Abstract
Despite efforts to generate new vaccines and antibiotics for tuberculosis, the disease remains a public health problem worldwide. The zebrafish Danio rerio has emerged as a useful model to investigate mycobacterial pathogenesis and treatment. Infection of zebrafish with Mycobacterium marinum, the closest relative of the Mycobacterium tuberculosis complex, recapitulates many aspects of human tuberculosis. The zebrafish model affords optical transparency, abundant genetic tools and in vivo imaging of the progression of infection. Here, we review how the zebrafish–M. marinum system has been deployed to make novel observations about the role of innate immunity, the tuberculous granuloma, and crucial host and bacterial genes. Finally, we assess how these findings relate to human disease and provide a framework for novel strategies to treat tuberculosis.
KEY WORDS: Disease models, Genetics, Mycobacterium, Pathogenesis, Tuberculosis, Zebrafish
Introduction
Tuberculosis pathology in humans
Since arising as a human pathogen an estimated 70,000 years ago, Mycobacterium tuberculosis (Mtb) has been a major cause of mortality and morbidity in human populations (Comas et al., 2013). Although Mtb was identified as the cause of tuberculosis (TB) in the 1880s, current therapeutic options have not been able to eradicate the disease. More than 8 million cases of TB were diagnosed in 2012, leading to 1.3 million deaths worldwide (WHO, 2013).
Clinically, the ongoing mortality of TB results in part from the difficulty in treating Mtb with existing antibiotics. Current frontline antibiotic combinations require months-long treatment courses, making treatment difficult, particularly in the developing world where access to healthcare can be limited (Sacchettini et al., 2008). This high rate of treatment failure has led to the appearance of multidrug-resistant (MDR) and extremely drug-resistant (XDR) strains, which further contribute to clinical burden and mortality (Sacchettini et al., 2008). Furthermore, although a number of promising new vaccine approaches have been described in recent years, the Mycobacterium bovis bacillus Calmette–Guérin (BCG) vaccine, in use for almost a century, remains the only vaccine deployed throughout the world (Ottenhoff and Kaufmann, 2012). BCG vaccination protects from disseminated TB, particularly TB meningitis in younger patients, but has little efficacy in adult populations (Ottenhoff and Kaufmann, 2012). Thus, although vaccine and treatment options exist for TB, their suboptimal performance contributes to the continued public health burden of tuberculosis. To address the limited efficacy of existing treatment options, ongoing research has sought to better understand the process of Mtb pathogenesis, and to identify novel host and bacterial genes that are crucial for Mtb infection.
During Mtb infection, the bacterium is taken up by host phagocytes and replicates within them (Wolf et al., 2007; Eum et al., 2010; Repasy et al., 2013). As infection progresses, macrophages and other immune cell types are recruited to the lung and other sites of infection, creating a structured aggregate of infected and uninfected cells called the granuloma (Philips and Ernst, 2012; Ramakrishnan, 2012; Russell, 2013). These granulomas consist of a tightly interdigitated inner core of macrophages termed ‘epithelioid’ macrophages, surrounded by additional immune cells, including T-cells, B-cells, dendritic cells and neutrophils (Philips and Ernst, 2012; Ramakrishnan, 2012; Russell, 2013). The inner core of these granulomas undergoes pronounced changes, including the induction of necrosis and hypoxia, resulting in a central core filled with lipid-rich cell debris termed caseum (Philips and Ernst, 2012; Ramakrishnan, 2012; Russell, 2013). The granuloma is the hallmark structure of tuberculosis and constitutes a crucial niche in which bacteria persist. Eventual rupture of granulomas is crucial to bacterial release into the lung and transmission of the disease.
Modeling Mtb infection in vitro and in animal models
A number of groups have used Mtb cultures in vitro or Mtb-infected macrophages to rapidly identify host and bacterial genes that constitute targets for novel anti-tubercular therapeutics (Sassetti et al., 2001; Sassetti et al., 2003; Jayaswal et al., 2010; Kumar et al., 2010). However, translating these results into patients is limited by major differences between broth-grown Mtb, cultured macrophages infected with Mtb and the in vivo reality of Mtb growth within the complex architecture of the granuloma. To these ends, groups have generated elegant mixed cell culture models that can give rise to granuloma-like structures in vitro (Puissegur et al., 2004; Kapoor et al., 2013). However, validation of host and bacterial genes involved in infection and novel TB drugs still requires the use of animal models of tuberculosis infection. Thus, progress in TB research has been linked to the development of animal models that can functionally reproduce the pathology and bacterial growth characteristics observed in humans.
Of the many animal models developed for Mtb infection, each has distinct strengths and weaknesses. The most popular has been the mouse infection model. The mouse model is particularly versatile because of the extensive range of tools and strains available for mice. However, it can be limited in its ability to reproduce the observed human pathologies (Flynn, 2006). Rather than the compact, caseating granulomas observed in humans, the most widely used mouse models (C57BL6 and BALB/c) form granulomas that are comparatively diffuse and fail to caseate (Flynn, 2006). Newer mouse models, including the C3HeB/FeJ ‘Kramnik’ model, Nos2−/− C57BL/6 mice and IL10−/− CBA/J mice, form granulomas that undergo caseating necrosis (Kramnik et al., 2000; Reece et al., 2010; Cyktor et al., 2013), but use of these models with existing knockout lines requires extensive crossing. In addition to mouse models, researchers have adopted other mammalian models that closely mimic human TB pathology, including guinea pigs and rabbits, which form necrotic granulomas (Flynn, 2006). However, the rabbit and guinea pig models lack the catalog of existing reagents available for mice, and are not nearly as amenable for transgenic and knockout line production as mice. The most clinically relevant model has been the primate infection model (Capuano et al., 2003). Nonetheless, the cost, time and ethical considerations of this model mean that it can only be used in a limited fashion. Thus, researchers continue to search for models of Mtb infection that can offer new insights into Mtb pathogenesis. The emergence of a zebrafish mycobacterial infection model, using the closely related mycobacterial pathogen M. marinum, complements these models. It recapitulates many aspects of human tuberculosis infection, and its unique characteristics have expanded experimental possibilities, leading to new insights about mycobacterial pathogenesis that have been validated in human disease.
The zebrafish model of mycobacterial infection
The zebrafish immune system has extensive homology with the human immune system, possessing both innate and adaptive arms (Renshaw and Trede, 2012). Systemic infection of zebrafish with M. marinum results in phagocytosis of infecting bacteria by macrophages (Fig. 1A) and formation of caseating granulomas that are histologically similar to human Mtb granulomas (Davis et al., 2002; Swaim et al., 2006). Functionally, crucial virulence factors, host genes and immune cell types implicated in human Mtb pathogenesis have conserved functions within the zebrafish–M. marinum model (Gao et al., 2004; Volkman et al., 2004; Cosma et al., 2006; Swaim et al., 2006; Clay et al., 2008; Stoop et al., 2011; van der Woude et al., 2012; Kanwal et al., 2013; Stoop et al., 2013; van der Vaart et al., 2013).
