Abstract
AIM: To evaluate laparoscopic re-sleeve gastrectomy as a treatment of weight regain after Sleeve.
METHODS: Laparoscopic sleeve gastrectomy is a common bariatric procedure. Weight regain after long-term follow-up is reported. Patients were considered for laparoscopic re-sleeve gastrectomy when we observed progressive weight regain and persistence of comorbidities associated with evidence of dilated gastric fundus and/or antrum on upper gastro-intestinal series. Follow-up visits were scheduled at 1, 3, 6 and 12 mo after surgery and every 6 mo thereafter. Measures of change from baseline at different times were analyzed with the paired samples t test.
RESULTS: We observed progressive weight regain after sleeve in 11 of the 201 patients (5.4%) who had a mean follow-up of 21.1 ± 9.7 mo (range 6-57 mo). Three patients started to regain weight after 6 mo following Sleeve, 5 patients after 12 mo, 3 patients after 18 m. Re-sleeve gastrectomy was always performed by laparoscopy. The mean time of intervention was 55.8 ± 29.1 min. In all cases, neither intra-operative nor post-operative complications occurred. After 1 year follow-up we observed a significant (P < 0.05) mean body mass index reduction (-6.6 ± 2.7 kg/m2) and mean % excess weight loss (%EWL) increase (+31.0% ± 15.8%). An important reduction of antihypertensive drugs and hypoglycemic agents was observed after re-sleeve in those patients affected by hypertension and diabetes. Joint problems and sleep apnea syndrome improved in all 11 patients.
CONCLUSION: Laparoscopic re-sleeve gastrectomy is a feasible and effective intervention to correct weight regain after sleeve.
Keywords: Obesity, Bariatric surgery, Laparoscopic surgery, Stomach stapling, Gastrectomy, Surgery, Repeat
Core tip: Laparoscopic sleeve gastrectomy is gaining an important role in bariatric surgery because it may have similar results to gastric by-pass and duodenal switch, without problems of malabsorption and digestive anastomosis. However, weight regain after a long-term follow-up is reported. In this paper we show that re-sleeve gastrectomy is a valid and effective intervention to correct weight regain after sleeve.
INTRODUCTION
Laparoscopic sleeve gastrectomy (LSG) is a bariatric procedure that may allow similar results to Roux-en-Y gastric bypass (RYGB) and duodenal switch (DS), without problem of malabsorption[1-4]. Several studies, reporting large series, show that LSG is safe and effective in terms of weight loss[5-8] and improvement of comorbidities[9-11] in the first post-operative years. For these reasons and for the fact that it does not imply any digestive anastomosis, LSG has become very popular among surgeons. It appears that there are less complications after LSG compared to those seen after RYGB[7,12-14]. The most worrisome complication of LSG is a leak of the long suture line, reported in 1%-7% of patients[6]. Despite its wide diffusion, LSG’s long-term weight loss data are not uniform. Some authors report a regain of weight after LSG[15-17]. This data could be in line with the fact that weight regain has been found after all bariatric operations[18,19]. One of the main advantages of LSG is that it may also work as a bridge procedure before a laparoscopic DS[20-22] or a laparoscopic RYGB[23], in case of insufficient weight loss or progressive weight regain. Recently, some authors suggested treating the inadequate weight loss, resulting from a large stomach or neofundus after LSG, with laparoscopic re-sleeve gastrectomy (LRSG)[24-27]. In this paper we present our series of 11 LRSG procedures with 1 year follow up.
MATERIALS AND METHODS
Patient characteristics
All patients who underwent LSG in our institution from December 2007 to September 2011 were considered for LRSG when we observed progressive weight regain and persistence of comorbidities, associated with evidence of persistence of gastric fundus and/or antrum on upper gastro-intestinal series (Figure 1B). No patients were considered for RYGB or DS after failed LSG because, in accordance with patients, we wanted to maintain the advantages of Sleeve in terms of avoiding post-operative malabsorption and in terms of preserving the possibility to easily explore the gastro-intestinal tract in the necessity of diagnostic or operative endoscopy. Institutional Review Board approval was obtained for the present study and all patients gave their informed consent prior to surgery. The presence of comorbidities, such as joint problems, was quantified according to anamnesis and use of specific medications before and after surgery. The presence of Diabetes was quantified by pre and post-operative fasting blood glucose (FBG) and glycosylated hemoglobin (HbA1c). The presence of blood hypertension was quantified by systolic and diastolic pressure before and after surgery. The presence of sleep apnea was quantified by sleep studies before surgery and post-operative resolution by discontinuation of the use of CPAP (Continuous Positive Airway Pressure) mask.
