Abstract
Previous methods for the quantitative analysis of phytosterols have usually used GC-MS and require elaborate sample preparation including chemical derivatization. Other common methods such as HPLC with absorbance detection do not provide information regarding the identity of the analytes. To address the need for an assay that utilizes mass selectivity while avoiding derivatization, a quantitative method based on LC-tandem mass spectrometry (LC-MS-MS) was developed and validated for the measurement of six abundant dietary phytosterols and structurally related triterpene alcohols including brassicasterol, campesterol, cycloartenol, β-sitosterol, stigmasterol, and lupeol in edible oils. Samples were saponified, extracted with hexane and then analyzed using reversed phase HPLC with positive ion atmospheric pressure chemical ionization tandem mass spectrometry and selected reaction monitoring. The utility of the LC-MS-MS method was demonstrated by analyzing 14 edible oils. All six compounds were present in at least some of the edible oils. The most abundant phytosterol in all samples was β-sitosterol, which was highest in corn oil at 4.35 ± 0.03 mg/g, followed by campesterol in canola oil at 1.84 ± 0.01 mg/g. The new LC-MS-MS method for the quantitative analysis of phytosterols provides a combination of speed, selectivity and sensitivity that exceed those of previous assays.
Keywords: Phytosterols, brassicasterol, campesterol, cycloartenol, β-sitosterol, stigmasterol, lupeol, edible oil, LC-MS-MS, quantitation
Introduction
Phytosterols occur in many food plants, nuts, seeds, vegetables, and edible oils. More than 250 phytosterols have been identified in botanical sources, with β-sitosterol being the most commonly reported. They can exist in plants as free form, as esters with fatty acids, as glycosides, or as acylated steryl glycosides. Other significant phytosterols and related triterpenes in edible plants include brassicasterol, campesterol, stigmasterol, cycloartenol, and lupeol (Figure 1) [1]. Phytosterols have been reported to exhibit anti-inflammatory, antibacterial, antifungal, and antitumor activities [2,3]. These compounds are structurally and biosynthetically related to cholesterol, a principle sterol in animal cells (Figure 1). Early mechanism of action studies indicated that dietary phytosterols block intestinal absorption and subsequent cholesterol re-secretion into chylomicrons [4]. More recently, phytosterols were also shown to reduce intestinal cholesterol absorption by regulating several transporters including the stimulation of trans-intestinal cholesterol excretion [5]. Although some clinical trials have suggested that individuals with elevatedlow-density lipoproteins and high cardiovascular disease risk might benefit from consuming dietary supplements or foods rich in phytosterols, other studies indicate that dietary phytosterols do not reduce the risks of cardiovascular disease [1,4-8]. Nevertheless, phytosterols remain an option for the management of serum cholesterol for those concerned about the side effects of statins [9,10].
Figure 1.
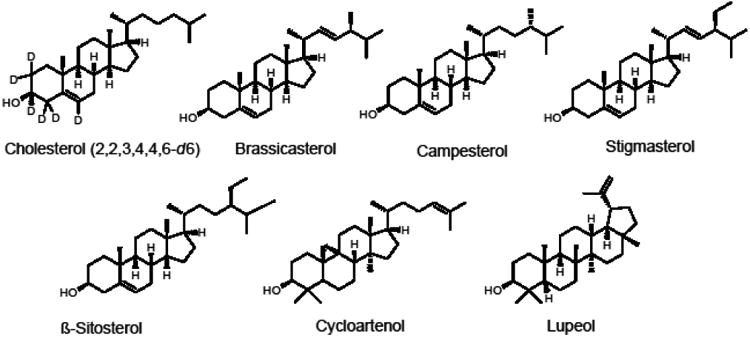
Chemical structures of the phytosterols and related triterpenes measured using LC-MS-MS including the internal standard [d6]-cholesterol.
The most popular technique for the analysis of phytosterols has been gas chromatography with flame ionization or mass spectrometric (MS) detection, and gas chromatography with flame ionization has usually been used to analyze only desmethylsterols such as β-sitosterol, campesterol and stimasterol [11-14]. However, gas chromatographic methods require labor-intensive sample preparation including chemical derivatization before analysis [15]. As an alternative to gas chromatography, normal-phase and reverse-phase high performance-liquid chromatography (HPLC) have been used for the separation of underivatized phytosterols coupled with detectors such as photodiode array absorbance, evaporative light scattering and mass spectrometry [16-21]. Due to the structural similarity of phytosterols, long HPLC separations (up to 80 min) are often required to resolve these compounds [20]. However, the selectivity of tandem mass spectrometric (MS-MS) detection can facilitate quantitative analysis of overlapping peaks and thereby allow shorter (although incomplete) chromatographic runs. Nevertheless, few LC-MS-MS methods have been reported that achieve separation of major phytosterols within 15 min [22]. To address the need for fast and selective quantitative analysis of phytosterols in edible oils, a method was developed and validated that is based on an LC-MS-MS assay with a total run time of only 4 min. The method was applied to the analysis of 14 edible oils for levels of 6 common dietary phytosterols and related triterpene alcohols, including β-sitosterol, campesterol, brassicasterol, stigmasterol, cycloartenol, and lupeol.
