Abstract
Semiconductor nanocrystals are tiny fluorescent particles that have recently made a major impact in the biological and medical sciences by enabling high-sensitivity imaging of biomolecules, cells, and tissues. Spherical quantum dots are the prototypical material for these applications but recent synthetic advances have led to a diverse range of nanostructures with controllable sizes, shapes, and materials combinations that offer new dimensions of optical and structural tunability. Uniform anisotropic shapes with linearly polarized light emission allow optical imaging of particle orientation, planar structures have large flexible surfaces and ultra-narrow electronic transitions, and compact nanoparticles have enhanced diffusion in crowded biological environments. These properties are providing unique opportunities to probe basic biological processes, cellular structures, and organismal physiology.
Introduction
Semiconductor nanocrystals are light-absorbing, light-emitting colloids that are the subject of widespread interest for diverse applications spanning bioimaging, light-emitting devices, solar energy conversion, photocatalysis, and quantum computing [1•,2]. They are particularly valuable as fluorescent bioimaging probes because their light emission intensity and stability far surpasses what is possible with organic dyes and fluorescent proteins. Thereby a protein or nucleic acid attached to a semiconductor nanocrystal can be imaged and tracked with single-molecule sensitivity for long durations in living cells and tissues, which has recently allowed the the discovery of a host of novel biological phenomena [3•].
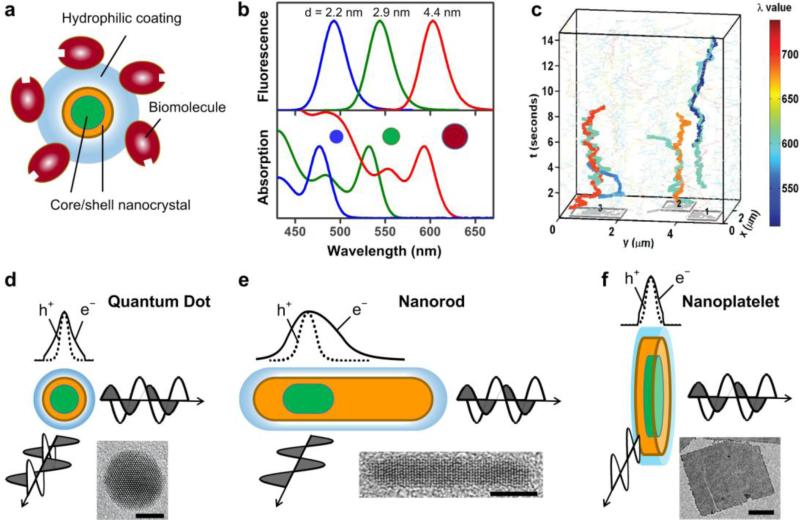
The prototypical semiconductor nanocrystals used for bioimaging are quantum dots (QDs), which are nearly spherical in shape with diameters between 2-10 nm [2]. Modern QD formulations are complex heterostructures depicted schematically in Figure 1a. The nanocrystal core, most often composed of CdSe, has size-tunable optical properties as shown in Figure 1b. This arises from the quantum confinement effect: charge carriers (negatively charged electrons, e-, and positively charged holes, h+) are confined in a crystal volume that is smaller than the intrinsic carrier sizes in the bulk semiconductor crystal, causing the absorption spectrum and fluorescence wavelength to depend on the crystal domain size. The core is coated with a crystalline shell (most often ZnS or CdS) to enhance fluorescence emission intensity and stability, coated with an outer organic layer that enables stable dispersion in biological media, and is attached to biological molecules to provide specific interactions with biological systems. The end result is a stable colloid with wavelength-tunable emission that can be monitored down to the level of single molecules (Figure 1c) [4••]. But over the last several years, QDs have evolved into a wide assortment of composite nanostructures with diverse combinations of shapes, sizes, materials, and dimensions of quantum confinement with novel photophysics that are driving biological explorations. This review highlights new nanocrystal architectures in development and useful optical properties that have emerged, and discusses implications for biological imaging and early-stage efforts to interface these materials with biological systems.
Figure 1.
Structural and optical properties of semiconductor nanocrystals for advanced bioimaging applications. (a) A prototypical core/shell quantum dot with hydrophilic coating bound to biomolecules such as antibodies or streptavidin. (b) Fluorescence emission (top) and optical absorption (bottom) spectra of different sizes of CdSe nanocrystals. (c) Narrow emission spectra enable multiplexed imaging: single-molecule traces show nanocrystals diffusing in the x-y plane of a cell membrane, and positions are monitored over time in the z-axis [4••]. (d-f) Schematic illustrations of nanocrystal structures show wavefunctions of electrons (e-) and holes (h+) and depictions of emission light polarization, as well as representative electron micrographs. Scale bars are 5 nm (d), 5 nm (e) and 100 nm (f). Electron micrographs are extracted with permission from references [1•] (d), [5] (e), and [6] (f).