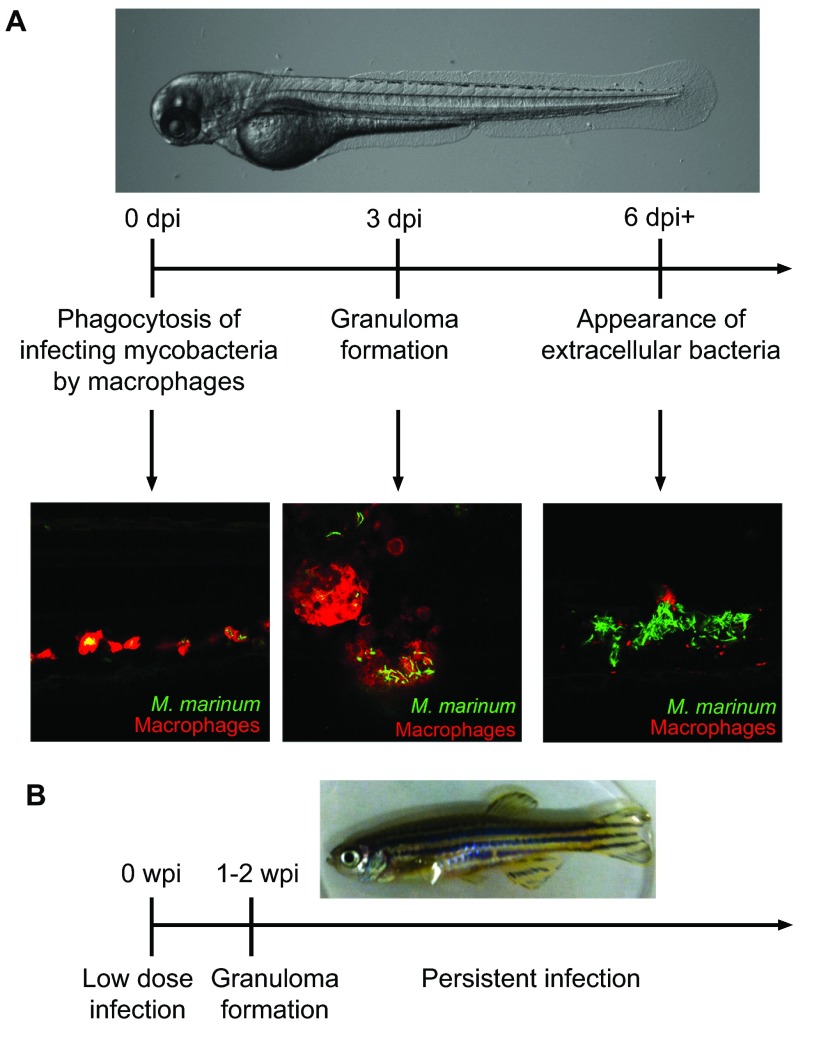
Fig. 1.
Modeling mycobacterial infection in larval and adult zebrafish. (A) From top – brightfield image of a zebrafish larva. Middle – an approximate timeline of progression of infection in zebrafish larvae, in days post-infection (dpi). Bottom – confocal images of zebrafish lines, with fluorescently labeled macrophages shown in red and infecting fluorescent mycobacteria visualized in green. Representative images display from left – scattered infected macrophages; center – macrophages aggregated into granulomas; right – the appearance of extracellular bacteria as containment fails at isolated granulomas. (B) Top – image of an adult zebrafish. Bottom – a timeline of infection progression in weeks post-infection (wpi) in the adult persistent infection model (based on the work of Parikka et al., 2012).
The similarities between human and zebrafish mycobacterial infection are striking, and the zebrafish–M. marinum model presents additional advantages that are distinct from those of other mycobacterial infection models. First, using optically clear zebrafish larvae, researchers are able to directly observe the process of active infection in real time (Fig. 1A) (Davis et al., 2002). Second, zebrafish larvae possess only the innate immune system, but still show robust granuloma formation (Fig. 1A) (Davis et al., 2002). Thus, infection of zebrafish larvae enables researchers to study relevant pathology using a subgroup of cell types. Third, both host and bacteria are genetically tractable and easy to obtain in large numbers. Zebrafish transgenesis methods have been well characterized, and the recent breakthroughs in gene editing with transcription-activator-like effector nucleases (TALENs) and CRISPR/Cas9 have enabled rapid and facile gene knockouts in zebrafish (Kawakami et al., 2004; Huang et al., 2011; Sander et al., 2011; Hwang et al., 2013). On the bacterial side, the rapid generation time (4 hours for M. marinum versus ~20–24 hours for Mtb) facilitates construction of knockout and transposon-containing strains, and results in an infection model in which experiments are conducted within the time frame of days to weeks rather than weeks to months. Furthermore, zebrafish have been experimentally infected with a wide range of bacterial pathogens, allowing comparison of mycobacterial infection phenotypes with other pathogenic bacteria (Meijer and Spaink, 2011; Torraca et al., 2014). Finally, in conjunction with facile transgenesis and optical clarity, a number of promoters specific for distinct immune lineages have recently been described in zebrafish, enabling direct observation of these cell types by fluorescent protein expression and interrogation of gene function by cell-type-specific expression constructs (Langenau et al., 2004; Renshaw et al., 2006; Hall et al., 2007; Ellett et al., 2011; Torraca et al., 2014). Beyond the larvae, adult zebrafish infections have enabled researchers to investigate mycobacterial infection in the context of both the innate and adaptive immune systems. Low-dose infection of adult zebrafish with the Aronson strain of M. marinum leads to persistent infections resembling latency that can be reactivated by immunocompromise (Fig. 1B) (Parikka et al., 2012). In the following sections, we will discuss how researchers taking advantage of the unique benefits of the zebrafish–M. marinum system have been able to answer longstanding questions and open new fields of inquiry regarding innate immunity, granuloma dynamics, and host and bacterial genetics within mycobacterial pathogenesis.
Innate immunity
As an early host cell of mycobacteria, macrophages play a central role in mycobacterial pathogenesis and granuloma formation. Despite the well-known phagocytic capacity of macrophages, mycobacteria readily persist within these cells (Clay et al., 2007). Macrophages do serve to restrict proliferation of these persistent mycobacteria; extracellular M. marinum grow rapidly in zebrafish specifically depleted of macrophages (Clay et al., 2007). However, studies in macrophage-depleted zebrafish also indicate that mycobacteria co-opt this highly motile cell type to seed new infection foci in surrounding tissues within the host (Clay et al., 2007).
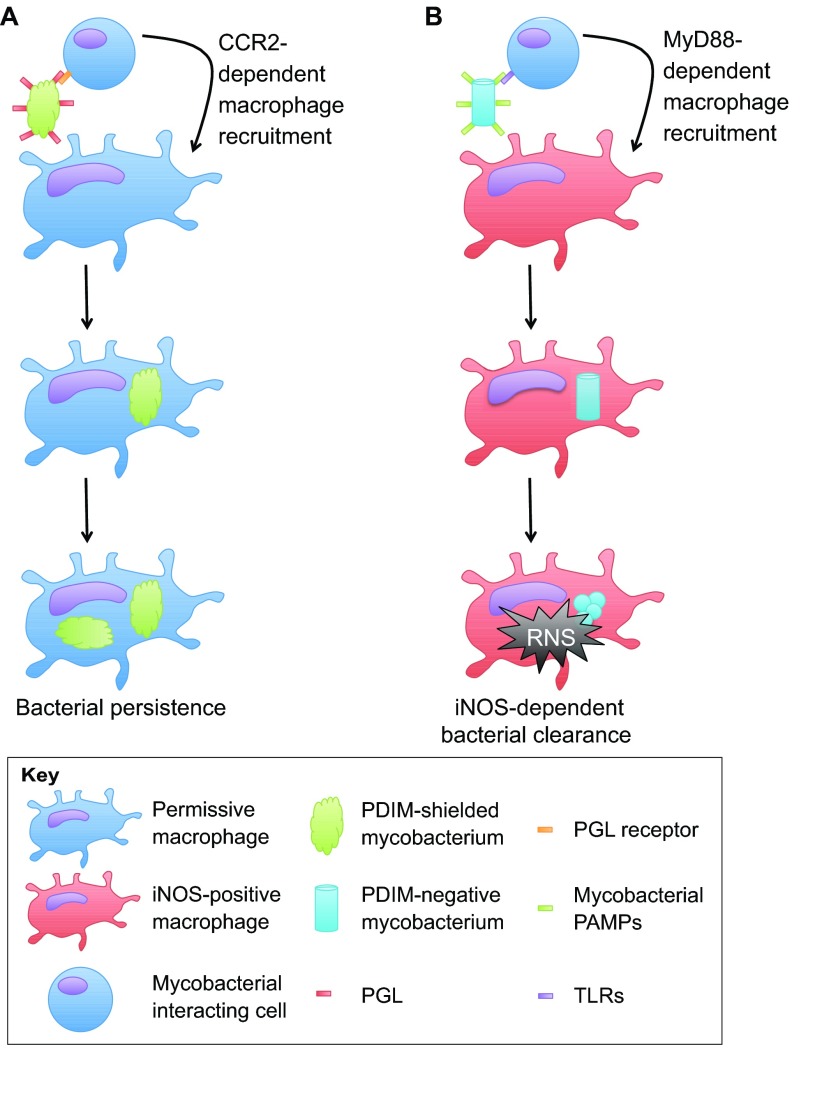
Researchers have sought to understand how mycobacteria persist within a professional phagocyte. Findings in the zebrafish–M. marinum model suggest that mycobacteria promote their persistence by selectively enhancing phagocytosis into subsets of macrophages deficient for inducible nitric oxide synthase (iNOS), which have reduced microbicidal activity (Cambier et al., 2014). Using mycobacteria lacking cell-wall components phthiocerol dimycoceroserate (PDIM) and phenolic glycolipids (PGLs), two lipid species previously found to be involved in Mtb virulence (Cox et al., 1999; Reed et al., 2004), and zebrafish depleted for the toll-like receptor (TLR) adaptor MyD88, the mechanistic underpinnings of this selective trafficking have been mapped in vivo (Cambier et al., 2014). PDIM served to hide mycobacteria from pattern-recognition receptors, avoiding TLR-dependent recognition of mycobacterial pathogen-associated molecular patterns (PAMPs), macrophage recruitment, phagocytosis by iNOS-positive macrophages and reactive nitrogen species (RNS)-mediated killing (Cambier et al., 2014). PGL, by contrast, recruited iNOS-deficient macrophages to bacteria in a manner dependent upon the chemokine receptor CCR2 (Fig. 2) (Cambier et al., 2014). In this and other studies, M. marinum deficient in PDIM and/or PGL were attenuated in zebrafish (Alibaud et al., 2011; Yu et al., 2012; Cambier et al., 2014). These findings indicate that mycobacterial phagocytosis, rather than being exclusively host driven, is instead a process tuned by the mycobacteria themselves to establish a productive niche for their growth and dissemination.
