Abstract
Background
To provide an estimate for the burden of neural tube defects (NTD) in low– and middle–income countries (LMIC) and explore potential public health policies that may be implemented. Although effective interventions are available to prevent NTD, there is still considerable childhood morbidity and mortality present in LMIC.
Methods
A search of Medline, EMBASE, Global Health Library and PubMed identified 37 relevant studies that provided estimates of the burden of NTD in LMIC. Information on burden of total NTD and specific NTD types was separated according to the denominator into two groups: (i) estimates based on the number of live births only; and (ii) live births, stillbirths and terminations. The data was then extracted and analysed.
Results
The search retrieved NTD burden from 18 countries in 6 WHO regions. The overall burden calculated using the median from studies based on livebirths was 1.67/1000 (IQR = 0.98–3.49) for total NTD burden, 1.13/1000 (IQR = 0.75–1.73) for spina bifida, 0.25/1000 (IQR = 0.08–1.07) for anencephaly and 0.15/1000 (IQR = 0.08–0.23) for encephalocele. Corresponding estimates based on all pregnancies resulting in live births, still births and terminations were 2.55/1000 (IQR = 1.56–3.91) for total NTD burden, 1.04/1000 (IQR = 0.67–2.48) for spina bifida, 1.03/1000 (IQR = 0.67–1.60) for anencephaly and 0.21 (IQR = 0.16–0.28) for encephalocele. This translates into about 190 000neonates who are born each year with NTD in LMIC.
Conclusion
Limited available data on NTD in LMIC indicates the need for additional research that would improve the estimated burden of NTD and recommend suitable aid policies through maternal education on folic acid supplementation or food fortification.
Every year, more than 300 000 children are born with neural tube defects (NTD) [1-6]. NTD are a group of congenital abnormalities that still cause hundreds of thousands of deaths in 0–4 years age group, while similar number of surviving children remain disabled for life [1-6]. One of the Millennium Development Goals initiated by the United Nations was dedicated to reducing global mortality rates of children in this age group. Since 1990, global child mortality has been declining largely due to the focus on communicable diseases, which included the expansion of immunisation programmes, promotion of breast–feeding and increased provision of mosquito bednets in many countries worldwide [2]. This reduction of mortality has led to the neglected causes of child mortality to be exposed, including that of congenital abnormalities [3-6].
NTD are one of the most common presenting birth defects, arising as a result of incomplete closure of the brain or spinal cord in the 3rd and/or 4th week of pregnancy [3]. NTD can be classified as open or closed, depending on whether neural tissues are exposed or covered by skin, respectively. Open NTD are more frequent and include spina bifida, anencephaly and encephalocele. Closed NTD, such as tethered cord syndrome, are less frequent in comparison [4].
The best known risk factor for foetal NTD is maternal folate deficiency, arising from low levels of vitamin B9 (folic acid) [7,8]. Maternal vitamin B12 deficiency has only recently been shown to independently contribute to risk of NTD [9,10]. Additional risk factors for NTD development include a positive family history, smoking and indoor air pollution from coal and biomass heating used predominantly in developing countries [11-15]. Moreover, NTD are related to maternal socio–economic status, education, area of residence, and maternal nutrient deficiency or obesity [16,17].
NTD can be identified through simple prenatal testing using ultrasound imaging or maternal serum alpha–fetoprotein (MSAFP) level screening [18]. Abnormal elevation of MSAFP is a relatively specific and sensitive test for detection of NTD [18]. The abnormal presence of acetylcholinesterases (AChE) in amniotic fluid determined through amniocentesis can also be used for screening of NTD. However, a higher–than–normal test result is often not diagnostic and further evaluation should always be undertaken [19,20].
Since the discovery of folic acid as an effective intervention for prevention of neural tube defects [21], many countries have recommended folic acid intake before conception and during pregnancy. However, the dramatic 20% decrease in NTD birth burden after mandatory folic acid fortification (FAF) of enriched products in the US in 1998 showed that there may be more practical ways to administer this intervention [22]. Since this example, many countries such as Chile, Saudi Arabia and South Africa have implemented similar measures to staple food [23-25]. Despite folic acid being a well–known, cost–effective intervention, many developing countries continue to have either ineffective or no policy to increase maternal uptake of folic acid to prevent NTD.
The aims and objectives of this systematic review were:
1. To provide an estimate of NTD burden in LMIC by systematically reviewing literature available in public domain;
2. To examine and discuss the significance of these findings and consider clinical and cost–effective interventions and health policies with regards to NTD.
METHODS
A systematic literature review was conducted to search for published literature regarding population–based NTD burden estimates in LMIC, through the use of electronic databases: Medline, Embase, Global Health Library and PubMed. Potential further data were searched for on Google Scholar and by crosschecking reference lists from review articles. The search used Medical Subject Headings (MeSH) and key words for the burden of NTD in LMIC, as outlined by the World Bank. Limits of “human” and “2000–current” were used to obtain the most up to date NTD burden information. The last searches of the four databases were conducted on 6 February 2013. Search terms for Medline are shown in Table 1 and were modified for other databases as required.
Table 1.