Quantum Nanorods
QDs are quasi-spherical in shape and their charge carriers are confined almost equally in all dimensions, which results in isotropic photophysical properties (Figure 1d). Quantum nanorods (NRs), on the other hand, are elongated structures that are only strongly confined in two dimensions and have been of great interest for their physical and optical anisotropy [7]. The electron and hole are polarized along the long axis, leading to unique interactions with polarized light (Figure 1e): light absorbed and emitted perpendicular to the long axis is polarized linearly along the axis, and the degree of polarization can be tuned by the nanorod aspect ratio [8]. Light emitted parallel to the long axis (from the end) is plane-polarized like that of a QD. A “seeded growth” technology developed in 2007 substantially expanded the diversity of NRs by allowing the incorporation of multiple domains of materials within highly uniform composite structures [9,10]. By modulating the composition and structures of both the core “seed” and the outer rod “shell,” the electron and hole can be shaped to widely tune their locations and optical properties. Colocalizing the electron and hole yields bright fluorescence whereas spatial segregation of the charge carriers to different domains extends the excited state lifetime and can enhance energy transfer and charge transfer [11]. The crystal structure of the core can also be used to tune the number and size of the outer rods, allowing the production of single or multiple rod branches protruding from the core. Cubic lattices, for example, have 4 tetrahedral symmetric facets that yield tetrapod nanostructures with 4 rods elongating out from the core; each arm has anisotropic properties but the overall tetrahedral symmetry results in a net isotropy for each particle [10].
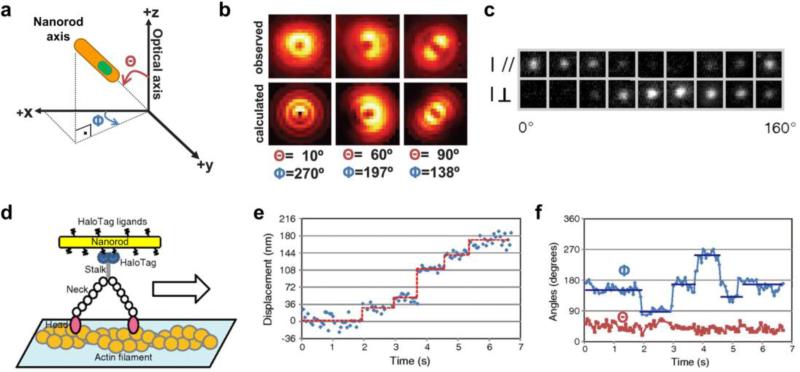
For bioimaging applications nanorods are intriguing due to their large extinction coefficients that yield exceptionally bright single-molecule emission [12,13], and their linearly polarized light emission that can be used to detect particle orientation by a variety of optical imaging techniques. For example with defocused light microscopy, the image is slightly defocused from the focal plane of interest; when light is emitted from an isolated polarized light source in the focal plane, the shape of the image spot is elongated with respect to the direction of polarization (see Figure 2a-b). Also fluorescence polarization microscopy can be used to detect the nanorod orientation using polarization filters (Figure 2c). With these technique, researchers have monitored the location and orientation of NR-tagged CD36 membrane receptors diffusing on cell membranes [14] and imaged and tracked the 3D orientation of a motor protein (myosin V) as it walked along an actin filament (Figure 2d-f) [15•]. By observing both location and orientation, a 90 degree rotation was resolved in association with each ~36 nm motor step, revealing how the two necks of the myosin motor swing back and forth during translocation.
Figure 2.
Imaging the orientation of nanorods at the single-molecule level. (a) Schematic diagram of nanorod orientation with respect to the optical axis (z) of the imaging system, defined by the axial (Θ) and azimuthal (Φ) angles. (b) Defocused imaging of nanorods with different orientations, showing nearly vertical, inclined, and parallel emission dipoles, both calculated and observed experimentally. (c) Imaging the intensity of a single nanorod in two polarization directions perpendicular to one another. (d) A nanorod attached to a myosin motor protein walking on an actin filament can be imaged in its displacement of its centroid (e) as well as its angular rotation with respect to the image plane through polarization microscopy (f). Panels a-b are from reference [16] and c-f are from reference [15•].
Quantum Nanoplatelets
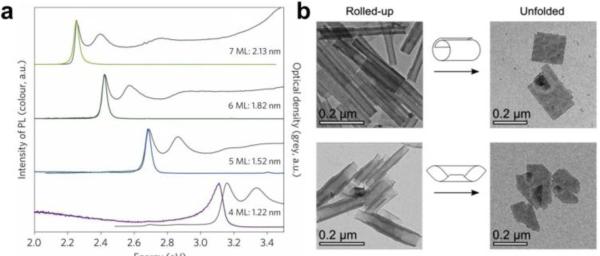
Nanoplatelets (NPs) are a new family of semiconductor nanocrystals that are nearly planar and atomically flat (see Figure 1f) [17,18], and are colloidal analogues of quantum well films that serve as lasing and light-harvesting layers in optoelectronic devices [19]. Recent synthetic advances have led to the growth of colloidal NPs composed of CdS, CdSe, or CdTe with atomic-layer precision in thickness, from 4 to 11 molecular monolayers, and with widely tunable lateral dimensions of ~10 nm to more than 100 nm and shapes resembling discs, flat squares, and long ribbons (although the homogeneity in lateral dimensions is usually poor) [17,18,20]. The atomic homogeneity in the confinement dimension is unprecedented in a nanostructure with this many atoms which, in conjunction with very small optical coupling with crystal lattice vibrations [21], results in exceptionally narrow excitation and emission bands (see Figure 3a) with enhanced capacity for detecting multiple spectrally distinct signals for multiplexed bioimaging applications.