Materials and Methods
Reagents and chemicals
HPLC-grade methanol, acetonitrile, n-hexane, and isopropanol were purchased from Thermo Fisher (Hanover Park, IL). Purified water was prepared by using a Millipore (Billerica, MA) Milli-Q purification system. Ethanol and potassium hydroxide as well as the standards brassicasterol (>98%), campesterol (≥97%), cycloartenol (≥90%), β-sitosterol (≥95%), stigmasterol (≥97%), and lupeol (≥94%) were purchased from Sigma-Aldrich (St. Louis, MO). [2,2,3,4,4,6-d6]-Cholesterol (97-98%) was purchased from Cambridge Isotope Laboratories (Andover, MA) for use as an internal standard. Samples of edible oils were purchased from commercial sources as indicated in Table 1.
Table 1.
Concentrations of phytosterols in edible oils.
| Sample name | β-Sitosterol (μg/g) | Campesterol (μg/g) | Brassicasterol (μg/g) | Stigmasterol (μg/g) | Cycloartenol (μg/g) | Lupeol (μg/g) |
|---|---|---|---|---|---|---|
| Canola oil (Richfood) | 3597 ± 596a | 1837 ± 10 | 487.9 ± 6.8 | 34.0 ± 0.6 | 87.0 ± 1.8 | <LLOQ |
| Olive oil (Richfood) | 1209 ± 30 | 54.2 ± 1.6 | <LLOQ | 23.6 ± 0.7 | 338 ± 30 | 19.6 ± 1.1 |
| Hemp oil (Living Harvest) | 3160 ± 109 | 706 ± 15 | <LLOQ | 113.6 ± 4.7 | 169.0 ± 4.8 | <LLOQ |
| Almond oil (International Collection) | 940 ± 32 | 184.2 ± 6.0 | 14.6 ± 1.0 | 156.9 ± 2.4 | 139 ± 10 | 19.5 ± 0.7 |
| Hazelnut oil (International Collection) | 472 ± 28 | 30.1 ± 0.9 | 1.5 ± 0.1 | 9.7 ± 0.1 | 5.4 ± 0.1 | 3.5 ± 0.1 |
| Walnut oil (International Collection) | 1222 ± 25 | 89.9 ± 4.4 | 2.6 ± 0.0 | 13.1 ± 0.9 | 244 ± 10 | <LLOQ |
| Grape seed oil (International Collection) | 1913 ± 63 | 393.5 ± 2.7 | <LLOQ | 340.0 ± 2.3 | 261.7 ± 9.9 | 28.5 ± 1.8 |
| Sesame oil (Spectrum) | 3215 ± 463 | 880 ± 38 | <LLOQ | 418 ± 27 | 156.4 ± 2.0 | 12.8 ± 0.8 |
| Avocado oil (Spectrum) | 2629 ± 92 | 404.3 ± 8.3 | <LLOQ | 172.6 ± 5.6 | 347.1 ± 1.6 | 18.1 ± 2.3 |
| Sunflower oil (Richfood) | 2178 ± 266 | 317.8 ± 8.9 | 13.5 ± 0.5 | 301.0 ± 6.8 | 174.5 ± 3.9 | 30.2 ± 0.1 |
| Peanut oil (Richfood) | 1030 ± 35 | 278 ± 16 | 1.9 ± 0.5 | 154.1 ± 9.8 | 58.2 ± 4.2 | 12.8 ± 0.7 |
| Corn oil (Richfood) | 4354 ± 33 | 1555 ± 25 | <LLOQ | 703 ± 12 | 273.1 ± 4.0 | <LLOQ |
| Macadamia nut oil (Olivado) | 1915 ± 16 | 137.8 ± 4.1 | <LLOQ | 7.1 ± 0.3 | 8.5 ± 0.2 | <LLOQ |
| Sunflower oil (Hollywood) | 1349 ± 37 | 369 ± 16 | 11.1 ± 0.4 | 159.4 ± 9.2 | 140 ± 22 | 42.0 ± 0.1 |
Mean ± Std. Dev.
Sample preparation
The extraction procedure of Bedner, et al. [20] was modified and used for sample preparation. Briefly, 20 mg of edible oil was spiked with 10 μL of internal standard [d6]-cholesterol (250 μg/mL in isopropanol) and then saponified in 2 mL of 2 M ethanolic KOH at 80 °C for 60 min. Deionized water (2 mL) and 3 mL of n-hexane were added, and the mixture was vortex mixed for 1 min. After centrifugation at 3000×g and 4 °C for 5 min, the upper hexane layer was collected, and the extraction process was repeated twice more. The hexane extracts were combined and evaporated to dryness. The residue was reconstituted in 2 mL of isopropanol for analysis using LC-MS-MS.
