Abstract
A fistula formation between the esophagus and an aberrant right subclavian artery is a rare but fatal complication that has been mostly described in the setting of prolonged nasogastric intubation and foreign body erosion. We report a case of a young morbidly obese patient who underwent sleeve gastrectomy that was complicated by a postoperative leak at the level of the gastroesophageal junction. A covered esophageal stent was placed endoscopically to treat the leak. The patient developed massive upper gastrointestinal bleeding secondary to the erosion of the stent into an aberrant retroesophageal right subclavian artery twelve days after stent placement. She was ultimately treated by endovascular stenting of the aberrant right subclavian artery followed by thoracotomy and esophageal repair over a T-tube. This case report highlights the multidisciplinary approach needed to diagnose and manage such a devastating complication. It also emphasizes the need for imaging studies prior to stent deployment to delineate the vascular anatomy and rule out the possibility of such an anomaly in view of the growing popularity of esophageal stents, especially in the setting of a leak.
Keywords: Aberrant subclavian artery, Arterioesophageal fistula, Esophageal stent, Esophageal repair, Angioplasty, Sleeve gastrectomy, Leak
Core tip: The use of esophageal covered stents to treat leaks following sleeve gastrectomy has increased significantly over the past years. However, their possible complications have not yet been fully explored. As demonstrated by our case report, the presence of an aberrant retroesophageal Subclavian artery can predispose to the formation of a fistula with the esophagus secondary to stent erosion, thereby leading to catastrophic hemorrhage and death. Our approach in this case was to start with stent angioplasty of the Subclavian artery followed by thoracotomy and esophageal repair over a T-tube and this approach proved successful in saving the patient’s life.
INTRODUCTION
Aorto-esophageal and arterial-esophageal fistulae have both been described in the literature. These usually arise in the setting of arterial aneurysms and esophageal malignancy. An aberrant subclavian arterioesophageal fistula, on the other hand, is an exceedingly rare occurrence and has been mostly reported in association with prolonged nasogastric intubation and erosion of esophageal instrumentation into the retroesophageal vessel. The mortality rate in such cases is extremely high as the bleeding is usually massive and sudden and ultimately leads to hemorrhagic shock and death. There are only a handful of reported cases of successful management of such a fistula. This paper describes the case of a young female who developed this devastating complication following the placement esophageal covered stent to treat a leak after sleeve gastrectomy. It also highlights the multidisciplinary approach used to diagnose and treat this complication.
CASE REPORT
A 29-year-old morbidly obese female developed a leak at the gastroesophageal junction following laparoscopic sleeve gastrectomy and underwent jejunostomy feeding tube insertion to allow spontaneous closure of the fistula. A gastrografin swallow performed a month later showed persistence of the leak. The patient then underwent an uneventful endoscopic stenting to exclude the leak and was discharged home on the same day. Twelve days later, she developed massive hematemesis and was taken to a district general hospital in a state of shock, where she was intubated and resuscitated. She underwent endoscopic removal of the stent followed by right thoracotomy, esophageal exploration and suturing of the fistula opening from the esophageal mucosal side thus obliterating and controlling the bleeding, followed by primary repair of the esophagus. Twelve hours later, the patient had a second episode of massive upper gastrointestinal (GI) bleeding and was in shock. She was taken back to the operating room where she was re-explored through a right thoracotomy. The esophagus was opened again and the bleeding site at the posterior wall of the esophagus was overrun and buttressed with a Teflon patch. She received a total of 29 units of packed red blood cells and 15 units of fresh frozen plasma. She was transferred to the intensive care unit at our medical center in critical condition. Computed tomography (CT) angiography revealed the presence of an esophageal perforation and a large pseudoaneurysm involving a right aberrant subclavian artery but there was no evidence of active bleeding at that point in time (Figure 1). Upper endoscopy revealed the presence of a Teflon patch within the lumen of the esophagus. The Teflon patch measured 2 cm × 3 cm and was placed at 22 cm from the incisors (Figure 2). After stabilizing the patient, she was taken to the interventional radiology suite for endovascular stenting and exclusion of the subclavian pseudoaneurysm to be followed by definitive surgical repair of the esophagus. Thoracic aorta angiography was performed and it revealed the presence of a right aberrant subclavian artery and a diverticulum of Kommerell as well as a 7 mm pseudoaneurysm approximately 1 cm from the origin of the right aberrant subclavian artery (Figure 3A). A right brachial artery cut down was then performed and a 6F sheath was used to deploy an 8 mm × 38 mm ATRIUM covered stent over the targeted area. Sequential balloon dilatation of the stent was then performed using 12 mm × 2 mm and 12 mm × 3 mm balloons and completion angiography demonstrated total occlusion of the pseudoaneurysm (Figure 3B). Following angioplasty, a right posterolateral thoracotomy with esophageal exploration was performed. The esophagotomy site was located above the division of the azygous vein. It was opened longitudinally and the Teflon patch was released from the posterior wall of the esophagus on the mucosal side (Figure 4). There was no evidence of bleeding through the surgically obliterated fistulous opening. The esophagus and its mucosa looked healthy and therefore primary closure over a 19F T-tube was performed. The esophagotomy was closed as a single layer using interrupted Polydioxanone sutures and was then buttressed with a fourth intercostal muscle flap.
