Abstract
Human noroviruses (HuNoVs) are the major cause of nonbacterial gastroenteritis epidemics. The culturable feline calicivirus and murine norovirus have been used extensively as surrogates to study HuNoV biology, as HuNoV does not grow in vitro. Additional efforts to identify new surrogates are needed, because neither of these common surrogates are truly intestinal pathogens. The newly described Tulane virus (TV) is a typical calicivirus, it is isolated from macaque stools, is cultivable in vitro, and recognizes human histo-blood group antigens. Therefore, TV is a promising surrogate for HuNoVs. In this study, we evaluated the resistance or stability of TV under various physical and environmental conditions by measuring a 50% reduction of tissue culture infective dose (TCID50) by using a TV cell culture system. Due to the nature of this virus, it is hard to produce a high-titer stock through tissue culture. In our study, the maximal reduction in virus titers was 5 D (D = 1 log) in heat-denaturation and EtOH experiments, and 4 D in UV, chlorine, and pH-stability experiments. Therefore in this study, we defined the inactivation of TV as reaching a TCID50/ml of 0 (a 4- to 5-D reduction in TCID50, depending on the detection limit). TV was inactivated after incubation at 63°C for 5 min, incubation at 56°C for 30 min (5 D), exposure to 60 mJ/cm2 of UVC radiation (4 D), or incubation at 300 ppm of free chlorine for 10 min (4 D). TV was shown to be stable from pH 3.0 to 8.0, though an obvious reduction in virus titer was observed at pH 2.5 and 9.0, and was inactivated at pH 10.0 (4 D). TV was resistant to a low concentration of EtOH (40% or lower) but was fully inactivated (5 D) by 50 to 70% EtOH after a short exposure (20 s). In contrast, quantitative real-time PCR was unable to detect, or poorly detected, virus titer reductions between treated and untreated samples described in this study.
Caliciviruses are important human and animal pathogens, causing a wide variety of diseases in different hosts. The family Caliciviridae consists of five genera (Norovirus, Sapovirusa, Lagovirus, Vesivirus, and Nebovirus) (14, 15). Recently, two new calicivirus genera were proposed, Recovirus and Valovirus, which are represented by the Tulane virus and the St. Valerien–like viruses (11, 23), respectively. Human noroviruses (HuNoVs) mainly cause acute gastroenteritis and they account for approximately 60% of foodborne illness in the United States (30).
Infection is caused by oral ingestion of HuNoVs. HuNoVs replicate in the small intestine, where it causes transient lesions of the intestinal mucosa and is shed in the feces (3). The histo-blood group antigens (HBGAs) were identified as receptors for HuNoVs, and mediate virus attachment and entry (35). Different strains of HuNoVs recognize variable HBGAs in the three major families of HBGAs: Lewis, ABO, and secretor (36). Clearly, receptors play an important part in host susceptibility. The presence of human-like HBGAs in oysters was demonstrated as an important factor for bioaccumulation of HuNoVs in oysters (22, 24, 25, 38). Gandhi et al. (12) reported that recombinant HuNoV virus–like particles localized primarily on the leaf veins when applied to the surface of romaine lettuce. Therefore, bioaccumulation of HuNoVs in produce can also occur when produce is exposed to contaminated irrigation water.
Although HuNoVs cause significant problems in food safety and public health, many aspects of HuNoVs are poorly understood due to the current inability to culture HuNoVs and the lack of efficient small animal models. Only limited data pertaining to the survival of HuNoVs were obtained from human volunteer challenge studies (8). Therefore, proper surrogates for HuNoVs are required to study the fundamental biology of the virus. Prior to the discovery of animal caliciviruses, poliovirus, hepatitis A virus, and bacteriophages were used as surrogates for virus inactivation studies (1). Recently, studies were performed with animal caliciviruses, including the feline calicivirus (FCV) and murine norovirus (MNV). Both FCV and MNV can be cultured and amplified in their respective hosts (20, 41). However, neither of them possesses receptors similar to that of HuNoVs, nor do they cause gastrointestinal disease. FCV belongs to the Vesivirus genus, uses sialic acid on the host cell surface as a receptor (37), and causes infectious upper respiratory tract disease in cats (29, 31). In addition, the junctional adhesion molecule 1 is apparently required for FCV infection, likely aiding virus penetration after initial attachment (26). The first isolated murine norovirus, MNV-1, was discovered in severely immunocompromised mice and causes systemic infection and lethal disease in STAT1−/− mice (18). Although MNV shares many genetic characteristics with HuNoVs, it also differs significantly at the molecular level. MNV also employs a sialic acid receptor for virus entry and infection (34) and unlike HuNoVs, targets macrophages and dendritic cells (37, 41).
The Tulane virus (TV), also known as the monkey calicivirus, was isolated from the stools of rhesus macaques at the Tulane National Primate Research Center (11). TV replicates in vitro in rhesus monkey kidney (LLC-MK2) cells and causes typical cytopathic effects. In contrast to the abovementioned surrogates, TV recognizes type-B HBGAs as receptors for infection (10). This property makes TV a potentially useful surrogate for HuNoVs. Although the inactivation conditions for FCV and MNV have been established (1), to our knowledge, similar conditions for TV have not been reported. In this study, we tested the inactivation conditions for TV including temperature, UV dose, chlorine concentration, pH stability, and resistance to EtOH by using in vitro assays, 50% reduction of tissue culture infective dose (TCID50), and quantitative real-time PCR (qRT-PCR).