Figure 3.
Cadmium selenide nanoplatelets. (a) Absorption spectra (black lines) and fluorescence emission spectra (colored lines) of four thicknesses of nanoplatelets (ML = monolayer of CdSe) [22••]. (b) Rolling of large nanoplatelets into cylindrical structures, as observed by electron microscopy [23].
Like carbon-based graphene sheets, thin NPs have a tendency to roll into cylindrical structures [23,24], a tunable surface-mediated effect that exemplifies mechanical flexibility not available in most colloidal crystalline materials (see Figure 3b). These materials are also unique because of their combination of high quantum efficiency of fluorescence and very short emission lifetime (~1 ns compared to ~20 ns for typical QDs) [22••], as well as surface-tunable optical properties. Together these properties suggest utility as scaffolds for novel nanostructures that can be modulated optically, photochemically, or optoelectrically. Recently it has become possible to cap these materials with insulating shells to yield high stability and bright fluorescence (~80% quantum efficiency) [6,25•] while maintaining planar morphology, setting the stage for their implementation in bioimaging applications.
Composition-Tunable Nanomaterials through Cation Exchange
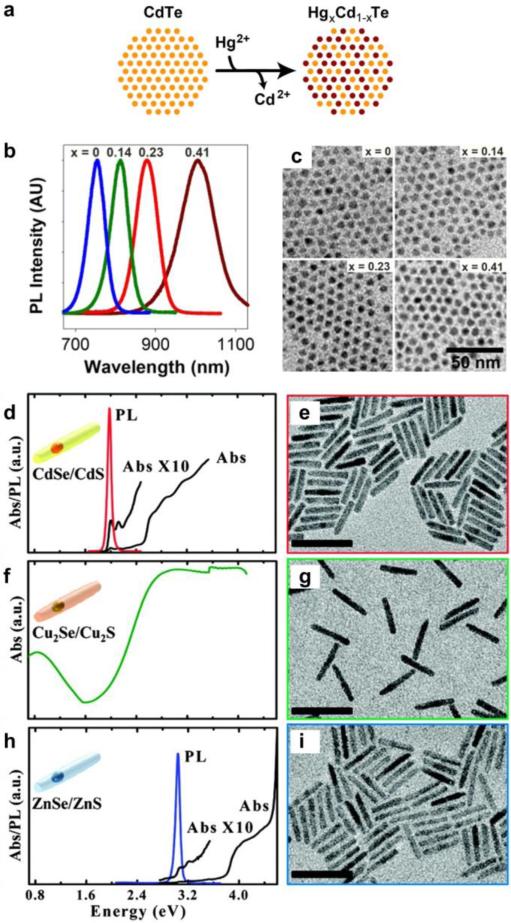
Recently the ability to create uniform nanostructures with a diverse range of materials has become possible through advances in cation exchange [26-28]. Under certain thermodynamically driven conditions, ionic semiconductors such as CdSe will rapidly exchange cations in the crystalline lattice and with soluble ions such as Ag+ or Cu+ to yield Ag2Se or Cu2Se. This process is highly efficient in nanocrystals compared with bulk materials due to high surface area to volume ratios [28], and the nanocrystal shape and crystal structure are maintained by the anionic lattice [29,30]. This technology has recently enabled the production of many nanocrystals with combinations of shapes and materials that could not have been synthesized using current de novo methods. For example, mercury chalcogenide materials (HgS, HgSe, and HgTe) are desirable for imaging applications due to their long wavelengths of emission and absorption in the near-infrared, but the inability to control colloidal synthesis reactions has limited their development. Instead by starting with well-defined CdS, CdSe, or CdTe cores, partial cation exchange with Hg2+ ions controllably leads to HgxCd1-xS, HgxCd1-xSe, and HgxCd1-xTe nanocrystal alloys with near-infrared emission (Figure 4a-c) [31,32•]. The wavelengths are tuned without altering the size, a major advantage compared with size-tuned QDs, because all colors have nearly identical diffusion coefficients in biological environments.
Figure 4.
