Abstract
The primary cilium is a well-established target in the pathogenesis of numerous developmental and chronic disorders, and more recently is attracting interest as a structure relevant to cancer. Here we discuss mechanisms by which changes in cilia can contribute to the formation and growth of tumors. We emphasize the cancer-relevance of cilia-dependent signaling pathways and proteins including mTOR, VHL, TSC, WNT, Aurora-A, NEDD9, and Hedgehog, and highlight the emerging role of ciliary dysfunction in renal cell carcinoma, medulloblastoma, and breast cancer.
1. Introduction
Almost all eukaryotic cell types can assemble a hair-like structure from their cell surface called the primary cilium (summarized in [1]). This immotile cellular organelle senses the extracellular environment to regulate intracellular signaling of multiple cell processes. The mechanistic importance of this structure in tissue development and the maintenance of tissue homeostasis is now becoming well established, yielding insights into why ciliary function is altered or lost in many different genetic diseases, including polycystic kidney disease (PKD), additional cystic kidney diseases, and ciliopathies affecting other tissues (summarized in [2]). Increasingly, studies of ciliary signaling suggest that deregulation of cilia may also contribute to the pathogenesis of common and clinically significant diseases such as cancer. This review will discuss the general mechanisms by which cilia can impact tumorigenesis, with particular focus on the signaling pathways most involved in clear cell renal cell carcinoma (ccRCC), medulloblastoma and breast cancer (BCa).
2. The relevance of cilia-specified processes to cancer
Altered cell cycle dynamics, including loss of response to external signals that inhibit cell growth, increased proliferation, loss of cell polarity control, and altered interaction with the cellular microenvironment, including increased or defective secretion of extracellular matrix (ECM) leading to fibrosis are among the hallmarks distinguishing a cancer cell from an untransformed normal cell [3]. Many of these defects also characterize classic ciliopathies (Figure 1). In this context, it is particularly interesting that loss of cilia has been described in many types of solid tumor, including renal cell carcinoma [4], basal cell carcinoma [5], and breast cancer [6,7], although in some specific tumor types, such as medulloblastoma [8], the presence of cilia is required for cancer initiation. It is very plausible that these changes in ciliary integrity are linked to the process of tumor formation, as cell cycle regulation [9,10], planar cell polarity (PCP, also referred to as noncanonical Wnt signaling) [11,12,13], ECM deposition [14], and other tumor-relevant defects are now known to be specified in part by signaling proteins localized at cilia. Some studies reveal a necessity for cilia that is reminiscent of a rheostat, in which too little or too much signaling by cilia-localized protein can perturb normal cell biology [5,8], while other data indicate the oncogenic context can dictate whether loss of cilia suppresses or accelerates tumor growth [5,8]. It is currently a question of considerable interest as to whether changes in the cilia typically precede and contribute to cell transformation, or if oncogenic transformation subsequently induces changes in cilia that may or may not enhance the malignant potential of tumors. In this review, we will describe a number of cilia-linked signaling pathways in the context of tumor formation or progression.
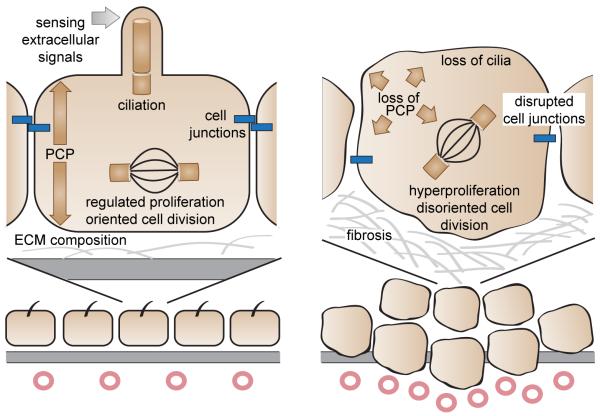
Figure 1. Cilia-associated hallmarks of transformed epithelial cells.
Left panel: The integrity of cell junctions, planar cell polarity (PCP), composition of the extracellular matrix (ECM), proliferation and oriented cell division, and sensing of some soluble extracellular signals are important features for the homeostasis of epithelial cells. Right panel: Loss of cilia is associated with loss of PCP, hyperproliferation, disoriented cell division, disrupted cell junctions, and fibrosis. Together, these defects are important hallmarks of the formation of solid tumors.
3. Specific signaling pathways in carcinogenesis with ciliary links
a. Control of ciliary disassembly
Over the past 5 years, several proteins with well-established roles in tumor growth have been established as regulators of ciliary resorption (Figure 2A). In untransformed, quiescent cells, low levels of the protein AURKA (Aurora-A kinase) localizes to the basal body of the primary cilium of quiescent cells [15]. Stimuli leading to initiation of the cell division cycle induce expression and basal body localization of the AURKA activator NEDD9. Activated AURKA phosphorylates HDAC6 and other substrates, thereby triggering ciliary disassembly [15]. The AURKA-activating interaction with NEDD9 is stimulated by the binding of Ca2+-liganded calmodulin to AURKA [15,16]. AURKA-NEDD9 interactions are also stabilized by an interacting PLK1-DVL2 complex at the basal body [11] and the adjacent ciliary transition zone [17]; in the normal initiation of ciliogenesis after mitosis, BUBR1 restrains DVL2 expression [18].
