Abstract
Background
There have been attempts to develop universally applicable nomenclature for pancreatic fluid collections (PFCs) in acute pancreatitis. But PFCs following acute necrotizing pancreatitis (ANP) has not been studied by sensitive imaging techniques like endoscopic ultrasound (EUS). The aim of the study was to prospectively study morphological structure of pancreatic fluid collections occurring after ANP by serial EUS.
Methods
Patients with ANP having PFC at ≥4 weeks of onset of symptoms seen at our center from October 2011 to November 2012 were prospectively followed up with EUS at 6 weeks, 3 months and 6 months respectively and the amount of solid content in the collection was quantified as percentage amount of echogenic material. The symptomatic patients undergoing EUS/percutaneous drainage also underwent EUS prior to drainage for assessment of solid content.
Results
Of the 54 patients enrolled, 7 patients were lost to follow up or refused EUS. Forty seven patients (34 males; mean age 36.7±11.6 years) were studied. Etiology of acute pancreatitis was alcohol (n=22), gallstones (n=10), idiopathic (10), gallstones+alcohol (n=3) and drug induced (n=2). Contrast enhanced computed tomography done after 3 days of onset of ANP had shown less than 30%, 30-50% and more than 50% necrosis in 6 (13%), 14 (30%) and 27 (57%) patients respectively. On EUS at 6 weeks, 41/47 (87%) patients had fluid collection with solid debris. Follow up EUS at 3 and 6 months revealed progressively decreasing solid content in PFCs.
Conclusions
All PFCs following ANP may not have solid necrotic content and over a period of time necrotic content tends to liquefy. This may have therapeutic implications.
Keywords: Endoscopic ultrasound, walled off pancreatic necrosis, acute pancreatitis, computed tomography
Introduction
Acute pancreatitis is an acute inflammatory disease process of the pancreas and is associated with a wide spectrum of local and systemic complications. With better understanding of the pathophysiological process and the availability of sophisticated cross sectional imaging techniques like computed tomography (CT) and magnetic resonance imaging (MRI) various local complications of acute necrotizing pancreatitis (ANP) are being increasingly recognized. Pancreatic fluid collections (PFCs) are one of the important local complications of acute pancreatitis and continuous understanding of their morphology as well as the natural history has led to the use of various terminologies. The Atlanta symposium in 1992 suggested universally applicable terminologies for various PFCs [1].
Although the Atlanta classification has been guiding pancreatologists for the last two decades, some of the definitions of various PFCs have been found to be confusing and there have been increasing attempts to revise this classification [2,3]. Recently, the Acute Pancreatitis Classification Working Group proposed a revision of the Atlanta classification taking into consideration the recent concepts of the disease with the aim of standardizing reported data, and facilitating communication between treating physicians and institutions [3]. This revision subclassified the PFCs into various subtypes and one of the most important factors considered was whether the collections are composed of fluid alone or contain a solid component admixed with varying amount of liquid content. The pancreatic pseudocyst was defined as a collection surrounded by a well-defined wall and containing essentially no or minimal solid material. Walled off pancreatic necrosis (WOPN) was defined as encapsulated collection of solid necrotic material with varying amount of liquid content. According to this classification pseudocyst is seen with acute interstitial edematous pancreatitis only and does not evolve from acute necrotic collections (ANCs) that occur following ANP [3].
The WOPN contains a mixture of both fluid and solid necrotic debris and these are usually difficult to distinguish from pure liquid collections on contrast enhanced CT (CECT) [4]. MRI and endoscopic ultrasound (EUS) have been shown to be more sensitive than CECT in detecting the solid debris [4-6]. The PFCs following ANP have not been systematically studied by the more sensitive imaging techniques like EUS and therefore even in the revised Atlanta classification all collections occurring in the delayed phase of ANP are presumed to have solid necrotic material and are labeled as WOPN. Also in our clinical practice while draining the PFCs following ANP, we have encountered PFCs with no or minimal solid debris. Therefore, we planned this study to systematically study the morphology of PFCs following ANP with EUS. In this study, we prospectively followed up patients with PFCs following ANP and studied their morphology including the amount of the solid content by serial EUS at 6 weeks, 3 months and 6 months after the onset of ANP.