MATERIALS AND METHODS
Virus cultivation
LLC-MK2 cells (American Type Culture Collection, Manassas, VA) were used for culturing and titration of virus (39). The Tulane virus was kindly provided by Dr. Jiang (Division of Infectious Diseases, Cincinnati Children’s Hospital Medical Center). Cells were grown in HyClone M199–Earle’s balanced salt solution medium (Thermo Fisher Scientific, Waltham, MA) supplemented with 10% fetal bovine serum (Atlanta Biologicals, Norcross, GA) and 2 × Gibco antibiotic-antimycotic (Life Technologies, Carlsbad, CA). One day after plating cells, confluent cell cultures (100% confluency) were inoculated with virus at a multiplicity of infection of 0.1 in M199 media without serum, and were harvested 2 days postinoculation by scraping. The cultures were then transferred to sterile, 50-ml centrifuge tubes (BD, Franklin Lakes, NJ) and freeze-thawed (cycles of −20°C and 25°C) three times to release cell-associated virus. Cellular debris was removed by centrifugation at 2,500 × g for 10 min; the virus-enriched supernatant was aliquoted, and then stored at −20°C.
Thermal inactivation
Virus-stock aliquots of 150 μl in microcentrifuge tubes were incubated in 37, 56, 63, and 72°C heat blocks for durations of 1, 5, 10, and 30 min. At the end of each treatment time, each tube was removed and quickly cooled on ice. The treated virus stocks were each diluted with 1,350 μl of maintenance media (M199 without fetal bovine serum) for initial 1:10 dilutions for the TCID50 assay. One hundred microliters of virus was used per well, with a final titer of 6.4 × 105 TCID50 per well. For qRT-PCR, the same procedure was used to test conditions of 72 and 100°C for 2 min, with the final dilution adjusted to 1:100.
UV irradiation inactivation
Virus-stock aliquots of 250 μl were placed into the wells of a 24-well tissue culture plate, which in turn was placed into a shallow ice bath. A thin layer of virus stock was evenly distributed onto the bottom of the well, resulting in a surface area–to–volume ratio of 7.6. The plate-in-bath assembly was UV irradiated with energies of 10, 20, 30, 40, 50, 60, and 70 mJ/cm2 by using a Stratagene UV Stratalinker 1800 (emitted as UVC at 254 nm at ~3 mW/cm2). One hundred fifty microliters of treated virus stock was removed from each well and diluted with 1,350 μl of maintenance media for initial 1:10 dilutions for the TCID50 assay. One hundred microliters of virus was used per well, with a final virus titer of 4 × 104 TCID50 per well. For qRT-PCR, the same procedure was used to test conditions of 60 and 600 mJ/cm2, with the final dilution adjusted to 1:100.
Chlorine inactivation
Bleach (Pure Bright, Concord, Ontario, Canada) was used to make chlorine stocks ranging from 600 to 30,000 ppm of free chlorine. Similarly, stock solutions of sodium thiosulfate (Fisher Scientific) were made to span the same range (600 to 30,000 ppm). Three hundred microliters of virus stock in each 15-ml tube was brought to experimental free-chlorine conditions (10 to 500 ppm) by the addition of 5 μl of each bleach dilution and allowed to incubate for 10 min. The free-chlorine treatment conditions were neutralized by the addition of 5 μl of each respective sodium thiosulfate solution, and then diluted with 2,690 μl of maintenance media for initial 1:10 dilutions for the TCID50 assay. One hundred microliters of virus was used per well, with a final virus titer of 4.9 × 104 TCID50 per well. Medium with corresponding chlorine with neutralizer was used to rule out the possibility of nonspecific toxic effects caused by the reagents. For qRT-PCR, the same procedure was used to test conditions of 500 and 3,000 ppm, with the final dilution adjusted to 1:100.
pH stability
Aliquots of maintenance media were adjusted with HCl or NaOH to span the range from pH 2.0 to 10.0. One hundred fifty microliters of virus stock in each 15-ml tube was brought to experimental pH conditions by dilution with 1,350 μl of each pH-adjusted medium, and allowed to incubate for 30 min. The pH-treatment conditions were neutralized by further dilution with 13.5-ml aliquots of maintenance medium for initial 1:100 dilutions for the TCID50 assay. One hundred microliters of virus was used per well, with a final virus titer of 4.5 × 104 TCID50 per well.
EtOH inactivation
Virus-stock aliquots of 150 μl in microcentrifuge tubes were diluted with a range of 37.5 to 350 μl of 100% EtOH to achieve EtOH-treatment concentrations of 20 to 70% and allowed to incubate for 20 s. Each treatment condition was brought to a total volume of 500 μl, with maintenance medium, and then neutralized by further dilution with 4.5 ml of maintenance medium for initial 1:33.3 dilutions for the TCID50 assay. One hundred microliters of virus was used per well, with a final virus titer of 1.5 × 105 TCID50 per well. Media with corresponding EtOH concentrations were used to rule out the possibility of nonspecific toxic effects caused by EtOH. For qRT-PCR, the same procedure was used to test 70% EtOH, with the final dilution adjusted to 1:100.
