Abstract
Aims
We evaluated the immunogenicity, efficacy, and safety of succinylnorcocaine conjugated to cholera toxin B protein as a vaccine for cocaine dependence.
Methods
This 6-site, 24 week Phase III randomized double-blind placebo-controlled trial assessed efficacy during weeks 8 to 16. We measured urine cocaine metabolites thrice weekly as the main outcome.
Results
The 300 subjects (76% male, 72% African-American, mean age 46 years) had smoked cocaine on average for 13 days monthly at baseline. We hypothesized that retention might be better and positive urines lower for subjects with anti-cocaine IgG levels of ≥ 42 μg/mL (high IgG), which was attained by 67% of the 130 vaccine subjects receiving five vaccinations. Almost 3-times fewer high than low IgG subjects dropped out (7% vs 20%). Although for the full 16 weeks cocaine positive urine rates showed no significant difference between the three groups (placebo, high, low IgG), after week 8, more vaccinated than placebo subjects attained abstinence for at least two weeks of the trial (24% vs 18%), and the high IgG group had the most cocaine-free urines for the last 2 weeks of treatment (OR=3.02), but neither were significant. Injection site reactions of induration and tenderness differed between placebo and active vaccine, and the 29 serious adverse events did not lead to treatment related withdrawals, or deaths.
Conclusions
The vaccine was safe, but it only partially replicated the efficacy found in the previous study based on retention and attaining abstinence.
Keywords: cocaine, vaccine, clinical trial, immunotherapy
1. INTRODUCTION
Stimulant use disorders remain a significant public health concern (SAMHSA, 2012). For example, cocaine was noted more often than any other illicit drug among emergency department visits in the United States (SAMHSA, 2013). Currently, there are no medications that have regulatory approval for cocaine addiction leading to an urgent need for novel therapeutic approaches. TA-CD is a vaccine being developed for the treatment of cocaine dependence. Cocaine is a molecule that, by itself, is too small to elicit an antibody response. However, conjugation to larger, immunogenic protein carriers can enable production of antibodies specific to small molecules. The B subunit of cholera toxin (CTB) is a highly immunogenic protein known to elicit a potent antibody response.
TA-CD vaccine is designed to induce formation of anti-cocaine antibodies. This cocaine vaccine covalently links succinylnorcocaine (SNC) to cholera toxin B (rCTB), which has a worldwide safety record for cholera immunization (Jertborn et al., 1992, 1994; Holmgren et al., 1994; Svennerholm et al., 1984). The anti-drug antibodies elicited by TA-CD bind to cocaine entering the bloodstream, forming antigen-antibody complexes too large to cross the blood-brain barrier. In sufficient quantity and appropriate affinity, such antibodies can therefore prevent high concentrations of cocaine from reaching the mid-brain. The absence of reward stimulus in the brain should reduce the reinforcing psychoactive effects of cocaine. By blocking the pleasurable response to cocaine, it is expected that cocaine usage could be reduced in subjects undergoing treatment for cocaine dependence.
The concentration of anti-cocaine antibody in the blood must be sufficient to bind a significant amount of the drug in order to be effective. The peak plasma amount of cocaine that users need to experience pleasure in human laboratory studies is approximately 0.5 μM. (Jenkins et al., 2002), and to bind 90% of this amount of cocaine requires approximately 42 ug/ml of moderately high affinity antibody (Fox et al., 1996, 1997; Orson et al., 2007). We therefore compared reductions in cocaine use for the placebo group to two groups of vaccinated subjects: those with peak IgG antibody levels above (high IgG) versus below (low IgG) 42 ug/ml IgG. We also know from previous work that the window of optimal IgG levels would be after week 8 and that after week 16 these IgG levels would fall. (Kosten et al., 2002; Martell et al., 2005, 2009). Thus, we hypothesized that subjects with high IgG levels above 42 ug/ml should have more cocaine-free urines, more sustained abstinence (>2 weeks) and greater treatment retention than the subjects getting placebo or having low IgG responses to the vaccine.
2. METHODS
2.1 Site and population
We recruited cocaine dependent subjects (DSM IV-TR criteria; American Psychiatric Association, 1994) into outpatient clinical programs at six sites: Baylor/Houston MED VAMC, Columbia University, Johns Hopkins, New York University, University of Pennsylvania and University of Cincinnati. This study followed Good Clinical Practices, and subjects signed informed consents that included financial inducements for study retention and were approved by the institutional review boards of each participating institution.
2.2 Participants
Between October, 2010 and March, 2012 we randomized 300 of 736 screened cocaine dependent men and women who had cocaine metabolites in their urine and were aged 18 to 55. Figure 1 summarizes subject recruitment, screening exclusions and retention. Subjects were excluded primarily for lack of cocaine positive urines during screening, for no motivation to stop using cocaine or for major medical or psychiatric illness, current infection, psychotropic or corticosteroid medications, or a history of other vaccinations or blood product use within thirty days. Female participants had to be on birth control or be incapable of child bearing. Complete medical examinations included routine blood testing and an electrocardiogram.