Cation-exchange of semiconductor nanocrystals. (a) Cation exchange occurs through the controlled replacement of one ion with another in the lattice; here Cd2+ ions in CdTe quantum dots are replaced with Hg2+ to yield a ternary HgxCd1-xTe alloy. (b) Fluorescence spectra can be tuned due to differences in bandgap between CdTe and HgTe and (c) the resulting nanocrystals are nearly identical in size [32•]. (d-i) Sequential cation exchange of CdSe/CdS NRs (d-e) allows the production of Cu2Se/Cu2S (f-g) and ZnSe/ZnS (h-i) NRs with widely tunable optical spectra [34].
Cation exchange can also be applied to complex anisotropic and multi-domain materials to generate novel NPs (Cu2Te NPs → CdTe NPs) and multi-domain quantum nanorods that maintain their core/shell structure and size distribution after exchange [33•]. Using this method scientists have used CdSe/CdS NRs (Figure 4d-e) as templates to prepare Cu2Se/Cu2S (Figure 4f-g), ZnSe/ZnS (Figure 4h-i) [34], and PbSe/PbS core/shell NRs [29], three families of composite materials with widely different bandgaps from the ultraviolet to the near-infrared that could not have been readily prepared otherwise. A key finding is that while not all cations spontaneously exchange with a high degree of homogeneity and/or efficiency (e.g. CdSe → PbSe), sequential cation exchange processes (e.g. CdSe → Cu2Se → PbSe) have enabled highly homogeneous and nearly complete exchange, leading to vast diversity of materials including III-V materials that have been notoriously challenging to synthesize (GaP, GaAs, InP, InAs) using direct colloidal synthesis [35•].
Nonblinking Compact Nanocrystals
When viewed as isolated particles through a fluorescence microscope, semiconductor nanoparticles like QDs randomly flicker on and off. This “blinking” effect is thought to be the result of ionization or trapping of charge carriers at defects on the surface [36•]. This random loss of fluorescence signal is problematic for tracking biomolecules in cells, so many experimental studies have focused on modulating this effect through the nanocrystals structure. It has recently been shown that blinking can be almost entirely eliminated by growing a thick shell on the core material that prevents charge loss and surface trapping [37,38]. However this also results in a larger overall size that can detrimentally impact mobility in biological media. Therefore balancing the two effects is important. One alternative mechanism is to use a gradient alloy structure, rather than a distinct core/shell structure, which may have a improved ability to emit light when the particle is ionized [39,40]. Another method is to improve shell homogeneity by using very high temperature deposition [41••]. Semiconductor nanocrystals made of carbon [42] or silicon [43••] have been shown to be nonblinking emitters and also have exceptional photostability, but they cannot yet be widely tunability in their photophysical properties, and their broad emission bands and weak extinction coefficients have so far limited their utility. But the potential of these materials is intriguing because they are composed of materials that are expected to be nontoxic, but they also requiring UV-to blue excitation wavelengths, which itself can be cytotoxic.
Size- and Shape-Dependent Interactions in Biology
The interactions between nanoparticles and biomolecules, cellular structures, and tissues have been widely studied, leading to consistent trends dictated by nanoparticle size and shape [44•,45] However most studies have focused on nanoparticles substantially larger (~50-500 nm) than new semiconductor nanocrystals with confined dimensions on the order of 2-10 nm. Shape-tunable materials on this scale are expected to exhibit different effects as the dimensions and curvature of the particles match those of proteins that comprise biology and the size approaches the grain size of the aqueous medium, so that diffusive forces dominate. In terms of pure diffusion, these particles will not only translate, rotate, and vibrate more rapidly than their larger counterparts, but they will also have enhanced access to crowded macromolecular environments in the cellular cytoplasm, extracellular matrix, and neuronal synapses, and can potentially penetrate through fenestrated endothelia and nuclear pores [40,46-48•]. Importantly a size-sieving effect in crowded macromolecular spaces traps larger structures; the size cutoff is on the order of 20-40 nm for the cytoplasm of mammalian cells and some tissues [49], which is a primary rationale for a current concerted effort to minimize the size of QDs.
The need for compact nanoparticles does not preclude the use of high-aspect ratio materials for a variety of bioimaging applications. For example, by total mass, rod-like structures have higher diffuse faster than dot-shaped particles in porous media [50] and may have lower nonspecific cellular uptake [51], attributes that can substantially improve imaging on cell plasma membranes. Elongated structures can also align their long axes in directions of flow such that local patterns of shear in vasculature systems or in interstitial tissues can be directly imaged through fluorescence polarization. NRs and NPs also have higher surface areas than QDs to allow more efficient interactions between charge carriers and the local environment. Applications like sensing of local changes in electric fields, for example during the firing of a neuronal action potential [52•], would be more appropriate for a high surface area particle with separated charges compared with a conventional QD. Indeed the optical emission from NRs can be efficiently modulated by electric field strengths similar to those that arise from physiological transmembrane potentials (~100 kV/cm) [53], but more investigations are needed, especially at physiological temperatures. The ability to provide unique optical modulation tools to the rapidly growing field of optogenetics [54], for which cellular signals can be turned on and off and monitored with light using optically switchable proteins, could lead to major advances in our understanding of neurobiology and cellular signaling.