Virus titering by TCID50 assay
The virus stock was titered with the TCID50 assay. One-eighth of the total LLC-MK2 cells trypsinized from a T-150 flask at 100% confluency was resuspended in 20 ml of growth medium, from which 200 μl of cells were seeded in each well of a 96-well plate and incubated overnight until 100% confluency was again reached. Growth medium was then removed from all wells and replaced with 200 μl of maintenance medium in control wells and 100 μl in quantitation wells. One hundred microliters of serially diluted virus stock ranging from 10−1 to 10−8 was added to each quantitation well, organized with each serial dilution occupying a row, at 10 quantitation wells per row. The plate was incubated for 5 to 6 days, and then examined for cell balling via microscopy. Balling counts were converted into TCID50 and TCID50/ml by using the Reed and Muench calculator spreadsheet (http://www.med.yale.edu/micropath/pdf/Infectivity%20calculator.xls). Similarly, viable virus counts after treatment with experimental conditions (described above) were also determined by TCID50 assay.
qRT-PCR
Treatment conditions of virus stock were performed as described above, and each was adjusted with maintenance medium to a final dilution of 1:100 relative to virus stock. One hundred fifty microliters of each treated (and control) virus dilution was extracted for RNA with a viral RNA extraction kit (QIAGEN, Valencia, CA). Extracted viral RNA was quantitated with probe-based qRT-PCR by using a qPCR system (MX3000P, Stratagene, La Jolla, CA) with a one-step qRT-PCR kit (QuantiTect Probe RT-PCR Kit, QIAGEN) in accordance with the manufacturer’s protocol. The P289-P290 amplicon of TV in the RNA-dependent RNA polymerase gene of caliciviruses (11, 17) was determined in silico with DNASTAR software (Madison, WI) and experimentally confirmed (results not shown here). A qRT-PCR probe and primer set were designed for this amplicon by using the RealTime PCR and PrimeTime qPCR tools (http://www.idtdna.com/scitools/Applications/RealTimePCR/) and evaluated in silico with DNASTAR. Primers and probes were synthesized with modified fluorophores and quenchers (Integrated DNA Technologies, Inc., San Diego, CA). The primers and probes used for detection of TV were TV forward (5’-TGACGATGACCTTGCGTG-3’), TV reverse (5’-TGGGATTCAACCATGATACAGTC-3’), TV probe (5’-HEX-ACCCCAAAGCCCCAGAGTTGATBHQ-1-3’). Each 25-μl reaction consisted of 12.5 μl of QuantiTect Probe RT-PCR Master Mix, 7.5 μl of RNAse-free water, 0.75 μl of each primer (TV forward and TV reverse, both at 10 μM), 0.25 μl of TV probe at 10 μM, 0.25 μl of QuantiTect RT mix, and 3 μl of extracted RNA. Cycling times and temperatures were 50°C for 30 min, 95°C for 15 min, followed by 45 cycles of 95°C for 15 s, 53°C for 20 s, and 60°C for 50 s. Fluorescence was read at the end of each 60°C extension step. Data acquisition was conducted by MxPro (Stratagene), with threshold determination at default, automated settings. The p289i-p290j RT-PCR TV amplicons in a TOPO vector (Life Technologies) was used to generate the standard curve. The plasmid DNA was serially diluted to a theoretical range of 107 to 10−1 copies. The dilution set was quantified by qRT-PCR in triplicate. This allowed the remaining data to be interpreted into a six-point standard curve spanning 1 to 105 copies. A linear trend line was generated (R2 = 0.992).
Data analysis and statistics
Each plating experiment was repeated three times as independent replicates. One-way analysis of variance was used for data analysis.
RESULTS AND DISCUSSION
Complete inactivation
To claim complete inactivation of bacteria, a 5-log reduction in titer is required. However, the criteria are not well established for complete inactivation of viruses. European standards for virucidal efficacy have suggested a 4-D (D = 1 log) reduction in virus titer (33). However, both 3- and 4-D reductions in virus titer were used as the criteria of inactivation in surrogates used for HuNoV. Unlike FCV and MNV, which can be easily grown in tissue culture to titers as high as 1 × 108 to 1 × 109 PFU/ml, the typical cultivated titer of TV is about 2 to 3 D lower. Considering the dilution factor (undiluted to 1:33) and inoculation dose (100 μl per well), the starting virus titer was between 4 × 104 and 6.4 × 105 TCID50 per well in this study. The maximal detection limit in this study was limited to 4- to 5-D reduction in virus titer. Therefore, in this study, we defined the inactivation of TV as reaching a TCID50/ml of 0 (4- to 5-D reduction in TCID50 depends on the detection limit).