Fig. 2.
Mycobacterial lipids facilitate bacterial uptake into permissive macrophage populations. (A) PDIM shields wild-type mycobacteria (green) from TLR-mediated recognition of mycobacteria by the host cell. Interaction of PGL on the bacterial cell surface with surrounding host cells drives CCR2-mediated recruitment of mycobacterial permissive macrophages. These permissive macrophages phagocytose the mycobacterium, which can subsequently form a persistent infection within the host. (B) In PDIM-deficient mycobacteria (light blue), bacterial PAMPs are recognized by TLRs, leading to MyD88-dependent recruitment of iNOS-positive macrophages. These iNOS-positive macrophages phagocytose the infecting mycobacteria and rapidly clear them through iNOS-dependent RNS.
The macrophage is a major cell type recruited to and infected by Mtb; however, other immune cell types are also infected at high rates in vivo, including neutrophils (Wolf et al., 2007; Eum et al., 2010; Repasy et al., 2013). In mammalian models, studies have suggested that neutrophils probably play a protective role in Mtb infection (Lowe et al., 2012). However, ex vivo studies of mycobacterial killing by neutrophils have been contentious, probably because of the short half-life of this cell type and its sensitivity to conditions during isolation from blood (Lowe et al., 2012). Transgenic zebrafish in which both neutrophils and mycobacteria are fluorescently tagged have enabled direct interrogation of the interactions between neutrophils and mycobacteria in vivo (Meijer et al., 2008; Yang et al., 2012). In contrast to cell culture studies, in which neutrophils readily phagocytose mycobacteria, in vivo neutrophils fail to phagocytose free bacteria (Jones et al., 1990; Majeed et al., 1998; Yang et al., 2012). Instead, neutrophils become infected upon recruitment to established granulomas, where they phagocytose dead and dying infected macrophages (Yang et al., 2012). Previous work in zebrafish has demonstrated that macrophages readily phagocytose bacteria in fluid environments, whereas neutrophils can only phagocytose surface-associated microbes (Colucci-Guyon et al., 2011). Preferential uptake of mycobacteria into macrophages in this model might be due to the venous route of infection used in this model (Yang et al., 2012). However, in natural Mtb infections, alveolar macrophages are thought to be the first cells to encounter and phagocytose Mtb (Philips and Ernst, 2012). Consistent with initial infection of alveolar macrophages, in experimental mouse infections it was observed that Mtb solely infects monocytic cells at the earliest time points (Repasy et al., 2013). Thus, venous delivery of mycobacteria in zebrafish seems to mimic human Mtb infection, whereby neutrophils encounter mycobacteria only after transit through alveolar macrophages.
Upon uptake of infected cell debris, a subset of motile neutrophils actively kills the phagocytosed mycobacteria by means of oxidative mechanisms (Yang et al., 2012). Oxidative killing of mycobacteria is consistent with another study in which signaling through the transcription factor hypoxia-inducible factor 1-alpha (Hif-1α) enhances production of RNS by neutrophils and hence mycobacterial killing (Elks et al., 2013). To assess functionally the contribution of neutrophil recruitment and subsequent mycobacterial killing to pathogenesis, Yang and colleagues took advantage of a zebrafish line in which neutrophil recruitment was inhibited by expression of a truncated form of the chemokine receptor CXCR4b in neutrophils. In this mutant line, the failure of neutrophil recruitment to granulomas resulted in an enhanced mycobacterial burden within infected animals (Yang et al., 2012). These results indicate that innate immune cell types other than macrophages also contribute to mycobacterial pathogenesis. The recent discovery of zebrafish dendritic cells, mast cells and eosinophils suggests that the zebrafish model could be used to help clarify the involvement of other innate immune cell types during mycobacterial infection (Lieschke et al., 2001; Dobson et al., 2008; Balla et al., 2010; Lugo-Villarino et al., 2010; Wittamer et al., 2011).
During the recruitment of innate immune cells, the balance of pro-and anti-inflammatory molecules controls innate immune function. Dysregulation of this inflammatory milieu can render the host susceptible to infection. Depletion of the tyrosine-protein phosphatase Ptpn6 leads to hyperinflammation in zebrafish and exacerbated infection with both M. marinum and Salmonella typhimurium (Kanwal et al., 2013). Transcriptional profiling of Ptpn6-depleted animals showed elevated levels of pro-inflammatory cytokines and pathogen-recognition molecules that were further potentiated by infection. These findings illustrate the importance of a balanced inflammatory state during mycobacterial pathogenesis.
Orthologs of macrophage-derived and mycobacteria-relevant cytokines, such as the interleukins IL-4, IL-6, IL-10, IL-12 and IL-13, plus interferon-gamma (IFN-γ) and tumor necrosis factor (TNF), have been identified in zebrafish (Zhang et al., 2005; Igawa et al., 2006; Clay et al., 2008; Ohtani et al., 2008; Holt et al., 2011; Varela et al., 2012). Only TNF, a cytokine that has long been recognized as a crucial host-protective factor in Mtb infection, has been extensively studied in the zebrafish–M. marinum model (Clay et al., 2008; Roca and Ramakrishnan, 2013). Experiments in the zebrafish demonstrated the significance of a balanced TNF response to Mtb pathogenesis. Induction of a hypoinflammatory, low-TNF signaling, state through morpholino knockdown of the TNF receptor led to enhanced mycobacterial growth and loss of bacterial containment through necrosis of highly infected macrophages (Clay et al., 2008). These findings are in agreement with findings in monkeys and humans, in which TNF is crucial in limiting mycobacterial growth during persistent infections (Keane et al., 2001; Lin et al., 2010). However, hyperinflammation in the zebrafish due to high TNF levels was also unfavorable for the host (Tobin et al., 2012; Roca and Ramakrishnan, 2013). Longitudinal imaging demonstrated that high TNF levels initially diminish the bacterial burden through enhanced mycobacterial killing, but bacterial restriction later fails as macrophages undergo programmed necrosis induced by mitochondrial reactive oxygen species (Roca and Ramakrishnan, 2013). Ultimately, low and high TNF states were found to be similarly unfavorable to the zebrafish host, owing to the subsequent rapid growth of extracellular bacteria (Clay et al., 2008; Tobin et al., 2010; Tobin et al., 2012; Roca and Ramakrishnan, 2013). However, the distinct mechanism of failure for the high-TNF state enabled the authors to uncouple the negative effects of high TNF (macrophage cell death) from the positive effects of high TNF (mycobacterial killing) by chemical inhibition of the mitochondrial pore complex and ceramide release (Roca and Ramakrishnan, 2013). Promisingly, high-TNF animals treated with this regimen restricted mycobacteria better than either wild-type or low-TNF animals (Roca and Ramakrishnan, 2013). Overall, these results demonstrate that the mechanistic understandings gained through the use of the zebrafish model can be used to generate new rationally designed drug therapies for mycobacterial infection.