Search terms for Medline
| 1. |
exp Developing Countries/ |
| 2. |
Developing countr*.tw |
| 3. |
(developing adj3 countr*).tw |
| 4. |
africa/ or africa, northern/ or algeria/ or egypt/ or libya/ or morocco/ or tunisia/ or “africa south of the sahara”/ or africa, central/ or cameroon/ or central african republic/ or chad/ or congo/ or “democratic republic of the congo”/ or equatorial guinea/ or gabon/ or africa, eastern/ or burundi/ or djibouti/ or eritrea/ or ethiopia/ or kenya/ or rwanda/ or somalia/ or sudan/ or tanzania/ or uganda/ or africa, southern/ or angola/ or botswana/ or lesotho/ or malawi/ or mozambique/ or namibia/ or south africa/ or swaziland/ or zambia/ or zimbabwe/ or benin/ or burkinafaso/ or cape verde/ or cote d'ivoire/ or gambia/ or ghana/ or guinea/ or guinea–bissau/ or liberia/ or mali/ or mauritania/ or niger/ or nigeria/ or senegal/ or sierra leone/ or togo/ or americas/ or caribbean region/ or west indies/ or “antigua and barbuda”/ or bahamas/ or barbados/ or cuba/ or dominica/ or dominican republic/ or grenada/ or guadeloupe/ or haiti/ or jamaica/ or martinique/ or netherlandsantilles/ or puertorico/ or “saint kitts and nevis”/ or saint lucia/ or “saint vincent and the grenadines”/ or “trinidad and tobago”/ or central america/ or belize/ or costa rica/ or el salvador/ or guatemala/ or honduras/ or nicaragua/ or panama/ or panama canal zone/ or latinamerica/ or mexico/ or south america/ or argentina/ or bolivia/ or brazil/ or chile/ or colombia/ or ecuador/ or frenchguiana/ or guyana/ or paraguay/ or peru/ or suriname/ or uruguay/ or venezuela/ or kazakhstan/ or kyrgyzstan/ or tajikistan/ or turkmenistan/ or uzbekistan/ or borneo/ or brunei/ or cambodia/ or east timor/ or indonesia/ or laos/ or malaysia/ or mekong valley/ or myanmar/ or philippines/ or thailand/ or vietnam/ or bangladesh/ or bhutan/ or india/ or sikkim/ or middle east/ or afghanistan/ or iran/ or iraq/ or israel/ or jordan/ or lebanon/ or saudiarabia/ or syria/ or turkey/ or united arab emirates/ or yemen/ or nepal/ or pakistan/ or srilanka/ or far east/ or china/ or tibet/ or “democratic people's republic of korea”/ or mongolia/ or taiwan/ or albania/ or lithuania/ or bosnia–herzegovina/ or bulgaria/ or “macedonia (republic)”/ or moldova/ or montenegro/ or romania/ or russia/ or bashkiria/ or dagestan/ or moscow/ or siberia/ or serbia/ or ukraine/ or armenia/ or atlantic islands/ or azores/ azerbaijan/ or “georgia (republic)”/ or comoros/ or madagascar/ or mauritius/ or seychelles/ or vanuatu/ or micronesia/ or palau/ or expsamoa/ or americansamoa/ or “independent state of samoa”/ or tonga/ |
| 5. |
exp neural tube defects/ or anencephaly/ or arnold–chiari malformation/ or encephalocele/ or meningocele/ or meningomyelocele/ or “pentalogy of cantrell”/ or exp spinal dysraphism/ or nervous system malformation |
| 6. |
“neural tube defect”.tw |
| 7. |
“neural tube defects”.tw |
| 8. |
NTD.tw |
| 9. |
exp Prevalence/ |
| 10. |
prevalen*.tw |
| 11. |
1 or 2 or 3 or 4 |
| 12. |
5 or 6 or 7 or 8 |
| 13. |
9 or 10 |
| 14. |
11 and 12 and 13 |
| 15. | Limit 14 to (humans and yr = ”2000 –Current”) |
Study selection
The inclusion criteria for relevant papers included population or hospital based studies conducted in LMIC, which were geographically defined taking into account both the World Health Organization's and the World Bank's classification and treating any discrepancies in an inclusive, rather than exclusive way. The studies needed to have clearly expressed NTD burden showing a denominator, published between 2000 and 2013. The searches were limited to the period after the year 2000 in order to generate an estimate that is reflective of reasonably recent NTD trends. No limit on language and publication type was set. Keeping in mind that many babies with NTD are stillborn or terminated through miscarriages and abortions, we decided to include studies with live births, stillbirths and terminations as a separate body of evidence, in addition to studies that used live births–based denominators to report the burden of NTD.
Studies conducted solely in specialist hospital units were excluded, as they are likely to report a burden enriched for severe cases that would not be representative of the general population. Studies with incomplete data or where NTD burden could not be calculated were also excluded.
Data extraction
For the 37 retained studies, relevant data were extracted and compiled into Microsoft Excel spreadsheets. Data including authors, country, study size and diagnostic criteria for specific NTD type and total NTD cases were extracted. Types of NTD included spina bifida, myelomeningocele, meningocele, anencephaly, encephalocele and “other NTD types”. Burden was expressed using the number of cases observed and a total sample of live births (or, alternatively, a total sample of live births, stillbirths and terminations).
Data analysis
When the number of affected children was not specifically provided in the study, it was calculated with the sample population using the following equation:
Estimated NTD burden = Number of observed NTD cases/Sample size (eg, number of live births) × 1000
The estimates of the burden provided in the retained studies were separated into two categories: those in which the denominator was based on the number of live births, and the other group in which live births, stillbirths and terminations were all included. Wherever this information was available for both categories, figures were separately added to the respective groups. The median estimate of the NTD burden and the inter–quartile range (IQR) for all LMIC regions was then determined, based on the retained 37 studies. Eventually, the median was multiplied by the number of livebirths in LMIC in the year 2010, according to UN Population Division's estimates (www.un.org/esa/population/), to determine the absolute number of NTD cases that has been introduced to the LMIC in 2010.
RESULTS
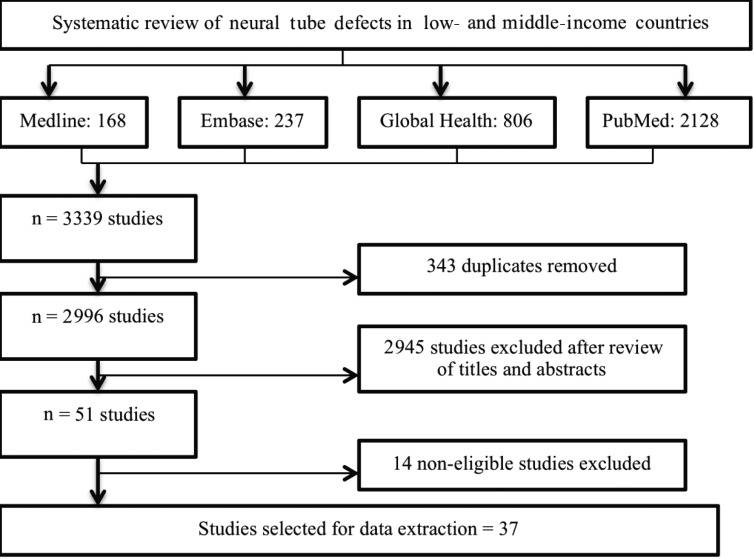
A review of relevant databases performed independently by two researchers (AL and SS) identified a total of 3339 studies, but only 37 satisfied all criteria for inclusion (as shown in Figure 1). Of the retained studies, 20 reported NTD rates in live births only, 14 reported rates in live births, stillbirths and terminations combined, and 3 studies reported both.