Thermal inactivation
Time-dependent thermal-inactivation experiments showed typical reductions of TV TCID50 titers in association with increased incubation temperatures and times. The TCID50 titers of TV were basically unchanged after incubation at 37°C for up to 30 min (6.4 × 105 TCID50 to 4.9 × 105 TCID50). At 56°C, a steep decrease in TCID50 was observed, reaching a 99.4% reduction at 5 min (5.1 × 103 TCID50) and 99.99% (1.1 × 102 TCID50) after 10 min (Fig. 1). A TCID50 of 0 (>5-D reduction in virus titer) was observed when TV was treated for 30 min at 56°C. Exposure of TV to 63 and 72°C for 1 min reduced TCID50 to 1.9 × 105 and 6.9 ×103 TCID50, respectively. A TCID50 of 0 was observed when TV was incubated at 63 and 72°C for only 5 min. The D-values (decimal reduction times) for the 56, 63, and 72°C groups were 11.8, 2.6, and 4.3 min, respectively. These thermal deactivation characteristics of TV are similar to those for FCV and MNV. For example, a 3-D reduction in virus titer was observed when FCV was exposed to 56°C for 8 min or 71.3°C for 1 min (9). A 3.1- and a 3.5-D reduction in virus titer were observed when MNV was exposed at 63°C for 5 min or 72°C for 1 min (16). Duizer et al. (9) reported that D-values for 3-D reductions in FCV titers were 8 min for 56°C and 1 min for 71.3°C. Due to the lack of both a cell culture system and an animal model for HuNoVs, alternative methods were used to study thermal inactivation of HuNoV RNA. Hewitt et al. (16) reported that HuNoVs are more heat resistant than FCV and MNV when measuring the virus titers by qRT-PCR, but Dancho et al. (5) reported that the thermal resistance of HuNoVs was similar to FCV and MNV when a qRT-PCR was preceded by a receptor-mediated capture assay. In the latter report, HuNoVs could also be completely inactivated at 73°C for 2 min.
FIGURE 1.

TCID50 quantitation of virus activity after treatment with elevated temperatures of 37, 56, 63, and 72°C for 1, 5, 10, and 30 min.
UV inactivation
A continual decrease in the TCID50 titers (from 104 to 0) TV was observed when the virus was treated with UV radiation of 10 to 70 mJ/cm2 (Fig 2). Although over half (less than 2 D) of viruses were inactivated by 10 mJ/cm2 of UVC radiation, there was no significant difference (P = 0.32) due to variation in the TCID50 assays. The titer of viruses inactivated by 20, 30, and 40 mJ/cm2 of UVC radiation was 6.9 × 103 (P = 0.002), 1.0 × 103 (P < 0.001), 5.8 × 102 (P < 0.001), and 2.4 × 102 (P<0.001) TCID50, respectively. No virus (TCID50 = 0) could be detected (>4-D reduction in TCID50) when TV was exposed to 60 and 70 mJ/cm2 of UVC radiation (Fig. 2). TV is seemingly more UV resistant than MNV and FCV. A 3- and a 4-D reduction in virus titer were reported when MNV was exposed to UVC at an energy dose of 25 mJ/cm2 (21) and 27 mJ/cm2 (27), respectively. A 3- and 4-D reduction in virus titer was reported when FCV was inactivated by UVC at energy doses of 12 mJ/cm2 (6) and 34 mJ/cm2 (9), respectively. There are no direct data regarding the UV inactivation of HuNoVs. Dancho et al. (5) recently estimated that the UV energy dose necessary for complete inactivation of HuNoVs could be as high as 2 J/cm2 when a receptor-mediated capture assay is combined with qRT-PCR.
FIGURE 2.

TCID50 quantitation of virus activity after treatment with UVC radiation at 10, 20, 30, 40, 50, and 60 mJ/cm2.
Chlorine inactivation
A continual decrease in the TCID50 titers (from 104 to 0) of TV was observed when the virus was treated with increasing concentrations of chlorine, from 10 to 500 ppm (Fig. 3). The titer of viruses inactivated by 10, 25, 50, 200, and 300 ppm of chlorine was 3.0 × 104 (P = 0.338), 2.6 × 104 (P = 0.221), 2.0 × 104 (P = 0.147), 7.5 ×102 (P = 0.008), and 9.0 × 101 (P = 0.008) TCID50, respectively. No virus (TCID50 = 0) could be detected with 500 ppm of chlorine-treated virus (>4-D reduction in virus titer). The sensitivity of TV to chlorine is similar to other surrogates. A 3- and a 4-D reduction in virus titer were reported when FCV was treated with chlorine at 5,000 ppm for 1.9 min (28) and 2,500 ppm for 10 min (7). Belliot et al. (2) reported a 4-D reduction in virus titer when MNV was treated with chlorine at 2,600 ppm for 30 s, and Park et al. (27) reported a 3.2-D reduction in virus titer when MNV was treated with 5,000 ppm of chlorine for 3.2 min. A >2-D reduction in infectivity was observed when FCV was treated with 100 ppm of chlorine for 1 min (40). HuNoVs are believed to be more resistant to chlorine than other gastrointestinal viruses (18). The only study regarding chlorine sensitivity of HuNoVs was performed by Keswick et al. (19) in human volunteers, in which 3.75 to 6.25 mg/liter chlorine, a concentration found in drinking water distribution systems, was insufficient to inactivate the virus. Employing a molecular approach such as traditional RT-PCR, Duizer et al. (9) reported that HuNoVs could be inactivated by exposing the viruses to 6,000 ppm of chlorine for 10 min.
FIGURE 3.