Figure 1.
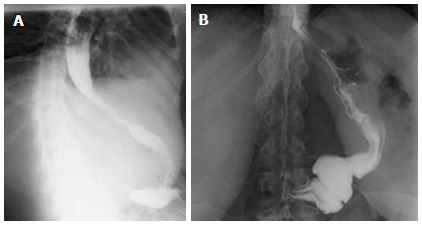
Comparison of upper gastro-intestinal series in the same patient on post-operative day 2 after sleeve gastrectomy (A) and one year later (B). After one year we see a dilated stomach (B) that allows weight regain.
Surgical technique
Surgery was performed according to our usual technique for laparoscopic Sleeve Gastrectomy. A Veress needle was used to accomplish pneumoperitoneum. The first trocar (12 mm, Endopath XCEL, Ethicon Endo-surgery, Cincinnati, OH, United States) was placed in the left subcostal space. The second and the third trocars (5 mm, Endopath XCEL, Ethicon Endo-surgery, Cincinnati, OH, United States) were placed in the subxiphoid space and in the right flank respectively. The last trocar (15 mm, Endopath XCEL, Ethicon Endo-surgery, Cincinnati, OH, United States) was placed above the umbilicus. The stomach was separated from the gastrocolic ligament and gastrosplenic ligament by Harmonic ACE 5 mm (Ethicon Endo-Surgery, Cincinnati, OH, United States). The left diaphragmatic crus was freed. The excessive part of the stomach was cut over a 12.7 mm (38 Fr) Gastric Calibration Tube (Ethicon Endo-Surgery, Cincinnati, OH, United States) starting from 6 cm proximal to the pylorus and proceeding up toward the diaphragmatic left crus. An articulating endoscopic linear cutter (Echelon Flex 60, Ethicon Endo-Surgery, Cincinnati, OHIO, United States) with 4.1 mm (Green) and 3.8 mm (Gold), 6 row cartridges (Endoscopic Linear Cutter Reloads, Ethicon Endosurgery, Cincinnati, OH, United States), was used to staple the stomach. In LRSG we rarely used 3.5 mm (Blue) cartridges because of the tissue’s density after the prior stapling. Running suture with PDS 3/0 (MIC55E, PDS*II, Ethicon Endo-Clip Suture, Cincinnati, OH, United States) was used to reinforce the stapled line. Surgical technique was the same in LSG and LRSG. The same calibration tube was used for all the patients in LSG and LRSG.
Post-operative management
Patients were started on an oral fluid diet on post-operative day 3 after upper gastro-intestinal series had shown no leak. Patients were discharged on day 5 if no post-operative complications occurred. Follow-up visits were scheduled at 1, 3, 6 and 12 mo after surgery and every 6 mo thereafter. Data were entered into a prospectively held database including age, gender, body mass index (BMI), excess of weight (EW), % of excess weight loss (%EWL), comorbidities before and after surgery, post-operative complications.
Statistical analysis
Data were obtained by review of the prospectively maintained database. Quantitative variables were reported as mean and standard deviation (SD); qualitative variables were described as number and percentages. Measures of change from baseline at 3, 6, 12 mo after surgery were analyzed with the paired t test. Statistical significance was set at P ≤ 0.05. All statistical analyses were performed with the Statistical Product and Service Solutions (SPSS) software package (version 19, SPSS-IBM, Chicago, IL, United States).
RESULTS
Patients characteristics
From December 2007 to September 2011, 201 patients underwent LSG at our Institution. We observed progressive weight regain in 11 patients (5.4%). Three patients started to regain weight after 6 mo post-LSG, 5 patients after 12 mo, 3 patients after 18 mo. An upper gastro-intestinal series showed gastric dilatation in all 11 patients. Three patients (27.3%) had another bariatric surgery prior to LSG: 2 patients had an adjustable gastric band (AGB) already removed before LSG and one patient underwent surgical intervention of laparoscopic Band removal and LSG at the same time. The AGB was removed because of dysfunction associated with weight regain.