Figure 1.
A summary of the process of literature search.
The median sample size from all reviewed papers was 36 331, which corresponded well to a typical study size. The median sample population in studies based on live births was 35 974, compared to 49 534 in studies based on live births, stillbirths and terminations.
The search retrieved NTD burden from 18 countries in 6 WHO regions (Table 2). The overall burden calculated using the median from studies based on live births was 1.67/1000 (IQR = 0.98–3.49) for total NTD burden (Table 3 and 4), 1.13/1000 (IQR = 0.75–1.73) for spina bifida (Table 3 and 5), 0.25/1000 (IQR = 0.08–1.07) for anencephaly (Table 3 and 6) and 0.15/1000 (IQR = 0.08–0.23) for encephalocele (Table 3 and 7).Corresponding estimates based on all pregnancies resulting in live births, still births and terminations were 2.55/1000 (IQR = 1.56–3.91) for total NTD burden (Table 3 and 8), 1.04/1000 (IQR = 0.67–2.48) for spina bifida (Table 3 and 9), 1.03/1000 (IQR = 0.67–1.60) for anencephaly (Table 3 and 10) and 0.21 (IQR = 0.16–0.28) for encephalocele (Table 3 and 11). This translates into about 190 000 neonates who are born each year with NTD in LMICs.
Table 2.
Distribution of retained studies by WHO regions
| Number of studies | ||||
|---|---|---|---|---|
|
WHO region |
Country |
Total studies |
Live births only |
Live births, stillbirths & terminations |
| Western Pacific | China |
6 |
1 |
5 |
| Malaysia |
1 |
0 |
1 |
|
| South East Asia | India |
3 |
2 |
1 |
| Pakistan |
1 |
1 |
0 |
|
| Thailand |
1 |
1 |
0 |
|
| Eastern Mediterranean | Jordan |
2 |
2 |
0 |
| Saudi Arabia |
2 |
2 |
0 |
|
| Iran |
4 |
1 |
3 |
|
| Europe | Azerbaijan |
1 |
0 |
1 |
| Russia |
1 |
1 |
0 |
|
| Ukraine |
1 |
0 |
1 |
|
| Turkey |
3 |
2 |
1 |
|
| Africa | Cameroon |
1 |
1 |
0 |
| South Africa |
1 |
1 |
0 |
|
| Americas | Brazil |
3 |
2 |
1 |
| Colombia |
1 |
1 |
0 |
|
| Peru |
1 |
1 |
0 |
|
| Chile | 4 | 1 | 3 | |
Table 3.
A summary of estimates of the burden of neural tube defects and its sub–types from 37 retained studies from low and middle–income countries
| Studied outcome | Denominator | Number of Studies | Median (per 1000) | Inter–quartile range (per 1000) | Minimum(per 1000) | Maximum (per 1000) |
|---|---|---|---|---|---|---|
| All neural tube defects | LB |
23 |
1.67 |
0.98–3.49 |
0.50 |
12.41 |
| LB+SB+TP |
17 |
2.55 |
1.56–3.91 |
0.86 |
19.94 |
|
| Spina bifida | LB |
14 |
1.13 |
0.75–1.73 |
0.38 |
5.90 |
| LB+SB+TP |
15 |
1.04 |
0.67–2.48 |
0.35 |
5.81 |
|
| Anencephaly | LB |
13 |
0.25 |
0.08–1.07 |
0.01 |
11.33 |
| LB+SB+TP |
16 |
1.03 |
0.67–1.60 |
0.30 |
8.26 |
|
| Encephalocele | LB |
9 |
0.15 |
0.08–0.23 |
0.03 |
0.39 |
| LB+SB+TP | 13 | 0.21 | 0.16–0.28 | 0.07 | 2.65 |
LB – live births; LB+SB+TP – live births, stillbirths and terminated pregnancies
Table 4.
Studies that reported rates for total NTD burden based on live births only
| Author and reference | Sample size | Cases | Rate (per 1000 live births) |
|---|---|---|---|
| Amarin et al. [26] |
61 447 |
16 |
0.95 |
| Aqrabawi [11] |
5088 |
33 |
6.50 |
| Asindi et al. [27] |
82 176 |
64 |
0.78 |
| Bademci et al. [28] |
5499 |
17 |
3.09 |
| Behrooz et al. [29] |
13 262 |
56 |
4.22 |
| Chen et al. [30] |
26 599 |
48 |
1.80 |
| Cherian et al. [31] |
1218 |
10 |
8.21 |
| Cortes et al. [32] |
59 627 |
67 |
1.12 |
| Costa et al. [33] |
9386 |
11 |
1.17 |
| Gu et al. [13] |
6420 |
25 |
3.89 |
| Hertrampf et al. [34] |
117 740 |
114 |
0.97 |
| Kaur et al. [35] |
7400 |
5 |
0.68 |
| Khattak et al. [36] |
5560 |
69 |
12.41 |
| Mandiracioglu et al. [37] |
36 331 |
56 |
1.54 |
| Njamnshi et al. [38] |
52 710 |
98 |
1.86 |
| Pachajoa et al. [39] |
32 995 |
55 |
1.67 |
| Petrova et al. [40] |
141 159 |
298 |
2.11 |
| Pacheco et al. [41] |
24 964 |
124 |
4.97 |
| Ricks et al. [42] |
35 974 |
72 |
2.00 |
| Safdar et al. [24] |
33 489 |
42 |
1.25 |
| Sayed et al. [25] |
46 021 |
45 |
0.98 |
| Wasant et al. [43] |
180 000 |
114 |
0.63 |
| Yuskiv et al. [33] | 75 609 | 38 | 0.50 |
Table5.
Studies that reported rates for the burden of spina bifida based on live births only
| Author and reference | Sample size | Cases | Rate(per 1000 live births) |
|---|---|---|---|
| Aqrabawi[11] |
5088 |
30 |
5.90 |
| Asindi et al. [27] |
82 176 |
46 |
0.56 |
| Bademci et al. [28] |
5499 |
11 |
2.00 |
| Behrooz et al. [29] |
13 262 |
23 |
1.73 |
| Cherian et al. [31] |
1218 |
6 |
4.93 |
| Costa et al. [44] |
9386 |
7 |
0.75 |
| Khattak et al. [36] |
5560 |
5 |
0.90 |
| Mandiracioglu et al. [37] |
36 331 |
43 |
1.18 |
| Njamnshi et al. [38] |
52 710 |
65 |
1.23 |
| Petrova et al. [40] |
141 159 |
147 |
1.04 |
| Ricks et al. [42] |
35 974 |
62 |
1.72 |
| Safdar et al. [24] |
33 489 |
36 |
1.07 |
| Sayed et al. [25] |
46 021 |
25 |
0.54 |
| Yuskiv et al. [33] | 75 609 | 29 | 0.38 |
Table 6.