Patients and methods
Patients with a PFC demonstrated on abdominal ultrasound/CECT/MRI at 6 weeks of onset of ANP were eligible for the study and were prospectively studied by serial EUS. All the enrolled patients had been earlier diagnosed with ANP based on the Atlanta classification [1]. The patients enrolled had been admitted with us or were treated for ANP at a referring center earlier and referred later to us for management of PFC. Informed consent was obtained from all the patients before enrollment in the study and the study protocol was approved by the institute ethics committee. Patients with pregnancy, age less than 18 years, presence of congestive cardiac failure, compromised pulmonary status or any contraindication to EUS were excluded. All patients provided procedural informed consent at the time of EUS.
All the enrolled patients underwent EUS examination at the time of enrollment (i.e. at 6 weeks of onset of ANP), 3 and 6 months of onset of ANP. The EUS examination was done with either a radial scanning echoendoscope (EG-3670 URK radial echoendoscope, Pentax Inc., Tokyo, Japan) or a linear scanning echoendoscope (EG-3870 UTK linear echoendoscope, Pentax Inc., Tokyo, Japan) at 7.5MHz. On EUS, the size as well as the detailed morphology of the PFC was studied with special emphasis on the presence of the solid necrotic debris. Any echogenic material present in the PFC was suggestive of solid debris. The depth of the imaging was altered to visualize larger collections. However, collections extending into paracolic gutters could not be properly evaluated.
Results
Fifty four patients with a PFC at 6 weeks following an attack of ANP seen at our center from October 2011 to November 2012 were enrolled in the study. Of these 54 patients enrolled, 7 patients were lost to follow up or refused EUS on follow up and forty seven patients (34 males; mean age 36.7±11.6 years) were finally included in the current study. The etiology of ANP was: alcohol (22 patients), gallstones (10 patients), idiopathic (10 patients), gallstones and alcohol (3 patients) and drug-induced (2 patients). CECT done >3 days of onset of ANP had shown less than 30%, 30-50% and more than 50% necrosis in 6 (13%), 14 (30%) and 27 (57%) patients respectively. Also, during the hospital stay (<4 weeks after onset of ANP) all of these 47 patients had demonstrable PFC either on ultrasound or CECT of the abdomen (acute fluid collection or ANC as per the revised Atlanta classification).
On EUS at 6 weeks, 41/47 (87%) patients had fluid collection with solid debris. The mean size of the fluid collection was 9.6±4.5 cm (range: 3.4-22.0 cm). Importantly, 6/47 (13%) patients had fluid collection with no solid debris and all these 6 patients had <30% necrosis in the CECT done during the acute phase of ANP. Follow-up EUS at 3 and 6 months revealed progressively decreasing solid content in PFCs (Fig. 1). Thirty one patients completed three months follow up and 29/31 (94%) patients had a PFC at 3 months. EUS done at that time revealed that 14/29 (48%) patients had a PFC with no solid debris. The mean diameter of the PFC at three months follow up was 6.9±2.9 cm (range: 3.5-14.0 cm). Twelve patients completed 6 months follow up and EUS at 6 months follow up revealed that PFC was present in 9/12 patients and of these nine patients, five (56%) patients had a PFC with no solid debris (Fig. 2, 3). The mean diameter of the PFC at six months follow up was 5.4±2.1 cm (range: 3.0-8.0 cm).
Figure 1.
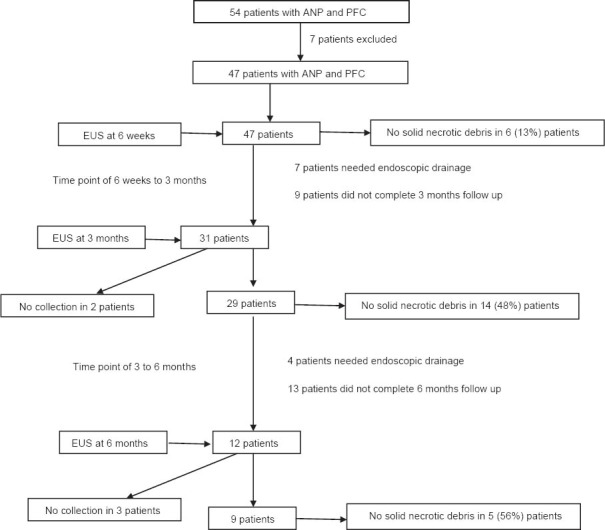
Flow chart showing the number of patients having PFC with no solid debris at 6 weeks, 3 and 6 months respectively
ANP, acute necrotizing pancreatitis; PFC, pancreatic fluid collection; EUS, endoscopic ultrasound
Figure 2.