Four patients (45.5%) were affected by at least 1 comorbidity (Table 1). Two of them (a female with BMI = 54.1 kg/m2 and a male with BMI = 48.5 kg/m2) were affected by blood hypertension, type II diabetes and joint problems. A third patient, a female with BMI = 52.7 kg/m2, was affected by blood hypertension and joint problems. A fourth patient, a male with BMI = 43.3 kg/m2, was affected by sleep apnea syndrome. In all patients, pre-operative blood hypertension was well controlled by drugs (mean systolic 123.3 ± 2.9 mmHg and mean diastolic 78.3 ± 2.9 mmHg). Two patients were in therapy with combination diuretics and ACE inhibitors; one patients with ACE inhibitors alone. Regarding the treatment of diabetes, the two patients affected used oral hypoglycemic agents. The average FBG before surgery was 147.5 ± 3.5 mg/dL and HbA1c averaged 6.9% ± 0.1%. The mean age of the patients (3 males and 8 females) was 40.6 ± 10.2 years (Table 1).
Table 1.
Pre-operative patients’ characteristics n (%)
| Characteristics | Value, mean ± SD n = 11 |
| Age (yr) | 40.6 ± 10.2 |
| Height (m) | 1.60 ± 0.1 |
| Ideal body weight (kg) | 57.4 ± 8.9 |
| Excess body weight (kg) | 59.3 ± 16 |
| Excess body weight (%) | 104.2 ± 25.5 |
| Body mass index (kg/m2) | 45.2 ± 5.6 |
| Gender | |
| Male | 3 (27.3) |
| Female | 8 (72.7) |
| Comorbidities | |
| At least 1 comorbidity | 4 (36.4) |
| Blood hypertension | 3 (27.3) |
| Type 2 diabetes mellitus | 2 (18.2) |
| Sleep apnea syndrome | 1 (9.1) |
| Joint problems | 3 (27.3) |
Findings after LSG
Before LSG, mean absolute weight was 116.4 ± 21.5 kg, mean EW was 59.3 ± 16 kg and mean BMI was 45.2 ± 5.6 kg/m2 (Table 1). One patient developed a high gastric leak after LSG and underwent a second operation six days later. She was a female with BMI = 41 kg/m2 and no comorbidities. She had surgical revision of the gastric staple line without resewing it. A perigastric abscess was drained and a drain tube was left in place. The leak resolved in 15 d and the patient was discharged on day 18. BMI and %EWL variations after LSG are collected in Figure 2. After an initial decrease, mean BMI start to increase after 6 mo.
Figure 2.
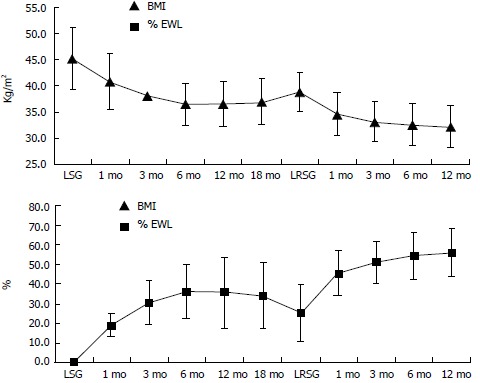
Body mass index and % of excess weight loss before and after laparoscopic sleeve gastrectomy and laparoscopic re-sleeve gastrectomy. Data are expressed as means and standard deviations. BMI decrease and %EWL increases for few months (mo) after LSG, then patients start to regain weight. After LRSG, BMI decreases and %EWL increases again. BMI: Body mass index; %EWL: % of excess weight loss; LSG: Laparoscopic sleeve gastrectomy; LRSG: Laparoscopic re-sleeve gastrectomy.
After LSG, systolic and diastolic pressure values did not differ significantly to prior LSG; however a reduction in requirement of antihypertensive drugs was observed. One patient suspended therapy and the others 2 reduced therapy. After LSG, FBG and HbA1c showed an important decrease (respectively 105.5 ± 28.9 mg/dL and 6.2% ± 0.5%). One of two patients (50%) suspended oral hypoglycemic agents. Joint problems and sleep apnea syndrome improved in all (100%).