LC-MS-MS measurement of phytosterols
LC-MS-MS analysis of phytosterols in the saponified extracts of edible oils was carried out using an Applied Biosystems (Foster City, CA) API 4000 triple quadrupole mass spectrometer equipped with positive ion atmospheric pressure chemical ionization (APCI), a Shimadzu (Columbia, MD) Prominence HPLC system and an Agilent (Santa Clara, CA) Zorbax XDB-C18 column (2.1 × 50 mm, 5 μm). An isocratic mobile phase of acetonitrile/methanol (99:1, v/v) was used at a flow rate of 600 μL/min, and the injection volume was 3 μL. Positive ion APCI tandem mass spectrometric analysis was carried out with collision-induced dissociation and selective reaction monitoring (SRM). The ion source temperature was 300 °C, the corona current was 4 μA, and the declustering potential was 75 V. Data were acquired and analyzed using Analyst 1.5 software.
Calibration curves
Stock solutions of standard compounds were prepared by dissolving each compound at 1.000 mg/mL in isopropanol. Working solutions with concentrations of 0.001, 0.010, 0.025, 0.250, 0.500, 1.000, 5.000, 10.000, 25.000, and 50.000 μg/mL were prepared by diluting the stock solutions of standards with isopropanol. A working solution of the internal standard was prepared at a concentration of 250 μg/mL in isopropanol. Aliquots (200 μL each) of standard working solution and 10 μL internal standard working solution were mixed with 2 mL of 2 M ethanolic KOH (note that no blank matrix was available), extracted using the sample preparation method described above, reconstituted in 200 μL isopropanol and then analyzed using LC-MS-MS for the preparation of calibration curves and the determination of linearity, limit of detection (LOD) and lower limit of quantitation (LLOQ).
Recovery
[d6]-Cholesterol at concentrations of 1.25, 12.5 and 50.0 μg/mL was spiked into corn oil and then extracted as described above to assess the recovery of the method.
Detection limit and linearity
Standard curves were constructed by plotting the peak area ratio of each standard and internal standard against the expected concentrations of the calibration standards. A 1/x weighting factor was used. The limit of detection and the lower limit of quantification were defined as the concentrations with signal-to-noise ratios of 3 and 10, respectively. The linear range was investigated up to an analyte concentration of 50 μg/mL.
Results and Discussion
LC-MS-MS measurement of phytosterols
Since phytosterols are highly lipophilic, APCI provided higher sensitivity than did electrospray, and no derivatization was required. Consistent with Cañabate-Díaz and coworkers [23], positive ion APCI provided higher sensitivity than did negative ion mode and was therefore used for all measurements. The base peak of each positive ion APCI mass spectrum (Figure 2) corresponded to the loss of a water molecule from the protonated phytosterol, which is consistent with the literature [18,19,22,23]. Therefore, this ion was used as the precursor for product ion tandem mass spectrometry (Figure 2) and SRM.
Figure 2.
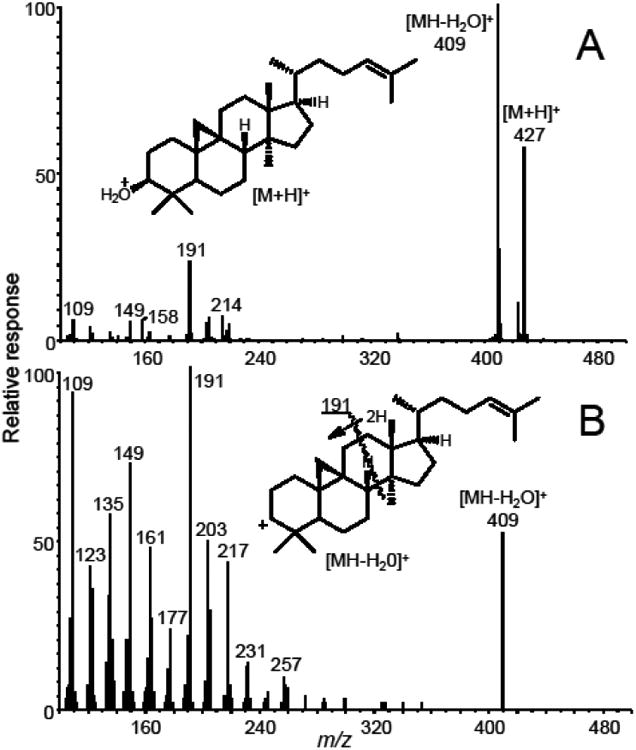
(A) Positive ion APCI mass spectrum of cycloartenol. Note that the most abundant cycloartenol ion of m/z 409, corresponding to [MH-H2O]+, was used as the precursor ion for selected reaction monitoring and that the abundance of this ion was optimized for SRM. (B) Positive ion APCI product ion tandem mass spectrum of the [MH-H2O]+ ion (m/z 409) of cycloartenol. The abundant fragment ion of m/z 191 was used as the product ion for SRM.