Conclusions
Semiconductor nanocrystals offer an immense degree of freedom to tune optical, electronic, and structural properties of dispersible colloidal materials that are on the same size scale as biological building blocks. The ability to interface these synthetic materials with living biological systems is now moving beyond simple fluorescence imaging applications to take advantage of the more sophisticated attributes of advanced nanocrystal designs. In the near term balancing the advantageous properties of high-aspect ratio materials with the need for an overall compact design is an outstanding challenge, requiring the construction of uniform, complex structures using fewer and fewer atoms. At the same time, it is critical to come to understand how these novel classes of materials impact cells and interact with proteins and other biomolecules so that their implementation can proceed beyond proof-of-concept studies to generate stable nanoscopic tools that minimally perturb the biological system under examination.
Highlights.
The spatial orientation of a nanorod can be monitored due to its linearly polarized light emission.
Nanoplatelets are flexible semiconductor sheets with atomically precise thicknesses.
Cation exchange has vastly expanded our ability to tune nanocrystal composition and optics.
Compact nanocrystals with continuous bright light emission are a major current focus research.
The small sizes of semiconductor nanocrystals may yield unexpected interactions with cells and proteins.
Acknowledgements
A.M.S. acknowledges funding from the National Institutes of Health (R00CA153914). S.M.N. acknowledges support from the National Institutes of Health (R01CA163256, RC2CA148265, and HHSN268201000043C).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Papers of particular interest, published within the period of review, have been highlighted as:
• Of special interest
•• Of outstanding interest
- 1•.Smith AM, Nie SM. Semiconductor nanocrystals: Structure, properties, and bandgap engineering. Acc Chem Res. 2010;43:190–200. doi: 10.1021/ar9001069. [Educational review of photophysical properties of quantum dots and related semiconductor nanostructures focused on bandgap engineering and wavefunction engineering.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kairdolf BA, Smith AM, Stokes TH, Wang MD, Young AN, Nie SM. Semiconductor quantum dots for bioimaging and biodiagnostic applications. Ann Rev Anal Chem, vol. 2013;6:143–162. doi: 10.1146/annurev-anchem-060908-155136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3•.Pinaud F, Clarke S, Sittner A, Dahan M. Probing cellular events, one quantum dot at a time. Nat Methods. 2010;7(4):275–285. doi: 10.1038/nmeth.1444. [Insightful review on how quantum dots are used for single molecule imaging in living cells, imaging and analysis methodologies and challenges, and key recent findings.] [DOI] [PubMed] [Google Scholar]
- 4••.Cutler PJ, Malik MD, Liu S, Byars JM, Lidke DS, Lidke KA. Multi-color quantum dot tracking using a high-speed hyperspectral line-scanning microscope. PLoS ONE. 2013;8(5):e64320. doi: 10.1371/journal.pone.0064320. [Demonstration of the massive multiplexing capabilities of semiconductor nanocrystals for single-molecule imaging applications, using specrally-resolved fluorescence microscopy to image 8 spectrally-resolved quantum dot colors with 10 nm precision near video rate.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manna L, Scher EC, Alivisatos AP. Synthesis of soluble and processable rod-, arrow-, teardrop-, and tetrapod-shaped CdSe nanocrystals. J Am Chem Soc. 2000;122(51):12700–12706. [Google Scholar]
- 6.Mahler B, Nadal B, Bouet C, Patriarche G, Dubertret B. Core/shell colloidal semiconductor nanoplatelets. J Am Chem Soc. 2012;134(45):18591–18598. doi: 10.1021/ja307944d. [DOI] [PubMed] [Google Scholar]
- 7.Peng XG, Manna L, Yang WD, Wickham J, Scher E, Kadavanich A, Alivisatos AP. Shape control of CdSe nanocrystals. Nature. 2000;404(6773):59–61. doi: 10.1038/35003535. [DOI] [PubMed] [Google Scholar]
- 8.Hu JT, Li LS, Yang WD, Manna L, Wang LW, Alivisatos AP. Linearly polarized emission from colloidal semiconductor quantum rods. Science. 2001;292(5524):2060–2063. doi: 10.1126/science.1060810. [DOI] [PubMed] [Google Scholar]