TCID50 quantitation of virus activity after treatment with free chlorine at 10, 25, 50, 200, and 300 ppm.
pH stability
The TCID50 results vary between pH 3.0 and 8.0, but without a significant difference. TCID50 for pH values of 3.0 to 8.0 were 4.3 × 104, 3.0 × 104, 2.9 × 104, 4.2 × 104, 3.0 × 104, and 1.1 × 104, respectively (Fig. 4). TCID50 = 0 (>4-D reduction in virus titer) was observed when virus was incubated at pH 10.0 for 10 min. Obvious reductions in TV titers were observed when the virus was treated below pH 2.5 and above pH 9.0. TCID50 values for pH 2.0, 2.5, and 9.0 were 2.2 × 103 (±2.6), 1.1 × 104 (±0.93), and 5.5 × 103 (±0.88), respectively. However, these changes were not statistically significant. FCV is sensitive to extreme pH conditions (9) and could be completely inactivated (>5-D reduction) at and beyond both pH 2.0 and 10.0. However, MNV is stable from pH 2.0 to 10.0 (4). A <1-D reduction in infectivity was observed when MNV was treated at pH 2.0 (4). In this respect, TV has comparable pH stability to MNV, with the exception of pH 10.0. HuNoVs remained infective after a treatment at pH 2.7 for 3 h in a challenge study in human volunteers (8). Stability of HuNoVs was also studied with mass spectrometry and atomic force microscopy to investigate the (dis)assembly of Norwalk virus–like particles. Shoemaker et al. (32) demonstrated that Norwalk virus–like particles remained intact at low pH (2.5) but dissembled when pH was above 9.0. Compared with other experimental conditions, significant variations between variable pH groups were observed, probably due to the influence of the nonspecific interaction between electronic charges on the viral capsid proteins and surface proteins of the cell.
FIGURE 4.
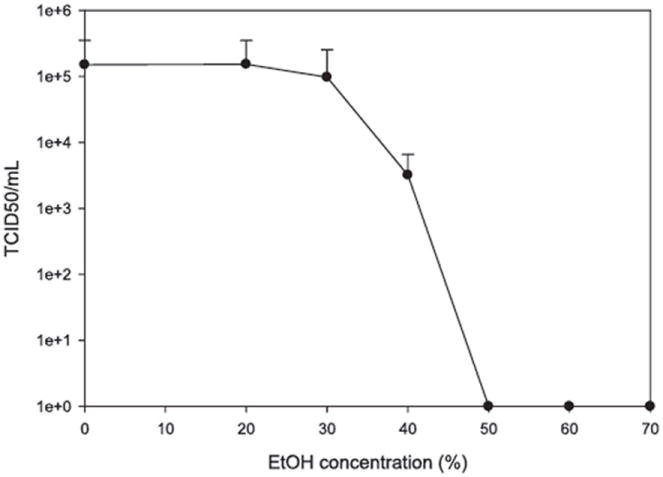
TCID50 quantitation of virus activity after treatment with 20, 30, 40, 50, 60, and 70% EtOH.
EtOH inactivation
TCID50 assay results show that TV is resistant to a low level of EtOH, with little effect at 20 and 30% EtOH (Fig. 5). TCID50 values for 0, 20, 30, and 40% EtOH were 1.5 × 105, 1.5 × 105, 9.7 × 104, and 3.2 × 103, respectively. A TCID50 of 0 (>5-D reduction in virus titer) was observed when TV was treated with 50 to 70% EtOH for as short as 20 s. (Fig. 5). These results were consistent with those for FCV and MNV. Both FCV and MNV are sensitive to 70% EtOH (2, 13). FCV could also be inactivated by 50% EtOH for 30 s (>3-D reduction in virus titer) or 70% EtOH for 30 s (>4-D reduction in virus titer) (13). MNV could be inactivated by exposure to 60% EtOH for 30 s (>4-D reduction in virus titer), but could not be inactivated by 30% EtOH for 3 min (2). Currently, there are no data for EtOH inactivation of HuNoVs.
FIGURE 5.
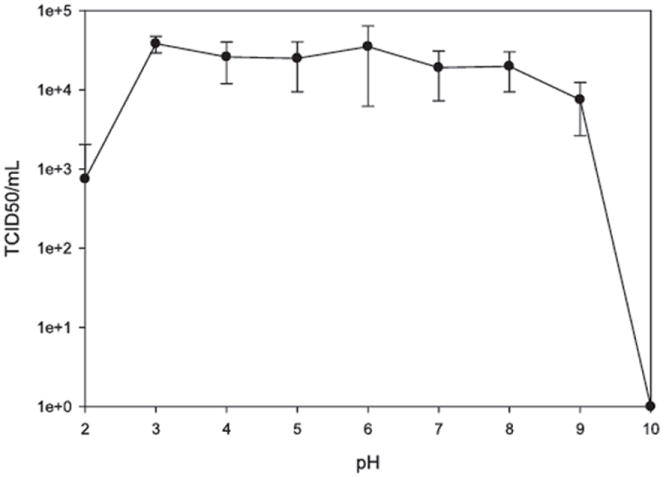
TCID50 quantitation of virus activity after treatment with pH values of 2.0, 2.5, 3.0, 4.0, 5.0, 6.0, 7.0, 8.0, 9.0, 9.5, and 10.0.
qRT-PCR
A subset of samples treated by variable conditions described above was also tested by qRT-PCR to determine a correlation to the TCID50 assay. Two inactivation conditions were selected in this study for qRT-PCR assays. One is the highest dose or treatment used in our study that obtained a 4- to 5-D reduction in virus titer in our TCID50 assays. The other conditions are extremes (boiling for heat inactivation, 600 mJ/cm2 for UV, and 3,000 ppm for chlorine) that were demonstrated having a great impact on RNA structure. Quantifying by qRT-PCR assay, a <1-D reduction in viral RNA copies was observed when TV virus was treated at conditions that would otherwise cause 4-D reduction in TCID50 titers. In addition, qRT-PCR measured <3-D reduction in copy numbers at conditions that should cause severe damage to RNA (Table 1). These data suggest that qRT-PCR does not seem to reflect virus activity as quantified by TCID50.