An abundant product ion of each [M+H-H2O]+ precursor was selected and optimized as the quantifier for SRM. The proposed structures of these precursor and product ions are shown in Figure 3. Most fragment ions selected for SRM were formed by cleavage of the steroid C-ring (Figures 2 and 3) with the exceptions of brassicasterol and stigmasterol, which eliminated 84 and 98 mass units from the C17 side chain, respectively, to form more abundant product ions of m/z 297 (Figure 4). An ion of m/z 161, corresponding to cleavage of the C-rings of brassicasterol and stigmasterol was detected, but at lower abundance than the ion of m/z 297. The following SRM transitions were used for optimum sensitivity and selectivity for quantitative analysis: m/z 381 to m/z 297 for brassicasterol, m/z 383 to m/z 161 for campesterol, m/z 409 to m/z 191 for cycloartenol, m/z 397 to m/z 161 for β-sitosterol, m/z 395 to m/z 297 for stigmasterol, m/z 409 to m/z 137 for lupeol, and m/z 375 to m/z 167 for the internal standard, [d6]-cholesterol. The optimized collision energies for collision-induced dissociation were 30 V for β-sitosterol, campesterol and lupeol, 24 V for brassicasterol and stigmasterol, 21 V for cycloartenol, and 27 V for [d6]-cholesterol.
Figure 3.
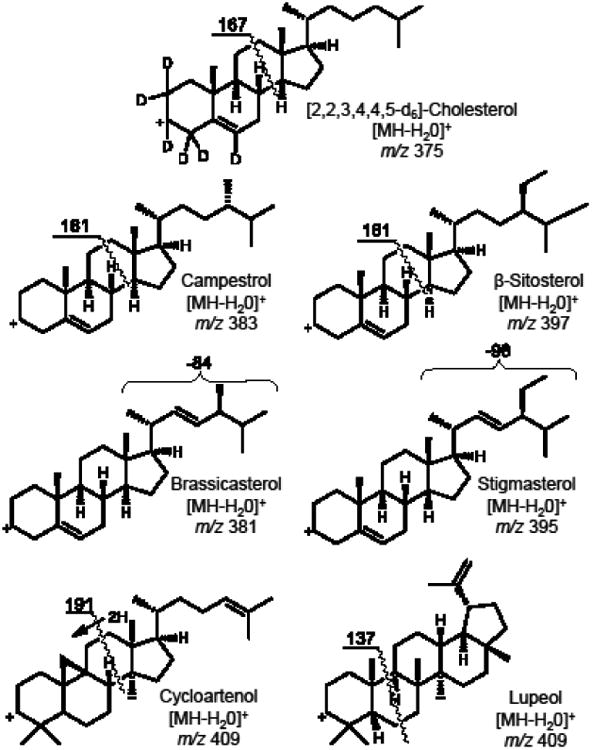
Proposed structures of precursor ions and product ions used during selected reaction monitoring (SRM) LC-MS-MS of phytosterols and related triterpene alcohols.
Figure 4.
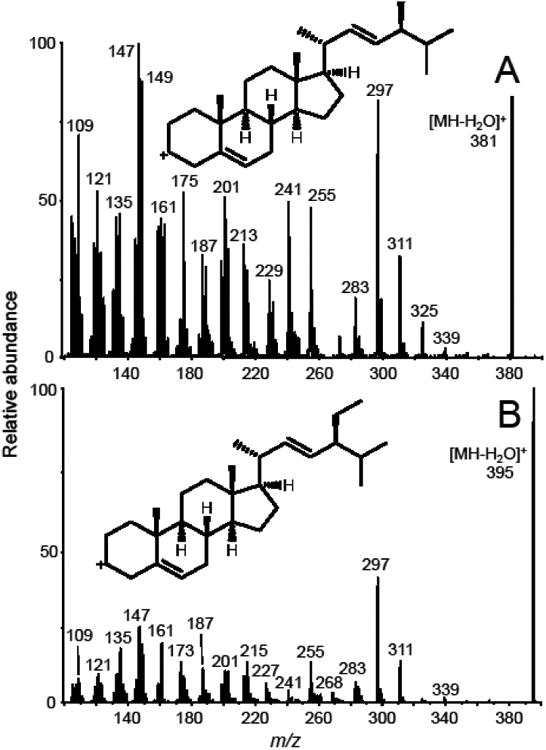
Positive ion APCI product ion tandem mass spectra of the [MH-H2O]+ions of (A) brassicasterol (m/z 381); and (B) stigmasterol (m/z 395). The abundant fragment of m/z 297, which was observed in the tandem mass spectra of both phytosterols, was used as the product ion for SRM.
As shown in Figure 5, the LC-MS-MS analysis of all 7 phytosterols and other triterpenes was complete within 4 min. Because isocratic instead of gradient separation was used, no column re-equilibration was required between analyses. Baseline separation was obtained for some of the analytes, but brassicasterol (tR 1.9 min) and cycloartenol (tR 2.1 min), as well as campesterol (tR 2.3 min) and stigmasterol (tR 2.4 min), were only partially resolved from each other. However, the high selectivity of MS-MS SRM detection facilitated the quantitative analysis of these partially co-eluting compounds without cross-interference.
Figure 5.