Figure 1.
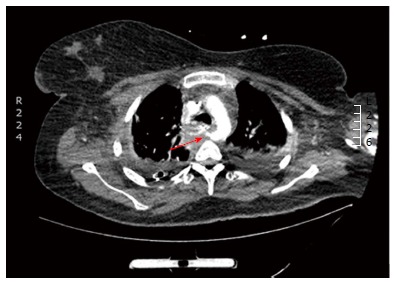
Computed tomography angiography showing a retroesophageal right suclavian artery with a pseudoaneurysm (arrow) around 1cm from its takeoff.
Figure 2.
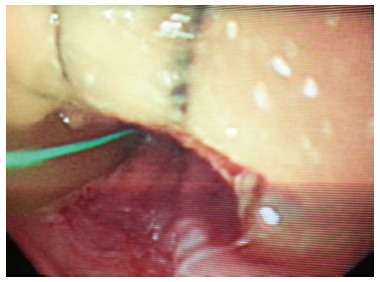
Upper endoscopy showing a 2 cm × 3 cm Teflon patch 22 cm from the incisor.
Figure 3.
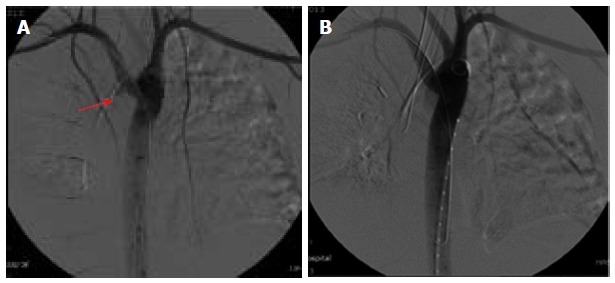
Thoracic aortic angiography. A: Showing the pseudoaneurysm (arrow); B: Angiography after stent deployment.
Figure 4.
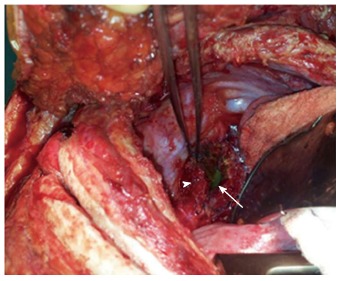
Thoracotomy with the Teflon patch (arrowhead) and nasogastric tube (arrow) visible through the esophagotomy.
The patient had a complicated postoperative course as she developed Acute Respiratory Distress Syndrome and was kept on mechanical ventilation for 10 d. She was fed through the previously inserted jejunostomy feeding tube. A gastrografin swallow performed two weeks after her surgery showed no evidence of contrast leak at the level of the esophageal repair (Figure 5). She was discharged home 33 d after her last operation. Twelve weeks later, the T tube was removed and she was started on regular oral feeding after a CT scan with oral contrast revealed no evidence of contrast extravasation (Figure 6).
Figure 5.
Gastrografin swallow performed postoperatively showing no evidence of a contrast leak at the site of the esophageal repair.
Figure 6.
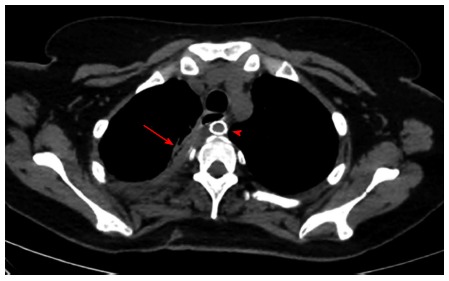
Computed tomography scan with oral contrast done after removal of the T-Tube with both the remnant tract of the T-tube (arrow) and the subclavian artery stent (arrowhead) seen.