Figure 1.
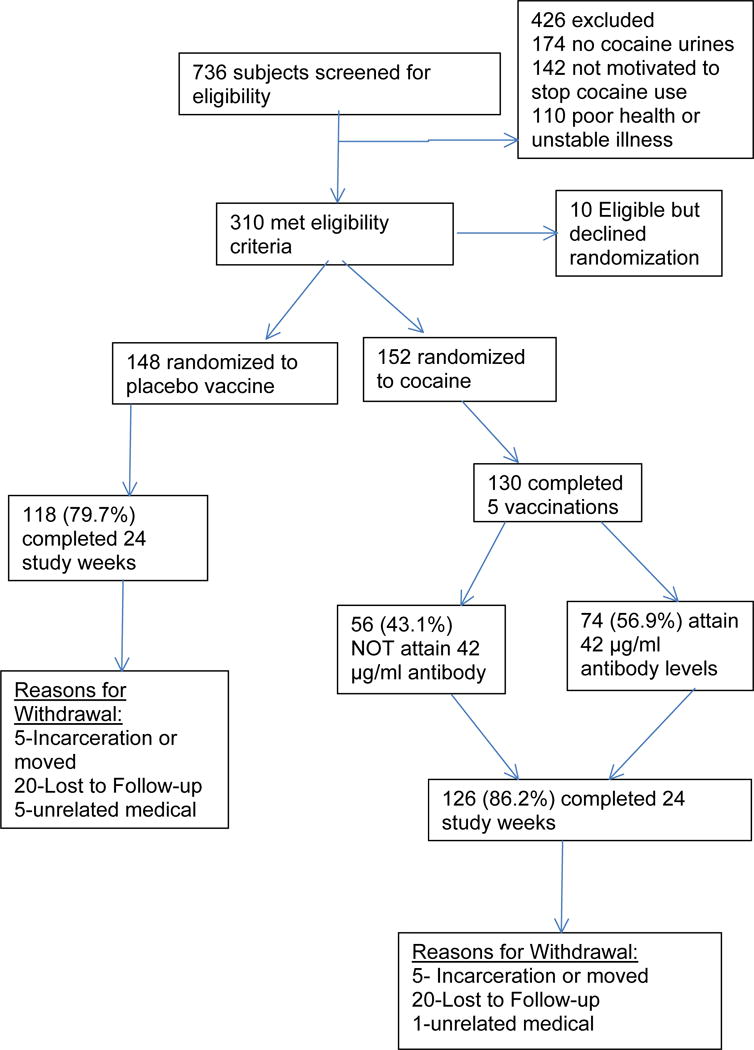
Flow chart of study participants screened, randomized and completing study according to CONSORT guidelines.
2.3 Interventions
2.3.1 Vaccination
The TA-CD vaccine is succinylnorcocaine covalently linked to cholera toxin B (SNC-rCTB) that is adsorbed onto aluminum hydroxide adjuvant. The placebo contained saline and aluminum hydroxide. Subjects were randomized to five 0.5 ml intramuscular vaccinations of 400 micrograms of active (SNC-rCTB) or placebo vaccine at weeks 1, 3, 5, 9, and 13 (Kosten et al., 2002; Martell et al., 2009).
2.3.2 Randomization
Subjects were randomized equally to each treatment and all subjects were analyzed. Randomization assignments were securely stored centrally, and research staff, investigators, and subjects were blinded until the database was unlocked in January 2013.
2.3.3 Counseling
Masters level substance abuse counselors provided optional individual cognitive behavioral therapy (CBT) sessions for 30–45 minutes (SAMSHSA, Treatment Improvement Protocol 33, 2010). The patients participated in an average of 11.2 (SD 4.4) out of 16 therapy sessions with no difference between the active and placebo groups.
2.4 Safety monitoring
Medical staff evaluated the vaccination site for erythema, induration, and/or tenderness after injection and monitored subjects for general health and subjective adverse events. Hematology and clinical chemistries were drawn at baseline and weeks 12, 16 and 24. Medical interventions or medications started after vaccination were considered possible adverse events (AE) and along with reasons for study termination were tabulated by treatment group.
2.5 Objectives
The overall goal of the study was to compare the effect of TA-CD to placebo on the degree of modulation of cocaine use in cocaine-dependent individuals (DSM-IV-TR) who are motivated to quit or reduce use of cocaine. The primary objective of this study is to evaluate the effect of 5 doses of TA-CD 400 μg compared to placebo on cocaine use over 8 weeks (Weeks 9 to 16 inclusive).
Secondary objectives included evaluating the safety and tolerability of vaccination through the week 24 study follow-up.