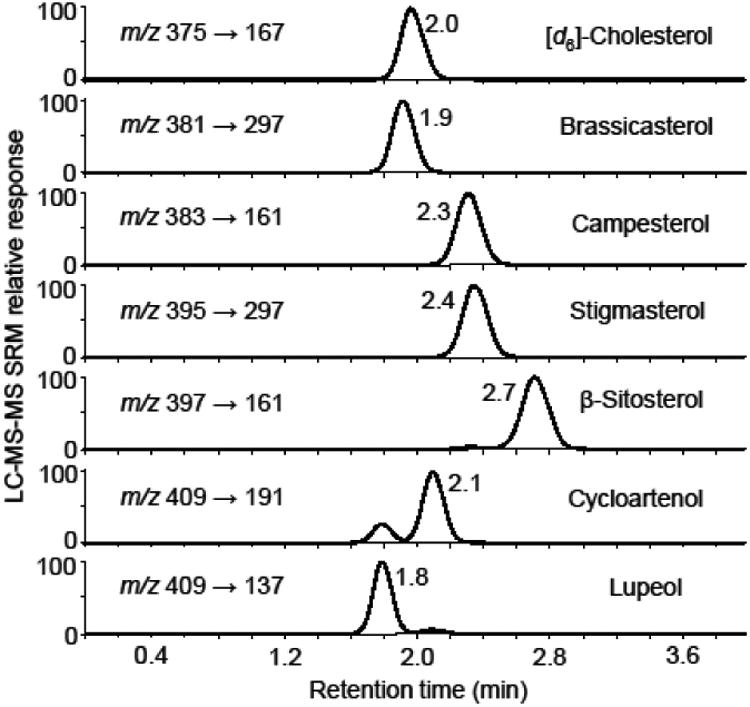
LC-MS-MS analysis of a mixture of phytosterol and related triterpene standards at 5 μg/mL each. The chromatograms were obtained using positive ion APCI, collision-induced dissociation and SRM of the [MH-H2O]+ ions of each analyte.
Calibration curve, linearity and detection limits
Calibration curves were obtained for each standard with good linearity (r2 > 0.99) over the concentration range 1 ng/mL to 50 μg/mL The LOD values ranged from 2 ng/mL to 25 ng/mL for each phytosterol, and the LLOQ values were from 10 ng/mL to 100 ng/mL (Table 2).
Table 2.
Limits of detection (LOD) and lower limits of quantitation (LLOQ) for the LC-MS-MS analysis of phytosterols in edible oils.
| Phytosterol | LOD | LLOQ | ||
|---|---|---|---|---|
|
| ||||
| ng/mL (nM) extract | μg/g oil | ng/mL (nM) extract | μg/g oil | |
| Brassicasterol | 10 (25.1) | 1.0 | 20 (50.3) | 2.0 |
| Campesterol | 5 (12.5) | 0.5 | 10 (25.0) | 1.0 |
| Cycloartenol | 5 (11.7) | 0.5 | 15 (35.2) | 1.5 |
| Lupeol | 2 (4.7) | 2.0 | 10 (23.5) | 1.0 |
| β-Sitosterol | 25 (60.4) | 2.5 | 100 (241.5) | 10. |
| Stigmasterol | 10 (24.3) | 1.0 | 25 (60.7) | 2.5 |
Recovery
The recovery of each analyte was evaluated by comparing the concentration of three replicates of low, medium and high concentrations (1.25, 12.50, and 50.0 μg/mL) of [d6]-cholesterol spiked into corn oil before and after extraction. The recoveries of [d6]-cholesterol from corn oil were 100 ± 5.1%, 111 ± 4.6% and 104 ± 6.0%, respectively. Therefore, there was no concern regarding the efficiency of phytosterol recovery from edible oil.
Quantification of phytosterols in edible oils
Phytosterols and related triterpenes in 14 edible oils were quantified using the new LC-MS-MS method. A major dietary source of phytosterols, edible oils were found to contain substantial levels of all 6 analytes (Table 1 and Figure 6). β-Sitosterol was the most abundant phytosterol in each of the edible oils with levels ranging from a minimum of 472 μg/g in hazelnut oil to a maximum of 4.35 mg/g in corn oil. In addition to high β-sitosterol, levels of campesterol and stigmasterol were also high in corn oil. Canola oil contained the second highest level of β-sitosterol and the highest levels of campesterol (1.84 mg/g) and brassicasterol (488 μg/g) compared with other edible oils (Figure 6). Most of the 6 analytes were present in all of the edible oils, but some, like lupeol (<45 μg/g in all oils), were detected at only low levels in some oils (Table 1). Lupeol has been proposed as a marker compound for the detection olive oil that has been adulterated with less expensive oils, such as hazelnut oil. The presence of lupeol in the olive oil sample used in this study suggests that it had been diluted with one or more other oils (Table 1).
Figure 6.