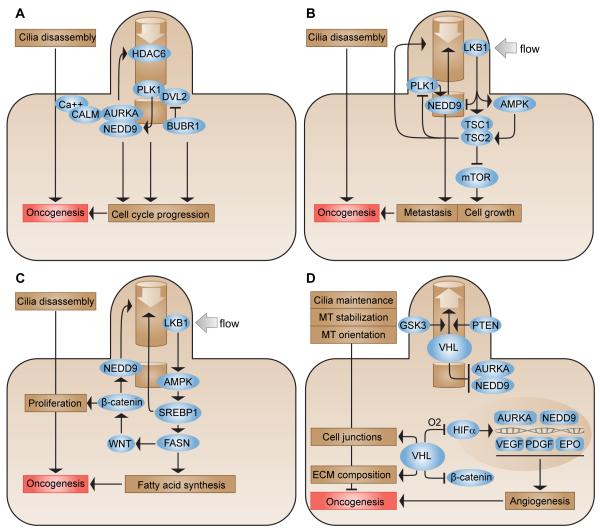
Figure 2. Cilia-associated signaling pathways relevant to cancer.
A. NEDD9 and AURKA at the basal body initiate ciliary disassembly through the activation of HDAC6 and other substrates. Ca2+-liganded CALM and a PLK1-DVL2 complex support the activation of AURKA, while BUBR1 limits DVL2 expression during ciliogenesis. Altered function of AURKA, NEDD9, PLK1 and BUBR1 contributes to oncogenesis by targeting cell cycle progression and other cellular processes. HDAC6, histone deacetylase 6; PLK1, polo-like kinase 1; DVL2, dishevelled-2; AURKA, aurora-A kinase; NEDD9, neural precursor expressed downregulated in development 9; BUBR1, budding uninhibited by benzimidazoles 1-related protein kinase; CALM, calmodulin. B. Renal fluid flow activates ciliary LKB1, which signals through AMPK and the TSC1/TSC2 heterodimer to limit mTOR signaling and cell growth. Ablation of the cilium stimulates cell growth by relieving mTOR inhibition. LKB1 negatively regulates NEDD9; TSC2 limits PLK1. LKB1, liver kinase B1 (also known as serine/threonine-protein kinase STK11); AMPK, 5′-AMP-activated protein kinase; TSC1, hamartin; TSC2, tuberin; mTOR, mammalian target of rapamycin. C. LKB1 signaling to AMPK is in part mediated through the activity of SREBPs, which regulate ciliary stability and activate FASN. Active FASN modifies and alters activity of the ciliary-responsive protein WNT, influencing activity of cytosolic β-catenin, NEDD9, and other oncogenic proteins. SREBP1, sterol regulatory element-binding protein 1; FASN, fatty acid synthase. D. In the presence of oxygen, VHL degrades HIFα; during hypoxia, HIFα induces transcription of angiogenesis promoting factors including VEGF, PDGF, and EPO, as well as NEDD9 and AURKA. VHL in cooperation with PTEN and GSK3 maintains the cilium by stabilizing and orienting microtubules (MT). Additional functions of VHL include the regulation of cell junctions and ECM composition. The depletion of VHL can drive oncogenesis via these various cell processes. GSK3, glycogen synthase kinase 3; PTEN, phosphatase and tensin homolog; VHL, von Hippel-Lindau; HIFα, hypoxia-inducible factor alpha; VEGF, vascular endothelial growth factor; PDGF, platelet derived growth factor; EPO, erythropoietin.
NEDD9, AURKA, PLK1, and BUBR1 all have previously described roles as regulators of mitotic progression, and in some cases additional functions, such as NEDD9 in integrin-dependent cell migration and invasion. Significantly, overexpression of each of these proteins also promotes tumor formation or progression. For instance, AURKA overexpression or gene amplification is associated with malignancies of breast, colon, pancreas, ovarian, bladder, liver, and gastric origins; extensive interplay between AURKA and PLK1 in cancer has led each to be heavily investigated as drug targets (reviewed in [19,20]). High levels of NEDD9 enhance metastasis of numerous types of human cancer as well as multiple human cancer cell types of various origin [21]. BUBR1 dysregulation is also associated with cancer, with this predominantly attributed to dysregulation-associated defects in genomic stability and spindle checkpoints (reviewed in [22]). However, it is possible that part of the oncogenic contribution of AURKA and its interacting proteins arises from the persistent loss of cilia, cilia-associated cell cycle restrictions, and growth-inhibitory signaling through cilia-localized receptors, when these are overexpressed. In support of this idea, AURKA activation at the ciliary basal body has recently been shown to rely on interactions with another partner, trichoplein [23]; interestingly, depletion of AURKA and trichoplein results in G1 cell cycle arrest unless formation of cilia is prevented by a simultaneous mutation in the IFT20 gene, suggesting resorption of cilia may be essential for cell cycle advancement.