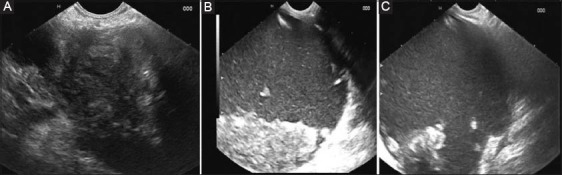
Endoscopic ultrasound in a patient of acute gall stone pancreatitis. (A) At 6 weeks showing a PFC with predominantly solid content. (B) At 3 months showing a PFC with decreasing solid content. (C) At 6 months showing a PFC with predominantly liquid content
PFC, pancreatic fluid collection
Figure 3.
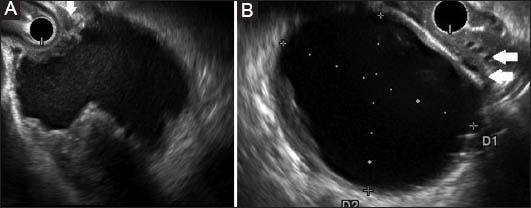
Endoscopic ultrasound in a patient with idiopathic acute pancreatitis. (A) At 6 weeks shows a pancreatic fluid collection (PFC) with ~35% solid content. (B) At 3 months completely liquefied PFC. Collaterals are noted (arrows)
PFC, pancreatic fluid collection
Discussion
The PFCs following acute pancreatitis have been varyingly named as acute fluid collections, infected or sterile necrosis, acute pseudocyst, pancreatic abscess, phlegmon, and more recently as organized necrosis or WOPN. The lack of uniform terminology and presence of number of terminologies for PFCs has led to confusion and lack of proper communication between physicians, gastroenterologists, surgeons and radiologists. In order to have standard definitions, the Acute Pancreatitis Classification Working Group recently proposed a revision of the Atlanta classification taking into consideration the recent concepts of the disease [3]. This revision sub classified the PFCs into various subtypes with one of the most important factors considered being the morphology and content of the PFCs. It has been proposed that PFCs following an attack of ANP have varying amounts of solid necrotic content and should be labeled as WOPN and acute pseduocysts are seen with acute interstitial edematous pancreatitis only and do not evolve from ANCs that occur following ANP. In the current study, we prospectively studied the detailed morphology of the fluid collections following an attack of ANP by EUS and obtained interesting results.
We found that even at 6 weeks following an attack of ANP, all PFCs did not have solid necrotic debris with 13% of patients having a PFC with complete liquid content. The follow up EUS at 3 and 6 months revealed progressively decreasing size as well as the amount of solid content in the PFCs and at six months of follow up the mean diameter of the PFCs had decreased to half of the original size and more than 50% of patients had a PFC with no solid content. Therefore, our results suggests that all the PFCs following an attack of ANP cannot be labeled as WOPN in a strict sense as many of them do have solid necrotic debris demonstrable on EUS. The liquefaction of the solid necrotic debris within the PFCs has been reported earlier but has not been systematically studied with sensitive imaging techniques like EUS [3,4,7,8]. Thoeni et al suggested that liquefaction of the necrotic tissue in the PFC occurs gradually (usually within 2-6 weeks) [8]. Our results also suggest that liquefaction is a slow process with almost 50% of the PFCs completely liquefying within 6 months of an attack of ANP. However, the factors that determine the process of liquefaction of solid necrotic debris are poorly understood. It has been suggested that the amount of necrotic tissue present in the ANC may be one of the factors that determines the rate of liquefaction [4,7]. Our results also suggest that the amount of necrotic tissue present at the outset is probably one of the factors that could determine the rate of liquefaction as all the patients with <30% necrosis at the onset of ANP had a completely liquefied PFC at 6 weeks follow up.