- 9.Talapin DV, Nelson JH, Shevchenko EV, Aloni S, Sadtler B, Alivisatos AP. Seeded growth of highly luminescent CdSe/CdS nanoheterostructures with rod and tetrapod morphologies. Nano Lett. 2007;7(10):2951–2959. doi: 10.1021/nl072003g. [DOI] [PubMed] [Google Scholar]
- 10.Carbone L, Nobile C, De Giorg M, Sala FD, Morello G, Pompa P, Hytch M, Snoeck E, Fiore A, Franchini IR, Nadasan M, et al. Synthesis and micrometer-scale assembly of colloidal cdse/cds nanorods prepared by a seeded growth approach. Nano Lett. 2007;7(10):2942–2950. doi: 10.1021/nl0717661. [DOI] [PubMed] [Google Scholar]
- 11.Dorfs D, Salant A, Popov I, Banin U. ZnSe quantum dots within CdS nanorods: A seeded-growth type-ii system. Small. 2008;4(9):1319–1323. doi: 10.1002/smll.200800084. [DOI] [PubMed] [Google Scholar]
- 12.Fu A, Gu WW, Boussert B, Koski K, Gerion D, Manna L, Gros ML, Larabell CA, Alivisatos AP. Semiconductor quantum rods as single molecule fluorescent biological labels. Nano Lett. 2007;7(1):179–182. doi: 10.1021/nl0626434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yong KT, Qian J, Roy I, Lee HH, Bergey EJ, Tramposch KM, He SL, Swihart MT, Maitra A, Prasad PN. Quantum rod bioconjugates as targeted probes for confocal and two-photon fluorescence imaging of cancer cells. Nano Lett. 2007;7(3):761–765. doi: 10.1021/nl063031m. [DOI] [PubMed] [Google Scholar]
- 14.Watanabe TM, Fujii F, Jin T, Umemoto E, Miyasaka M, Fujita H, Yanagida T. Four- dimensional spatial nanometry of single particles in living cells using polarized quantum rods. Biophys J. 2013;105(3):555–564. doi: 10.1016/j.bpj.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15•.Ohmachi M, Komori Y, Iwane AH, Fujii F, Jin T, Yanagida T. Fluorescence microscopy for simultaneous observation of 3D orientation and movement and its application to quantum rod-tagged myosin v. Proc Natl Acad Sci - USA. 2012;109(14):5294–5298. doi: 10.1073/pnas.1118472109. [Single-molecule imaging and tracking with orientation measurements using highly elongated CdSe/CdS nanorods to monitor the motion of myosin V motor proteins on actin filaments.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Toprak E, Enderlein J, Syed S, McKinney SA, Petschek RG, Ha TJ, Goldman YE, Selvin PR. Defocused orientation and position imaging (DOPI) of myosin V. Proc Natl Acad Sci USA. 2006;103(17):6495–6499. doi: 10.1073/pnas.0507134103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ithurria S, Dubertret B. Quasi 2D colloidal cdse platelets with thicknesses controlled at the atomic level. J Am Chem Soc. 2008;130(49):16504–16505. doi: 10.1021/ja807724e. [DOI] [PubMed] [Google Scholar]
- 18.Son JS, Wen XD, Joo J, Chae J, Baek SI, Park K, Kim JH, An K, Yu JH, Kwon SG, Choi SH, et al. Large-scale soft colloidal template synthesis of 1.4 nm thick cdse nanosheets. Angew Chem Int Ed. 2009;48(37):6861–6864. doi: 10.1002/anie.200902791. [DOI] [PubMed] [Google Scholar]
- 19.Pelton M, Ithurria S, Schaller RD, Dolzhnikov DS, Talapin DV. Carrier cooling in colloidal quantum wells. Nano Lett. 2012;12:6158–6163. doi: 10.1021/nl302986y. [DOI] [PubMed] [Google Scholar]
- 20.Joo J, Son JS, Kwon SG, Yu JH, Hyeon T. Low-temperature solution-phase synthesis of quantum well structured CdSe nanoribbons. J Am Chem Soc. 2006;128:5632–5633. doi: 10.1021/ja0601686. [DOI] [PubMed] [Google Scholar]
- 21.Achtstein AW, Schliwa A, Prudnikau A, Hardzei M, Artemyev MV, Thomsen C, Woggon U. Electronic structure and exciton-phonon interaction in two-dimensional colloidal CdSe nanosheets. Nano Lett. 2012;12(6):3151–3157. doi: 10.1021/nl301071n. [DOI] [PubMed] [Google Scholar]
- 22••.Ithurria S, Tessier MD, Mahler B, Lobo R, Dubertret B, Efros A. Colloidal nanoplatelets with two-dimensional electronic structure. Nat Mater. 2011;10(12):936–941. doi: 10.1038/nmat3145. [Synthesis and photophysical properties of nanoplatelets with a wide range of dimensions and compositions demonstrating giant transition oscillator strengths.] [DOI] [PubMed] [Google Scholar]
- 23.Bouet C, Mahler B, Nadal B, Abecassis B, Tessier MD, Ithurria S, Xu XZ, Dubertret B. Two-dimensional growth of CdSe nanocrystals, from nanoplatelets to nanosheets. Chem Mater. 2013;25(4):639–645. [Google Scholar]
- 24.Katsnelson MI, Fasolino A. Graphene as a prototype crystalline membrane. Acc Chem Res. 2013;46(1):97–105. doi: 10.1021/ar300117m. [DOI] [PubMed] [Google Scholar]