b. cilia and cell growth controls
The mTOR pathway (Figure 2B) integrates numerous signals related to growth factors, energy, cell stress, and genotoxic damage that are highly associated with acceleration of cancer development. In this pathway, the LKB1 kinase activates AMPK, which phosphorylates and activates the protein TSC2. TSC then inhibits the mTORC1 (mTOR/raptor) complex, limiting translation [24,25]. In the kidney, LKB1 localizes to the cilium and basal body of epithelial cells and is regulated by flow, which stimulates AMPK activity at the ciliary basal body; ablation of the cilium inhibits AMPK signaling, relieving inhibition of mTORC1 and increasing cell size [26]. Mutations eliminating activity of TSC2 and its heterodimerizing partner TSC1 hyperactivate mTOR signaling and simultaneously result in lengthening of cilia [27,28]. Mechanistically, TSC negatively regulates PLK1 [29], and LKB1 negatively regulates NEDD9 [21,30], suggesting mechanisms for dialogue between regulation of the mTOR pathway and presence of cilia. These connections may be important for cancer development. Mutations in TSC1/TSC2 and LKB1 are associated with familial cancer syndromes (reviewed in [31,32]). Given the function of cilia in extracellular sensing, it is conceivable that at least some of the extracellular signals regulating the mTOR pathway in tumorigenesis may be mediated through the cilia. Many non-small cell lung cancers (NSCLC) lacking LKB1 overexpress NEDD9, which contributes to increased metastasis, and is predicted to activate AURKA and support ciliary disassembly [21,30].
c. cilia, cell polarity controls, and tumor metabolism
For solid tumors, common features include the loss of cell polarity and lateral attachments, as part of the epithelial-mesenchymal transition (EMT) that allows invasion, and changes in energy metabolism. The activation of AMPK by LKB1 is required for cell polarization, and essential for formation of epithelial tight junctions [33]. Further, LKB1 signaling to AMPK is in part mediated through the activity of sterol response element binding proteins (SREBPs), which regulate cell metabolic processes (Figure 2C) [34]. Increased expression of SREBP1 causes loss of cilia, and also activates fatty acid synthesis; intriguingly, inhibition of fatty acid synthesis per se is sufficient to restore cilia [35]. SREBP1 signals through a downstream partner, fatty acid synthase (FASN), that promotes palmitoylation of a group of protein targets that includes Wnt [36] a key regulator of planar cell polarity (PCP). Palmitoylated Wnt is stabilized at the cell and ciliary membrane, thereby stabilizing cytoplasmic expression of its major canonical effector β-catenin [36]. Stabilization of β-catenin in the cytoplasm contributes to cell cycle entry, and limits the cilia-based Hedgehog/Sonic Hedgehog (Hh/Shh) signaling that supports differentiation rather than proliferation. β-catenin can also increase expression of NEDD9 [37], which may contribute to the loss of cilia associated with SREBP1. Abnormally high levels of FASN are associated with poor prognosis and reduced survival of every tumor type investigated [38]; no work to date has directly investigated FASN in the context of ciliary regulation.
d. cilia and hypoxia
As tumors grow, they typically experience hypoxic conditions because of initial limitations in angiogenesis. Under hypoxic conditions, hydroxylation of the transcription factor HIF1α prevents its binding to the E3 ubiquitin ligase and tumor suppressor VHL (Figure 2D). This inhibits degradation of HIF1α, allowing it to promote the transcription of downstream hypoxia-adaptive and growth signals, which include vascular endothelial growth factor (VEGF), platelet derived growth factor (PDGF), and erythropoietin (EPO), as well as the cilia resorption-promoting proteins AURKA and NEDD9 [40]. VHL itself localizes to the primary cilium. Besides its role in regulating HIF1α, VHL interacts with the proteins GSK3β [41] and the tumor suppressor PTEN [42] in ciliary maintenance. In addition, VHL interacts directly with microtubules, supporting the stabilization and orientation that is critical for maintaining the ciliary axoneme [43,44]. VHL−/− epithelial cells typically reduce expression of E-cadherin and have disrupted cell-cell contacts, providing further evidence of connection with the cell polarity machinery [45,46]. VHL also regulates fibronectin assembly within the ECM, expression of the survival factor NFκB activity, and cell surface expression of receptor tyrosine kinases, and also influences Wnt signaling and stability of β-catenin (summarized in [47]). Hence, loss of VHL in tumors provides multiple stimuli for loss of cilia but also indirectly induces other processes relevant to tumor growth: the extent to which these are interdependent or represent separate, parallel activities is a target of active investigation.
4. Ciliary signaling, tumors, and treatment strategies
Space restrictions do not allow a comprehensive discussion of the impact of defective cilia or ciliary signaling in all common cancers. The three examples provided represent well-established, emerging, or plausible linkages in the context of clinical disease.