Studies that reported rates for the burden of anencephaly based on live births only
| Author and reference | Sample size | Cases | Rate (per 1000 live births) |
|---|---|---|---|
| Asindi et al. [27] |
82 176 |
3 |
0.04 |
| Behrooz et al. [29] |
13 262 |
30 |
2.26 |
| Cherian et al. [31] |
1218 |
3 |
2.46 |
| Costa et al. [44] |
9386 |
1 |
0.11 |
| Khattak et al. [36] |
5560 |
63 |
11.33 |
| Mandiracioglu et al. [45] |
36 331 |
4 |
0.11 |
| Njamnshi et al. [38] |
52 710 |
4 |
0.08 |
| Petrova et al. [40] |
141 159 |
151 |
1.07 |
| Ricks et al. [42] |
35 974 |
10 |
0.28 |
| Safdar et al. [24] |
33 489 |
1 |
0.03 |
| Sayed et al. [25] |
46 021 |
17 |
0.37 |
| Wasant et al. [43] |
180 000 |
45 |
0.25 |
| Yuskiv et al. [33] | 75 609 | 1 | 0.01 |
Table 7.
Studies that reported rates for the burden of encepalocele based on live births only
| Author and reference | Sample size | Cases | Rate (per 1000 live births) |
|---|---|---|---|
| Aqrabawi [11] |
5088 |
2 |
0.39 |
| Asindi et al. [27] |
82 176 |
15 |
0.18 |
| Behrooz et al. [29] |
13 262 |
3 |
0.23 |
| Costa et al. [44] |
9386 |
3 |
0.32 |
| Mandiracioglu et al. [45] |
36 331 |
1 |
0.03 |
| Njamnshi et al. [38] |
52 710 |
5 |
0.09 |
| Safdar et al. [24] |
33 489 |
5 |
0.15 |
| Wasant et al. [43] |
180 000 |
14 |
0.08 |
| Yuskiv et al. [33] | 75 609 | 3 | 0.03 |
Table 8.
Studies that reported rates for total NTD burden based on live births, stillbirths and terminations of pregnancy
| Author and reference | Sample size | Cases | Rate (per 1000 live births stillbirths and terminations) |
|---|---|---|---|
| Aguiar et al. [46] |
18 807 |
89 |
4.73 |
| Cortes et al. [32] |
60 072 |
94 |
1.56 |
| Cortes et al. [12] |
486 779 |
419 |
0.86 |
| Dai et al. [46] |
2 281 616 |
2873 |
1.30 |
| Golalipour et al. [47] |
37 951 |
109 |
2.87 |
| Golalipour et al. [12] |
30 639 |
78 |
2.55 |
| Golalipour et al. [48] |
49 534 |
194 |
3.91 |
| Gu et al. [13] |
6420 |
128 |
19.94 |
| Li et al. [10] |
11 534 |
159 |
13.79 |
| Liu et al. [49] |
99 888 |
122 |
1.22 |
| Mahadevan et al. [45] |
54 738 |
310 |
5.66 |
| Nazer et al. [50] |
434 624 |
740 |
1.70 |
| Noraihan et al. [51] |
34 109 |
37 |
1.08 |
| Onrat et al. [52] |
8631 |
31 |
3.59 |
| Rad et al. [53] |
14 121 |
117 |
2.57 |
| Yuskiv et al. [33] |
75 928 |
159 |
2.09 |
| Zhang et al. [54] | 62 373 | 126 | 2.02 |
Table 9.
Studies that reported rates for the burden of spina bifida based on live births, stillbirths and terminations of pregnancy
| Author and reference | Sample size | Cases | Rate (per 1000 live births, stillbirths and terminations) |
|---|---|---|---|
| Cortes et al. [32] |
60 072 |
46 |
0.77 |
| Cortes et al. [12] |
486 779 |
204 |
0.42 |
| Golalipour et al. [47] |
37 951 |
62 |
1.63 |
| Golalipour et al. [12] |
30 639 |
39 |
1.27 |
| Gu et al. [13] |
6420 |
25 |
3.89 |
| Li et al. [10] |
11 534 |
67 |
5.81 |
| Liu et al. [49] |
99 888 |
59 |
0.59 |
| Mahadevan et al. [45] |
54 738 |
170 |
3.11 |
| Noraihan et al. [51] |
34 109 |
12 |
0.35 |
| Onrat et al. [52] |
8631 |
9 |
1.04 |
| Yuskiv et al. [33] |
75 928 |
64 |
0.84 |
| Aguiar et al. [55] |
18 807 |
47 |
2.49 |
| Dai et al. [46] |
2 281 616 |
1369 |
0.60 |
| Nazer et al. [50] |
434 624 |
374 |
0.86 |
| Rad et al. [53] | 14 121 | 35 | 2.48 |
Table 10.
Studies that reported rates for the burden of anencephaly based on live births, stillbirths and terminations of pregnancy
| Author and reference | Sample size | Cases | Rate (per 1000 live births, stillbirths and terminations) |
|---|---|---|---|
| Aguiar et al. [55] |
18 807 |
24 |
1.28 |
| Cortes et al. [32] |
60 072 |
37 |
0.62 |
| Cortes et al. [12] |
486 779 |
147 |
0.30 |
| Dai et al. [46] |
2 281 616 |
1140 |
0.50 |
| Golalipour et al. [47] |
37 951 |
43 |
1.40 |
| Golalipour et al. [12] |
30 639 |
35 |
0.92 |
| Golalipour et al. [48] |
49 534 |
56 |
1.13 |
| Gu et al. [13] |
6420 |
53 |
8.26 |
| Li et al. [10] |
11 534 |
75 |
6.50 |
| Liu et al. [49] |
99 888 |
42 |
0.42 |
| Mahadevan et al. [45] |
54 738 |
98 |
1.80 |
| Nazer et al. [50] |
434 624 |
311 |
0.72 |
| Noraihan et al. [51] |
34 109 |
25 |
0.73 |
| Onrat et al. [52] |
8631 |
12 |
1.39 |
| Rad et al. [53] |
14 121 |
78 |
5.52 |
| Yuskiv et al. [33] | 75 928 | 62 | 0.82 |
Table 11.