DISCUSSION
An aberrant retroesophageal subclavian artery, also known as arteria lusoria, is the most common aortic arch anomaly. It has an incidence of 0.2% to 2% in the general population and is even more prevalent in patients with Down syndrome[1]. The persistence of the right dorsal aorta (known as Kommerell’s diveritculum) and involution of the right 4th embryologic arch causes the advent of an aberrant right subclavian artery (ARSA)[2]. It is usually asymptomatic but may cause dysphagia (dysphagia lusoria) and may demonstrate symptomatic atherosclerotic and aneurysmal degeneration[3].
Aorto-esophageal, arterial-esophageal, and aorto-enteric fistulae have all been described in the literature mainly in the setting aortic aneurysms, aortitis, atherosclerosis, and gastrointestinal malignancies[4]. Such fistulae have also been described in otherwise healthy people secondary to sharp trauma to the esophagus after swallowing animal bones, dentures, coins and other sharp objects[5]. A fistula between the esophagus and an ARSA, however, remains an exceptional event that has mostly been described in association with prolonged NG intubation[6]. The abnormal anatomic proximity to the esophagus or trachea likely renders the aberrant subclavian artery vulnerable to extrinsic compression and pressure necrosis by indwelling NG or endotracheal (ET) tubes[7]. The sequence of events may involve occlusion and thrombosis of the vasa vasorum, leading to vessel wall infarction and eventual wall dissolution. Moreover, ischemia and bacterial invasion of the vessel wall have also been suggested as important etiologic factors[8]. Unlike aorto-esophageal fistulae which present with sentinel bleeding in around 63% of cases, ARSA-esophageal fistulae present with massive upper GI bleeding with an associated mortality approaching 100%[5]. As such, early diagnosis is of paramount importance.
In our literature review, we were able to find 17 reported cases of ARSA-esophageal fistulae out of which, only 4 patients survived the acute event[9-12]. In all reported cases, bleeding was sudden, unheralded by prior symptoms, and massive. In the surviving patients, the upper GI bleeding was controlled by a multidisciplinary approach consisting of endoscopic balloon compression or angiographic control or both, and the definitive therapy was provided by urgent surgery. Described successful surgical interventions include ligation of the subclavian artery after angiographic or endoscopic control followed mostly by subclavian artery bypass. Left thoracotomy has been advocated for satisfactory control of the aorta and ligation of the origin of the ARSA, but other recommendations include right thoracotomy for optimal exposure for both the arterial resection and the esophageal repair[10]. Miller et al[9] described the first successful repair of such a fistula in a young girl who developed massive hematemesis two weeks after insertion of an Endotracheal (ET) and NG tubes. She underwent left thoracotomy with esophagotomy and intraesophageal balloon tamponade by positioning a 30F Foley catheter at the bleeding point to control the hemorrhage and allow the patient to be stabilized hemodynamically. Following that, aortic arch arteriography through a right femoral approach was performed demonstrating an aberrant right subclavian artery as well as contrast extravasation consistent with a fistula between the anomalous vessel and the esophagus. The involved segment of the aberrant vessel was then ligated and resected via right thoracotomy, and the esophagus was repaired primarily. No revascularization of the right upper extremity was performed and the patient was reported to be symptom free. A similar successful repair was reported by Feugier et al[10] in which a 24-year-old male with prolonged Intensive Care Unit stay following polytrauma developed massive upper GI bleeding 31 d after insertion of ET and NG tubes. Urgent CT angiography revealed an ARSA in direct contact with the NG tube. Angiography was then performed through the right brachial artery confirming contrast extravasation and the bleeding was controlled by inflating a 7 mm × 20 mm balloon. The patient then underwent left anterolatral thoracotomy with ligation of the subclavian origin and carotid to subclavian bypass.
Embolization of the bleeding vessel has been described but it may not always be successful in stopping the bleeding and may even create further complications. This was demonstrated by Vanden Eynden et al[11] in a patient with an esophageal stent that was placed to manage an esophageal stricture and eroded into a retroesophageal subclavian artery. Attempted endovascular coil embolization failed to control the bleeding vessel and the patient underwent urgent exploration with ligation of the artery at its takeoff while the endoesophageal prosthesis was left in place followed by construction of an aorto-axillary bypass using an 8-mm polytetrafluoroethylene graft constructed between the lateral aspect of the ascending aorta and the axillary artery. Postoperative endoscopy revealed erosion of the deployed endovascular coils into the lumen of the esophagus.