Clear cell Renal Cell Carcinoma (ccRCC)
ccRCC is the most common form of kidney cancer, and is estimated to be newly diagnosed in about 52,000 individuals in the US in 2012 [48]. Increased risk of ccRCC is associated with the familial cancer syndrome Von Hippel-Lindau (VHL), involving mutations in the VHL gene. Although only a small percentage of ccRCC cases arise in these families, approximately 90% of spontaneously arising ccRCC cases have genetic alterations in either the short arm of chromosome 3 where VHL is localized, or targeting VHL itself, with 50% of cases exhibiting loss of wild type VHL expression [47]. In addition to experiencing high rates of ccRCC, members of VHL families also have elevated probability of developing kidney cysts, benign vascular tumors, and pheochromocytomas. Further, families with heterozygous deletions of the mTOR repressor protein TSC1 are also predisposed to ccRCC, which evolves from pre-malignant kidney cysts. Frequent loss of heterozygosity (LOH) for TSC1 is observed in RCC but not in the pre-malignant cysts, [49].
Regulation of VHL has been widely investigated within the context of its role in regulating HIF1α, with some newer studies also addressing HIF1α-independent mechanisms (summarized in [47,50]). Since 2005, novel molecular therapies have been approved that interfere with the HIF1α-mediated response to hypoxia that are upregulated subsequent to mutation of VHL (Figure 3). mTOR activation is clearly linked to the pathology of the ccRCC and indirectly supports HIF1α-dependent transcription: mTOR inhibitors (temsirolimus, everolimus) are now commonly used in the clinic. Alternative therapeutics employed for ccRCC include multi-targeted tyrosine kinase inhibitors (TKIs) (including sunitinib, sorafinib, pazopanib and axitinib), which block mTOR activation by inhibiting upstream factors such as VEGF, PDGF and C-KIT; bevacizumab specifically targets VEGF (summarized in [http://www.nature.com/nrclinonc/journal/v9/n6/full/nrclinonc.2012.59.html]). Given the roles of VHL and TSC1 as regulators of ciliary maintenance, and the fact that the PDGF receptor PDGFRα and numerous other growth regulatory receptors function specifically at cilia, it is worth considering the impact of ciliary loss in predicting which tumor growth pathways are most likely to be impacted in a class of cancers where the common initiating event destabilizes cilia.
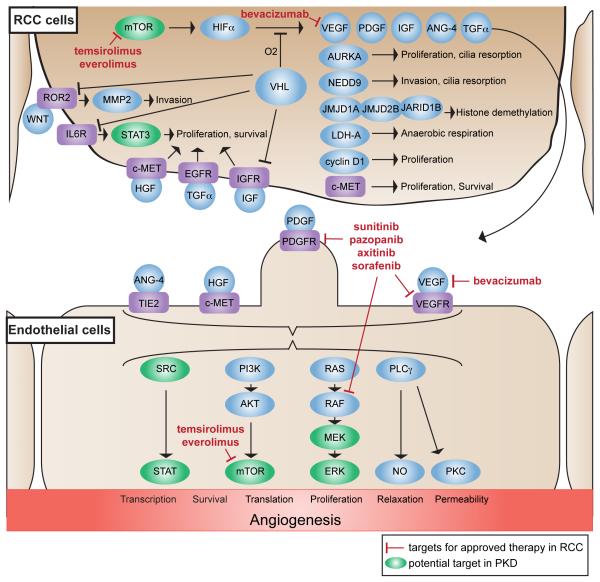
Figure 3. Targeted therapies in renal cell carcinoma (RCC).
Inactivation of VHL in RCC cells triggers a cascade of signaling and HIFα-dependent transcription that promote tumor growth cell autonomously, and by influencing angiogenesis in adjacent endothelial cells. Drugs currently approved in the therapy of RCC interfere with the downstream effectors of HIFα, including VEGF (bevacizumab), receptor tyrosine kinases (RTKs) like VEGFR and PDGFR (sunitinib, sorafenib, pazopanib, axitinib), and the m-TOR pathway (temsirolimus, everolimus). Ongoing preclinical studies suggest further potential targets with some of them overlapping with the potential targets in the treatment of ciliopathies such as polycystic kidney disease (green). These targets include mTOR, SRC, STAT and the RAS/RAF/MEK/ERK pathway. VEGF(R), vascular endothelial growth factor (receptor); PDGF(R), platelet derived growth factor (receptor); IGF(R), insulin-like growth factor (receptor); ANG-4, angiopoetin-4; TGFα, transforming growth factor alpha; JMJD1A/JMJD2B, jumonji domain-containing protein 1A/2B; JARID1B, lysine-specific demethylase 5B; LDH, lactate dehydrogenase A chain; c-MET, hepatocyte growth factor receptor; ROR2, RTK-like orphan receptor 2; MMP2, matrix metalloproteinase-2; IL6R, interleukin-6 receptor; STAT3, signal transducer and activator of transcription 3; HGF, hepatocyte growth factor; EGFR, epidermal growth factor receptor; TIE2, Tyrosine-protein kinase receptor-2; PI3K, phosphatidylinositol 3-kinase; MEK, mitogen-activated protein kinase kinase 1; ERK, extracellular-regulated kinase; PLCγ phospholipase C-gamma; NO, nitric oxide; PKC, protein kinase C.