Granuloma formation and dynamics
Two longstanding assumptions within the tuberculosis community have shaped our expectations of the granuloma – first, that the granuloma is exclusively a host-driven protective structure and second that the granuloma is a static, wall-like structure. However, taking advantage of the visual accessibility of the zebrafish–M. marinum model, researchers have started to find that our traditional ideas based on pathology might not tell the whole story.
The first assumption – that of the host-driven granuloma – has been tested in longitudinal imaging experiments in zebrafish. Using mycobacteria lacking the RD1 virulence locus, the bacterial RD1 locus was found to be required for efficient granuloma formation, indicating that a virulence factor works to accelerate granuloma formation (Volkman et al., 2004). RD1 deficiency also led to attenuated M. marinum infection, indicating that loss of RD1-mediated granuloma formation might limit mycobacterial virulence as well (Volkman et al., 2004). The RD1 region, originally identified as a crucial pathogenicity locus that is lost in the attenuated vaccine strain BCG, encompasses a type VII secretion system called ESX-1 that is conserved in M. marinum (Volkman et al., 2004). Further examination of M. marinum ESX-1 components identified multiple bacterial genes within the ESX-1 locus required for optimal granuloma formation and virulence in vivo, including the early secretory antigenic target ESAT-6, the ATPase EccA1 and the ESX-1 secretion-associated protein EspL (Gao et al., 2004; Volkman et al., 2010; Stoop et al., 2011; Joshi et al., 2012). Secretion systems other than the ESX-1 machinery are also crucial to mycobacterial granuloma formation. For example, M. marinum strains lacking SecA2 secretion were also found to have diminished granuloma formation (Watkins et al., 2012). These findings indicate that mycobacteria have evolved to use multiple secretion systems to facilitate granuloma formation.
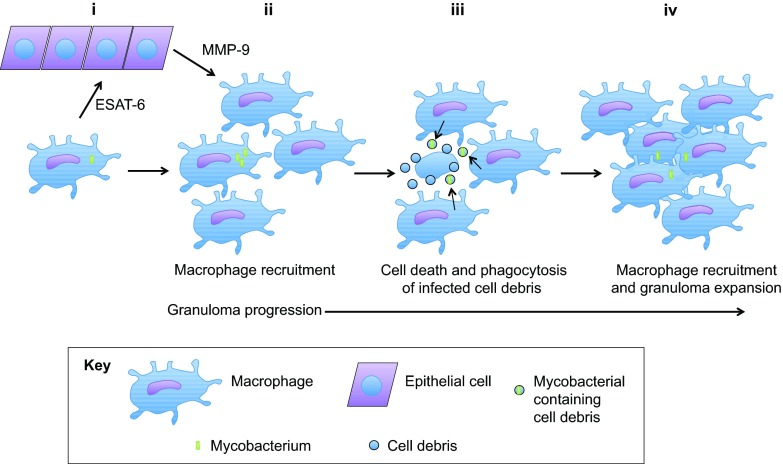
To better understand how mycobacterial ESX-1 secretion drives granuloma formation, individual infected macrophages were followed in zebrafish. It was found that the ESX-1 system drives cell death of infected macrophages (Volkman et al., 2004; Davis and Ramakrishnan, 2009). This locally expands the population of infected macrophages through phagocytosis of the associated mycobacterium-containing cell debris by multiple incoming macrophages (Fig. 3) (Davis and Ramakrishnan, 2009). The ESX-1 machinery also enhances recruitment of surrounding macrophages to the nascent infection (Davis and Ramakrishnan, 2009). ESX-1-dependent macrophage recruitment is driven by secretion of ESAT-6 into the surrounding tissue. Secreted ESAT-6 induces the inflammatory matrix metalloprotease MMP-9 in adjacent epithelial cells, acting as a guidance cue for nearby macrophages (Volkman et al., 2010). Successive waves of RD1-dependent macrophage cell death and recruitment further enhance bacterial expansion and granuloma formation (Fig. 3) (Davis and Ramakrishnan, 2009; Volkman et al., 2010). Granuloma formation coincides with a rapid bacterial expansion, indicating that this bacterially driven structure probably enhances this subsequent outgrowth of bacteria (Volkman et al., 2004). Thus, in contrast to the established model of an exclusively host-protective granuloma, the visual access afforded by the zebrafish–M. marinum model has allowed researchers to recognize that the early granuloma is also a bacterially influenced structure that can enhance mycobacterial pathogenicity. However, the granuloma might serve a dual purpose whereby, during later stages of infection, it can also exert a host-protective role.
Fig. 3.
Macrophage recruitment and granuloma formation during mycobacterial infection. (i) ESAT-6 released from an infected macrophage drives MMP-9 production in surrounding epithelial cells. (ii) MMP-9-dependent and -independent signals drive recruitment of surrounding macrophages to the infected macrophage. (iii) The infected macrophage undergoes cell death, releasing infected cell debris. This infected cell debris is phagocytosed by recruited macrophages, expanding the population of infected macrophages. (iv) The newly infected macrophages continue to recruit additional macrophages through MMP-9-dependent and -independent mechanisms, as in panels i and ii, and ongoing cell death of infected macrophages continues to expand bacterial infection to recruited macrophage populations. As these processes continue, these infected and uninfected macrophages aggregate to form granulomas.
Visualization of granulomas in zebrafish has revealed that the granuloma is a dynamic structure. Differential interference contrast (DIC) imaging of the granuloma revealed that, despite its tightly packed structure, there was considerable movement of cells into and within the granuloma (Davis et al., 2002). This was later confirmed in superinfection experiments, in which an infected animal is subsequently infected with a second strain. Using multiple fluorescent strains of M. marinum, it was found that superinfecting mycobacteria readily home to existing granulomas, indicating that this structure could dynamically rearrange to accommodate incoming macrophages and their phagocytosed mycobacteria (Cosma et al., 2004). Homing of superinfecting mycobacteria into established granulomas was subsequently confirmed in mouse–Mtb infections, demonstrating that this behavior is conserved across pathogenic mycobacteria (Cosma et al., 2008). These data, taken together with the active RD1-dependent recruitment of macrophages during infection, characterizes a dynamic granuloma within the zebrafish, at odds with the staid granulomas of medical textbooks. Researchers have also observed the emigration of infected macrophages from the granuloma (Davis and Ramakrishnan, 2009). These departing macrophages alight in other tissues, seeding new granulomas at the distal locations (Davis and Ramakrishnan, 2009). Thus, rather than a static host-protective structure, the granuloma can be dynamically remodeled to the benefit of infecting mycobacteria. In agreement with these findings from the zebrafish–M. marinum model, positron emission tomography imaging of lung granulomas in Mtb-infected macaques found that individual lesions were dynamic, freely progressing, regressing or coalescing with neighboring lesions over time (Lin et al., 2014).