Findings after LRSG
LRSG was performed at a mean interval of 21.1 ± 9.7 mo after LSG. The mean BMI before LRSG was 38.9 ± 3.8 kg/m2 and the mean %EWL was 25.3% ± 14.2% (Figure 2). LRSG was completed laparoscopically in all cases and no intra-operative or post-operative complications occurred. The mean time of intervention was 55.8 ± 29.1 min. The mean operative time for LSG in the same patients was longer: 65.4 ± 17.4 min. This finding could be related to the fact that when we perform LRSG the stomach is already dissected and prepared. We only cut off the exceeding part of the stomach over the boogie. No significative blood loss occurred either in LSG or in LRSG. No patient showed leakage from the stapled line at upper gastro-intestinal series scheduled on day 2. All patients resumed an oral liquid diet on day 3 and they were discharged from the hospital on day 5 after LRSG. At 1, 6, 12 mo after LRSG the BMI progressively decreased and %EWL increased in each patient. As shown in Table 2, after 1 year of follow-up the mean BMI significantly decreased from 38.9 ± 3.4 kg/m2 to 32.2 ± 3.9 kg/m2 (P < 0.05) and the %EWL significantly increased from 25.3 ± 14.2 to 56.3 ± 12.4 (P < 0.05).
Table 2.
Weight loss at 12 mo after laparoscopic re-sleeve gastrectomy
| Value (n = 11) before LRSG mean ± SD | After LRSG mean ± SD | Mean change (SD) | 95%CI | P value | |
| Absolute weight (kg) | 100.3 ± 17.5 | 82.9 ± 14.7 | -17.4 (± 7.8) | -12.0--22.6 | < 0.001 |
| BMI (kg/m2) | 38.9 ± 3.8 | 32.2 ± 3.9 | -6.6 (± 2.7) | -4.8--8.5 | < 0.001 |
| Excess weight (kg) | 43.2 ± 10.1 | 25.8 ± 9.5 | -17.4 (± 7.8) | -12.0--22.6 | < 0.001 |
| Excess weight (%) | 75.4 ± 11.6 | 45.4 ± 16.5 | -29.2 (± 12.4) | -20.8--37.6 | < 0.001 |
| Excess weight loss (%) | 25.3 ± 14.2 | 56.8 ± 12.4 | +31.0 (± 15.8) | 41.6-20.4 | < 0.001 |
P value is calculated with paired t test assessing change from LRSG. BMI: Body mass index; LRSG: Laparoscopic re-sleeve gastrectomy.
After LRSG only 1 of 3 patients continued to use antihypertensive drugs and only 1 of 2 continued to use oral hypoglycemic agents. Mean systolic pressure was 121.6 ± 1.5 mmHg and mean diastolic was 78 ± 2 mmHg. Mean FBG was 98.5 ± 16.2 mg/dL and mean HbA1c was 6.1 ± 0.6 %. Neither joint problems nor sleep apnea were noted after LRSG.
DISCUSSION
LSG is gaining an important role in bariatric surgery because it may have similar results to RYGB and DS, without malabsorbitive problems and digestive anastomosis[1-3]. Moreover, after LSG there are no problems exploring the upper gastro-intestinal tract. Some authors have noted a weight regain after LSG[15-17]. In our series we observed weight regain in 11 out of 201 patients (5.4%), who had a mean follow-up of 21.1 ± 9.7 mo (range 6-57 mo). Three patients started to regain weight after 6 mo post-LSG, 5 patients after 12 mo, 3 patients after 18 mo. An upper gastro-intestinal series was performed and showed a dilatation of the antrum and/or gastric fundus in all the 11 patients (Figure 1). The causes of gastric dilatation are not clear[27]. It could be related to a technical problem or to a natural process of stomach tissue dilatation. The main technical cause for a dilated antrum might be a dissection started farther than 6 cm from the pylorus. In these cases the patients will regain weight after a few months. The main cause for a dilated fundus might be a dissection farther than 1 cm to the left of the esophagus. A complete dissection of the gastric fundus is difficult when the patient underwent prior AGB placement or removal. Other causes of stomach tissue dilatation after LSG, besides technical problems, could be related to patient’s psychological problems or negligence in following the post-surgical diet recommendations. We believe that these factors, technical and not, are often both involved in the process of weight regain after LSG. For example, an incomplete section of the gastric fundus will not decrease the secretion of ghrelin[27], which can explain the incapacity of the patient to follow diet recommendations, therefore permitting stomach dilatation and weight regain.
