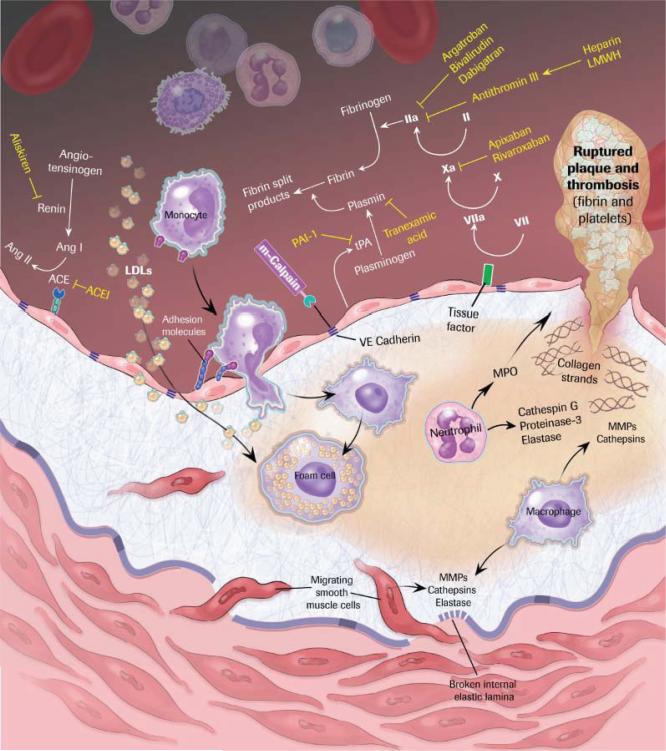
The initiation, progression, and complications of atherosclerosis are mediated by a complex interplay of hematopoietic cells, vascular cells, and the extracellular matrix in response to metabolic (eg, hyperlipidemia, diabetes mellitus) and environmental (eg, smoking) injury.1 Antiatherosclerotic therapies are currently limited to statins and their lipid-lowering and pleiotropic anti-inflammatory effects, to angiotensin-converting enzyme inhibitors and their local effects on vascular cell proliferation and plaque progression independently of blood pressure lowering, and to antiplatelet agents and their role in preventing the thrombotic complications of plaque rupture and superficial endothelial cell (EC) erosion.2,3 However, although these therapies have been shown to reduce event rates by ≈20% to 40% in randomized, placebo-controlled clinical trials, absolute event rates remain high. For example, recurrent ischemic events after index acute coronary syndrome presentation occur in >20% of patients by 36 months despite optimal medical therapy and revascularization.4 This failure of drug and device therapies represents an enormous unmet clinical need. In contrast to effective disease-modifying therapies in psoriasis, rheumatoid arthritis, and multiple sclerosis, targeted antiatherosclerotic therapies have remained elusive. In this issue of Circulation, Miyazaki and colleagues5 have identified m-calpain (calpain 2 and its regulatory domain, calpain 4) as a novel protease target for the modulation of atherogenesis.
After 7 transmembrane superfamily receptors (G protein– coupled receptors), proteases are one of the most attractive pharmacological targets.6 The US market for protease-targeted drugs in 2009 was approximately $11 billion, with the major focus on cardiovascular (hypertension: angiotensin-converting enzyme and renin; thrombosis: thrombin and factor Xa), inflammatory (caspase 1), infectious (HIV pro-tease), and metabolic (diabetes mellitus: dipeptidyl peptidase IV) diseases. Proteases using different mechanisms of substrate hydrolysis (eg, serine, cysteine, threonine, aspartic proteases, metalloproteases) modulate virtually all aspects of atherosclerotic lesion formation and subsequent plaque activation and thrombosis secondary to direct effects on extra-cellular matrix degradation; vascular cell apoptosis, proliferation, and migration; and initiation of coagulation (the Figure). The blood coagulation cascade described by Davie and Ratnoff7 and MacFarland8 is the prototypical example of protease signaling through the activation of zymogens by limited proteolysis. Final activation of this cascade results in the generation of the serine protease thrombin, one of the most intensely targeted proteases in medicine with indirect (unfractionated and low-molecular-weight heparins promoting antithrombin III binding) and direct (bivalirudin, argatroban, dabigatran) mechanisms of pharmacological inhibition. Importantly, there is precedence for 1 protease having far-reaching, multifunctional roles in atherosclerosis. Leukocyte-derived myeloperoxidase generates HOCl, which promotes the formation of oxidized low-density lipoprotein,9 dysfunctional high-density lipoprotein,10 and impaired EC function, in part by altering the bioavailability of nitric oxide. Myeloperoxidase may also contribute directly to EC desquamation, which is associated with thrombosis by promoting EC apoptosis and detachment.11
Figure.