2.6 Outcome measures
2.6.1 Efficacy analysis
We compared serum IgG anti-cocaine levels from baseline to week 24 in actively vaccinated subjects and then compared the weekly rate of cocaine-free urines between the placebo and two groups of actively vaccinated subjects – those above 42 ug/ml and those less than or equal to 42 ug/ml of anti-cocaine IgG. The primary statistical analyses followed the intention to treat principle. All efficacy and retention analyses controlled for potential site differences using fixed effects. Retention analyses used Kaplan-Meier Curves and Cox Proportional Hazards. Longitudinal analysis of the probability of cocaine-positive urines as a function of time, treatment and their interaction used generalized linear mixed modeling. Secondary, per protocol analyses of longitudinal cocaine-positive urines evaluated each of these outcomes as a function of antibody titre (i.e. ≥ 42 μg/ml versus < 42 μg/ml). All statistical analyses used SAS v. 9.3.
2.6.2 Quantitative antibody measurement
Serum anti-cocaine IgG was measured by an Enzyme-Linked ImmunoSorbent Assay (ELISA). Briefly, cocaine conjugated to bovine serum albumin (BSA) was bound to ELISA plates overnight (2 μg/well) and then incubated with dilutions of patient serum. After washing, human IgG antibodies bound to the plates were detected with anti-human IgG antibody conjugated with horseradish peroxidase and an appropriate substrate (Kosten et al., 2002; Martell et al., 2009). Background antibody binding to the target conjugate protein (BSA) alone was subtracted, and each ELISA plate included wells with serially diluted polyclonal human IgG to provide an internal standard curve. This ELISA’s specificity and reproducibility was validated using serial dilutions of a humanized monoclonal antibody to cocaine, 2E2, from Dr. Andrew Norman at the University of Cincinnati (Paula et al., 2004).
2.6.3 Urine cocaine metabolites
Urine was qualitatively tested for benzoylecgonine (BE) at the time of screening and thrice weekly for the duration of the 16 week study and 8 week follow-up. Urine BE values of at least 300 ng/ml were considered positive. Pharmacokinetic studies have shown that anti-cocaine antibodies in the circulation did not change BE excretion, and our subjects’ anti-cocaine IgG had less than 15% cross-reactivity with BE (Norman et al., 2007; Martell et al., 2009).
2.7 Sample size estimate
The primary analysis compared the TA-CD group to placebo. With a two-sided t-test of the difference in the weekly fraction of cocaine-free urines between TA-CD (0.65) and placebo (0.50) with a common standard deviation of 0.40, the sample size needed with an 80% power is 113 for TA-CD and 113 for placebo, at a two-sided alpha of 0.05. Thus, the total sample size needed for the study would be 226 subjects in the efficacy evaluable sample. However, assuming approximately 20% of randomized subjects will drop out by the start of the efficacy evaluable period and be excluded from the efficacy evaluable population, a total of 300 subjects were randomized (resulting in an increase to 150 subjects for TA-CD and 150 subjects for placebo). Although the primary analysis did not use the t-test, it is anticipated that the primary analysis was more powerful than the t-test.
3. RESULTS
3.1 Baseline characteristics and retention
The vaccine and placebo subjects were comparable in age, gender and ethnicity, but most were African American preventing ethnic sub-analysis (Table 1). They had a long history of cocaine use (17 years) and on average used 13 days in the month prior to treatment (Table 2). Alcohol use was relatively low with an average of 4 days per month with few subjects reporting use to intoxication. Cannabis use was uncommon. For intention-to-treat analysis, Cox Proportional Hazard Analysis failed to demonstrate differential retention as a function of treatment condition (83% vs 80% completed both 16 and 20 weeks) (Wald χ2(1) = 0.07, p ≤ 0.79). Among participants receiving active vaccine, those reaching an antibody titre ≥ 42 μg/ml showed greater retention (93% vs 80%) and a decreased hazard of attrition (HR = 0.39, 95% CI 0.16–0.97).
Table 1.
Baseline clinical characteristics of sample
| Variable | Active (n = 152) | Placebo (n = 148) |
|---|---|---|
| Mean (Std.) | Mean (Std.) | |
| Age | 45.76 (7.25) | 46.40 (5.75) |
| Education (yrs.) | 12.96 (1.90) | 12.67 (2.05) |
| Employment (Days in Last 30) | 4.96 (3.14) | 5.66 (3.04) |
| n (%) | n (%) | |
| Male | 114 (75) | 114 (77) |
| Hispanic | 12 (7.9) | 11 (7.4) |
| Caucasian | 33 (22) | 33 (22) |
| African-American | 109 (72) | 108 (73) |
| Asian | 1 (0.7) | 2 (1.4) |
| American Indian/Alaskan Native | 2 (1.3) | 1 (0.7) |
| Unknown | 7 (4.6) | 4 (2.7) |
Table 2.