In order to resume weight loss in patients for which LSG failed, there are few surgical options. LSG can be converted to RYGB or DS[20-23], or a LRSG can be performed[24-27]. In our series, no patients underwent RYGB or DS because we wanted to maintain the advantages of LSG in terms of avoiding malabsorption or gastro-intestinal anastomosis, and of preserving the possibility to easily explore the gastro-intestinal tract in the necessity of diagnostic or operative endoscopy. Our patients underwent LRSG over the same calibration tube of LSG: 12.7 cm boogie (38 Fr). After LSG, the gastric tissue around the stapled line is denser because of the scar’s healing and remodeling. For this reason, we used only Green (4.1 mm) and Gold (3.8 mm) cartridges to dissect the stomach in the LRSG. An oversewing suture of the stapled line was always performed.
LRSG was feasible in all patients in our series. Although we had neither intra-operative nor post-operative complications, the main post-operative problem after LRSG may be the same of LSG: leakage from the long stapled line. LRSG was effective for weight loss in all patients (Table 2). After one year follow-up we noted a significant decrease in mean BMI from 38.9 ± 3.4 kg/m2 to 32.2 ± 3.9 kg/m2 (P < 0.05) and a significant increase in mean %EWL from 25.3 ± 14.2 to 56.3 ± 12.4 (P < 0.05) (Figure 2). Although median BMI was > 30 kg/m2 after one year, we noted a significative trend in decreasing weight after LRSG. We need a longer follow-up to determine if the treatment is really effective, but these preliminary data are encouraging in not substituting LSG with a malabsorbitive intervention, loosing the advantages of LSG, especially in terms of quality of life. If weight regain will still occur after a longer follow-up post-LRSG, patients will undergo upper gastro-intestinal series. If the series will show a dilatation of the stomach, the patient will undergo another LRSG. If patients will regain weight without signs of stomach dilatation, he will be led to a malabsorbitive intervention.
Comorbidities improved: sleep apneas and joint problems disappeared completely 12 mo after LSG. Blood hypertension values did not differ significantly before surgery, after LSG and after LRSG, but a significant reduction in the requirement of antihypertensive drugs was observed. FBG and HbA1c gradually decreased after LSG and LRSG. One of two patient suspended oral hypoglycemic agents.
In conclusion, like the other major bariatric interventions[18,19] LSG can result in weight regain. Other bariatric procedures can be performed to correct it[20-27]. In our series of patients who regained weight after LSG, LRSG was performed. The result was a safe procedure which allowed a significant weight loss in each patient. LRSG appears to be a valid correction for post-LSG weight regain.
Our study is limited by the fact that it is retrospective, involves few patients and has a limited follow-up (12 mo after LRSG). We believe that these preliminary data can be a promising start for further studies, which are needed to confirm the initial results.
COMMENTS
Background
Obesity is an increasing problem in modern western society. In the past few years, different types of surgery have been developed to resolve this problem when it could not be treated by diets or drugs. Sleeve gastrectomy is an intervention that allows for good results in term of weight loss without problems of malabsorption. Sleeve became very popular among surgeons. Despite its wide diffusion, sleeve’s long-term weight loss data are not uniform. Some authors report a regain of weight after Sleeve.
Research frontiers
To show that it is feasible and effective to correct weight regain after sleeve through a re-sleeve gastrectomy.
Innovations and breakthroughs
Re-sleeve gastrectomy can correct weight regain after sleeve. It avoids converting sleeve in a malabsorbitive intervention, loosing the advantages of sleeve. They describe the surgical technique, which is valid and without major complications.
Applications
Sleeve gastrectomy has advantages in terms of quality of life for obese patients, avoiding problems of malabsorption and allowing weight lost. The possibility of managing weight regain with a re-sleeve, without converting it in a malabsorbitive intervention, can allow surgeons to choose this type of surgery in the cure of obesity.
Terminology
Sleeve gastrectomy is a vertical resection of stomach. Re-sleeve gastrectomy is the resection of those parts of stomach that underwent dilatation after sleeve.
Peer review
This is a case series of laparoscopic re-sleeve gastrectomy in obese patients who showed weight regain after laparoscopic sleeve gastrectomy. The present study is important because there are few data on this type of bariatric surgery.