Studies that reported rates for the burden of encephalocele based on live births, stillbirths and terminations of pregnancy
| Author and reference | Sample size | Cases | Rate (per 1000 live births stillbirths and terminations) |
|---|---|---|---|
| Aguiar et al. [55] |
18 807 |
5 |
0.27 |
| Cortes et al. [32] |
60 072 |
11 |
0.18 |
| Dai et al. [46] |
2 281 616 |
365 |
0.16 |
| Golalipour et al. [47] |
37 951 |
4 |
0.11 |
| Golalipour et al. [12] |
30 639 |
4 |
0.13 |
| Gu et al. [13] |
6420 |
17 |
2.65 |
| Li et al. [10] |
11 534 |
17 |
1.47 |
| Liu et al. [49] |
99 888 |
7 |
0.07 |
| Mahadevan et al. [45] |
54 738 |
36 |
0.66 |
| Nazer et al. [50] |
434 624 |
91 |
0.21 |
| Onrat et al. [52] |
8631 |
2 |
0.23 |
| Rad et al. [53] |
14 121 |
4 |
0.28 |
| Yuskiv et al. [33] | 75 928 | 12 | 0.16 |
As expected, when comparing IQRs as the robust predictions, overall NTD burden estimates were found to be higher in the live births, stillbirths and terminations group in comparison to studies that included only live births, while spina bifida was the most commonly reported NTD type. Moreover, there is internal consistency in the presented estimates, because the sum of the specific NTD types always fits into the “envelope” of all NTD.
DISCUSSION
This systematic literature review aimed to examine the burden of NTD in LMIC. It is, to our knowledge, the first study to quantitatively estimate the total NTD burden in LMIC. As such, the burden estimates can be successfully used in the much–needed preventive policy development in LMIC with high risk of NTD.
The results from the 37 selected studies [10-13,23-55] suggest that NTD burden is approximately twice as high, if not higher, in LMIC than in high–income countries [56-58]. The findings from live birth–only studies showed that the median total NTD burden is 1.67 per 1000 live births, although there were reports of significantly higher values, with a maximum burden as high as 12.41/1000. The overall median is greater in studies where live births, stillbirths and terminations were taken into account, where the burden is 2.55 per 1000 and maximum reported burden of 19.94/1000. This is expected, as a considerable proportion of NTD result in stillbirths and terminations [59,60].
Significant discrepancies between reported burdens from the same country were sometimes observed. These differences were attributed to different study settings, for example in rural and urban India [31,35,45], or different time periods as seen in two studies from Jordan [11,26]. Extremely high burden of NTD of 13.79 and 19.94 was observed in two studies from China, although the samples were rather small, indicating a possible selection bias [10,13].
Regardless of the progress in control of NTDs observed in high–income countries, NTD continue to be a problem of significant public health impact in LMIC. NTD have detrimental physical and emotional effects on the affected children and their caregivers, and may present a life–long important and often insurmountable economic problem, especially to poor families [52]. The cost of raising a child with spina bifida from birth to 18 years of age in Chile was estimated to be around US$ 120 000 [34]. These expenses, apart from causing individual deprivation, are a significant economic burden on the level of the whole society, causing a vicious circle of poverty in the LMIC.
Hundreds of thousands of live born babies are affected by NTD in LMIC, which remain an important and preventable cause of morbidity and mortality. Thus, effective policies for prevention are vital to reduce the burden of NTD on individuals and on society. Up to now, more than 59 countries have committed to mandatory fortification programmes [59,61,62]. However, many LMIC still have ineffective recommendations and policies towards folic acid uptake. Some countries have recommended the improvement of daily diet and folic acid supplement use, but do not have a mandatory policy [59,61,62]. Recommendation provides a good starting point for reducing NTD burden in LMIC. However, many households in LMIC may not be able to afford folic acid supplementation throughout pregnancy [63]. As shown by the example from the US where NTD burden had fallen by 20% after mandatory fortification, recommendation alone, even without the economic constraint, is not likely to provide a feasible and effective solution [22].Interestingly, survey conducted in the UK found that there was only a marginal increase in folic acid intake in women who were planning pregnancy [64]. Additionally, around half of all pregnancies in the US are unexpected [58,62], and this figure may be even higher in LMIC where there may be limited availability of contraception.
Despite obvious benefits, before promoting folic acid fortification, many factors must be considered. Currently no country in the European Union has compulsory fortification schemes due to risk consideration and campaigns against ‘mass medication’ [65,66]. Safety, ethics and economic feasibility of a FAF programme must be taken into account before implementing such a policy, especially on a whole–country level. Nevertheless, current high burden of NTD in LMIC stresses the need for a comprehensive prevention program.
For consistent and reliable estimates on burden of NTD, it is important to set up vital and birth registration documentation programs in countries that lack coherent information on NTD burden. Not only will this aid in the prevention and treatment of NTD, but it will also enable policy makers to monitor the benefits of implemented prevention programs. This may be particularly important for countries in the African WHO region, where a high NTD burden is expected, but from which only a few studies have been published [67,68].
The reported NTD burden was estimated based on a limited number of available studies, some with very variable sample sizes that differed in inclusion of stillbirths and terminated births in the study design. We could not use meta–analysis, because studies came from such heterogeneous contexts that we didn't feel it was justified to present anything beyond simple median and IQR in this initial estimate. This is partly because not all studies adhered to ICD–10 classification of NTD and were not uniformly conducted regarding method of diagnosis and reporting of NTD type, enabling potential over– or under–estimation of NTD burden through misdiagnosis. Also, the technical restrictions of accounting for all stillbirths and terminations in the examined studies limited the precision of our estimated burden in that population [68].
Finally, the data was available from studies conducted in only 18 countries, implying that the studied sample is unlikely to be representative of all the LMIC globally. Regardless of these significant limitations, it is our opinion that the estimated burdens reported in the results provide useful data for initial assessment of NTD burden in LMIC. An increase in high quality research on NTD, especially with regards to gender and geographical regions, should be prioritised to allow more accurate NTD estimates. This would make the burden of the problem easier to estimate in a more credible way, and allow effective planning of prevention and intervention to minimise the risks for NTD.