Baseline drug use characteristics of sample
| Variable | Active (n = 152) | Placebo (n = 148) |
|---|---|---|
| Median (25th, 75th) | Median (25th, 75th) | |
| Alcohol (any; past 30 days) | 4.0 (1.0, 15.0) | 4.5 (1.0, 12.0) |
| Alcohol (to intoxication; past 30 days) | 0.0 (0.0, 4.0) | 0.0 (0.0, 5.0) |
| Cocaine (past 30 days) | 14.0 (7.0, 22.5) | 12.0 (7.0, 20.0) |
| Cannabis (past 30 days) | 0.0 (0.0, 2.0) | 0.0 (0.0, 1.0) |
| > 1 Substance per Day (past 30 days) | 4.0 (0.5, 13.0) | 3.0 (0.0, 10.0) |
| Alcohol (any; lifetime years) | 19.0 (4.5, 30.0) | 15.0 (3.0, 26.0) |
| Alcohol (to intoxication; lifetime years) | 4.5 (0.0, 19.0) | 4.0 (0.0, 17.0) |
| Cocaine (lifetime years) | 16.5 (10.0, 22.0) | 18.0 (10.0, 23.0) |
| Cannabis (lifetime years) | 6.0 (0.0, 15.0) | 5.0 (0.0, 15.0) |
| > 1 Substance per Day (lifetime years) | 10.0 (1.5, 20.0) | 10.0 (1.0, 20.0) |
| n (%) | n (%) | |
| Heroin (past 30 days) | 8 (5.59) | 6 (4.35) |
| Other opiates/analgesics (past 30 days) | 9 (6.25) | 8 (5.80) |
| Sedatives/Hypnotics/Tranquilizers (past30 days) | 4 (2.92) | 5 (3.76) |
| Barbituates (lifetime) | 1 (0.74) | 3 (2.27) |
| Sedatives/Hypnotics/Tranquilizers | 4 (2.92) | 7 (5.30) |
| Other opiates/analgesics (lifetime) | 13 (9.03) | 16 (11.76) |
| Heroin (lifetime) | 10 (6.99) | 12 (8.76) |
| Amphetamines (lifetime) | 13 (9.49) | 10 (7.46) |
| Inhalants (lifetime) | 4 (2.96) | 3 (2.27) |
| Hallucinogens (lifetime) | 14 (10.00) | 18 (13.33) |
3.2 Cocaine vaccine specific IgG levels
Of the 152 subjects randomized to receive active vaccine, the mean peak antibody levels were 46 ug/ml at week 9 and 59 ug/ml at week 16 with a 95% confidence interval of 0 to 186 ug/ml. Figure 2 is the histogram showing the peak antibody levels for the vaccinated subjects. Every subject’s IgG levels fell during weeks 16 to 24 indicating that potential vaccine efficacy in blocking cocaine was decreasing.
Figure 2.
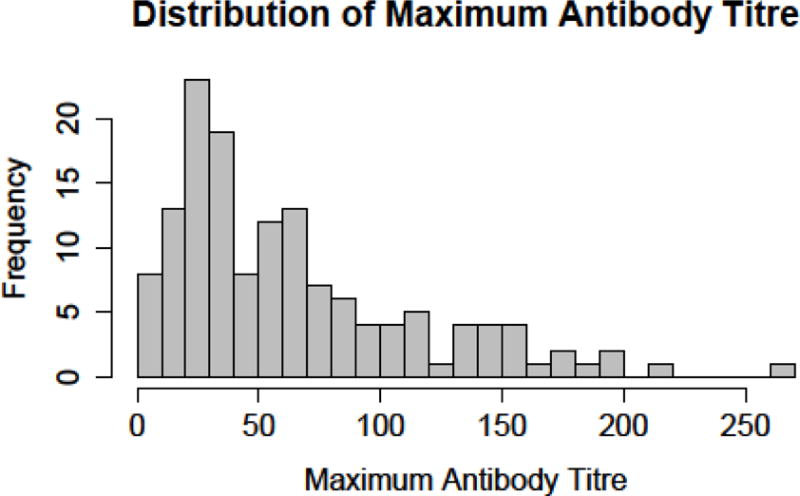
Distribution of maximum antibody titers for actively vaccinated subjects (n=152)
3.3 Cocaine-free urines by IgG response to vaccine versus placebo
We found no significant differences between the vaccine and placebo groups in an intention to treat analysis using generalized linear mixed modeling. Figure 3 shows the biweekly urine toxicology results for the placebo vs active vaccine groups. Considering the full 16 weeks of the trial, analyses failed to identify a difference in the probability of cocaine-positive urines as a function of time (F(1,287) = 0.15, p≤0.70), treatment (F(1,1551) = 0.31, p≤0.58) or the interaction of time and treatment (F(1,1551) ≤ 0.01, p≤0.95). Among participants in the active treatment condition generalized linear mixed modeling failed to identify a statistically reliable effect of antibody titre (<42 μg/ml v. ≥ 42 μg/ml) (F(1,785) = 3.38, p≤0.07) after controlling for time (F(1,140) = 0.14, p≤0.71). In addition to the cut-off of ≥42μg/ml we reanalyzed the data utilizing the 50th, 75th, and 90th percentiles of the whole sample as antibody titre cut-points, and similarly found no differences between the high and low antibody titer groups.