Ongoing investigations into the initiating events of RCC have found that homozygous deletion of APC (commonly mutated in Gardner’s syndrome, also known as familial adenomatous polyposis) predisposes mice to renal cancer, particularly in the context of depleted p21 [51,52]. APC has recently been identified as a regulator of ciliary function, and Gardner’s syndrome proposed to be at least partly caused by cilia dysfunction (summarized in [53,54]). Finally, despite the high rate of VHL alteration in ccRCC, inactivation of VHL is insufficient for tumor formation (summarized in [47]) and new genes are continually being identified as mutated in ccRCC [55,56,57]. Recent genomic sequencing studies of ccRCC tumors have revealed common mutations affecting the genes PBRM1, AHNAK and SRGAP3 [55,56,57,58]. Whether and how these genes interact with cilia-based functions is as yet unknown.
Cilia in medulloblastoma
Medulloblastomas are the most common brain tumor in children. A subset of these tumors is characterized by activated Hh or Wnt signaling, while others lack this pathway activation [8]. Hh signaling at the cilium involves Hh binding to its receptor, Patched (Ptch1), which signals in additional cilia-localized interactions through the intermediate Smoothened (Smo) to regulate activity of the Gli2 transcription factor. Using mouse models, Han et al showed that Smo-driven medulloblastomas required intact cilia for transformation; in contrast, Gli2-driven tumors required the loss of cilia. Further supporting the idea that there is clinical relevance to the observation of differing requirements for ciliation, the authors determined that primary medulloblastomas from patients segregate into cilia-positive and -negative cohorts. Ciliated medulloblastomas are characterized by activated Hh and/or Wnt signaling: tumors with high Wnt signaling also have mutations in CTNNB1, encoding β-catenin [8]. Whether non-ciliated tumors are particularly dependent on Gli is not known, although amplification of Gli1 and Gli2 has been described as a rare event in medulloblastomas. The authors point out that these results suggest that therapies targeted at ciliary signaling systems may be particularly efficacious in the ciliated, Hh/Wnt-high group of tumors [8].
Cilia in breast cancer
Breast cancers fall into discrete categories, reflecting origin from luminal or myoepithelial (basal) cell types. Luminal tumors fall into two subtypes, A and B, typically express estrogen receptor (ER), and often respond to ER-targeting therapies. Basal cell-derived tumors are also known as triple negative based on their failure to express ER, progesterone receptor, or HER2/ERBB2: these tumors have more limited response to treatment. Very recent work analyzing ciliation of mammary cells during breast morphogenesis showed striking differences among different cell populations and at different stages of development, with both myoepithelial/basal and luminal precursors ciliated in early morphogenesis, but myoepithelial/basal cells remaining highly ciliated, and luminal cells losing cilia in differentiated mammary glands [59]. Cilia played an active role in the differentiation process, as mutations leading to loss of cilia caused defects in morphogenesis and signaling, including unusual increases in canonical Wnt signaling and depressed Hh activation [59]. Abnormal Wnt signaling characterizes a subset of triple negative breast cancers (reviewed in [60]), and elevated Hedgehog signaling has been associated with poor outcome in one study of triple negative breast cancer [61]. It seems likely that ciliation status plays a role in breast cancer pathogenesis, although this idea awaits definitive experimentation.
5. Conclusions
As discussed elsewhere in this issue, defective ciliary regulation of Wnt, Hedgehog, β-catenin, mTOR, and other signaling proteins (see Figures) is central to the pathology of multiple ciliopathies. In the past, less attention has been devoted to the study of the consequences of ciliary status in cancer. However, it is becoming evident that they are likely to impact this disease in multiple ways, including 1) regulation of cell cycle progression, 2) regulation of growth and differentiation signals, and 3) regulation of response to therapeutics targeted to proteins that localize to cilia or are controlled by cilia-localized receptors. In addition, it is important to consider that therapeutics targeting such proteins as AURKA, currently under assessment in the clinic as inhibitors of mitosis [62], may have unanticipated additional activities arising from their stabilization of cilia on the surface of both normal and transformed cells. Given this growing appreciation of the importance of cilia, it would be timely to begin incorporating assessment of ciliary status in measures of cancer aggressiveness. Further, as the trend towards “personalization” of cancer therapy gains acceptance, this information should figure into decisions as to which tumors are most likely to respond to therapies targeting Wnt, Hh, mTOR, or other relevant targets (see, for example, the discussion in [39].