Acknowledgments
Funding: There was no formal sponsorship of this research.
Ethical approval: None required.
Authorship declaration: AL performed the initial systematic review and literature search and wrote the first draft of the manuscript. DP has revised the first draft and performed calculations. SS has performed a parallel review, double–checked all calculations, revised the second draft and prepared the final version of the paper.
Competing interest: All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author). They declared no competing interests.
References
- 1.Shibuya K, Murray CJ. Congenital anomalies. In: Murray CJ, Lopez AD (eds). Health dimensions of sex and reproduction: the global burden of sexually transmitted diseases, HIV, maternal conditions, perinatal disorders, and congenital anomalies. Boston, Massachusetts, USA: Harvard School of Public Health, on behalf of the World Health Organization and the World Bank;1998. pp. 455–512. [Google Scholar]
- 2.United Nations. The Millennium Development Goals Report. 2012. Available at: http://mdgs.un.org/unsd/mdg/Resources/Static/Products/Progress2012/English2012.pdf#page=28 Accessed 20th April 2013.
- 3.Duke Centre for Human Genetics. Neural Tube Defects (NTDs). 2012. Available at: http://www.chg.duke.edu/diseases/ntd.html Accessed 20th April 2013.
- 4.Botto LD, Moore CA, Khoury MJ, Erickson JD. Neural tube defects. N Engl J Med. 1999;341:1509–19. doi: 10.1056/NEJM199911113412006. [DOI] [PubMed] [Google Scholar]
- 5.Spina Bifida Association. Spina Bifida Occulta. 2012. Available at: http://www.spinabifidaassociation.org/site/c.evKRI7OXIoJ8H/b.8277205/ Accessed 20th April 2013.
- 6.Christianson A, Howson CP, Modell B. Global Report on Birth Defects: The Hidden Toll of Dying and Disabled children. March of Dimes. 2006. Available at: http://www.marchofdimes.com/mission/globalprograms_birthdefectsreport.html Accessed 20th April 2013.
- 7.Kirke PN, Molloy AM, Daly LE, Burke H, Weir DG, Scott JM. Maternal plasma folate and vitamin B12 are independent risk factors for neural tube defects. Q J Med. 1993;86:703–8. [PubMed] [Google Scholar]
- 8.Bjorklund NK, Gordan R. A hypothesis linking low folate intake to neural tube defects due to failure of post–translation methylations of the cytoskeletion. Int J Dev Biol. 2006;50:135–41. doi: 10.1387/ijdb.052102nb. [DOI] [PubMed] [Google Scholar]
- 9.Wang ZP, Shang XX, Zhao ZT. Low maternal vitamin B12 is a risk factor for neural tube defects: a meta–analysis. J Matern Fetal Neonatal Med. 2012;25:389–94. doi: 10.3109/14767058.2011.580800. [DOI] [PubMed] [Google Scholar]
- 10.Li Z, Ren A, Zhang L, Ye R, Li S, Zheng J, et al. Extremely high prevalence of NTD in a 4–county area of Shanxi Province, China. Birth Defects Res A Clin Mol Teratol. 2006;76:237–40. doi: 10.1002/bdra.20248. [DOI] [PubMed] [Google Scholar]
- 11.Aqrabawi HE. Incidence of neural tube defects among neonates at King Hussein Medical Centre, Jordan. East Mediterr Health J. 2005;11:819–23. [PubMed] [Google Scholar]
- 12.Golalipour M, Najafi L, Keshtkar A. Neural tube defects in native Fars ethnicity in Northern Iran. Iran J Public Health. 2010;39:116–23. [PMC free article] [PubMed] [Google Scholar]
- 13.Gu X, Lin L, Zheng X, Zhang T, Song X, Wang J, et al. High prevalence of NTDs in Shanxi Province: a combined epidemiological approach. Birth Defects Res A Clin Mol Teratol. 2007;79:702–7. doi: 10.1002/bdra.20397. [DOI] [PubMed] [Google Scholar]
- 14.Suarez L, Felkner M, Brender JD, Canfield M, Hendricks K. Maternal exposures to cigarette smoke, alcohol and street drugs and neural tube defect occurrence in offspring. Matern Child Health J. 2008;12:394–401. doi: 10.1007/s10995-007-0251-y. [DOI] [PubMed] [Google Scholar]
- 15.Li Z, Zhang L, Ye R, Pei L, Liu J, Zheng X, et al. Indoor air pollution from coal combustion and the risk of neural tube defects in a rural population in Shanxi Province, China. Am J Epidemiol. 2011;174:451–8. doi: 10.1093/aje/kwr108. [DOI] [PubMed] [Google Scholar]
- 16.Tunçbilek E, Boduroglu K, Alikasifoglu M. Neural tube defects in Turkey: prevalence, distribution and risk factor. Turk J Pediatr. 1999;41:299–305. [PubMed] [Google Scholar]
- 17.Watkins ML, Rasmussen SA, Honein MA, Botto LD, Moore CA. Maternal obesity and risk for birth defects. Pediatrics. 2003;111:1152–8. [PubMed] [Google Scholar]
- 18.Mayo Medical Laboratories. Test ID: MAFP. 2013. Available at: http://www.mayomedicallaboratories.com/test–catalog/Clinical+and+Interpretive/81169 Accessed 21 April 2013.
- 19.Mayo Medical Laboratories. Test ID: ACHE. 2013. Available at: http://www.mayomedicallaboratories.com/test–catalog/Clinical+and+Interpretive/9287 Accessed 21 April 2013.
- 20.National Cancer Institute. Computed Tomography (CT): A guide for Health Care. 2012. Available at: http://www.cancer.gov/cancertopics/causes/radiation/radiation–risks–pediatric–CT Accessed 21st April 2013.