Bacterial and host genetics
Mycobacterial factors
A dizzying array of Mtb virulence factors have been described in cell-culture and animal-infection models. However, reconciling virulence factor phenotypes in these distinct models and mechanistically unwinding their functions is challenging. The extensive conservation between M. marinum and Mtb virulence factors enables researchers to exploit both the faster generation time of M. marinum and the visual accessibility of zebrafish to interrogate virulence mutant phenotypes within weeks to months rather than months to years. Earlier in this Review, we highlighted bacterial mutants in the RD1 locus, SecA2 and EspL, for their roles in granuloma formation, and mutants in PDIM and PGL synthesis for their role in mycobacterial recognition by host macrophages. Beyond these proteins and lipids, the zebrafish–M. marinum model has been applied more broadly to identify other bacterial mutants that are attenuated in virulence in vivo. Cell-wall mutants are an important class of attenuated Mtb mutants. In addition to PDIM- and PGL-deficient strains, other M. marinum mutants with cell-wall deficiencies have been identified, such as the iipA, iipB and erp mutants (Cosma et al., 2006; Gao et al., 2006). These mutants have all been found to have increased sensitivity to antibiotics and are readily killed by macrophages, leading to attenuated infections in zebrafish models (Cosma et al., 2006; Gao et al., 2006). Mycobacterial strains either lacking mannose core branching of the glycolipid lipoarabinomannan (LAM) or with reduced mycolic acid synthesis were also attenuated in vivo (Joshi et al., 2012; Stoop et al., 2013). However, not all cell-wall mutants are hypovirulent. A transposon screen identified a cluster of genes involved in the biosynthesis of the cell-wall component lipooligosaccharide (van der Woude et al., 2012). Knockout of a member of this biosynthetic cluster, wecE, resulted in selective loss of one of four species of lipooligosaccharide – lipooligosaccharide-IV – leading to hypervirulence in vivo (van der Woude et al., 2012).
Mycobacteria possess two distinct groups of proteins called PE and PPE proteins, named for their N-terminal sequence motifs (either proline-glutamate or proline-proline-glutamate, respectively). These families are thought to participate in virulence, and large numbers of these proteins are present in both Mtb (169 potential coding sequences) and M. marinum (281 potential coding sequences) (Stinear et al., 2008). A transposon insertion in M. marinum disrupting the PPE38 gene (a PPE protein that is also present in Mtb) led to attenuation of M. marinum in zebrafish. This attenuated phenotype correlated with reduced tissue pathology in animals infected with the PPE38 mutant (Dong et al., 2012). Consistent with the reduced pathology in infected zebrafish, cell-culture experiments interrogating cytokine release found that the PPE38 mutant had diminished TNF and IL-6 expression (Dong et al., 2012). More broadly, the combined function of PE and PPE proteins was addressed using an ESX-5 mutant that is defective in PE and PPE protein secretion (Weerdenburg et al., 2012). The ESX-5 mutant was found to be modestly attenuated in larval zebrafish, but highly virulent in adult zebrafish (Weerdenburg et al., 2012). Increased virulence in adult zebrafish was accompanied by increased inflammatory gene expression and granuloma formation (Weerdenburg et al., 2012). These findings indicate that PE and PPE proteins can influence virulence, and play both pro- and anti-inflammatory roles during pathogenesis.
Host factors
On the host side, work has uncovered a number of host factors crucial for mycobacterial recognition and growth, and ongoing screening efforts continue to identify novel host loci involved in the process of infection. As a host, zebrafish are particularly amenable to screening approaches, enabling researchers to screen in an in vivo system. The large number of embryos obtained from a single cross, simple infection protocol, small size and aforementioned optical clarity greatly facilitate screening efforts. To further facilitate high-throughput screening, Carvalho and colleagues have designed a machine allowing automated infection of zebrafish (Carvalho et al., 2011). Infected zebrafish arrayed in 96- or 384-well plates can be readily imaged by high-content microscopy, flow cytometry or automated plate reader approaches, allowing automated detection of mycobacterial burden and other phenotypes defined by fluorescent markers (Carvalho et al., 2011; Takaki et al., 2012). Other than visual assays, changes in gene expression can be investigated at either the whole transcriptome level or in more focused subsets of genes (Hegedűs et al., 2009; Rotman et al., 2011; Kanwal et al., 2013; van der Vaart et al., 2013; Veneman et al., 2013).
Illustrating the merits of zebrafish screening efforts, a forward-genetic screen using larvae infected with M. marinum from 355 distinct ENU-mutagenized founders identified a novel role for Lta4h-dependent lipoxin signaling in mycobacterial pathogenesis (Tobin et al., 2010). Based on these findings, LTA4H variants were investigated in human patients. Genotypes associated with either high or low LTA4H levels were both found to be detrimental in disseminated TB meningitis (Tobin et al., 2010). In contrast, heterozygous patients with intermediate levels of LTA4H were protected from TB meningitis, a finding that was subsequently modeled in zebrafish (Tobin et al., 2010; Tobin et al., 2012). Dexamethasone serves as standard-of-care adjunctive therapy in TB meningitis (Thwaites et al., 2004). Combined with modeling of human LTA4H genotypes and dexamethasone treatment in zebrafish, further studies in human patients revealed that the survival effect of dexamethasone in TB meningitis associated strongly with the high-activity (and not the low-activity) LTA4H variant, suggesting that genotype-guided therapies might be useful in humans (Tobin et al., 2012). The ability to translate zebrafish genetic screens into novel clinical insights demonstrates fundamental conservation between the zebrafish–M. marinum model and Mtb infection. Additionally, these findings suggest that data from this model can be translated from ‘fish tank to bedside’, as has previously been achieved with other zebrafish models (Ablain and Zon, 2013). Complementing traditional forward-genetic screening approaches, reverse-genetic techniques have become easier in zebrafish with the description of TILLING, TALENs and CRISPR RNAs, and the growing list of existing zebrafish mutants, allowing researchers to rapidly interrogate specific host genes (Wienholds et al., 2003; Huang et al., 2011; Sander et al., 2011; Hwang et al., 2013). Thus, the host-driven response represents a promising target of antimycobacterial therapies. Additionally, the targeting of host pathways represents a valuable strategy to limit the risks associated with the emergence of treatment-resistant mycobacteria. The array of host-directed tools available for zebrafish should facilitate in vivo screening for potential host targets.
Conclusions and outlook
Studies of host–pathogen interactions that give rise to the complex pathology of tuberculosis infection have long benefited from effective use of animal models. Traditional mammalian models of Mtb infection have provided important insights into disease, but do not pair a pathogen with its natural host and/or lack the reagents to ask the desired experimental questions. Furthermore, visual access to live animals is relatively limited in mammalian models, restricting the questions that researchers can ask effectively. In the zebrafish–M. marinum model, infection of zebrafish with the closest relative of the Mtb complex recapitulates the major pathologic feature of Mtb infection – the granuloma – within a genetically tractable and optically clear native host. The zebrafish–M. marinum model is not intended to replace the mammalian models of Mtb infection. However, the particular strengths of the zebrafish–M. marinum model enable researchers to ask questions that are difficult, impossible or simply too expensive to answer in mammalian models. These benefits have allowed researchers to gain a new understanding of mycobacterial infection, host response and pathology, and many of these findings have now been extended to mammalian models and human populations. Going forward, the clinical reality of tuberculosis requires the development of new therapeutics. Facile manipulation of host and bacterial genetics within the zebrafish–M. marinum system, and the ability to translate findings from the zebrafish into therapeutic strategies for patients indicate that the zebrafish–M. marinum model provides an excellent platform for understanding basic questions of pathogenesis and a unique resource for developing new approaches to antimycobacterial therapies.
Acknowledgments
The authors thank members of the Tobin lab, particularly Rebecca Beerman, Kim Dohlich and Molly Matty, for critical reading of this manuscript.
Footnotes
This article is part of a Special Issue, Spotlight on Zebrafish: Translational Impact. See all the articles in the issue at http://dmm.biologists.org/content/7/7.toc.
Competing interests
The authors declare no competing financial interests.
Funding
M.R.C. is supported by a postdoctoral fellowship, PF-13-223-01–MPC, from the American Cancer Society. The Tobin laboratory is funded through a Mallinckrodt Scholar Award, a Searle Scholar Award, a National Institutes of Health Director’s New Innovator Award (1DP2OD008614-01), a Vallee Foundation Young Investigator Award, a Whitehead Scholar Award and the Duke University Center for AIDS Research (5P30 AI064518).