Figure 3.
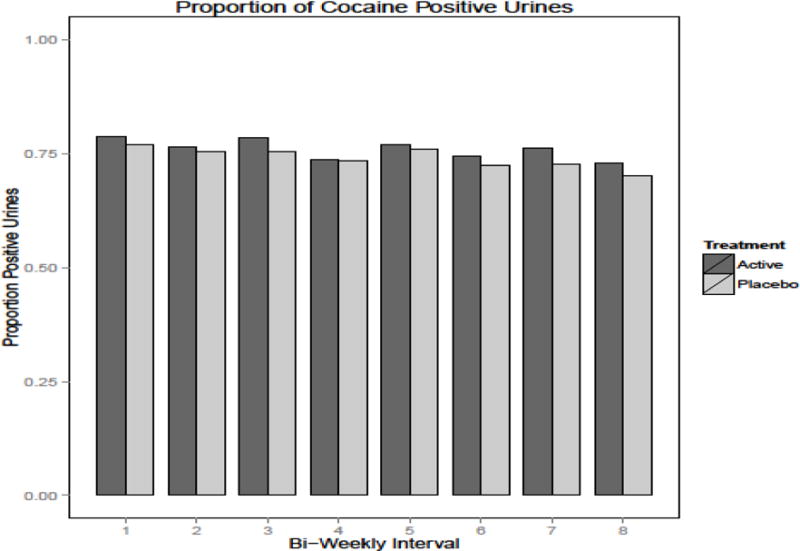
Proportion of cocaine positive urines between active vs. placebo groups by biweekly treatment intervals (16 weeks of treatment, n=300)
Remaining abstinent from cocaine for at least two weeks of treatment occurred more often in the active than placebo vaccine group (24% vs 18%), but not significantly (O.R. = 1.67, 95% CI = 0.90–3.51). Among the vaccinated patients those with the high antibody titer (>42 ug/ml) had a greater odds ratio of being cocaine-free for their final 2-weeks in treatment compared to the low antibody titer group (O.R. = 3.02, 95% CI = 0.92–10.18), although only 15 patients attained abstinence for their last 2 weeks of treatment. Reanalyzing the data utilizing the 50th, 75th, and 90th percentiles as antibody titre cut-points yielded the same relative odds ratios between the high and low antibody groups.
3.4 Adverse events (AE)
Table 3 shows the number of individuals reporting side-effects from specific body systems. Also provided are raw p-values and False Discovery Rates (FDR) evaluating these counts as a function of treatment condition. The only category showing differential reporting as a function of treatment condition is “General disorders and administration site conditions” (Table 3). Further evaluation of preferred diagnostic terms occurring under “General disorders and administration site conditions” indicated differential reporting for an “Injection Site Reaction” (12 vs. 0; P<0.0004; FDR 0.015).
Table 3.
Summary of Adverse Events (AEs)
| Organ System | Active (n) | Placebo (n) | p-value | FDR |
|---|---|---|---|---|
| Blood and Lymphatic System | 6 | 2 | 0.2828 | 0.8176 |
| Cardiac | 5 | 7 | 0.5687 | 0.8176 |
| Ear and Labyrinth | 5 | 3 | 0.7230 | 0.8315 |
| Eye | 9 | 12 | 0.5035 | 0.8176 |
| Gastrointestinal | 52 | 47 | 0.7129 | 0.8315 |
| General and Administrative Site Conditions | 87 | 64 | 0.0208 | 0.4773 |
| Immune System | 9 | 5 | 0.4133 | 0.8176 |
| Infections and Infestations | 47 | 40 | 0.5248 | 0.8176 |
| Injury, Poisoning and Procedural Complications | 26 | 20 | 0.4255 | 0.8176 |
| Investigations | 22 | 25 | 0.6346 | 0.8315 |
| Metabolism and Nutrition | 8 | 6 | 0.7857 | 0.8606 |
| Musculoskeletal and Connective Tissue | 65 | 56 | 0.4113 | 0.8176 |
| Neoplasms | 2 | 0 | 0.4984 | 0.8176 |
| Nervous System | 44 | 56 | 0.1123 | 0.8176 |
| Pregnancy, Puerperium and Perinatal | 0 | 1 | 0.4933 | 0.8176 |
| Psychiatric | 45 | 38 | 0.5189 | 0.8176 |
| Renal and Urinary | 1 | 5 | 0.1170 | 0.8176 |
| Reproductive System and Breast Disorders | 5 | 7 | 0.5688 | 0.8176 |
| Respiratory, Thoracic and Mediastinal | 36 | 28 | 0.3274 | 0.8176 |
| Skin and Subcutaneous Tissue | 20 | 21 | 0.8671 | 0.9065 |
| Social Circumstances | 2 | 1 | 1 | 1 |
| Surgical and Medical procedures | 3 | 4 | 0.7202 | 0.8315 |
| Vascular | 4 | 6 | 0.5372 | 0.8176 |
All these AEs were mild or moderate and none led to dropout from treatment. There were also no differences in side-effects related to peak antibody levels. Treatment-emergent severe AEs among randomized subjects included 29 hospitalizations: 14 in the active vaccine and 15 in the placebo group. Two in the placebo group were considered possibly related to the vaccine, but none in the active vaccine group were considered treatment related. Depression with suicidality was the most common event: 6 in the active vaccine and 5 in the placebo group. Cocaine use accounted for 3 in the vaccine and 2 in the placebo group. Other hospitalizations in the active vaccine group were for a leg ulcer, chronic obstructive lung disease, erectile dysfunction, and alcohol intoxication. Other hospitalizations in the placebo groups were for loss of consciousness, coronary vasospasm, foot pain, leg cellulitis, chest pain and a pelvic bone fracture.