- 21.Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. Lancet. 1991;338:131–7. doi: 10.1016/0140-6736(91)90133-A. [DOI] [PubMed] [Google Scholar]
- 22.Honein MA, Paulozzi LJ, Mathews TJ, Erickson JD, Wong LY. Impact of folic acid fortification of the US food supply on the occurrence of neural tube defect. JAMA. 2001;285:2981–6. doi: 10.1001/jama.285.23.2981. [DOI] [PubMed] [Google Scholar]
- 23.Cortés F, Mellado C, Pardo RA, Villarroel LA, Hertrampf E. Wheat flour fortification with folic acid: changes in neural tube defect rates in Chile. Am J Med Genet A. 2012;158A:1885–90. doi: 10.1002/ajmg.a.35430. [DOI] [PubMed] [Google Scholar]
- 24.Safdar OY, Al–Dabbagh AA, Abuelieneen WA, Kari JA. Decline in the incidence of neural tube defects after the national fortification of flour (1997–2005). Saudi Med J. 2007;28:1227–9. [PubMed] [Google Scholar]
- 25.Sayed AR, Bourne D, Pattinson R, Nixon J, Henderson B. Decline in the prevalence of neural tube defects following folic acid fortification and its cost–benefit in South Africa. Birth Defects Res A Clin Mol Teratol. 2008;82:211–6. doi: 10.1002/bdra.20442. [DOI] [PubMed] [Google Scholar]
- 26.Amarin ZO, Obeidat AZ. Effect of folic acid fortification on the incidence of neural tube defects. Paediatr Perinat Epidemiol. 2010;24:349–51. doi: 10.1111/j.1365-3016.2010.01123.x. [DOI] [PubMed] [Google Scholar]
- 27.Asindi A, Al–Shehri A. Neural tube defects in the Asir Region of Saudi Arabia. Ann Saudi Med. 2001;21:26–9. doi: 10.5144/0256-4947.2001.26. [DOI] [PubMed] [Google Scholar]
- 28.Bademci G, Saygun M, Batay F, Cakmak A, Basar H, Anbarci H, et al. Prevalence of primary tethered cord syndrome associated with occult spinal dysraphism in primary school children in Turkey. Pediatr Neurosurg. 2006;42:4–13. doi: 10.1159/000089503. [DOI] [PubMed] [Google Scholar]
- 29.Behrooz A, Gorjizadeh MH. Prevalence and correlates of neural tube defects in South West Iran: Retrospective analysis. Sultan Qaboos Univ Med J. 2007;7:31–4. [PMC free article] [PubMed] [Google Scholar]
- 30.Chen G, Song Z, Ji Y, Zhang L, Pei L, Chen J, et al. Prevention of NTDs with periconeptional multivitamin supplementation containing folic acid in China. Birth Defect Res A Clin Mol Teratol. 2008;82:592–6. [DOI] [PubMed]
- 31.Cherian A, Seena S, Bullock RK, Antony AC. Incidence of neural tube defects in the least–developed area of India: a population–based study. Lancet. 2005;366:930–1. doi: 10.1016/S0140-6736(05)67319-9. [DOI] [PubMed] [Google Scholar]
- 32.Cortés F, Mellado C, Hertrampf E, Alliende A, Castillo S. Frequency of neural tube defects in public maternity during 1999 in Santiago, Chile. Rev Med Chil. 2001;129:277–84. [PubMed] [Google Scholar]
- 33.Yuskiv N, Andelin CO, Polischuk S, Shevchuk O, Sosynyuk Z, Vihovska T, et al. High rates of neural tube defects in Ukraine. Birth Defects Res A Clin Mol Teratol. 2004;70:400–2. doi: 10.1002/bdra.20020. [DOI] [PubMed] [Google Scholar]
- 34.Hertrampf E, Cortés F. National food fortification program with folic acid in Chile. Food Nutr Bull. 2008;29(2) Suppl:S231–7. doi: 10.1177/15648265080292S128. [DOI] [PubMed] [Google Scholar]
- 35.Kaur G, Srivastav J, Kaur A, Huria A, Goel P, Kaur R, et al. Maternal serum second trimester screening for the chromosomal disorders and neural tube defects in a government hospital of North India. Prenat Diagn. 2012;32:1192–6. doi: 10.1002/pd.3984. [DOI] [PubMed] [Google Scholar]
- 36.Khattak ST, Khan M, Naheed T, Khattak IU, Ismali M. Prevalence and management of anencephaly at Saidu Teaching Hospital. J Ayub Med Coll Abbottabad. 2010;22:61–3. [PubMed] [Google Scholar]
- 37.Mandiracioğlu A, Ulman I, Lüleci E, Ulman C. The incidence and risk factors of neural tube defects in Izmir, Turkey: a nested case–control study. Turk J Pediatr. 2004;46:214–20. [PubMed] [Google Scholar]
- 38.Njamnshi AK, Djientcheu Vde P, Lekoubou A, Guemse M, Obama MT, Mbu R, et al. Neural tube defects are rare among black Americans but not in sub–Saharan black Africans: the case of Yaounde – Cameroon. J Neurol Sci. 2008;270:13–7. doi: 10.1016/j.jns.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 39.Pachajoa H, Ariza Y, Isaza C, Méndez F. Major birth defects in a third–level hospital in Cali, Colombia 2004–2008. Rev Salud Publica (Bogota) 2011;13:152–62. [PubMed] [Google Scholar]
- 40.Petrova JG, Vatskjold A. The incidence of neural tube defects in Norway and the Arkhangelskaja Oblast in Russia and the association with maternal age. Acta Obstet Gynecol Scand. 2009;88:667–72. doi: 10.1080/00016340902898008. [DOI] [PubMed] [Google Scholar]
- 41.Pacheco SS, Braga C, Souza AI, Figueiroa JN. Neural tube defects prevalence in newborn infants in the Women Care Center of the Instituto Materno Infantil Prof. Fernando Figueira. Rev Bras Saude Mater Infant. 2006;6(Suppl 1):S35–42. [Google Scholar]
- 42.Ricks DJ, Rees CA, Osborn KA, Crookston BT, Leaver K, Merrill SB, et al. Peru’s national folic acid fortification program and its effect on neural tube defects in Lima. Rev Panam Salud Publica. 2012;32:391–8. doi: 10.1590/S1020-49892012001400001. [DOI] [PubMed] [Google Scholar]
- 43.Wasant P, Sathienkijkanchai A. Neural tube defects at Sirirajhospital, Bangkok, Thailand – 10 years review (1990–1999). J Med Assoc Thai. 2005;88(Suppl 8):S92–9. [PubMed] [Google Scholar]