References
- Ablain J., Zon L. I. (2013). Of fish and men: using zebrafish to fight human diseases. Trends Cell Biol. 23, 584–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alibaud L., Rombouts Y., Trivelli X., Burguière A., Cirillo S. L., Cirillo J. D., Dubremetz J. F., Guérardel Y., Lutfalla G., Kremer L. (2011). A Mycobacterium marinum TesA mutant defective for major cell wall-associated lipids is highly attenuated in Dictyostelium discoideum and zebrafish embryos. Mol. Microbiol. 80, 919–934 [DOI] [PubMed] [Google Scholar]
- Balla K. M., Lugo-Villarino G., Spitsbergen J. M., Stachura D. L., Hu Y., Bañuelos K., Romo-Fewell O., Aroian R. V., Traver D. (2010). Eosinophils in the zebrafish: prospective isolation, characterization, and eosinophilia induction by helminth determinants. Blood 116, 3944–3954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambier C. J., Takaki K. K., Larson R. P., Hernandez R. E., Tobin D. M., Urdahl K. B., Cosma C. L., Ramakrishnan L. (2014). Mycobacteria manipulate macrophage recruitment through coordinated use of membrane lipids. Nature 505, 218–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuano S. V., III, Croix D. A., Pawar S., Zinovik A., Myers A., Lin P. L., Bissel S., Fuhrman C., Klein E., Flynn J. L. (2003). Experimental Mycobacterium tuberculosis infection of cynomolgus macaques closely resembles the various manifestations of human M. tuberculosis infection. Infect. Immun. 71, 5831–5844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho R., de Sonneville J., Stockhammer O. W., Savage N. D., Veneman W. J., Ottenhoff T. H., Dirks R. P., Meijer A. H., Spaink H. P. (2011). A high-throughput screen for tuberculosis progression. PLoS ONE 6, e16779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay H., Davis J. M., Beery D., Huttenlocher A., Lyons S. E., Ramakrishnan L. (2007). Dichotomous role of the macrophage in early Mycobacterium marinum infection of the zebrafish. Cell Host Microbe 2, 29–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay H., Volkman H. E., Ramakrishnan L. (2008). Tumor necrosis factor signaling mediates resistance to mycobacteria by inhibiting bacterial growth and macrophage death. Immunity 29, 283–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colucci-Guyon E., Tinevez J. Y., Renshaw S. A., Herbomel P. (2011). Strategies of professional phagocytes in vivo: unlike macrophages, neutrophils engulf only surface-associated microbes. J. Cell Sci. 124, 3053–3059 [DOI] [PubMed] [Google Scholar]
- Comas I., Coscolla M., Luo T., Borrell S., Holt K. E., Kato-Maeda M., Parkhill J., Malla B., Berg S., Thwaites G., et al. (2013). Out-of-Africa migration and Neolithic coexpansion of Mycobacterium tuberculosis with modern humans. Nat. Genet. 45, 1176–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosma C. L., Humbert O., Ramakrishnan L. (2004). Superinfecting mycobacteria home to established tuberculous granulomas. Nat. Immunol. 5, 828–835 [DOI] [PubMed] [Google Scholar]
- Cosma C. L., Klein K., Kim R., Beery D., Ramakrishnan L. (2006). Mycobacterium marinum Erp is a virulence determinant required for cell wall integrity and intracellular survival. Infect. Immun. 74, 3125–3133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosma C. L., Humbert O., Sherman D. R., Ramakrishnan L. (2008). Trafficking of superinfecting Mycobacterium organisms into established granulomas occurs in mammals and is independent of the Erp and ESX-1 mycobacterial virulence loci. J. Infect. Dis. 198, 1851–1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J. S., Chen B., McNeil M., Jacobs W. R., Jr (1999). Complex lipid determines tissue-specific replication of Mycobacterium tuberculosis in mice. Nature 402, 79–83 [DOI] [PubMed] [Google Scholar]
- Cyktor J. C., Carruthers B., Kominsky R. A., Beamer G. L., Stromberg P., Turner J. (2013). IL-10 inhibits mature fibrotic granuloma formation during Mycobacterium tuberculosis infection. J. Immunol. 190, 2778–2790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. M., Ramakrishnan L. (2009). The role of the granuloma in expansion and dissemination of early tuberculous infection. Cell 136, 37–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. M., Clay H., Lewis J. L., Ghori N., Herbomel P., Ramakrishnan L. (2002). Real-time visualization of mycobacterium-macrophage interactions leading to initiation of granuloma formation in zebrafish embryos. Immunity 17, 693–702 [DOI] [PubMed] [Google Scholar]
- Dobson J. T., Seibert J., Teh E. M., Da’as S., Fraser R. B., Paw B. H., Lin T. J., Berman J. N. (2008). Carboxypeptidase A5 identifies a novel mast cell lineage in the zebrafish providing new insight into mast cell fate determination. Blood 112, 2969–2972 [DOI] [PubMed] [Google Scholar]
- Dong D., Wang D., Li M., Wang H., Yu J., Wang C., Liu J., Gao Q. (2012). PPE38 modulates the innate immune response and is required for Mycobacterium marinum virulence. Infect. Immun. 80, 43–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elks P. M., Brizee S., van der Vaart M., Walmsley S. R., van Eeden F. J., Renshaw S. A., Meijer A. H. (2013). Hypoxia inducible factor signaling modulates susceptibility to mycobacterial infection via a nitric oxide dependent mechanism. PLoS Pathog. 9, e1003789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellett F., Pase L., Hayman J. W., Andrianopoulos A., Lieschke G. J. (2011). mpeg1 promoter transgenes direct macrophage-lineage expression in zebrafish. Blood 117, e49–e56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eum S. Y., Kong J. H., Hong M. S., Lee Y. J., Kim J. H., Hwang S. H., Cho S. N., Via L. E., Barry C. E., III(2010). Neutrophils are the predominant infected phagocytic cells in the airways of patients with active pulmonary TB. Chest 137, 122–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn J. L. (2006). Lessons from experimental Mycobacterium tuberculosis infections. Microbes Infect. 8, 1179–1188 [DOI] [PubMed] [Google Scholar]
- Gao L. Y., Guo S., McLaughlin B., Morisaki H., Engel J. N., Brown E. J. (2004). A mycobacterial virulence gene cluster extending RD1 is required for cytolysis, bacterial spreading and ESAT-6 secretion. Mol. Microbiol. 53, 1677–1693 [DOI] [PubMed] [Google Scholar]
- Gao L. Y., Pak M., Kish R., Kajihara K., Brown E. J. (2006). A mycobacterial operon essential for virulence in vivo and invasion and intracellular persistence in macrophages. Infect. Immun. 74, 1757–1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall C., Flores M. V., Storm T., Crosier K., Crosier P. (2007). The zebrafish lysozyme C promoter drives myeloid-specific expression in transgenic fish. BMC Dev. Biol. 7, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegedűs Z., Zakrzewska A., Agoston V. C., Ordas A., Rácz P., Mink M., Spaink H. P., Meijer A. H. (2009). Deep sequencing of the zebrafish transcriptome response to mycobacterium infection. Mol. Immunol. 46, 2918–2930 [DOI] [PubMed] [Google Scholar]
- Holt A., Mitra S., van der Sar A. M., Alnabulsi A., Secombes C. J., Bird S. (2011). Discovery of zebrafish (Danio rerio) interleukin-23 alpha (IL-23α) chain, a subunit important for the formation of IL-23, a cytokine involved in the development of Th17 cells and inflammation. Mol. Immunol. 48, 981–991 [DOI] [PubMed] [Google Scholar]
- Huang P., Xiao A., Zhou M., Zhu Z., Lin S., Zhang B. (2011). Heritable gene targeting in zebrafish using customized TALENs. Nat. Biotechnol. 29, 699–700 [DOI] [PubMed] [Google Scholar]
- Hwang W. Y., Fu Y., Reyon D., Maeder M. L., Tsai S. Q., Sander J. D., Peterson R. T., Yeh J. R., Joung J. K. (2013). Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat. Biotechnol. 31, 227–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igawa D., Sakai M., Savan R. (2006). An unexpected discovery of two interferon gamma-like genes along with interleukin (IL)-22 and -26 from teleost: IL-22 and -26 genes have been described for the first time outside mammals. Mol. Immunol. 43, 999–1009 [DOI] [PubMed] [Google Scholar]