Complete endovascular repair with covered stents without dividing the fistulous tract theoretically carries a risk of graft infection and should be reserved to extremely critical patients when a rapid and minimally invasive procedure could positively affect patient’s outcome. Magagna et al[12] reported the placement of a 14 mm endovascular prosthesis in an ARSA to cover a fistula between the artery and the oesophagus in an elderly female with a tracheostomy and history of laryngectomy and radiotherapy who presented with massive upper GI bleeding. The procedure was performed after the bleeding was controlled by positioning a Sengstaken-Blakemore tube and the patient was stabilized.
The surgical rationale followed in our case which included stenting of the artery followed by thoracotomy and primary esophageal repair over T tube and buttressing with an intercostal muscle flap has never been attempted in the literature but appears to be an equally effective and successful option. Although our patient’s initial operation to control her bleeding stabilized her enough to allow for endovascular stenting of the pseudoaneurysm, it also necessitated another esophageal exploration for removal of the prosthetic patch and definitive esophageal repair. As the patient had two previous thoracotomies, it was of paramount importance to make sure that the esophageal repair is solid and to rule out a communication between the posterior wall of the esophagus and the stent that was placed in the subclavian artery. The Teflon patch that was previously placed in the lumen of the esophagus prevented us from assessing the posterior lumen of the esophagus and the integrity of the esophageal wall. It is also important to point to the fact that our patient had a sleeve gastrectomy, which precluded the option of using the stomach as conduit in the future should the esophageal repair fail. It was therefore extremely important to preserve the esophagus and as such, exploration and repair over a T-tube rather than a diverting cervical esphagostomy seemed the only treatment option.
ARSA-esophageal fistula is a rare but devastating complication of prolonged esophageal stenting and intubation and should be included in the differential diagnosis when investigating upper GI bleeding in patients with prolonged NG and ET intubation. It requires a multidisciplinary approach of different specialties including general and cardiothoracic surgery, interventional radiology, vascular surgery and intensive care. Angiography is an essential diagnostic and therapeutic tool as endoluminal balloons and stent grafts can be used as temporizing measures when feasible. Thoracotomy with ligation of the bleeding vessel and repair of the esophagotomy followed by revascularization of the right arm appears to be the most successful approach described in the literature. However, as demonstrated in our case, endovascular stenting of the fistulous tract followed by esophageal repair offers an alternate effective treatment modality. Taking into account the poor survival after massive bleeding caused by an esophageal stent erosion into a major mediastinal vessels, and given the fact that esophageal stenting is gaining ground in the management of esophageal and gastroesophageal junction leaks post bariatric surgery[13], we recommend CT angiography of the chest to rule out the presence of the not uncommon ARSA in patients considered for prolonged esophageal stent placement.
COMMENTS
Case characteristics
Twenty-nine years old obese female with history of leak following sleeve gastrectomy underwent esophageal stent placement.
Clinical diagnosis
Massive upper gastrointestinal bleeding with hemorrhagic shock 12 d after stent placement.
Differential diagnosis
Bleeding gastric ulcer, esophageal bleeding, erosion of the stent into a major vessel.
Laboratory diagnosis
White blood cells 10.1 k/μL; HGB 6 mg/dL; hematocrit 18%; the electrolytes and metabolic panel were within normal limits.
Imaging diagnosis
Computed tomography angiography showed a communication between the esophagus and an aberrant retroesophageal right subclavian artery around 1 cm from the takeoff of the artery.
Pathological diagnosis
The esophageal covered stent eroded through the layers of the esophagus posteriorly reaching the aberrant Subclavian artery.
Treatment
Endovascular stenting of the Subclavian artery was first performed followed by thoracotomy and repair of the esophageal perforation over a T tube with intercostal muscle flap coverage.
Related reports
Aberrant right Subclavian rterioesophageal fistulas have been reported in the setting of prolonged nasogastric and endotracheal intubation causing erosion of the tube through the esophageal wall into the adjacent vessel with a high associated mortality rate.
Term explanation
An aberrant right Subclavian artery describes an anatomic variant in which the right Subclavian artery arises distal to the left Subclavian artery and courses behind the esophagus.