TABLE 1. qRT-PCR quantitation of signal after treatment with inactivation conditions.
| Condition | Mean CTa | SD | ΔCT | Proportion of copy (%) |
|---|---|---|---|---|
| Control | 32.024 | 0.283 | 0.00 | 100.0 |
| 72°C, 2 min | 33.549 | 0.744 | 1.53 | 47.34 |
| 100°C, 2 min | 41.530 | 2.414 | 9.51 | 0.945 |
| Bleach, 300 ppm | 33.346 | 1.293 | 1.32 | 52.29 |
| Bleach, 3,000 ppm | 44.347 | 1.305 | 12.32 | 0.237 |
| EtOH | 33.390 | 0.913 | 1.366 | 51.17 |
| pH 2.0 | 32.039 | 1.161 | 0.015 | 99.27 |
| pH 10.0 | 32.743 | 1.395 | 0.719 | 70.28 |
| UV, 60 mJ/cm2 | 32.30 | 0.564 | 0.276 | 87.34 |
| UV, 600 mJ/cm2 | 33.895 | 0.497 | 1.571 | 39.94 |
CT, threshold cycle.
To the best of our knowledge, this is the first study on inactivation of TV. The inactivation characteristics of TV by elevated temperature and EtOH are comparable to FCV and MNV. However, TV seemed to be more resistant to UV radiation, but more sensitive to chlorine than FCV and MNV. The pH stability of TV is also similar to FCV than MNV. These results are informative in assessment of HuNoVs in food industry using the TV as a surrogate.
In this study, we assessed the correlation of qRT-PCR in measuring viral infectivity with TCID50, and an overall poor correlation was observed. While a rough correlation was obtained for heat treatment, significantly lower TCID50 titers were still detected by qRT-PCR for most of the other treatments including chlorine, EtOH, UV, and pH. These results are consistent with the reports that qRT-PCR does not correctly reflect the status of inactivation (9, 16).
The lack of correlation is apparently due to different mechanisms of viral inactivation by different treatments. For example, the treatment of TV with UV radiation, chlorine, or EtOH could result in damage to the viral capsid but not, or less, to the viral RNA. Thus, a virus could lose infectivity with a damaged viral capsid, but the viral RNA could still be intact and capable of detection. In addition, even if a treatment damages the viral RNA, there are likely many hits on the viral genome, and each hit could be lethal to the virus; however, qRT-PCR only amplifies a short region of the viral genome and might still be detected. Thus, we conclude that alternative methods such as HBGA-capture RT-PCR and RNase-coupled qRT-PCR should be used.
The recently reported HBGA-capture qRT-PCR is a promising alternative to discriminate between infectious and noninfectious HuNoVs. In this method, a porcine gastric mucin is used to capture HuNoVs, followed by qRT-PCR to detect viral RNA (5). With the establishment of parameters for TV inactivation, we are currently in the process of correlating infectivity measured by tissue culture–based quantitation methods such as plaque assay and TCID50 against molecular-based quantitation approaches such as qRT-PCR and receptor-mediated capture qRT-PCR. With the establishment of such correlations through surrogates, the infectivity of HuNoVs could then be quantitated with such molecular approaches.
Acknowledgments
This work was supported by the U.S. Department of Agriculture (USDA), Agricultural Research Service, Current Research Information System project 5325-42000-046-06R and the Agriculture and Food Research Initiative projects to X. Jiang. The USDA is an equal opportunity provider and employer.