- Jayaswal S., Kamal M. A., Dua R., Gupta S., Majumdar T., Das G., Kumar D., Rao K. V. (2010). Identification of host-dependent survival factors for intracellular Mycobacterium tuberculosis through an siRNA screen. PLoS Pathog. 6, e1000839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. S., Amirault H. J., Andersen B. R. (1990). Killing of Mycobacterium tuberculosis by neutrophils: a nonoxidative process. J. Infect. Dis. 162, 700–704 [DOI] [PubMed] [Google Scholar]
- Joshi S. A., Ball D. A., Sun M. G., Carlsson F., Watkins B. Y., Aggarwal N., McCracken J. M., Huynh K. K., Brown E. J. (2012). EccA1, a component of the Mycobacterium marinum ESX-1 protein virulence factor secretion pathway, regulates mycolic acid lipid synthesis. Chem. Biol. 19, 372–380 [DOI] [PubMed] [Google Scholar]
- Kanwal Z., Zakrzewska A., den Hertog J., Spaink H. P., Schaaf M. J., Meijer A. H. (2013). Deficiency in hematopoietic phosphatase ptpn6/Shp1 hyperactivates the innate immune system and impairs control of bacterial infections in zebrafish embryos. J. Immunol. 190, 1631–1645 [DOI] [PubMed] [Google Scholar]
- Kapoor N., Pawar S., Sirakova T. D., Deb C., Warren W. L., Kolattukudy P. E. (2013). Human granuloma in vitro model, for TB dormancy and resuscitation. PLoS ONE 8, e53657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami K., Takeda H., Kawakami N., Kobayashi M., Matsuda N., Mishina M. (2004). A transposon-mediated gene trap approach identifies developmentally regulated genes in zebrafish. Dev. Cell 7, 133–144 [DOI] [PubMed] [Google Scholar]
- Keane J., Gershon S., Wise R. P., Mirabile-Levens E., Kasznica J., Schwieterman W. D., Siegel J. N., Braun M. M. (2001). Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N. Engl. J. Med. 345, 1098–1104 [DOI] [PubMed] [Google Scholar]
- Kramnik I., Dietrich W. F., Demant P., Bloom B. R. (2000). Genetic control of resistance to experimental infection with virulent Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 97, 8560–8565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar D., Nath L., Kamal M. A., Varshney A., Jain A., Singh S., Rao K. V. (2010). Genome-wide analysis of the host intracellular network that regulates survival of Mycobacterium tuberculosis. Cell 140, 731–743 [DOI] [PubMed] [Google Scholar]
- Langenau D. M., Ferrando A. A., Traver D., Kutok J. L., Hezel J. P., Kanki J. P., Zon L. I., Look A. T., Trede N. S. (2004). In vivo tracking of T cell development, ablation, and engraftment in transgenic zebrafish. Proc. Natl. Acad. Sci. USA 101, 7369–7374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieschke G. J., Oates A. C., Crowhurst M. O., Ward A. C., Layton J. E. (2001). Morphologic and functional characterization of granulocytes and macrophages in embryonic and adult zebrafish. Blood 98, 3087–3096 [DOI] [PubMed] [Google Scholar]
- Lin P. L., Myers A., Smith L., Bigbee C., Bigbee M., Fuhrman C., Grieser H., Chiosea I., Voitenek N. N., Capuano S. V., et al. (2010). Tumor necrosis factor neutralization results in disseminated disease in acute and latent Mycobacterium tuberculosis infection with normal granuloma structure in a cynomolgus macaque model. Arthritis Rheum. 62, 340–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin P. L., Ford C. B., Coleman M. T., Myers A. J., Gawande R., Ioerger T., Sacchettini J., Fortune S. M., Flynn J. L. (2014). Sterilization of granulomas is common in active and latent tuberculosis despite within-host variability in bacterial killing. Nat. Med. 20, 75–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe D. M., Redford P. S., Wilkinson R. J., O’Garra A., Martineau A. R. (2012). Neutrophils in tuberculosis: friend or foe? Trends Immunol. 33, 14–25 [DOI] [PubMed] [Google Scholar]
- Lugo-Villarino G., Balla K. M., Stachura D. L., Bañuelos K., Werneck M. B., Traver D. (2010). Identification of dendritic antigen-presenting cells in the zebrafish. Proc. Natl. Acad. Sci. USA 107, 15850–15855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeed M., Perskvist N., Ernst J. D., Orselius K., Stendahl O. (1998). Roles of calcium and annexins in phagocytosis and elimination of an attenuated strain of Mycobacterium tuberculosis in human neutrophils. Microb. Pathog. 24, 309–320 [DOI] [PubMed] [Google Scholar]
- Meijer A. H., Spaink H. P. (2011). Host-pathogen interactions made transparent with the zebrafish model. Curr. Drug Targets 12, 1000–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer A. H., van der Sar A. M., Cunha C., Lamers G. E., Laplante M. A., Kikuta H., Bitter W., Becker T. S., Spaink H. P. (2008). Identification and real-time imaging of a myc-expressing neutrophil population involved in inflammation and mycobacterial granuloma formation in zebrafish. Dev. Comp. Immunol. 32, 36–49 [DOI] [PubMed] [Google Scholar]
- Ohtani M., Hayashi N., Hashimoto K., Nakanishi T., Dijkstra J. M. (2008). Comprehensive clarification of two paralogous interleukin 4/13 loci in teleost fish. Immunogenetics 60, 383–397 [DOI] [PubMed] [Google Scholar]
- Ottenhoff T. H., Kaufmann S. H. (2012). Vaccines against tuberculosis: where are we and where do we need to go? PLoS Pathog. 8, e1002607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikka M., Hammarén M. M., Harjula S. K., Halfpenny N. J., Oksanen K. E., Lahtinen M. J., Pajula E. T., Iivanainen A., Pesu M., Rämet M. (2012). Mycobacterium marinum causes a latent infection that can be reactivated by gamma irradiation in adult zebrafish. PLoS Pathog. 8, e1002944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips J. A., Ernst J. D. (2012). Tuberculosis pathogenesis and immunity. Annu. Rev. Pathol. 7, 353–384 [DOI] [PubMed] [Google Scholar]
- Puissegur M. P., Botanch C., Duteyrat J. L., Delsol G., Caratero C., Altare F. (2004). An in vitro dual model of mycobacterial granulomas to investigate the molecular interactions between mycobacteria and human host cells. Cell. Microbiol. 6, 423–433 [DOI] [PubMed] [Google Scholar]
- Ramakrishnan L. (2012). Revisiting the role of the granuloma in tuberculosis. Nat. Rev. Immunol. 12, 352–366 [DOI] [PubMed] [Google Scholar]
- Reece S. T., Loddenkemper C., Askew D. J., Zedler U., Schommer-Leitner S., Stein M., Mir F. A., Dorhoi A., Mollenkopf H. J., Silverman G. A., et al. (2010). Serine protease activity contributes to control of Mycobacterium tuberculosis in hypoxic lung granulomas in mice. J. Clin. Invest. 120, 3365–3376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed M. B., Domenech P., Manca C., Su H., Barczak A. K., Kreiswirth B. N., Kaplan G., Barry C. E., III(2004). A glycolipid of hypervirulent tuberculosis strains that inhibits the innate immune response. Nature 431, 84–87 [DOI] [PubMed] [Google Scholar]
- Renshaw S. A., Trede N. S. (2012). A model 450 million years in the making: zebrafish and vertebrate immunity. Dis. Model. Mech. 5, 38–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renshaw S. A., Loynes C. A., Trushell D. M., Elworthy S., Ingham P. W., Whyte M. K. (2006). A transgenic zebrafish model of neutrophilic inflammation. Blood 108, 3976–3978 [DOI] [PubMed] [Google Scholar]