Protease targets in atherothrombosis. Ang indicates angiotensin; ACE, angiotensin-converting enzyme; ACEI, ACE inhibitor; LDL, low-density lipoprotein; PAI, plasminogen activator inhibitor; tPA, tissue-type plasminogen activator; LMWH, low-molecular-weight heparin; MPO, myeloperoxidase; and MMP, matrix metalloproteinase.
m-Calpain is a member of the calpain family of calcium-activated, nonlysosomal cysteine protease active at neutral pH.12,13 Calpains have ubiquitous and tissue-specific isoforms that exhibit broad substrate specificity and regulate cellular migration, proliferation, and apoptosis. Importantly, calpain activity in vivo is tightly regulated by its endogenous inhibitor calpastatin, and in this regard, it is similar to other protease systems (ie, serpin inhibitors of serine proteases and tissue inhibitors of metalloproteinases). Miyazaki and colleagues5 investigated the role of m-calpain in proteolytic disorganization of VE-cadherin and the subsequent progression of atherosclerosis. Increased expression of m-calpain was observed in aortic ECs in atherosclerotic lesions, and proteolysis of VE-cadherin was shown in aortic ECs in ldlr−/− and apoE−/− mice. Long-term administration of the nonspecific calpain inhibitors N-acetyl-leu-leu-methional and calpeptin into these mice attenuated atherosclerotic lesion development and vascular inflammation independently of lipids. Specificity for m-calpain was inferred by the finding that in vivo transfection of m-calpain siRNA to ldlr−/− mice prevented disorganization of VE-cadherin and proatherogenic hyperpermeability in aortic ECs. Interestingly, treatment of cultured ECs with oxidized low-density lipoprotein, lysophosphatidylcholine, or low-density lipoprotein pretreated with secreted phospholipase A2 led to selective induction of m-calpain but not μ-calpain (calpain I). The authors conclude that m-calpain directly cleaves a juxtamembrane region of VE-cadherin, resulting in dissociation of β-catenin from the VE-cadherin complex, disorganization of adherence junctions, and hyperpermeability in ECs, thereby promoting extravasation of inflammatory cells or proatherogenic macromolecules into the vascular wall.
The proposed strategy of selective inhibition of m-calpain for the prevention/treatment of atherosclerotic disease is intriguing and raises important questions. First, is selective inhibition of m-calpain achievable in vivo? No inhibitor is completely selective for a single protease target. The calpain inhibitors (N-acetyl-leu-leu-methional and calpeptin) used in this study lack specificity among cysteine proteases with, for example, significant inhibitory activity against calpain 1, calpain 2, and cathepsins B, K, L, and S.14 Cathepsins K15 and S16 play critical roles in atherogenesis, possibly confounding the interpretation of N-acetyl-leu-leu-methional and calpeptin experiments. Recent advances in elucidating the tertiary structures of calpain 2 and its regulatory domain calpain 4 have resulted in the development of a new class of calpain inhibitors that interact with domains outside the catalytic site and may provide greater specificity and an improved safety profile secondary to reversible inhibition.17 Second, what is the most appropriate timing of m-calpain inhibition? The role of m-calpain in disrupting endothelial barrier cell function suggests that inhibitors will need to be administered early in the disease process, focusing primarily on asymptomatic individuals with risk factors alone or subclinical disease. Third, is the induction of m-calpain also accompanied by a reduction in the endogenous inhibitor calpastatin, which further increases proteolytic activity? Fourth, are other mechanisms besides altered EC barrier function responsible for the proatherogenic effects of m-calpain? There is experimental evidence that calpains also promote vascular cell proliferation18 and platelet activation.19 In addition, the dissociation of β-catenin from VE-cadherin provides a plausible mechanism linking proteolysis and altered gene expression. Interestingly, β-catenin activity has been identified in atherosclerosis-prone regions of the arterial tree and shown, through interactions with the T-cell–specific transcription factor complex, to alter the expression of targets (eg, fibronectin, monocyte chemoattractant protein-1, interleukin-8, bone morphological protein-4) that promote lesion formation.20 Finally, are the calpain 2 genes (CAPN2 and its regulatory domain, CAPN4) disease-susceptibility genes for atherosclerosis? Prior studies demonstrating the association of calpain 10 gene haplotypes with both diabetes mellitus21 and carotid intima-media thickness22 suggest that this is likely worthy of consideration.