4. DISCUSSION
In contrast to our previous study with this cocaine vaccine TA-CD (Martell et al., 2009), we did not find a significant attenuation of outpatient cocaine use, even when adequate antibody levels were attained. Instead, the patients with higher antibody levels had more cocaine-positive urines than those with the lower levels of 42 ug/ml or less throughout the first 16 weeks of the study. However, several findings suggested some efficacy for the vaccine. First, treatment dropout was significantly different and almost 3-times lower for the high IgG than for the low IgG or placebo groups (7% vs 20%). Second, the active vaccine group was more likely than placebo to attain sustained abstinence during the last two weeks of treatment, particularly those with the higher antibody levels (>42 ug/ml). The odds ratio of 3.02 suggests that having more abstinent patients than the 5% (15/300 patients) obtained in this study or having a greater sample size of 466 would have demonstrated the vaccine efficacy for those attaining the higher antibody levels with a statistical power of 80%. Third, based on the mean antibody levels at weeks 9 and 16 the vaccine showed sufficient immunogenicity with two thirds of the patients attaining peak levels above 42 ug/ml. Thus, the vaccine may have therapeutic value for a portion of cocaine dependent patients, although many will try to override the partial blockade that is possible with this vaccine. Furthermore, it took us 17 months from multiple trial sites to recruit 300 cocaine addicted persons who were nominally motivated to cease using cocaine. This suggests that even an effective vaccine may not be much more attractive to the cocaine abusing treatment population than are current psychosocial treatments.
We are concerned that adequately immunized subjects may have increased their cocaine use to overcome the competitive anti-cocaine antibody blockade. As Haney and colleagues (2010) showed, these antibody levels were only sufficient to block modest doses of cocaine. Overall, this vaccine is designed to reduce relapse among cocaine dependent patients who are motivated to stop using cocaine. The high rate of cocaine use and low rate of sustained abstinence shown by these study patients clearly reflected a population that was using cocaine several times per week and rarely attempted abstinence during the clinical trial. In the previous study the high antibody level patients achieved 48% cocaine-free urines during weeks 9 to 16 compared to only 25% in the present study. Having only half the rate of cocaine-free urines in the present Phase III study compared to the previous Phase IIb study in methadone maintained patients may reflect several non-pharmacological differences in design and programmatic structure between the two studies. Some salient differences include first the study setting, which involved a daily methadone program with monitoring 6 days per week vs three times weekly monitoring in the current study. Second, delivery of Cognitive Behavioral Therapy was mandated in the Phase IIb methadone study and optional in this Phase III study. The participation rates in the therapy involved about two-thirds of the sessions in this study. Third, the single study site of opiate addicts who had secondary cocaine use was more homogenous than the primary cocaine users drawn from six sites in different regions of the country. Fourth, financial incentives of $55 per week plus $65 for each of the five vaccinations were approximately three times higher in this Phase III study than the compensation in the previous methadone based study, which may have made more funds available to purchase or trade for cocaine. In view of these differences future studies would benefit from a more structured study setting such as a day-treatment program that helps the patients become motivated for abstinence. This should lead to a more homogeneous population of patients who have been at least 2-weeks cocaine-free prior to starting the TA-CD vaccinations. In this way the study would shift from an abstinence initiation to a relapse-prevention design and potentially greater integration of CBT to facilitate the maintenance of abstinence. Future studies also may benefit from the development of high activity cholinesterase enzymes that will more rapidly metabolize the cocaine to inactive metabolites (Gao et al., 2004, 2010). Because these enzymes appear to act relatively slowly compared to the rapid entry of cocaine into the brain, the fast kinetics of antibody-drug interactions are ideally suited to being combined with these enhanced enzymes to produce extremely effective blockers of cocaine’s actions on all organ systems including both the brain and heart (Carroll et al., 2014, in press).