References
- 1.Baert L, Debevere J, Uyttendaele M. The efficacy of preservation methods to inactivate foodborne viruses. Int J Food Microbiol. 2009;131:83–94. doi: 10.1016/j.ijfoodmicro.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 2.Belliot G, Lavaux A, Souihel D, Agnello D, Pothier P. Use of murine norovirus as a surrogate to evaluate resistance of human norovirus to disinfectants. Appl Environ Microbiol. 2008;74:3315–3318. doi: 10.1128/AEM.02148-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blacklow NR. Norwalk virus and other caliciviruses. In: Baron S, editor. Medical microbiology. 4. University of Texas Medical Branch; Galveston: 1996. [PubMed] [Google Scholar]
- 4.Cannon JL, Papafragkou E, Park GW, Osborne J, Jaykus LA, Vinje J. Surrogates for the study of norovirus stability and inactivation in the environment: a comparison of murine norovirus and feline calicivirus. J Food Prot. 2006;69:2761–2765. doi: 10.4315/0362-028x-69.11.2761. [DOI] [PubMed] [Google Scholar]
- 5.Dancho BA, Chen H, Kingsley DH. Discrimination between infectious and non-infectious human norovirus using porcine gastric mucin. Int J Food Microbiol. 2012;155:222–226. doi: 10.1016/j.ijfoodmicro.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 6.De Roda Husman AM, Bijkerk P, Lodder W, Van Den Berg H, Pribil W, Cabaj A, Gehringer P, Sommer R, Duizer E. Calicivirus inactivation by nonionizing (253.7-nanometer-wavelength [UV]) and ionizing (gamma) radiation. Appl Environ Microbiol. 2004;70:5089–5093. doi: 10.1128/AEM.70.9.5089-5093.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Martino B, Ceci C, Di Profio F, Marsilio F. In vitro inactivation of feline calicivirus (FCV) by chemical disinfectants: resistance variation among field strains. Arch Virol. 2010;155:2047–2051. doi: 10.1007/s00705-010-0795-9. [DOI] [PubMed] [Google Scholar]
- 8.Dolin R, Blacklow NR, DuPont H, Buscho RF, Wyatt RG, Kasel JA, Hornick R, Chanock RM. Biological properties of Norwalk agent of acute infectious nonbacterial gastroenteritis. Proc Soc Exp Biol Med. 1972;140:578–583. doi: 10.3181/00379727-140-36508. [DOI] [PubMed] [Google Scholar]
- 9.Duizer E, Bijkerk P, Rockx B, De Groot A, Twisk F, Koopmans M. Inactivation of caliciviruses. Appl Environ Microbiol. 2004;70:4538–4543. doi: 10.1128/AEM.70.8.4538-4543.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farkas T, Cross RW, Hargitt E, III, Lerche NW, Morrow AL, Sestak K. Genetic diversity and histo-blood group antigen interactions of rhesus enteric caliciviruses. J Virol. 2010;84:8617–8625. doi: 10.1128/JVI.00630-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farkas T, Sestak K, Wei C, Jiang X. Characterization of a rhesus monkey calicivirus representing a new genus of Caliciviridae. J Virol. 2008;82:5408–5416. doi: 10.1128/JVI.00070-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gandhi KM, Mandrell RE, Tian P. Binding of virus-like particles of Norwalk virus to romaine lettuce veins. Appl Environ Microbiol. 2010;76:7997–8003. doi: 10.1128/AEM.01566-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gehrke C, Steinmann J, Goroncy-Bermes P. Inactivation of feline calicivirus, a surrogate of norovirus (formerly Norwalk-like viruses), by different types of alcohol in vitro and in vivo. J Hosp Infect. 2004;56:49–55. doi: 10.1016/j.jhin.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 14.Green KY. Summary of the first international workshop on human caliciviruses. J Infect Dis. 2000;181(Suppl):S252–S253. doi: 10.1086/315592. [DOI] [PubMed] [Google Scholar]
- 15.Green KY. Caliciviridae: the noroviruses. In: Knipe DM, Howley DM, editors. Fields virology. 5. Wolters Kluwer Health–Lippincott Williams and Wilkins; Philadelphia: 2007. pp. 949–978. [Google Scholar]
- 16.Hewitt J, Rivera-Aban M, Greening GE. Evaluation of murine norovirus as a surrogate for human norovirus and hepatitis A virus in heat inactivation studies. J Appl Microbiol. 2009;107:65–71. doi: 10.1111/j.1365-2672.2009.04179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang X, Huang PW, Zhong WM, Farkas T, Cubitt DW, Matson DO. Design and evaluation of a primer pair that detects both Norwalk- and Sapporo-like caliciviruses by RT-PCR. J Virol Methods. 1999;83:145–154. doi: 10.1016/s0166-0934(99)00114-7. [DOI] [PubMed] [Google Scholar]
- 18.Karst SM, Wobus CE, Lay M, Davidson J, Virgin HW., IV STAT1-dependent innate immunity to a Norwalk-like virus. Science. 2003;299:1575–1578. doi: 10.1126/science.1077905. [DOI] [PubMed] [Google Scholar]
- 19.Keswick BH, Satterwhite TK, Johnson PC, DuPont HL, Secor SL, Bitsura JA, Gary GW, Hoff JC. Inactivation of Norwalk virus in drinking water by chlorine. Appl Environ Microbiol. 1985;50:261–264. doi: 10.1128/aem.50.2.261-264.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kreutz LC, Seal BS, Mengeling WL. Early interaction of feline calicivirus with cells in culture. Arch Virol. 1994;136:19–34. doi: 10.1007/BF01538814. [DOI] [PubMed] [Google Scholar]