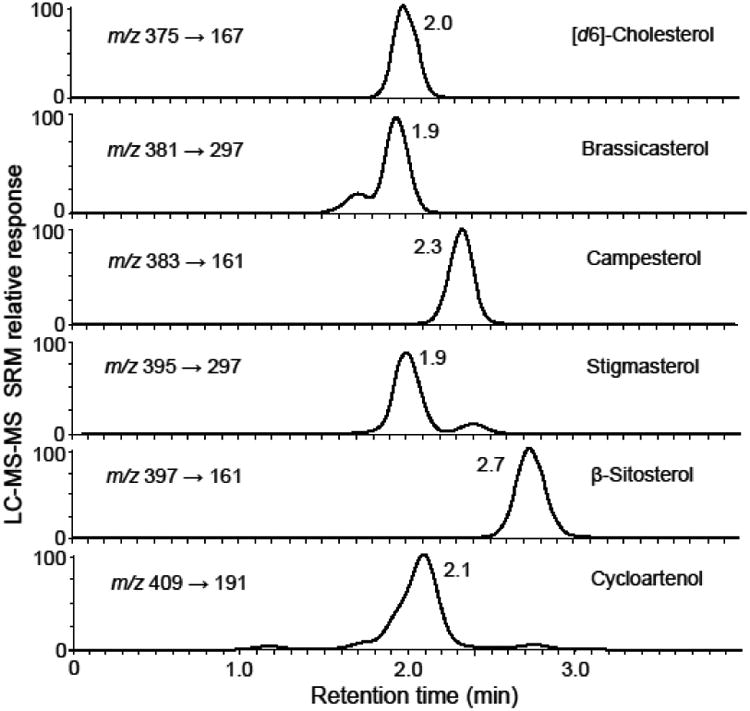
Positive ion APCI LC-MS-MS SRM chromatograms of phytosterols in canola oil. Lupeol (not shown) was below the limit of quantitation.
Several unknown physterols or related compounds were detected at low abundance during the LC-MS-MS SRM analysis of the edible oils. For example in canola oil, an isomer of stigmaterol was detected eluting at ∼2.4 min (Figure 6) that might correspond to avenasterol. A possible isomer of brassicasterol was observed eluting at 1.7 min, and several compounds that might include 24-methylene-cycloartenol were detected eluting just before or after cycloartenol (Figure 6). By preparing additional standard curves and in some cases including additional SRM transitions during LC-MS-MS, this method can be extended to include additional phytosterols such as avenasterol or cholesterol.
Comparison with existing methods
Among the analytical methods reported for the quantitative analysis of phytosterols in botanical oils and other edible matrices, GC-MS of silylated phytosterols is more popular than GC-flame ionization detection or HPLC-UV [24]. This popularity is due, at least in part, to the higher selectivity and/or sensitivity of mass spectrometry-based detection. Following extraction and derivatization, GC-MS separations of phytosterol derivatives require 18 min or more, and LLOQ values only as low as 100 ng/mL have been reported [25]. Besides eliminating the need for phytosterol derivatization, our LC-MS-MS separation requires only 4 min per analysis and has LLOQ values ranging from 10 ng/mL to 100 ng/mL (Table 2).
Several approaches based on electrospray mass spectrometry have been reported for the quantitiative analysis of phytosterols in edible oils and serum. For example, Wewer, et al. [26] used electrospray mass spectrometry without chromatographic separation to measure phytosterols in plant extracts after derivatization with N-chlorobetainyl chloride. However, isomeric phytosterols such as cycloartenol and lupeol cannot be distinguished using this approach or any phytosterols that produce ions isomeric with matrix constituents. Honda, et al. [27] used electrospray LC-MS-MS to measure phytosterols extracted from serum and then derivatized them to picolinyl esters. This approach used a 40 min chromatographic separation.
Unlike electrospray, APCI mass spectrometry-based assays of phytosterols have been reported that do not require derivatization. Lu, et al. [28] used UHPLC-MS with APCI to analyze 10 phytosterols. Although UHPLC was used, the chromatographic separation still required 30 min, and the single stage MS was used instead of tandem mass spectrometry. Mendiara, et al. [29] used LC-MS with APCI but no MS-MS with a 20-min cycle time between analyses, and Zarrouk, et al. [19] used APCI LC-MS-MS with a 65-min separation. In perhaps the only application of atmospheric pressure photoionization LC-MS-MS to the analysis of phytosterols to date, Lembcke, et al. [22] measured 4 phytosterols in human serum but not edible oils. A gradient HPLC separation was used that required only 10-min per run.
In conclusion, we have developed a fast method based on LC-MS-MS for the quantitative analysis of 6 phytosterols and triterpene alcohols in edible oils. The utility of our new method was demonstrated in the quantitative analysis of 6 phytosterols in edible oils from 14 botanicals. To the best of our knowledge, the presence of lupeol in almond oil has not been reported previously, and this finding should be verified by additional studies. Our method requires no sample derivatization and utilizes tandem mass spectrometry with SRM for high sensitivity and high selectivity. Finally, each LC-MS-MS analysis requires only 4 min.