- 44.Costa CM, da Gama SG, Leal Mdo C. Congenital malformations in Rio de Janeiro, Brazil: prevalence and associated factors. Cad Saude Publica. 2006;22:2423–31. doi: 10.1590/S0102-311X2006001100016. [DOI] [PubMed] [Google Scholar]
- 45.Mahadevan B, Bhat BV. Neural tube defects in Pondicherry. Indian J Pediatr. 2005;72:557–9. doi: 10.1007/BF02724177. [DOI] [PubMed] [Google Scholar]
- 46.Dai L, Zhu J, Zhou G, Wang Y, Wu Y, Miao L, et al. Dynamic monitoring of neural tube defects in China during 1996 to 2000. Zhonghua Yu Fang Yi Xue Za Zhi. 2002;36:402–5. [PubMed] [Google Scholar]
- 47.Golalipour MJ, Mobasheri E, Vakilil MA, Keshtkar AA. Epidemiology of neural tube defects in north Iran, 1998 – 2003. East Mediterr Health J. 2007;13:560–6. [PubMed] [Google Scholar]
- 48.Golalipour MJ, Najafi L, Keshtkar AA. Prevalence of anencephaly in Gorgan, Northern Iran. Arch Iran Med. 2010;13:34–7. [PubMed] [Google Scholar]
- 49.Liu J, Yang GZ, Zhou JL, Cao SP, Chau DH, Kung HF, et al. Prevalence of neural tube defects in economically and socially deprived area of China. Childs Nerv Syst. 2007;23:1119–24. doi: 10.1007/s00381-007-0344-3. [DOI] [PubMed] [Google Scholar]
- 50.Nazer H J. Congenital malformations in Latin America in the period 1995–2008. Rev Med Chil. 2011;139:72–8. [PubMed] [Google Scholar]
- 51.Noraihan MN, See MH, Raja R, Baskaran TP, Symonds EM. Audit of birth defects in 34,109 deliveries in a tertiary referral center. Med J Malaysia. 2005;60:460–8. [PubMed] [Google Scholar]
- 52.Onrat ST, Seyman H, Honuk M. Incidence of neural tube defects in Afyonkarahisar, Western Turkey. Genet Mol Res. 2009;8:154–61. doi: 10.4238/vol8-1gmr552. [DOI] [PubMed] [Google Scholar]
- 53.Rad IA, Farrokh–Islamlou HR, Khoshkalam M. Neural tube defects prevalence in a hospital–based study in Urmia. Iran J Child Neurol. 2008;2:19–23. [Google Scholar]
- 54.Xiao KZ, Zhang ZY, Su YM, Liu FQ, Yan ZZ, Jiang ZQ, et al. Central nervous system congenital malformations, especially neural tube defects in 29 provinces, metropolitan cities and autonomous regions of China: Chinese Birth Defects Monitoring Program. Int J Epidemiol. 1990;19:978–82. doi: 10.1093/ije/19.4.978. [DOI] [PubMed] [Google Scholar]
- 55.Aguiar MJ, Campos AS, Aguiar RA, Lana AM, Magalhăes RL, Babeto LT. Neural tube defects and associated factors in liveborn and stillborn infants. J Pediatr (Rio J) 2003;79:129–34. doi: 10.2223/JPED.964. [DOI] [PubMed] [Google Scholar]
- 56.Tinker SC, Cogswell ME, Devine O, Berry RJ. Folic acid intake among U.S. women aged 15–44 years, National Health and Nutrition Examination Survey, 2003–2006. Am J Prev Med. 2010;38:534–42. doi: 10.1016/j.amepre.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 57.Grosse SD, Waitzman NJ, Romano PS, Mulinare J. Re–evaluation the benefits of folic acid fortification in the United States: economic analysis, regulation and public health. Am J Public Health. 2005;95:1917–22. doi: 10.2105/AJPH.2004.058859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yen IH, Khoury MJ, Erickson JD, James LM, Waters GD, Berry RJ. The changing epidemiology of neural tube defects. United States, 1968–1989. Am J Dis Child. 1992;146:857–61. doi: 10.1001/archpedi.1992.02160190089028. [DOI] [PubMed] [Google Scholar]
- 59.World Health Organization. Weekly iron–folic acid supplementation (WIFS) in Women of Reproductive age: Its role in promoting optimal maternal and child health. 2009. Available at: http://www.who.int/nutrition/publications/micronutrients/weekly_iron_folicacid.pdf Accessed 21st April 2013. [PubMed]
- 60.National Library of Medicine. Spina Bifida. 2013. Available at: http://www.nlm.nih.gov/medlineplus/spinabifida.html Accessed 20th April 2013.
- 61.Berry RJ, Bailey L, Mulinare J, Bower C, Folic Acid Working Group Fortification of flour with folic acid. Food Nutr Bull. 2010;31(Suppl):S22–35. doi: 10.1177/15648265100311S103. [DOI] [PubMed] [Google Scholar]
- 62.Crider KS, Bailey LB, Berry RJ. Folic acid fortification – its history, effect, concerns, and future directions. Nutrients. 2011;3:370–84. doi: 10.3390/nu3030370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chopra M, Campbell H, Rudan I. Understanding the determinants of the complex interplay between cost–effectiveness and equitable impact in maternal and child mortality reduction. J Glob Health. 2012;2:010406. doi: 10.7189/jogh.01.010406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Crozier SR, Robinson SM, Borland SE, Godfrey KM, Cooper C, Inskip H, SWS study group Do women change their health behaviours in pregnancy? Findings from the Southampton Women’s Survey. Paediatr Perinat Epidemiol. 2009;23:446–53. doi: 10.1111/j.1365-3016.2009.01036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Russell A. The UK campaign on folic acid and flour fortification. Cerebrospinal Fluid Res. 2006;3(Suppl 1):S33. doi: 10.1186/1743-8454-3-S1-S33. [DOI] [Google Scholar]
- 66.FAO and WHO. Folate and folic acid. 2002. Available at: http://www.fao.org/docrep/004/Y2809E/y2809e0a.htm Accessed 25th April 2013.
- 67.Campbell A, Rudan I. Systematic review of birth cohort studies in Africa. J Glob Health. 2011;1:46–58. [PMC free article] [PubMed] [Google Scholar]
- 68.Frey L, Hauser WA. Epidemiology of neural tube defects. Epilepsia. 2003;44(Suppl 3):4–13. doi: 10.1046/j.1528-1157.44.s3.2.x. [DOI] [PubMed] [Google Scholar]