Acknowledgments
The authors acknowledge support from R01 CA063366 (to EAG) and DFG SE2280/1-1 (to TS-N).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Oh EC, Katsanis N. Cilia in vertebrate development and disease. Development. 2012;139:443–448. doi: 10.1242/dev.050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Novarino G, Akizu N, Gleeson JG. Modeling human disease in humans: the ciliopathies. Cell. 2011;147:70–79. doi: 10.1016/j.cell.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Schraml P, Frew IJ, Thoma CR, Boysen G, Struckmann K, et al. Sporadic clear cell renal cell carcinoma but not the papillary type is characterized by severely reduced frequency of primary cilia. Mod Pathol. 2009;22:31–36. doi: 10.1038/modpathol.2008.132. [DOI] [PubMed] [Google Scholar]
- 5.Wong SY, Seol AD, So PL, Ermilov AN, Bichakjian CK, et al. Primary cilia can both mediate and suppress Hedgehog pathway-dependent tumorigenesis. Nat Med. 2009;15:1055–1061. doi: 10.1038/nm.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reilova-Velez J, Seiler MW. Abnormal cilia in a breast carcinoma. An ultrastructural study. Arch Pathol Lab Med. 1984;108:795–797. [PubMed] [Google Scholar]
- 7.Yuan K, Frolova N, Xie Y, Wang D, Cook L, et al. Primary cilia are decreased in breast cancer: analysis of a collection of human breast cancer cell lines and tissues. J Histochem Cytochem. 2010;58:857–870. doi: 10.1369/jhc.2010.955856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han YG, Kim HJ, Dlugosz AA, Ellison DW, Gilbertson RJ, et al. Dual and opposing roles of primary cilia in medulloblastoma development. Nat Med. 2009;15:1062–1065. doi: 10.1038/nm.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim S, Zaghloul NA, Bubenshchikova E, Oh EC, Rankin S, et al. Nde1-mediated inhibition of ciliogenesis affects cell cycle re-entry. Nat Cell Biol. 2011;13:351–360. doi: 10.1038/ncb2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li A, Saito M, Chuang JZ, Tseng YY, Dedesma C, et al. Ciliary transition zone activation of phosphorylated Tctex-1 controls ciliary resorption, S-phase entry and fate of neural progenitors. Nat Cell Biol. 2011;13:402–411. doi: 10.1038/ncb2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee KH, Johmura Y, Yu LR, Park JE, Gao Y, et al. Identification of a novel Wnt5a-CK1epsilon-Dvl2-Plk1-mediated primary cilia disassembly pathway. EMBO J. 2012 doi: 10.1038/emboj.2012.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lienkamp S, Ganner A, Walz G. Inversin, Wnt signaling and primary cilia. Differentiation. 2012;83:S49–55. doi: 10.1016/j.diff.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 13.Anastas JN, Biechele TL, Robitaille M, Muster J, Allison KH, et al. A protein complex of SCRIB, NOS1AP and VANGL1 regulates cell polarity and migration, and is associated with breast cancer progression. Oncogene. 2011 doi: 10.1038/onc.2011.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jun JI, Lau LF. Taking aim at the extracellular matrix: CCN proteins as emerging therapeutic targets. Nat Rev Drug Discov. 2011;10:945–963. doi: 10.1038/nrd3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pugacheva EN, Jablonski SA, Hartman TR, Henske EP, Golemis EA. HEF1-dependent Aurora A activation induces disassembly of the primary cilium. Cell. 2007;129:1351–1363. doi: 10.1016/j.cell.2007.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Plotnikova OV, Nikonova AS, Loskutov YV, Kozyulina PY, Pugacheva EN, et al. Calmodulin activation of Aurora-A (AURKA) is required during ciliary disassembly and in mitosis. Mol Biol Cell. 2012 doi: 10.1091/mbc.E11-12-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seeger-Nukpezah T, Liebau MC, Hopker K, Lamkemeyer T, Benzing T, et al. The centrosomal kinase plk1 localizes to the transition zone of primary cilia and induces phosphorylation of nephrocystin-1. PLoS One. 2012;7:e38838. doi: 10.1371/journal.pone.0038838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyamoto T, Porazinski S, Wang H, Borovina A, Ciruna B, et al. Insufficiency of BUBR1, a mitotic spindle checkpoint regulator, causes impaired ciliogenesis in vertebrates. Hum Mol Genet. 2011;20:2058–2070. doi: 10.1093/hmg/ddr090. [DOI] [PubMed] [Google Scholar]
- 19.Lens SM, Voest EE, Medema RH. Shared and separate functions of polo-like kinases and aurora kinases in cancer. Nat Rev Cancer. 2010;10:825–841. doi: 10.1038/nrc2964. [DOI] [PubMed] [Google Scholar]