Acknowledgments
Celtic Pharma Development and their medical Director, Patrick O’Connor PhD, FRCP (Edin) for supplies of TA-CD vaccine
NIDA and VA Coop Studies staff: Liza Gorgon, Cristin Murtaugh
At the Baylor/VA site: Tim Shutter, Jose Perez
At the NYU/VA NYHHS Site: Bridget McClure, Renee Degener, Thomas Shirley, Agatha Kulaga, Patricia Novo, Joel Solomon, Katie Martindale, Paul Casadonte, Lenard Adler, Dylan Brock, Shirley Irons, Sarah Farkas, Marianne Guschwan, Jeffrey Lorin, Alexandra Schepens, Lauren Moy, Alexandra Kvernland, Elliot Schwartz, Kamelia Eltaher and Syeda Ahmed
Disclosures
Role of funding source Clinical trials.gov ID: NCT00969878,
Supported by NIDA grants: R01DA025223 (Kosten, Baylor/VA), R01 DA 025248 (Rotrosen, NYU/VA NYHHS), RO1DA025249 (Somoza, U. Cincinnati), R01DA025246 (Kampman, U. Pennsylvania), R01 DA033310 (Levin, Columbia U.), R01DA025251 (Stitzer, Johns Hopkins U.), T32DA007209, K23DA029609 (Tompkins, Johns Hopkins U.). Investigator sponsored by TR Kosten, who designed study and was responsible for data collection, analyses and interpretation of data, writing the report and decision to submit for publication. NIDA/VA had a role in data collection, but had no further role in analyses, interpretation, writing or decision to submit for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Thomas R. Kosten – Overall PI, designed study and was responsible for data collection, analyses and interpretation of data, writing the report and decision to submit for publication
Coreen Domingo – Houston site PI, was responsible for data collection, analyses and interpretation of data, writing the report and decision to submit for publication
Daryl Shorter – Houston site MD, was responsible for data collection, interpretation of data, writing the report and decision to submit for publication
Frank Orson – Houston immunologist, was responsible for immunological assays, analyses and interpretation of data, writing the report and decision to submit for publication
Charles Green – Statistician, was responsible for statistical analyses and interpretation of data, writing the report and decision to submit for publication
Eugene Somoza – Cincinnati site PI, was responsible for data collection, interpretation of data, writing the report and decision to submit for publication
Rachelle Sekerka – Cincinnati site, was responsible for data collection, and decision to submit for publication
Frances R. Levin – Columbia site PI, was responsible for data collection, analyses and interpretation of data, writing the report and decision to submit for publication
John Mariani – Columbia site, was responsible for data collection, writing the report and decision to submit for publication
Maxine Stitzer – Johns Hopkins site PI, was responsible for data collection, writing the report and decision to submit for publication
D. Andrew Tompkins, – Johns Hopkins site MD, was responsible for data collection, writing the report and decision to submit for publication
John Rotrosen – NYU/VA NYHHS site PI, was responsible for data collection, writing the report and decision to submit for publication
Vatsal Thakkar – NYU/VA NYHHS site MD, was responsible for data collection, writing the report and decision to submit for publication
Benjamin Smoak – NYU/VA NYHHS site MD, was responsible for data collection, writing the report and decision to submit for publication
Kyle Kampman – Univ. Pennsylvania site PI, was responsible for data collection, writing the report and decision to submit for publication
All authors approved the final manuscript for submission.
Conflicts of interest
No conflicts of interest for any authors.
Ethics of experimentation
This study was conducted in accordance with the Declaration of Helsinki and according to requirements of all applicable local and international standards.