Footnotes
P- Reviewers: Kanda T, Kim HH, Teo M S- Editor: Song XX L- Editor: A E- Editor: Wu HL
References
- 1.Moy J, Pomp A, Dakin G, Parikh M, Gagner M. Laparoscopic sleeve gastrectomy for morbid obesity. Am J Surg. 2008;196:e56–e59. doi: 10.1016/j.amjsurg.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 2.Nocca D, Krawczykowsky D, Bomans B, Noël P, Picot MC, Blanc PM, de Seguin de Hons C, Millat B, Gagner M, Monnier L, et al. A prospective multicenter study of 163 sleeve gastrectomies: results at 1 and 2 years. Obes Surg. 2008;18:560–565. doi: 10.1007/s11695-007-9288-7. [DOI] [PubMed] [Google Scholar]
- 3.Gagner M, Gumbs AA, Milone L, Yung E, Goldenberg L, Pomp A. Laparoscopic sleeve gastrectomy for the super-super-obese (body mass index & gt; 60 kg/m(2)) Surg Today. 2008;38:399–403. doi: 10.1007/s00595-007-3645-y. [DOI] [PubMed] [Google Scholar]
- 4.Fischer L, Hildebrandt C, Bruckner T, Kenngott H, Linke GR, Gehrig T, Büchler MW, Müller-Stich BP. Excessive weight loss after sleeve gastrectomy: a systematic review. Obes Surg. 2012;22:721–731. doi: 10.1007/s11695-012-0616-1. [DOI] [PubMed] [Google Scholar]
- 5.Boza C, Salinas J, Salgado N, Pérez G, Raddatz A, Funke R, Pimentel F, Ibáñez L. Laparoscopic sleeve gastrectomy as a stand-alone procedure for morbid obesity: report of 1,000 cases and 3-year follow-up. Obes Surg. 2012;22:866–871. doi: 10.1007/s11695-012-0591-6. [DOI] [PubMed] [Google Scholar]
- 6.Deitel M, Gagner M, Erickson AL, Crosby RD. Third International Summit: Current status of sleeve gastrectomy. Surg Obes Relat Dis. 2011;7:749–759. doi: 10.1016/j.soard.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 7.Brethauer SA, Hammel JP, Schauer PR. Systematic review of sleeve gastrectomy as staging and primary bariatric procedure. Surg Obes Relat Dis. 2009;5:469–475. doi: 10.1016/j.soard.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 8.Shi X, Karmali S, Sharma AM, Birch DW. A review of laparoscopic sleeve gastrectomy for morbid obesity. Obes Surg. 2010;20:1171–1177. doi: 10.1007/s11695-010-0145-8. [DOI] [PubMed] [Google Scholar]
- 9.Gill RS, Birch DW, Shi X, Sharma AM, Karmali S. Sleeve gastrectomy and type 2 diabetes mellitus: a systematic review. Surg Obes Relat Dis. 2010;6:707–713. doi: 10.1016/j.soard.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 10.Kehagias I, Karamanakos SN, Argentou M, Kalfarentzos F. Randomized clinical trial of laparoscopic Roux-en-Y gastric bypass versus laparoscopic sleeve gastrectomy for the management of patients with BMI & lt; 50 kg/m2. Obes Surg. 2011;21:1650–1656. doi: 10.1007/s11695-011-0479-x. [DOI] [PubMed] [Google Scholar]
- 11.Leyba JL, Aulestia SN, Llopis SN. Laparoscopic Roux-en-Y gastric bypass versus laparoscopic sleeve gastrectomy for the treatment of morbid obesity. A prospective study of 117 patients. Obes Surg. 2011;21:212–216. doi: 10.1007/s11695-010-0279-8. [DOI] [PubMed] [Google Scholar]
- 12.Bennett JM, Mehta S, Rhodes M. Surgery for morbid obesity. Postgrad Med J. 2007;83:8–15. doi: 10.1136/pgmj.2006.048868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frezza EE, Reddy S, Gee LL, Wachtel MS. Complications after sleeve gastrectomy for morbid obesity. Obes Surg. 2009;19:684–687. doi: 10.1007/s11695-008-9677-6. [DOI] [PubMed] [Google Scholar]