What are the implications of our results and do we need a new nomenclature for PFCs that develop following an attack of ANP? The revised Atlanta classification stressed that the PFCs should be described on the basis of location (pancreatic, peripancreatic, other), the nature of the content (liquid, solid, gas), and the thickness of the wall (thin, thick) [3]. The description of the PFCs occurring in the delayed phase of ANP as WOPN is based on the premise that it is a mature, encapsulated collection of pancreatic and/or peripancreatic necrosis and has a well defined inflammatory wall. But we have shown that many of these collections have completely liquefied on follow up and are completely devoid of solid necrotic debris.
In conclusion, all the PFCs following ANP may not have solid necrotic content and over a period of time the collection tends to decrease in size and the solid content tends to liquefy with almost half of PFCs completely liquefying at 6 months.
Summary Box.
What is already known:
An attempt has been made by the acute pancreatitis classification working group to revise the nomenclature for various types of pancreatic fluid collections (PFCs)
The PFC occurring in the delayed phase (>4 weeks) of acute necrotizing pancreatitis (ANP) have been named as walled off pancreatic necrosis that contains a mixture of both fluid and solid necrotic debris
According to this revised classification acute pseudocyst is seen with acute interstitial edematous pancreatitis only and does not evolve from acute necrotic collections that occur following ANP
What the new findings are:
Studying PFCs with endoscopic ultrasound, we have found that all PFCs following ANP do not have solid necrotic debris with some having a completely liquid content
Over a period of time the PFCs following ANP tend to decrease in size and solid content tends to liquefy with almost half of PFCs completely liquefying at 6 months
Biography
Post Graduate Institute of Medical Education and Research (PGIMER), Chandigarh, India
Footnotes
Conflict of Interest: None
References
- 1.Bradley EL., III A clinically based classification system for acute pancreatitis. Summary of the international symposium on acute pancreatitis, Atlanta, Ga, September 11 through 13, 1992. Arch Surg. 1993;128:586–590. doi: 10.1001/archsurg.1993.01420170122019. [DOI] [PubMed] [Google Scholar]
- 2.Bollen TL, van Santvoort HC, Besselink MG, et al. The Atlanta classification of acute pancreatitis revisited. Br J Surg. 2008;95:6–21. doi: 10.1002/bjs.6010. [DOI] [PubMed] [Google Scholar]
- 3.Banks PA, Bollen TL, Dervenis C, et al. Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis-2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–111. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 4.Zaheer A, Singh VK, Qureshi RO, Fishman EK. The revised Atlanta classification for acute pancreatitis: updates in imaging terminology and guidelines. Abdom Imaging. 2013;38:125–136. doi: 10.1007/s00261-012-9908-0. [DOI] [PubMed] [Google Scholar]
- 5.Morgan DE, Baron TH, Smith JK, Robbin ML, Kenney PJ. Pancreatic fluid collections prior to intervention: evaluation with MR imaging compared with CT and US. Radiology. 1997;203:773–778. doi: 10.1148/radiology.203.3.9169703. [DOI] [PubMed] [Google Scholar]
- 6.Rana SS, Bhasin DK, Rao C, Gupta R, Singh K. Non-fluoroscopic endoscopic ultrasound-guided transmural drainage of symptomatic non-bulging walled-off pancreatic necrosis. Dig Endosc. 2013;25:47–52. doi: 10.1111/j.1443-1661.2012.01318.x. [DOI] [PubMed] [Google Scholar]
- 7.Hariri M, Slivka A, Carr-Locke DL, Banks PA. Pseudocyst drainage predisposes to infection when pancreatic necrosis is unrecognized. Am J Gastroenterol. 1994;89:1781–1784. [PubMed] [Google Scholar]
- 8.Thoeni RF. The revised Atlanta classification of acute pancreatitis: its importance for the radiologist and its effect on treatment. Radiology. 2012;262:751–764. doi: 10.1148/radiol.11110947. [DOI] [PubMed] [Google Scholar]