References
- DSM-IV, editor. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Carroll ME, Zlebnik NE, Anker JJ, Kosten TR, Orson FM, Shen X, Kinsey B, Parks RJ, Gao Y, Brimijoin S. Combined cocaine hydrolase gene transfer and anti-cocaine vaccine synergistically block cocaine-induced locomotion. PLoS One. 2014 doi: 10.1371/journal.pone.0043536. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox BS. Development of a therapeutic vaccine for the treatment of cocaine addiction. Drug Alcohol Depend. 1997;48:153–158. doi: 10.1016/s0376-8716(97)00121-x. [DOI] [PubMed] [Google Scholar]
- Fox BS, Kantak KM, Edwards MA. Efficacy of a therapeutic cocaine vaccine in rodent models. Nat Med. 1996;2:1129–1132. doi: 10.1038/nm1096-1129. [DOI] [PubMed] [Google Scholar]
- Gao Y, Brimijoin S. An engineered cocaine hydrolase blunts and reverses cardiovascular responses to cocaine in rats. J Pharmacol Exp Thera. 2004;310:1046–1052. doi: 10.1124/jpet.104.068122. [DOI] [PubMed] [Google Scholar]
- Gao Y, Orson FM, Kinsey B, Kosten T, Brimijoin S. The concept of pharmacologic cocaine interception as a treatment for drug abuse. Chem Biol Interact. 2010;187:421–424. doi: 10.1016/j.cbi.2010.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Gunderson EW, Jiang H, Collins ED, Foltin RW. Cocaine-specific antibodies blunt the subjective effects of smoked cocaine in humans. 2010;67:59–65. doi: 10.1016/j.biopsych.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren J, Czerkinsky C, Lycke N, Svennerholm AM. Strategies for the induction of immune responses at mucosal surfaces making use of cholera toxin B subunit as immunogen, carrier, and adjuvant. Am J Trop Med Hyg. 1994;50:42–54. [PubMed] [Google Scholar]
- Jenkins AJ, Keenan RM, Henningfield JE, Cone EJ. Correlation between pharmacological effects and plasma cocaine concentrations after smoked administration. J Anal Toxicol. 2002;26:382–392. doi: 10.1093/jat/26.7.382. [DOI] [PubMed] [Google Scholar]
- Jertborn M, Svennerholm A-M, Holmgren J. Safety and immunogenicity of an oral recombinant cholera B subunit-whole cell vaccine in Swedish volunteers. Vaccine. 1992;10:130–132. doi: 10.1016/0264-410x(92)90030-n. [DOI] [PubMed] [Google Scholar]
- Jertborn M, Svennerholm AM, Holmgren J. Immunological memory after immunization with oral cholera B subunit-whole-cell vaccine in Swedish volunteers. Vaccine. 1994;12:1078–1082. doi: 10.1016/0264-410x(94)90176-7. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Rosen M, Bond J, Settles M, Roberts JS, Shields J, Jack L, Fox B. Human therapeutic cocaine vaccine: safety and immunogenicity. Vaccine. 2002;20:1196–1204. doi: 10.1016/s0264-410x(01)00425-x. [DOI] [PubMed] [Google Scholar]
- Martell B, Mitchell E, Poling J, Gonsai K, Kosten TR. Vaccine pharmacotherapy for the treatment of cocaine dependence. Biol Psychiatry. 2005;58:158–164. doi: 10.1016/j.biopsych.2005.04.032. [DOI] [PubMed] [Google Scholar]
- Martell BA, Orson FM, Poling J, Mitchell E, Rossen RD, Gardner T, Kosten TR. Cocaine vaccine for the treatment of cocaine dependence in methadone-maintained patients: a randomized, double-blind, placebo-controlled efficacy trial. Arch Gen Psychiatry. 2009;66:1116–1123. doi: 10.1001/archgenpsychiatry.2009.128. PMID:19805702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman AB, Tabet MR, Norman MK, Buesing WR, Pesce AJ, Ball WJ. A chimeric human/murine anti-cocaine monoclonal antibody inhibits the distribution of cocaine to the brain in mice. J Pharmacol Exp Thera. 2007;320:145–153. doi: 10.1124/jpet.106.111781. [DOI] [PubMed] [Google Scholar]
- Orson FM, Kinsey BM, Singh RAK, Wu Y, Gardner T, Kosten TR. The future of vaccines in the management of addictive disorders. Curr Psychiatry Rep. 2007;9:381–387. doi: 10.1007/s11920-007-0049-z. [DOI] [PubMed] [Google Scholar]
- Paula S, Tabet MR, Farr CD, Norman AB, Ball WJJ. Three-dimensional quantitative structure-activity relationship modeling of cocaine binding by a novel human monoclonal antibody. J Med Chem. 2004;47:133–142. doi: 10.1021/jm030351z. [DOI] [PubMed] [Google Scholar]
- SAMHSA. Results from the 2011 National Survey on Drug Use and Health: Summary of National Findings. Substance Abuse and Mental Health Services Administration; Rockville, MD: 2012. [Google Scholar]
- SAMHSA. Drug Abuse Warning Network, 2011: National Estimates of Drug-Related Emergency Department Visits. Substance Abuse and Mental Health Services Administration; Rockville, MD: 2013. [PubMed] [Google Scholar]
- SAMHSA. Treatment Improvement Protocol 33: Treatment for Stimulant Use Disorders. 2010 http://kap.samhsa.gov/products/manuals/index.htm. http://www.ncbi.nlm.nih.gov/books/bv.fcgi?rid=hstat5.section.57658 Accessed January, 2014.
- Svennerholm A, Gothefors L, Sack D, Bardhan P, Holmgren J. Local and systemic antibody responses and immunological memory after immunization with cholera B subunit by different routes. Bull WHO. 1984;62:909–918. [PMC free article] [PubMed] [Google Scholar]