- Repasy T., Lee J., Marino S., Martinez N., Kirschner D. E., Hendricks G., Baker S., Wilson A. A., Kotton D. N., Kornfeld H. (2013). Intracellular bacillary burden reflects a burst size for Mycobacterium tuberculosis in vivo. PLoS Pathog. 9, e1003190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca F. J., Ramakrishnan L. (2013). TNF dually mediates resistance and susceptibility to mycobacteria via mitochondrial reactive oxygen species. Cell 153, 521–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotman J., van Gils W., Butler D., Spaink H. P., Meijer A. H. (2011). Rapid screening of innate immune gene expression in zebrafish using reverse transcription – multiplex ligation-dependent probe amplification. BMC Res. Notes 4, 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D. G. (2013). The evolutionary pressures that have molded Mycobacterium tuberculosis into an infectious adjuvant. Curr. Opin. Microbiol. 16, 78–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchettini J. C., Rubin E. J., Freundlich J. S. (2008). Drugs versus bugs: in pursuit of the persistent predator Mycobacterium tuberculosis. Nat. Rev. Microbiol. 6, 41–52 [DOI] [PubMed] [Google Scholar]
- Sander J. D., Cade L., Khayter C., Reyon D., Peterson R. T., Joung J. K., Yeh J. R. (2011). Targeted gene disruption in somatic zebrafish cells using engineered TALENs. Nat. Biotechnol. 29, 697–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassetti C. M., Boyd D. H., Rubin E. J. (2001). Comprehensive identification of conditionally essential genes in mycobacteria. Proc. Natl. Acad. Sci. USA 98, 12712–12717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassetti C. M., Boyd D. H., Rubin E. J. (2003). Genes required for mycobacterial growth defined by high density mutagenesis. Mol. Microbiol. 48, 77–84 [DOI] [PubMed] [Google Scholar]
- Stinear T. P., Seemann T., Harrison P. F., Jenkin G. A., Davies J. K., Johnson P. D., Abdellah Z., Arrowsmith C., Chillingworth T., Churcher C., et al. (2008). Insights from the complete genome sequence of Mycobacterium marinum on the evolution of Mycobacterium tuberculosis. Genome Res. 18, 729–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoop E. J., Schipper T., Rosendahl Huber S. K., Nezhinsky A. E., Verbeek F. J., Gurcha S. S., Besra G. S., Vandenbroucke-Grauls C. M., Bitter W., van der Sar A. M. (2011). Zebrafish embryo screen for mycobacterial genes involved in the initiation of granuloma formation reveals a newly identified ESX-1 component. Dis. Model. Mech. 4, 526–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoop E. J., Mishra A. K., Driessen N. N., van Stempvoort G., Bouchier P., Verboom T., van Leeuwen L. M., Sparrius M., Raadsen S. A., van Zon M., et al. (2013). Mannan core branching of lipo(arabino)mannan is required for mycobacterial virulence in the context of innate immunity. Cell. Microbiol. 15, 2093–2108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaim L. E., Connolly L. E., Volkman H. E., Humbert O., Born D. E., Ramakrishnan L. (2006). Mycobacterium marinum infection of adult zebrafish causes caseating granulomatous tuberculosis and is moderated by adaptive immunity. Infect. Immun. 74, 6108–6117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaki K., Cosma C. L., Troll M. A., Ramakrishnan L. (2012). An in vivo platform for rapid high-throughput antitubercular drug discovery. Cell Rep. 2, 175–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thwaites G. E., Nguyen D. B., Nguyen H. D., Hoang T. Q., Do T. T., Nguyen T. C., Nguyen Q. H., Nguyen T. T., Nguyen N. H., Nguyen T. N., et al. (2004). Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N. Engl. J. Med. 351, 1741–1751 [DOI] [PubMed] [Google Scholar]
- Tobin D. M., Vary J. C., Jr, Ray J. P., Walsh G. S., Dunstan S. J., Bang N. D., Hagge D. A., Khadge S., King M. C., Hawn T. R., et al. (2010). The lta4h locus modulates susceptibility to mycobacterial infection in zebrafish and humans. Cell 140, 717–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin D. M., Roca F. J., Oh S. F., McFarland R., Vickery T. W., Ray J. P., Ko D. C., Zou Y., Bang N. D., Chau T. T., et al. (2012). Host genotype-specific therapies can optimize the inflammatory response to mycobacterial infections. Cell 148, 434–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torraca V., Masud S., Spaink H. P., Meijer A. H. (2014). Macrophage-pathogen interactions in infectious diseases: new therapeutic insights from the zebrafish host model. Dis. Model. Mech. 7, 785–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Vaart M., van Soest J. J., Spaink H. P., Meijer A. H. (2013). Functional analysis of a zebrafish myd88 mutant identifies key transcriptional components of the innate immune system. Dis. Model. Mech. 6, 841–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Woude A. D., Sarkar D., Bhatt A., Sparrius M., Raadsen S. A., Boon L., Geurtsen J., van der Sar A. M., Luirink J., Houben E. N., et al. (2012). Unexpected link between lipooligosaccharide biosynthesis and surface protein release in Mycobacterium marinum. J. Biol. Chem. 287, 20417–20429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela M., Dios S., Novoa B., Figueras A. (2012). Characterisation, expression and ontogeny of interleukin-6 and its receptors in zebrafish (Danio rerio). Dev. Comp. Immunol. 37, 97–106 [DOI] [PubMed] [Google Scholar]
- Veneman W. J., Stockhammer O. W., de Boer L., Zaat S. A., Meijer A. H., Spaink H. P. (2013). A zebrafish high throughput screening system used for Staphylococcus epidermidis infection marker discovery. BMC Genomics 14, 255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkman H. E., Clay H., Beery D., Chang J. C., Sherman D. R., Ramakrishnan L. (2004). Tuberculous granuloma formation is enhanced by a mycobacterium virulence determinant. PLoS Biol. 2, e367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkman H. E., Pozos T. C., Zheng J., Davis J. M., Rawls J. F., Ramakrishnan L. (2010). Tuberculous granuloma induction via interaction of a bacterial secreted protein with host epithelium. Science 327, 466–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins B. Y., Joshi S. A., Ball D. A., Leggett H., Park S., Kim J., Austin C. D., Paler-Martinez A., Xu M., Downing K. H., et al. (2012). Mycobacterium marinum SecA2 promotes stable granulomas and induces tumor necrosis factor alpha in vivo. Infect. Immun. 80, 3512–3520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerdenburg E. M., Abdallah A. M., Mitra S., de Punder K., van der Wel N. N., Bird S., Appelmelk B. J., Bitter W., van der Sar A. M. (2012). ESX-5-deficient Mycobacterium marinum is hypervirulent in adult zebrafish. Cell. Microbiol. 14, 728–739 [DOI] [PubMed] [Google Scholar]
- WHO (2013). global Tuberculosis Report 2013. Geneva, Switzerland: World Health Organisation [Google Scholar]
- Wienholds E., van Eeden F., Kosters M., Mudde J., Plasterk R. H., Cuppen E. (2003). Efficient target-selected mutagenesis in zebrafish. Genome Res. 13, 2700–2707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittamer V., Bertrand J. Y., Gutschow P. W., Traver D. (2011). Characterization of the mononuclear phagocyte system in zebrafish. Blood 117, 7126–7135 [DOI] [PubMed] [Google Scholar]
- Wolf A. J., Linas B., Trevejo-Nuñez G. J., Kincaid E., Tamura T., Takatsu K., Ernst J. D. (2007). Mycobacterium tuberculosis infects dendritic cells with high frequency and impairs their function in vivo. J. Immunol. 179, 2509–2519 [DOI] [PubMed] [Google Scholar]
- Yang C. T., Cambier C. J., Davis J. M., Hall C. J., Crosier P. S., Ramakrishnan L. (2012). Neutrophils exert protection in the early tuberculous granuloma by oxidative killing of mycobacteria phagocytosed from infected macrophages. Cell Host Microbe 12, 301–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Tran V., Li M., Huang X., Niu C., Wang D., Zhu J., Wang J., Gao Q., Liu J. (2012). Both phthiocerol dimycocerosates and phenolic glycolipids are required for virulence of Mycobacterium marinum. Infect. Immun. 80, 1381–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D. C., Shao Y. Q., Huang Y. Q., Jiang S. G. (2005). Cloning, characterization and expression analysis of interleukin-10 from the zebrafish (Danio rerion). J. Biochem. Mol. Biol. 38, 571–576 [DOI] [PubMed] [Google Scholar]