- 20.Dar AA, Goff LW, Majid S, Berlin J, El-Rifai W. Aurora kinase inhibitors--rising stars in cancer therapeutics? Mol Cancer Ther. 2010;9:268–278. doi: 10.1158/1535-7163.MCT-09-0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tikhmyanova N, Little JL, Golemis EA. CAS proteins in normal and pathological cell growth control. Cell Mol Life Sci. 2010;67:1025–1048. doi: 10.1007/s00018-009-0213-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bolanos-Garcia VM, Blundell TL. BUB1 and BUBR1: multifaceted kinases of the cell cycle. Trends Biochem Sci. 2011;36:141–150. doi: 10.1016/j.tibs.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inoko A, Matsuyama M, Goto H, Ohmuro-Matsuyama Y, Hayashi Y, et al. Trichoplein and Aurora A block aberrant primary cilia assembly in proliferating cells. J Cell Biol. 2012;197:391–405. doi: 10.1083/jcb.201106101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaw RJ, Bardeesy N, Manning BD, Lopez L, Kosmatka M, et al. The LKB1 tumor suppressor negatively regulates mTOR signaling. Cancer Cell. 2004;6:91–99. doi: 10.1016/j.ccr.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 25.Green AS, Chapuis N, Lacombe C, Mayeux P, Bouscary D, et al. LKB1/AMPK/mTOR signaling pathway in hematological malignancies: from metabolism to cancer cell biology. Cell Cycle. 2011;10:2115–2120. doi: 10.4161/cc.10.13.16244. [DOI] [PubMed] [Google Scholar]
- 26.Boehlke C, Kotsis F, Patel V, Braeg S, Voelker H, et al. Primary cilia regulate mTORC1 activity and cell size through Lkb1. Nat Cell Biol. 2010;12:1115–1122. doi: 10.1038/ncb2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hartman TR, Liu D, Zilfou JT, Robb V, Morrison T, et al. The tuberous sclerosis proteins regulate formation of the primary cilium via a rapamycin-insensitive and polycystin 1-independent pathway. Hum Mol Genet. 2009;18:151–163. doi: 10.1093/hmg/ddn325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DiBella LM, Park A, Sun Z. Zebrafish Tsc1 reveals functional interactions between the cilium and the TOR pathway. Hum Mol Genet. 2009;18:595–606. doi: 10.1093/hmg/ddn384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Astrinidis A, Senapedis W, Henske EP. Hamartin, the tuberous sclerosis complex 1 gene product, interacts with polo-like kinase 1 in a phosphorylation-dependent manner. Hum Mol Genet. 2006;15:287–297. doi: 10.1093/hmg/ddi444. [DOI] [PubMed] [Google Scholar]
- 30.Ji H, Ramsey MR, Hayes DN, Fan C, McNamara K, et al. LKB1 modulates lung cancer differentiation and metastasis. Nature. 2007;448:807–810. doi: 10.1038/nature06030. [DOI] [PubMed] [Google Scholar]
- 31.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dancey J. mTOR signaling and drug development in cancer. Nat Rev Clin Oncol. 2010;7:209–219. doi: 10.1038/nrclinonc.2010.21. [DOI] [PubMed] [Google Scholar]
- 33.Zheng B, Cantley LC. Regulation of epithelial tight junction assembly and disassembly by AMP-activated protein kinase. Proc Natl Acad Sci U S A. 2007;104:819–822. doi: 10.1073/pnas.0610157104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shackelford DB, Shaw RJ. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer. 2009;9:563–575. doi: 10.1038/nrc2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Willemarck N, Rysman E, Brusselmans K, Van Imschoot G, Vanderhoydonc F, et al. Aberrant activation of fatty acid synthesis suppresses primary cilium formation and distorts tissue development. Cancer Res. 2010;70:9453–9462. doi: 10.1158/0008-5472.CAN-10-2324. [DOI] [PubMed] [Google Scholar]
- 36.Fiorentino M, Zadra G, Palescandolo E, Fedele G, Bailey D, et al. Overexpression of fatty acid synthase is associated with palmitoylation of Wnt1 and cytoplasmic stabilization of beta-catenin in prostate cancer. Lab Invest. 2008;88:1340–1348. doi: 10.1038/labinvest.2008.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Y, Bavarva JH, Wang Z, Guo J, Qian C, et al. HEF1, a novel target of Wnt signaling, promotes colonic cell migration and cancer progression. Oncogene. 2011;30:2633–2643. doi: 10.1038/onc.2010.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Little JL, Kridel SJ. Fatty acid synthase activity in tumor cells. Subcell Biochem. 2008;49:169–194. doi: 10.1007/978-1-4020-8831-5_7. [DOI] [PubMed] [Google Scholar]
- 39.Hassounah NB, Bunch TA, McDermott KM. Molecular pathways: the role of primary cilia in cancer progression and therapeutics with a focus on hedgehog signaling. Clin Cancer Res. 2012;18:2429–2435. doi: 10.1158/1078-0432.CCR-11-0755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu J, Li H, Wang B, Xu Y, Yang J, et al. VHL inactivation induces HEF1 and Aurora kinase A. J Am Soc Nephrol. 2010;21:2041–2046. doi: 10.1681/ASN.2010040345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thoma CR, Frew IJ, Krek W. The VHL tumor suppressor: riding tandem with GSK3beta in primary cilium maintenance. Cell Cycle. 2007;6:1809–1813. doi: 10.4161/cc.6.15.4518. [DOI] [PubMed] [Google Scholar]