- 21.Lee J, Zoh K, Ko G. Inactivation and UV disinfection of murine norovirus with TiO2 under various environmental conditions. Appl Environ Microbiol. 2008;74:2111–2117. doi: 10.1128/AEM.02442-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Le Guyader F, Loisy F, Atmar RL, Hutson AM, Estes MK, Ruvoen-Clouet N, Pommepuy M, Le Pendu J. Norwalk virus-specific binding to oyster digestive tissues. Emerg Infect Dis. 2006;12:931–936. doi: 10.3201/eid1206.051519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.L’Homme Y, Sansregret R, Plante-Fortier E, Lamontagne AM, Ouardani M, Lacroix G, Simard C. Genomic characterization of swine caliciviruses representing a new genus of Caliciviridae. Virus Genes. 2009;39:66–75. doi: 10.1007/s11262-009-0360-3. [DOI] [PubMed] [Google Scholar]
- 24.Maalouf H, Schaeffer J, Parnaudeau S, Le Pendu J, Atmar RL, Crawford SE, Le Guyader FS. Strain-dependent norovirus bioaccumulation in oysters. Appl Environ Microbiol. 2011;77:3189–3196. doi: 10.1128/AEM.03010-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maalouf H, Zakhour M, Le Pendu J, Le Saux JC, Atmar RL, Le Guyader FS. Distribution in tissue and seasonal variation of norovirus genogroup I and II ligands in oysters. Appl Environ Microbiol. 2010;76:5621–5630. doi: 10.1128/AEM.00148-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Makino A, Shimojima M, Miyazawa T, Kato K, Tohya Y, Akashi H. Junctional adhesion molecule 1 is a functional receptor for feline calicivirus. J Virol. 2006;80:4482–44890. doi: 10.1128/JVI.80.9.4482-4490.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park GW, Linden KG, Sobsey MD. Inactivation of murine norovirus, feline calicivirus, and echovirus 12 as surrogates for human norovirus (NoV) and coliphage (F+) MS2 by ultraviolet light (254 nm) and the effect of cell association on UV inactivation. Lett Appl Microbiol. 2011;52:162–167. doi: 10.1111/j.1472-765X.2010.02982.x. [DOI] [PubMed] [Google Scholar]
- 28.Park GW, Sobsey MD. Simultaneous comparison of murine norovirus, feline calicivirus, coliphage MS2, and GII.4 norovirus to evaluate the efficacy of sodium hypochlorite against human norovirus on a fecally soiled stainless steel surface. Foodborne Pathog Dis. 2011;8:1005–1010. doi: 10.1089/fpd.2010.0782. [DOI] [PubMed] [Google Scholar]
- 29.Radford AD, Coyne KP, Dawson S, Porter CJ, Gaskell RM. Feline calicivirus. Vet Res. 2007;38:319–335. doi: 10.1051/vetres:2006056. [DOI] [PubMed] [Google Scholar]
- 30.Scallan E, Griffin PM, Angulo FJ, Tauxe RV, Hoekstra RM. Foodborne illness acquired in the United States—unspecified agents. Emerg Infect Dis. 2011;17:16–22. doi: 10.3201/eid1701.P21101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scipioni A, Mauroy A, Vinje J, Thiry E. Animal noroviruses. Vet J. 2008;178:32–45. doi: 10.1016/j.tvjl.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 32.Shoemaker GK, van Duijn E, Crawford SE, Uetrecht C, Baclayon M, Roos WH, Wuite GJ, Estes MK, Prasad BV, Heck AJ. Norwalk virus assembly and stability monitored by mass spectrometry. Mol Cell Proteomics. 2010;9:1742–1751. doi: 10.1074/mcp.M900620-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steinmann J. Surrogate viruses for testing virucidal efficacy of chemical disinfectants. J Hosp Infect. 2004;56(Suppl):S49–S54. doi: 10.1016/j.jhin.2003.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stuart AD, Brown TD. α2,6-Linked sialic acid acts as a receptor for feline calicivirus. J Gen Virol. 2007;88:177–186. doi: 10.1099/vir.0.82158-0. [DOI] [PubMed] [Google Scholar]
- 35.Tan M, Jiang X. Norovirus and its histo-blood group antigen receptors: an answer to a historical puzzle. Trends Microbiol. 2005;13:285–293. doi: 10.1016/j.tim.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 36.Tan M, Jiang X. Norovirus gastroenteritis, carbohydrate receptors, and animal models. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1000983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taube S, Perry JW, Yetming K, Patel SP, Auble H, Shu L, Nawar HF, Lee CH, Connell TD, Shayman JA, Wobus CE. Ganglioside-linked terminal sialic acid moieties on murine macrophages function as attachment receptors for murine noroviruses. J Virol. 2009;83:4092–4101. doi: 10.1128/JVI.02245-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tian P, Engelbrektson AL, Jiang X, Zhong W, Mandrell RE. Norovirus recognizes histo-blood group antigens on gastrointestinal cells of clams, mussels, and oysters: a possible mechanism of bioaccumulation. J Food Prot. 2007;70:2140–2147. doi: 10.4315/0362-028x-70.9.2140. [DOI] [PubMed] [Google Scholar]
- 39.Wei C, Farkas T, Sestak K, Jiang X. Recovery of infectious virus by transfection of in vitro–generated RNA from Tulane calicivirus cDNA. J Virol. 2008;82:11429–11436. doi: 10.1128/JVI.00696-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whitehead K, McCue KA. Virucidal efficacy of disinfectant actives against feline calicivirus, a surrogate for norovirus, in a short contact time. Am J Infect Control. 2009;38:26–30. doi: 10.1016/j.ajic.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 41.Wobus CE, Karst SM, Thackray LB, Chang KO, Sosnovtsev SV, Belliot G, Krug A, Mackenzie JM, Green KY, Virgin HW. Replication of Norovirus in cell culture reveals a tropism for dendritic cells and macrophages. PLoS Biol. 2004;2:e432. doi: 10.1371/journal.pbio.0020432. [DOI] [PMC free article] [PubMed] [Google Scholar]