- 25•.Ithurria S, Talapin DV. Colloidal atomic layer deposition (c-ALD) using self-limiting reactions at nanocrystal surface coupled to phase transfer between polar and nonpolar media. J Am Chem Soc. 2012;134(45):18585–18590. doi: 10.1021/ja308088d. [A new shell growth method that enables precise, single layer deposition that will have important implications in the drive toward compact, precise nanocrystals.] [DOI] [PubMed] [Google Scholar]
- 26.Robinson RD, Sadtler B, Demchenko DO, Erdonmez CK, Wang LW, Alivisatos AP. Spontaneous superlattice formation in nanorods through partial cation exchange. Science. 2007;317(5836):355–358. doi: 10.1126/science.1142593. [DOI] [PubMed] [Google Scholar]
- 27.Camargo PHC, Lee YH, Jeong U, Zou Z, Xia Y. Cation exchange: A simple and versatile route to inorganic colloidal spheres with the same size but different compositions and properties. Langmuir. 2007;23:2985–2992. doi: 10.1021/la0632070. [DOI] [PubMed] [Google Scholar]
- 28.Son DH, Hughes SM, Yin YD, Alivisatos AP. Cation exchange reactions in ionic nanocrystals. Science. 2004;306:1009–1012. doi: 10.1126/science.1103755. [DOI] [PubMed] [Google Scholar]
- 29.Jain PK, Amirav L, Aloni S, Alivisatos AP. Nanoheterostructure cation exchange: Anionic framework conservation. J Am Chem Soc. 2010;132(29):9997–9999. doi: 10.1021/ja104126u. [DOI] [PubMed] [Google Scholar]
- 30.Li H, Zanella M, Genovese A, Povia M, Falqui A, Giannini C, Manna L. Sequential cation exchange in nanocrystals: Preservation of crystal phase and formation of metastable phases. Nano Lett. 2011;11:4964–4970. doi: 10.1021/nl202927a. [DOI] [PubMed] [Google Scholar]
- 31.Smith AM, Nie SM. Compact quantum dots for single-molecule imaging in living cells. J Vis Exp. 2012;68:e4236. doi: 10.3791/4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32•.Smith AM, Nie S. Bright and compact alloyed quantum dots with broadly tunable near-infrared absorption and fluorescence spectra through mercury cation exchange. J Am Chem Soc. 2011;133(1):24–26. doi: 10.1021/ja108482a. [The use of a controlled cation exchange process to generate alloys between cadmium-based semiconductors and mercury-based semiconductors to tune the optical properties to the near-infrared.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li HB, Brescia R, Povia M, Prato M, Bertoni G, Manna L, Moreels I. Synthesis of uniform disk-shaped copper telluride nanocrystals and cation exchange to cadmium telluride quantum disks with stable red emission. J Am Chem Soc. 2013;135(33):12270–12278. doi: 10.1021/ja404694k. [DOI] [PubMed] [Google Scholar]
- 34.Li H, Brescia R, Krahne R, Bertoni G, Alcocer MJP, D'Andrea C, Scotognella F, Tassone F, Zanella M, De Giorgi M, Manna L. Blue-UV-emitting ZnSe(dot)/ZnS(rod) core/shell nanocrystals prepared from CdSe/CdS nanocrystals by sequential cation exchange. Acs Nano. 2012;6(2):1637–1647. doi: 10.1021/nn204601n. [DOI] [PubMed] [Google Scholar]
- 35•.Beberwyck BJ, Alivisatos AP. Ion exchange synthesis of III-V nanocrystals. J Am Chem Soc. 2012;134(49):19977–19980. doi: 10.1021/ja309416c. [Cation exchange provides a novel route to homogeneous III-V semiconductors that are very challenging to synthesize with narrow size dispersios otherwise.] [DOI] [PubMed] [Google Scholar]
- 36•.Galland C, Ghosh Y, Steinbruck A, Sykora M, Hollingsworth JA, Klimov VI, Htoon H. Two types of luminescence blinking revealed by spectroelectrochemistry of single quantum dots. Nature. 2011;479(7372):203–U275. doi: 10.1038/nature10569. [Two discrete mechanisms of fluorescence blinking/flickering are identified using single-molecule fluorescence in conjunction with electrochemical charging.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mahler B, Spinicelli P, Buil S, Quelin X, Hermier JP, Dubertret B. Towards non-blinking colloidal quantum dots. Nat Mater. 2008;2008(7) doi: 10.1038/nmat2222. [DOI] [PubMed] [Google Scholar]
- 38.Chen YF, Vela J, Htoon H, Casson JL, Werder DJ, Bussian DA, Klimov VI, Hollingsworth JA. “Giant” multishell cdse nanocrystal quantum dots with suppressed blinking. J Am Chem Soc. 2008;130(15):5026–5027. doi: 10.1021/ja711379k. [DOI] [PubMed] [Google Scholar]
- 39.Wang XY, Ren XF, Kahen K, Hahn MA, Rajeswaran M, Maccagnano-Zacher S, Silcox J, Cragg GE, Efros AL, Krauss TD. Non-blinking semiconductor nanocrystals. Nature. 2009;459(7247):686–689. doi: 10.1038/nature08072. [DOI] [PubMed] [Google Scholar]