Experiences and lessons
This case report is the fifth reported case in literature of successful treatment of an aberrant subclavian arterioesophageal fistula. This was achieved by adopting a multidisciplinary approach spanning over different medical and surgical specialties. To prevent such a devastating complication, computed tomography angiography should be done to rule out the presence of an aberrant retroesophageal artery when contemplating the use of an esophageal stent for a prolonged period of time.
Peer review
The case is very interesting and the subject clinically relevant. The manuscript is well written and the images are nice. The subject is nicely reviewed in the discussion.
Footnotes
P- Reviewers: Azevedo CF, Sarkodieh JE S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
References
- 1.Molz G, Burri B. Aberrant subclavian artery (arteria lusoria): sex differences in the prevalence of various forms of the malformation. Evaluation of 1378 observations. Virchows Arch A Pathol Anat Histol. 1978;380:303–315. doi: 10.1007/BF00431315. [DOI] [PubMed] [Google Scholar]
- 2.van Son JA, Konstantinov IE. Burckhard F. Kommerell and Kommerell’s diverticulum. Tex Heart Inst J. 2002;29:109–112. [PMC free article] [PubMed] [Google Scholar]
- 3.Fockens P, Kisman K, Tytgat GNJ. Endosonographic imaging of an aberrant right subclavian (lusorian) artery. Gastrointestinal Endosc. 1996;43:419. [Google Scholar]
- 4.Dossa CD, Pipinos II, Shepard AD, Ernst CB. Primary aortoenteric fistula: Part I. Ann Vasc Surg. 1994;8:113–120. doi: 10.1007/BF02133413. [DOI] [PubMed] [Google Scholar]
- 5.Hollander JE, Quick G. Aortoesophageal fistula: a comprehensive review of the literature. Am J Med. 1991;91:279–287. doi: 10.1016/0002-9343(91)90129-l. [DOI] [PubMed] [Google Scholar]
- 6.Inman JC, Kim P, McHugh R. Retroesophageal subclavian artery--esophageal fistula: a rare complication of a salivary bypass tube. Head Neck. 2008;30:1120–1123. doi: 10.1002/hed.20854. [DOI] [PubMed] [Google Scholar]
- 7.Heck HA, Moore HV, Lutin WA, Leatherbury L, Truemper EJ, Steinhart CM, Pearson-Shaver AL. Esophageal-aortic erosion associated with double aortic arch and tracheomalacia. Experience with 2 infants. Tex Heart Inst J. 1993;20:126–129. [PMC free article] [PubMed] [Google Scholar]
- 8.Gable DS, Stoddard LD. Acute bacterial aortitis resulting in an aortoesophageal fistula. A fatal complication of untreated esophageal carcinoma. Pathol Res Pract. 1989;184:318–324. doi: 10.1016/S0344-0338(89)80093-7. [DOI] [PubMed] [Google Scholar]
- 9.Miller RG, Robie DK, Davis SL, Cooley DA, Klish WJ, Skolkin MD, Kearney DL, Jaksic T. Survival after aberrant right subclavian artery-esophageal fistula: case report and literature review. J Vasc Surg. 1996;24:271–275. doi: 10.1016/s0741-5214(96)70103-9. [DOI] [PubMed] [Google Scholar]
- 10.Feugier P, Lemoine L, Beaudoin N, Chevalier JM. Aberrant right subclavian arterioesophageal fistula: endovascular occlusion via a transbrachial approach. Eur J Vasc Endovasc Surg. 2002;23:77–78. doi: 10.1053/ejvs.2001.1512. [DOI] [PubMed] [Google Scholar]
- 11.Vanden Eynden F, Devière J, Laureys M, de Cannière D. Erosion of a retroesophageal subclavian artery by an esophageal prosthesis. J Thorac Cardiovasc Surg. 2006;131:1183–1184.e1. doi: 10.1016/j.jtcvs.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 12.Magagna P, Abbiate N, Mansi G, D’Onofrio A, Auriemma S, Piccin C, Savastano S, Fabbri A. Endovascular treatment of aberrant right subclavian (lusorian) artery to oesophagus fistula: a case report. Vasc Endovascular Surg. 2008;42:394–396. doi: 10.1177/1538574408315993. [DOI] [PubMed] [Google Scholar]
- 13.Simon F, Siciliano I, Gillet A, Castel B, Coffin B, Msika S. Gastric leak after laparoscopic sleeve gastrectomy: early covered self-expandable stent reduces healing time. Obes Surg. 2013;23:687–692. doi: 10.1007/s11695-012-0861-3. [DOI] [PubMed] [Google Scholar]