Acknowledgments
These studies were supported by the Hershey Company and National Institutes of Health grant P50AT00155 from the Office of Dietary Supplements and the National Center for Complementary and Alternative Medicine. The authors acknowledge Mr. Yongchao Li for assistance with tandem mass spectrometry.
Abbreviations
- APCI
atmospheric pressure chemical ionization
- HPLC
high performance liquid chromatography
- GC-MS
gas chromatography-mass spectrometry
- LC-MS-MS
liquid chromatography-tandem mass spectrometry
- LLOQ
lower limit of quantitation
- LOD
limit of detection
- SRM
selected reaction monitoring
Footnotes
Conflict of Interest: The authors have no conflicts of interest to report.
References
- 1.Marangoni F, Poli A. Phytosterols and cardiovascular health. Pharmacol Res. 2010;61:193–199. doi: 10.1016/j.phrs.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Othman RA, Moghadasian MH. Beyond cholesterol-lowering effects of plant sterols: clinical and experimental evidence of anti-inflammatory properties. Nutr Rev. 2011;69:371–382. doi: 10.1111/j.1753-4887.2011.00399.x. [DOI] [PubMed] [Google Scholar]
- 3.Siddique HR, Saleem M. Beneficial health effects of lupeol triterpene: A review of preclinical studies. Life Sci. 2011;88:285–293. doi: 10.1016/j.lfs.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 4.Amiot MJ, Knol D, Cardinault N, Nowicki M, Bott R, Antona C, Borel P, Bernard JP, Duchateau G, Lairon D. Phytosterol ester processing in the small intestine: impact on cholesterol availability for absorption and chylomicron cholesterol incorporation in healthy humans. J Lipid Res. 2011;52:1256–1264. doi: 10.1194/jlr.M013730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Smet E, Mensink RP, Plat J. Effects of plant sterols and stanols onintestinal cholesterol metabolism: suggested mechanisms from past to present. Mol Nutr Food Res. 2012;56:1058–1072. doi: 10.1002/mnfr.201100722. [DOI] [PubMed] [Google Scholar]
- 6.Sanclemente T, Marques-Lopes I, Fajó-Pascual M, Cofán M, Jarauta E, Ros E, Puzo J, García-Otín AL. Naturally-occurring phytosterols in the usual diet influence cholesterol metabolism in healthy subjects. Nutr Metab Cardiovasc Dis. 2012;22:849–55. doi: 10.1016/j.numecd.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 7.Polagruto JA, Wang-Polagruto JF, Braun MM, Lee L, Kwik-Uribe C, Keen CL. Cocoa flavanol-enriched snack bars containing phytosterols effectively lower total and low-density lipoprotein cholesterol levels. J Am Diet Assoc. 2006;106:1804–1813. doi: 10.1016/j.jada.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 8.Genser B, Silbernagel G, De Backer G, Bruckert E, Carmena R, Chapman MJ, Deanfield J, Descamps OS, Rietzschel ER, Dias KC, März W. Plant sterols and cardiovascular disease: a systematic review and meta-analysis. Eur Heart J. 2012;33:444–451. doi: 10.1093/eurheartj/ehr441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weingärtner O, Böhm M, Laufs U. Controversial role of plant sterol esters inthe management of hypercholesterolemia. Eur Heart J. 2008;30:404–409. doi: 10.1093/eurheartj/ehn580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lerman RH, Minich DM, Darland G, Lamb JJ, Chang JL, Hsi A, Bland JS, Tripp ML. Subjects with elevated LDL cholesterol and metabolic syndrome benefit from supplementation with soy protein, phytosterols, hops rho iso-alpha acids, and Acacianilotica proanthocyanidins. J Clin Lipidol. 2010;4:59–68. doi: 10.1016/j.jacl.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Redberg RF, Katz MH. Healthy men should not take statins. JAMA. 2012;307:1491–1492. doi: 10.1001/jama.2012.423. [DOI] [PubMed] [Google Scholar]
- 12.Azadmard-Damirchi S. Review of the use of phytosterols as a detection tool for adulteration of olive oil with hazelnut oil. Food Addit Contam Part A. 2009;27:1–10. doi: 10.1080/02652030903225773. [DOI] [PubMed] [Google Scholar]
- 13.Cunha SS, Fernandes JO, Oliveira MB. Quantification of free and esterified sterols in Portuguese olive oils by solid-phase extraction and gas chromatography-mass spectrometry. J Chromatogr A. 2006;1128:220–227. doi: 10.1016/j.chroma.2006.06.039. [DOI] [PubMed] [Google Scholar]
- 14.Nang Lau H, Puah C, Choo Y, Ma A, Chuah C. Simultaneous quantification of free fatty acids, free sterols, squalene, and acylglycerol molecular species in palm oil by high-temperature gas chromatography-flame ionization detection. Lipids. 2005;40:523–528. doi: 10.1007/s11745-005-1413-1. [DOI] [PubMed] [Google Scholar]
