AF, Valabhji P. Radiological diagnosis and management of a solitary tubercular hepatic abscess. Br J Surg. 1990;63:902–904. doi: 10.1259/0007-1285-63-755-902. [DOI] [PubMed] [Google Scholar]
- 27.Chan SG, Pang J. Isolated giant tuberculomata of the liver detected by computed tomography. Gastrointest Radiol. 1989;14:305–307. doi: 10.1007/BF01889223. [DOI] [PubMed] [Google Scholar]
- 28.Reynolds TB, Campra JL, Peters RL. Hepatic granulomata. In: Zakim D, Boyer TD, editors. Hepatolgy - A textbook of liver disease. 2nd ed. Philadelphia: WB Saunders; 1990. p. 1098. [Google Scholar]
- 29.Plumber ST, Pipalia DH, Vora IM, et al. Hepatic granulomas: profile and follow up of 10 cases responding to tuberculous therapy. J Assoc Physician India. 1987;35:207. [PubMed] [Google Scholar]
- 30.Alvarez SZ. Hepatobiliary tuberculosis. Phil J Gastroenterol. 2006;2:1–10. [Google Scholar]
- 31.Korn RJ, Kellow WF, Heller P, et al. Hepatic involvement in extrapulmonary tuberculosis: histologic and functional characteristics. Am J Med. 1959;27:60–71. doi: 10.1016/0002-9343(59)90061-0. [DOI] [PubMed] [Google Scholar]
- 32.Saukkonen JJ, Cohn DL, Jasmer RM, et al. An official ATS statement: hepatotoxicity of antituberculous therapy. Am J Respir Crit Care Med. 2006;174:935–952. doi: 10.1164/rccm.200510-1666ST. [DOI] [PubMed] [Google Scholar]
- 33.Xing X, Li H, Liu WG. Hepatic segmentectomy for treatment of hepatic tuberculous pseudotumor. Hepatobiliary Pancreat Dis Int. 2005;4:565–568. [PubMed] [Google Scholar]