Only a fraction (≈50) of the estimated 500 to 600 proteases in the human genome23,24 are validated pharmacological targets or are currently considered potential targets.6 Therefore, there is great interest in both the identification of novel proteases and their endogenous substrates using advanced proteomics and genetic (ie, RNA interference) and in silico biology approaches. Miyazaki and coworkers used a candidate molecule approach by simply staining for protease expression in atherosclerotic versus nondiseased arterial segments. It is likely that continued focused and unbiased protease efforts will yield new biological insights that can be exploited for therapeutic gain.
Acknowledgments
Sources of Funding
This work was supported in part by National Institutes of Health grants to Dr Jain (HL72952, HL75427, HL76754, HL086548, HL084154) and Dr Simon (HL57506 MERIT Award).
Footnotes
Disclosures
None.
References
- 1.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 2.Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE, Jr, Chavey WE, 2nd, Fesmire FM, Hochman JS, Levin TN, Lincoff AM, Peterson ED, Theroux P, Wenger NK, Wright RS, Smith SC, Jr, Jacobs AK, Halperin JL, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction): developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons: endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. Circulation. 2007;116:e148–e304. doi: 10.1161/CIRCULATIONAHA.107.181940. [DOI] [PubMed] [Google Scholar]
- 3.Kushner FG, Hand M, Smith SC, Jr, King SB, 3rd, Anderson JL, Antman EM, Bailey SR, Bates ER, Blankenship JC, Casey DE, Jr, Green LA, Hochman JS, Jacobs AK, Krumholz HM, Morrison DA, Ornato JP, Pearle DL, Peterson ED, Sloan MA, Whitlow PL, Williams DO. 2009 Focused updates: ACC/AHA guidelines for the management of patients with ST-Elevation Myocardial Infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2009;120:2271–2306. doi: 10.1161/CIRCULATIONAHA.109.192663. [DOI] [PubMed] [Google Scholar]
- 4.Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, Joyal SV, Hill KA, Pfeffer MA, Skene AM. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–1504. doi: 10.1056/NEJMoa040583. [DOI] [PubMed] [Google Scholar]
- 5.Miyazaki T, Taketomi Y, Takimoto M, Lei X-F, Arita S, Kim-Kaneyama J, Arata S, Ohata H, Ota H, Murakami M, Miyazaki A. m-Calpain induction in vascular endothelial cells on human and mouse atheromas and its roles in VE-cadherin disorganization and atherosclerosis. Circulation. 2011;124:2522–2532. doi: 10.1161/CIRCULATIONAHA.111.021675. [DOI] [PubMed] [Google Scholar]
- 6.Turk B. Targeting proteases: successes, failures and future prospects. Nat Rev Drug Discov. 2006;5:785–799. doi: 10.1038/nrd2092. [DOI] [PubMed] [Google Scholar]
- 7.Davie EW, Ratnoff OD. Waterfall sequence for intrinsic blood clotting. Science. 1964;145:1310–1312. doi: 10.1126/science.145.3638.1310. [DOI] [PubMed] [Google Scholar]
- 8.MacFarland RG. An enzyme cascade in the blood clotting mechanism, and its function as a biochemical amplifier. Nature. 1964;202:498–499. doi: 10.1038/202498a0. [DOI] [PubMed] [Google Scholar]
- 9.Podrez EA, Schmitt D, Hoff HF, Hazen SL. Myeloperoxidase-generated reactive nitrogen species convert LDL into an atherogenic form in vitro. J Clin Invest. 1999;103:1547–1560. doi: 10.1172/JCI5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nicholls SJ, Zheng L, Hazen SL. Formation of dysfunctional high-density lipoprotein by myeloperoxidase. Trends Cardiovasc Med. 2005;15:212–219. doi: 10.1016/j.tcm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Sugiyama S, Kugiyama K, Aikawa M, Nakamura S, Ogawa H, Libby P. Hypochlorous acid, a macrophage product, induces endothelial apoptosis and tissue factor expression: involvement of myeloperoxidase-mediated oxidant in plaque erosion and thrombogenesis. Arterioscler Thromb Vasc Biol. 2004;24:1309–1314. doi: 10.1161/01.ATV.0000131784.50633.4f. [DOI] [PubMed] [Google Scholar]