- 15.Kpoviéssi DSS, Gbaguidi F, Gbénou J, Accrombessi G, Moudachirou M, Rozet E, Hubert P, Quetin-Leclercq J. Validation of a method for the determination of sterols and triterpenes in the aerial part of Justicia anselliana (Nees) T. Anders by capillary gas chromatography. J Pharm Biomed Anal. 2008;48:1127–1135. doi: 10.1016/j.jpba.2008.08.036. [DOI] [PubMed] [Google Scholar]
- 16.Careri M, Elviri L, Mangia A. Liquid chromatography-UV determination and liquid chromatography-atmospheric pressure chemical ionization mass spectrometric characterization of sitosterol and stigmasterol in soybean oil. J Chromatogr A. 2001;935:249–257. doi: 10.1016/s0021-9673(01)01079-2. [DOI] [PubMed] [Google Scholar]
- 17.Nair VDP, Kanfer I, Hoogmartens J. Determination of stigmasterol, β-sitosteroland stigmastanol in oral dosage forms using high performance liquid chromatographywith evaporative light scattering detection. J Pharm Biomed Anal. 2006;41:731–737. doi: 10.1016/j.jpba.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 18.Lerma-García MAJS, Concha-Herrera V, Herrero-Martínez JM, Simó-Alfonso EF. Classification of extra virgin olive oils produced at La Comunitat Valencianaaccording to their genetic variety using sterol profiles established by high-performanceliquid chromatography with mass spectrometry detection. J Agric Food Chem. 2009;57:10512–10517. doi: 10.1021/jf902322c. [DOI] [PubMed] [Google Scholar]
- 19.Zarrouk W, Carrasco-Pancorbo AA, Segura-Carretero A, Ferníndez-Gutiérrez A, Zarrouk M. Exploratory characterization of the unsaponifiable fraction of Tunisian virgin olive oils by a global approach with HPLC-APCI-IT MS/MS analysis. J Agric Food Chem. 2010;58:6418–6426. doi: 10.1021/jf100024c. [DOI] [PubMed] [Google Scholar]
- 20.Bedner M, Schantz MM, Sander LC, Sharpless KE. Development of liquid chromatographic methods for the determination of phytosterols in Standard Reference Materials containing saw palmetto. J Chromatogr A. 2008;1192:74–80. doi: 10.1016/j.chroma.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 21.Sánchez-Machado DI, López-Hernández J, Paseiro-Losada P, López-Cervantes J. An HPLC method for the quantification of sterols in edible seaweeds. Biomed Chromatogr. 2004;18:183–190. doi: 10.1002/bmc.316. [DOI] [PubMed] [Google Scholar]
- 22.Lembcke J, Ceglarek U, Fiedler GM, Baumann S, Leichtle A, Thiery J. Rapid quantification of free and esterified phytosterols in human serum using APPI-LC-MS/MS. J Lipid Res. 2005;46:21–26. doi: 10.1194/jlr.C400004-JLR200. [DOI] [PubMed] [Google Scholar]
- 23.Cañabate-Díaz B, Segura Carretero A, Fernández-Gutiérrez A, Belmonte Vega A, Garrido Frenich A, Martínez Vidal JL, Duran Martos J. Separation and determination of sterols in olive oil by HPLC-MS. Food Chemistry. 2007;102:593–598. [Google Scholar]
- 24.Noppe H, Le Bizec B, Verheyden K, De Brabander HF. Novel analytical methods for the determination of steroid hormones in edible matrices. Anal Chim Acta. 2008;611:1–16. doi: 10.1016/j.aca.2008.01.066. [DOI] [PubMed] [Google Scholar]
- 25.Saraiva D, Semedo R, Castilho MD, Silva JM, Ramos F. Selection of the derivatization reagent--the case of human blood cholesterol, its precursors and phytosterols GC-MS analyses. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879:3806–3811. doi: 10.1016/j.jchromb.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 26.Wewer V, Dombrink I, vom Dorp K, Dörmann P. Quantification of sterol lipids in plants by quadrupole time-of-flight mass spectrometry. J Lipid Res. 2011;52:1039–1054. doi: 10.1194/jlr.D013987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Honda A, Yamashita K, Miyazaki H, Shirai M, Ikegami T, Xu G, Numazawa M, Hara T, Matsuzaki Y. Highly sensitive analysis of sterol profiles in human serum by LC-ESI-MS/MS. J Lipid Res. 2008;49:2063–2073. doi: 10.1194/jlr.D800017-JLR200. [DOI] [PubMed] [Google Scholar]
- 28.Lu B, Zhang Y, Wu X, Shi J. Separation and determination of diversiform phytosterols in food materials using supercritical carbon dioxide extraction and ultraperformance liquid chromatography-atmospheric pressure chemical ionization-mass spectrometry. Anal Chim Acta. 2007;588:50–63. doi: 10.1016/j.aca.2007.01.067. [DOI] [PubMed] [Google Scholar]
- 29.Mendiara I, Domeño C, Nerin C. Development a fast sample treatment for the analysis of free and bonded sterols in human serum by LC-MS. J Sep Sci Oct. 2012;30 doi: 10.1002/jssc.201200519. [DOI] [PubMed] [Google Scholar]