- 42.Frew IJ, Thoma CR, Georgiev S, Minola A, Hitz M, et al. pVHL and PTEN tumour suppressor proteins cooperatively suppress kidney cyst formation. EMBO J. 2008;27:1747–1757. doi: 10.1038/emboj.2008.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thoma CR, Matov A, Gutbrodt KL, Hoerner CR, Smole Z, et al. Quantitative image analysis identifies pVHL as a key regulator of microtubule dynamic instability. J Cell Biol. 2010;190:991–1003. doi: 10.1083/jcb.201006059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schermer B, Ghenoiu C, Bartram M, Muller RU, Kotsis F, et al. The von Hippel-Lindau tumor suppressor protein controls ciliogenesis by orienting microtubule growth. J Cell Biol. 2006;175:547–554. doi: 10.1083/jcb.200605092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tikhmyanova N, Golemis EA. NEDD9 and BCAR1 negatively regulate E-cadherin membrane localization, and promote E-cadherin degradation. PLoS One. 2011;6:e22102. doi: 10.1371/journal.pone.0022102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harten SK, Shukla D, Barod R, Hergovich A, Balda MS, et al. Regulation of renal epithelial tight junctions by the von Hippel-Lindau tumor suppressor gene involves occludin and claudin 1 and is independent of E-cadherin. Mol Biol Cell. 2009;20:1089–1101. doi: 10.1091/mbc.E08-06-0566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li L, Kaelin WG., Jr. New insights into the biology of renal cell carcinoma. Hematol Oncol Clin North Am. 2011;25:667–686. doi: 10.1016/j.hoc.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 49.Wilson C, Bonnet C, Guy C, Idziaszczyk S, Colley J, et al. Tsc1 haploinsufficiency without mammalian target of rapamycin activation is sufficient for renal cyst formation in Tsc1+/−mice. Cancer Res. 2006;66:7934–7938. doi: 10.1158/0008-5472.CAN-06-1740. [DOI] [PubMed] [Google Scholar]
- 50.Li M, Kim WY. Two sides to every story: the HIF-dependent and HIF-independent functions of pVHL. J Cell Mol Med. 2011;15:187–195. doi: 10.1111/j.1582-4934.2010.01238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sansom OJ, Meniel V, Wilkins JA, Cole AM, Oien KA, et al. Loss of Apc allows phenotypic manifestation of the transforming properties of an endogenous K-ras oncogene in vivo. Proc Natl Acad Sci U S A. 2006;103:14122–14127. doi: 10.1073/pnas.0604130103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cole AM, Ridgway RA, Derkits SE, Parry L, Barker N, et al. p21 loss blocks senescence following Apc loss and provokes tumourigenesis in the renal but not the intestinal epithelium. EMBO Mol Med. 2010;2:472–486. doi: 10.1002/emmm.201000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gomez Garcia EB, Knoers NV. Gardner’s syndrome (familial adenomatous polyposis): a cilia-related disorder. Lancet Oncol. 2009;10:727–735. doi: 10.1016/S1470-2045(09)70167-6. [DOI] [PubMed] [Google Scholar]
- 54.Li Z, Li W, Song L, Zhu W. Cilia, adenomatous polyposis coli and associated diseases. Oncogene. 2012;31:1475–1483. doi: 10.1038/onc.2011.351. [DOI] [PubMed] [Google Scholar]
- 55.Dalgliesh GL, Furge K, Greenman C, Chen L, Bignell G, et al. Systematic sequencing of renal carcinoma reveals inactivation of histone modifying genes. Nature. 2010;463:360–363. doi: 10.1038/nature08672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Varela I, Tarpey P, Raine K, Huang D, Ong CK, et al. Exome sequencing identifies frequent mutation of the SWI/SNF complex gene PBRM1 in renal carcinoma. Nature. 2011;469:539–542. doi: 10.1038/nature09639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu X, Hou Y, Yin X, Bao L, Tang A, et al. Single-cell exome sequencing reveals single-nucleotide mutation characteristics of a kidney tumor. Cell. 2012;148:886–895. doi: 10.1016/j.cell.2012.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guo G, Gui Y, Gao S, Tang A, Hu X, et al. Frequent mutations of genes encoding ubiquitin-mediated proteolysis pathway components in clear cell renal cell carcinoma. Nat Genet. 2012;44:17–19. doi: 10.1038/ng.1014. [DOI] [PubMed] [Google Scholar]
- 59.McDermott KM, Liu BY, Tlsty TD, Pazour GJ. Primary cilia regulate branching morphogenesis during mammary gland development. Curr Biol. 2010;20:731–737. doi: 10.1016/j.cub.2010.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.King TD, Suto MJ, Li Y. The Wnt/beta-catenin signaling pathway: a potential therapeutic target in the treatment of triple negative breast cancer. J Cell Biochem. 2012;113:13–18. doi: 10.1002/jcb.23350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.O’Toole SA, Machalek DA, Shearer RF, Millar EK, Nair R, et al. Hedgehog overexpression is associated with stromal interactions and predicts for poor outcome in breast cancer. Cancer Res. 2011;71:4002–4014. doi: 10.1158/0008-5472.CAN-10-3738. [DOI] [PubMed] [Google Scholar]
- 62.Nikonova AS, Astsaturov I, Serebriiskii IG, Dunbrack J, Golemis EA. Aurora-A (AURKA) kinase in normal and pathological cell growth. Cellular and Molecular Life Sciences. 2012 doi: 10.1007/s00018-012-1073-7. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]