- 40.Smith AM, Nie SM. Next-generation quantum dots. Nat Biotechnol. 2009;27:732–733. doi: 10.1038/nbt0809-732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41••.Chen O, Zhao J, Chauhan VP, Cui J, Wong C, Harris DK, Wei H, Han HS, Fukumura D, Jain RK, Bawendi MG. Compact high-quality CdSe-CdS core-shell nanocrystals with narrow emission linewidths and suppressed blinking. Nat Mater. 2013;12(5):445–451. doi: 10.1038/nmat3539. [Relatively small core/shell CdSe/CdS quantum dots are prepared with exceptionally narrow emission peaks and dramatically reduced blinking. The key finding here is that a slow, high-temperature shell deposition mechanism produces very high quality shells, which is a major step forward toward compact and stable emitters.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mochalin VN, Shenderova O, Ho D, Gogotsi Y. The properties and applications of nanodiamonds. Nat Nanotech. 2012;7(1):11–23. doi: 10.1038/nnano.2011.209. [DOI] [PubMed] [Google Scholar]
- 43••.Nishimura H, Ritchie K, Kasai RS, Goto M, Morone N, Sugimura H, Tanaka K, Sase I, Yoshimura A, Nakano Y, Fujiwara TK, et al. Biocompatible fluorescent silicon nanocrystals for single-molecule tracking and fluorescence imaging. J Cell Biol. 2013;202(6):967–983. doi: 10.1083/jcb.201301053. [Extremely small Si nanocrystals with stable emission and no blinking are demonstrated to be effective probes for single-molecule imaging studies.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44•.Walkey CD, Chan WCW. Understanding and controlling the interaction of nanomaterials with proteins in a physiological environment. Chem Soc Rev. 2012;41(7):2780–2799. doi: 10.1039/c1cs15233e. [Comprehensive review of the complex nature of interactions between nanoparticles and proteins in cells and biological fluids.] [DOI] [PubMed] [Google Scholar]
- 45.Jiang W, Kim BYS, Rutka JT, Chan WCW. Nanoparticle-mediated cellular response is size-dependent. Nat Nanotech. 2008;3:145–150. doi: 10.1038/nnano.2008.30. [DOI] [PubMed] [Google Scholar]
- 46.Choi HS, Liu W, Misra P, Tanaka E, Zimmer JP, Ipe BI, Bawendi MG, Frangioni JV. Renal clearance of quantum dots. Nat Biotechnol. 2007;25(10):1165–1170. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith AM, Wen MM, Nie S. Imaging dynamic cellular events with quantum dots. Biochemist. 2010;32(3):12. [PMC free article] [PubMed] [Google Scholar]
- 48•.Groc L, Lafourcade M, Heine M, Renner M, Racine V, Sibarita JB, Lounis B, Choquet D, Cognet L. Surface trafficking of neurotransmitter receptor: Comparison between single-molecule/quantum dot strategies. J Neurosci. 2007;27(46):12433–12437. doi: 10.1523/JNEUROSCI.3349-07.2007. [Insightful review on discoveries in neuron biology enabled by fluorescence microscopy and the impact of the physical dimensions of fluorescent probes.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Verkman A. Solute and macromolecule diffusion in cellular aqueous compartments. Trends Biochem Sci. 2002;27(1):27–33. doi: 10.1016/s0968-0004(01)02003-5. [DOI] [PubMed] [Google Scholar]
- 50.Pluen A, Netti PA, Jain RK, Berk DA. Diffusion of macromolecules in agarose gels: Comparison of linear and globular configurations. Biophys J. 1999;77:542–552. doi: 10.1016/S0006-3495(99)76911-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barua S, Yoo JW, Kolhar P, Wakankar A, Gokarn YR, Mitragotri S. Particle shape enhances specificity of antibody-displaying nanoparticles. Proc Natl Acad Sci USA. 2013;110(9):3270–3275. doi: 10.1073/pnas.1216893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52•.Marshall JD, Schnitzer MJ. Optical strategies for sensing neuronal voltage using quantum dots and other semiconductor nanocrystals. ACS Nano. 2013;7(5):4601–4609. doi: 10.1021/nn401410k. [Theoretical study on optimizing semiconductor nanocrysatls for sensing neuronal action potentials.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rothenberg E, Kazes M, Shaviv E, Banin U. Electric field induced switching of the fluorescence of single semiconductor quantum rods. Nano Lett. 2005;5(8):1581–1586. doi: 10.1021/nl051007n. [DOI] [PubMed] [Google Scholar]
- 55.Fenno L, Yizhar O, Deisseroth K. The development and application of optogenetics. Annu Rev Neurosci. 2011;34:389–412. doi: 10.1146/annurev-neuro-061010-113817. [DOI] [PMC free article] [PubMed] [Google Scholar]