- 14.Hallowell PT, Stellato TA, Schuster M, Graf K, Robinson A, Jasper JJ. Avoidance of complications in older patients and Medicare recipients undergoing gastric bypass. Arch Surg. 2007;142:506–510; discussion 510-512. doi: 10.1001/archsurg.142.6.506. [DOI] [PubMed] [Google Scholar]
- 15.Himpens J, Dobbeleir J, Peeters G. Long-term results of laparoscopic sleeve gastrectomy for obesity. Ann Surg. 2010;252:319–324. doi: 10.1097/SLA.0b013e3181e90b31. [DOI] [PubMed] [Google Scholar]
- 16.Bohdjalian A, Langer FB, Shakeri-Leidenmühler S, Gfrerer L, Ludvik B, Zacherl J, Prager G. Sleeve gastrectomy as sole and definitive bariatric procedure: 5-year results for weight loss and ghrelin. Obes Surg. 2010;20:535–540. doi: 10.1007/s11695-009-0066-6. [DOI] [PubMed] [Google Scholar]
- 17.Santoro S. Technical aspects in sleeve gastrectomy. Obes Surg. 2007;17:1534–1535. doi: 10.1007/s11695-008-9417-y. [DOI] [PubMed] [Google Scholar]
- 18.Langer FB, Bohdjalian A, Shakeri-Leidenmühler S, Schoppmann SF, Zacherl J, Prager G. Conversion from sleeve gastrectomy to Roux-en-Y gastric bypass--indications and outcome. Obes Surg. 2010;20:835–840. doi: 10.1007/s11695-010-0125-z. [DOI] [PubMed] [Google Scholar]
- 19.Roslin M, Damani T, Oren J, Andrews R, Yatco E, Shah P. Abnormal glucose tolerance testing following gastric bypass demonstrates reactive hypoglycemia. Surg Endosc. 2011;25:1926–1932. doi: 10.1007/s00464-010-1489-9. [DOI] [PubMed] [Google Scholar]
- 20.Iannelli A, Dainese R, Piche T, Facchiano E, Gugenheim J. Laparoscopic sleeve gastrectomy for morbid obesity. World J Gastroenterol. 2008;14:821–827. doi: 10.3748/wjg.14.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iannelli A, Schneck AS, Dahman M, Negri C, Gugenheim J. Two-step laparoscopic duodenal switch for superobesity: a feasibility study. Surg Endosc. 2009;23:2385–2389. doi: 10.1007/s00464-009-0363-0. [DOI] [PubMed] [Google Scholar]
- 22.Gagner M, Deitel M, Kalberer TL, Erickson AL, Crosby RD. The Second International Consensus Summit for Sleeve Gastrectomy, March 19-21, 2009. Surg Obes Relat Dis. 2009;5:476–485. doi: 10.1016/j.soard.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 23.Regan JP, Inabnet WB, Gagner M, Pomp A. Early experience with two-stage laparoscopic Roux-en-Y gastric bypass as an alternative in the super-super obese patient. Obes Surg. 2003;13:861–864. doi: 10.1381/096089203322618669. [DOI] [PubMed] [Google Scholar]
- 24.Dapri G, Cadière GB, Himpens J. Laparoscopic repeat sleeve gastrectomy versus duodenal switch after isolated sleeve gastrectomy for obesity. Surg Obes Relat Dis. 2011;7:38–43. doi: 10.1016/j.soard.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 25.Iannelli A, Schneck AS, Noel P, Ben Amor I, Krawczykowski D, Gugenheim J. Re-sleeve gastrectomy for failed laparoscopic sleeve gastrectomy: a feasibility study. Obes Surg. 2011;21:832–835. doi: 10.1007/s11695-010-0290-0. [DOI] [PubMed] [Google Scholar]
- 26.Baltasar A, Serra C, Pérez N, Bou R, Bengochea M. Re-sleeve gastrectomy. Obes Surg. 2006;16:1535–1538. doi: 10.1381/096089206778869924. [DOI] [PubMed] [Google Scholar]
- 27.Noel P, Nedelcu M, Nocca D, Schneck AS, Gugenheim J, Iannelli A, Gagner M. Revised sleeve gastrectomy: another option for weight loss failure after sleeve gastrectomy. Surg Endosc. 2014;28:1096–1102. doi: 10.1007/s00464-013-3277-9. [DOI] [PubMed] [Google Scholar]