- 12.Ohno S, Emori Y, Imajoh S, Kawasaki H, Kisaragi M, Suzuki K. Evolutionary origin of a calcium-dependent protease by fusion of genes for a thiol protease and a calcium-binding protein? Nature. 1984;312:566–570. doi: 10.1038/312566a0. [DOI] [PubMed] [Google Scholar]
- 13.Huang Y, Wang KK. The calpain family and human disease. Trends Mol Med. 2001;7:355–362. doi: 10.1016/s1471-4914(01)02049-4. [DOI] [PubMed] [Google Scholar]
- 14.Sasaki T, Kishi M, Saito M, Tanaka T, Higuchi N, Kominami E, Katunuma N, Murachi T. Inhibitory effect of di- and tripeptidyl aldehydes on calpains and cathepsins. J Enzyme Inhib. 1990;3:195–201. doi: 10.3109/14756369009035837. [DOI] [PubMed] [Google Scholar]
- 15.Lutgens E, Lutgens SP, Faber BC, Heeneman S, Gijbels MM, de Winther MP, Frederik P, van der Made I, Daugherty A, Sijbers AM, Fisher A, Long CJ, Saftig P, Black D, Daemen MJ, Cleutjens KB. Disruption of the cathepsin K gene reduces atherosclerosis progression and induces plaque fibrosis but accelerates macrophage foam cell formation. Circulation. 2006;113:98–107. doi: 10.1161/CIRCULATIONAHA.105.561449. [DOI] [PubMed] [Google Scholar]
- 16.Sukhova GK, Zhang Y, Pan JH, Wada Y, Yamamoto T, Naito M, Kodama T, Tsimikas S, Witztum JL, Lu ML, Sakara Y, Chin MT, Libby P, Shi GP. Deficiency of cathepsin S reduces atherosclerosis in LDL receptor-deficient mice. J Clin Invest. 2003;111:897–906. doi: 10.1172/JCI14915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carragher NO. Calpain inhibition: a therapeutic strategy targeting multiple disease states. Curr Pharm Des. 2006;12:615–638. doi: 10.2174/138161206775474314. [DOI] [PubMed] [Google Scholar]
- 18.Ariyoshi H, Okahara K, Sakon M, Kambayashi J, Kawashima S, Kawasaki T, Monden M. Possible involvement of m-calpain in vascular smooth muscle cell proliferation. Arterioscler Thromb Vasc Biol. 1998;18:493–498. doi: 10.1161/01.atv.18.3.493. [DOI] [PubMed] [Google Scholar]
- 19.Randriamboavonjy V, Fleming I. The role of calpain in diabetes-associated platelet hyperactivation. Adv Pharmacol. 2010;59:235–257. doi: 10.1016/S1054-3589(10)59008-2. [DOI] [PubMed] [Google Scholar]
- 20.Gelfand BD, Meller J, Pryor AW, Kahn M, Bortz PD, Wamhoff BR, Blackman BR. Hemodynamic activation of beta-catenin and T-cell-specific transcription factor signaling in vascular endothelium regulates fibronectin expression. Arterioscler Thromb Vasc Biol. 2011;31:1625–1633. doi: 10.1161/ATVBAHA.111.227827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horikawa Y, Oda N, Cox NJ, Li X, Orho-Melander M, Hara M, Hinokio Y, Lindner TH, Mashima H, Schwarz PE, del Bosque-Plata L, Oda Y, Yoshiuchi I, Colilla S, Polonsky KS, Wei S, Concannon P, Iwasaki N, Schulze J, Baier LJ, Bogardus C, Groop L, Boerwinkle E, Hanis CL, Bell GI. Genetic variation in the gene encoding calpain-10 is associated with type 2 diabetes mellitus. Nat Genet. 2000;26:163–175. doi: 10.1038/79876. [DOI] [PubMed] [Google Scholar]
- 22.Goodarzi MO, Taylor KD, Jones MR, Fang B, Guo X, Xiang AH, Buchanan TA, Hodis HN, Raffel LJ, Rotter JI. Replication of calpain-10 genetic association with carotid intima-media thickness. Atherosclerosis. 2009;205:503–505. doi: 10.1016/j.atherosclerosis.2008.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Puente XS, Sanchez LM, Overall CM, Lopez-Otin C. Human and mouse proteases: a comparative genomic approach. Nat Rev Genet. 2003;4:544–558. doi: 10.1038/nrg1111. [DOI] [PubMed] [Google Scholar]
- 24.Rawlings ND, Tolle DP, Barrett AJ. MEROPS: the peptidase database. Nucleic Acids Res. 2004;32(Database issue):D160–164. doi: 10.1093/nar/gkh071. [DOI] [PMC free article] [PubMed] [Google Scholar]