Abstract
Following stroke, aberrant three dimensional multijoint gait impairments emerge that present in kinematic asymmetries such as circumduction. A precise pattern of cross-planar coordination may underlie abnormal hemiparetic gait as several studies have underscored distinctive neural couplings between medio-lateral control and sagittal plane progression during walking. Here we investigate potential neuromechanical constraints governing abnormal multijoint coordination post-stroke. 15 chronic monohemispheric stroke patients and 10 healthy subjects were recruited. Coupled torque production patterns were assessed using a volitional isometric torque generation task where subjects matched torque targets for a primary joint in 4 directions while receiving visual feedback of the magnitude and direction of the torque. Secondary torques at other lower limb joints were recorded without subject feedback. We find that common features of cross-planar connectivity in stroke subjects include statistically significant frontal to sagittal plane kinetic coupling that overlay a common sagittal plane coupling in healthy subjects. Such coupling is independent of proximal or distal joint control and limb biomechanics. Principal component analysis of the stroke aggregate kinetic signature reveals unique abnormal frontal plane coupling features that explain a larger percentage of the total torque coupling variance. This study supports the idea that coupled cross-planar kinetic outflow between the lower limb joints uniquely emerges during pathological control of frontal plane degrees of freedom resulting in a generalized extension of the limb. It remains to be seen if a pattern of lower limb motor outflow that is centrally mediated contributes to abnormal hemiparetic gait.
Keywords: Stroke, Lower limb, Hemiparesis, Neuromechanics, Gait
Introduction
Following stroke, the emergence of aberrant stereotypical three dimensional (3D) gait impairments become evident. Yet previous research has traditionally limited the study of contributions to asymmetric gait to isolated single joint deficiencies. For example putative mechanisms to compensate for paretic ankle plantarflexion weakness (Dietz et al. 1981; Higginson et al. 2006), impaired knee flexion weakness and velocity (Goldberg & Anderson 2004), and over activity of hip flexors (Sung et al. 2000, Piazza & Delp 1996) have been extensively investigated. Similar studies have focused their investigation to exclusively sagittal plane mechanics for specific gait rehabilitation outcome measures (Jonkers 2009; Daly 2004). However the unique 3D observations of post-stroke gait abnormalities include impaired dynamic coupling between multiple lower limb joints across sagittal and frontal planes such as circumduction (Kerrigan et al. 2001). It is not known if a basic pattern of lower limb motor outflow that is centrally mediated underlies an impaired coordination pattern.
The ability to meet the biomechanical demands of gait following stroke may be neurally constrained to produce a precise pattern of cross-planar multijoint coordination. More specifically, sensitivity to frontal plane control may potentially be especially salient as several lines of investigation have underscored distinctive cross-planar couplings between medio-lateral (ML) control and sagittal plane progression during normal walking (Mackinnon & Winter 93). Both experimental (Cruz & Dhaher 2008, Rogers et al 2004) and simulation data (Allen et al 2013, Finley et al 2008) have implicated distinctive cross-planar neural couplings as potential contributors to stroke gait pathologies. Evidence for reciprocal heteronymous reflex meditated connectivity between hip adductors and knee extensors are observed when single joint angular perturbations were separately applied to the hip and knee (Finely et al. 2008). Frontal plane hip abduction in stroke gait is persistently observed even after applying assistive knee flexion torque (Sulzer et al 2009). This suggests that paretic frontal plane movements are a potential reflection of altered neural constraints rather than the result of voluntary kinematic compensations. Key experimental evidence from healthy subjects also show that lower limb sagittal plane muscles RF and VL make contributions to frontal plane hip acceleration (Hunter et al. 2009). Coupling of cross-planar kinetic outflow is further supported by modeling studies that demonstrate how muscles that primarily contribute to anterior-posterior (AP) COM acceleration also contribute to the ML acceleration (Pandy et al. 2010). It remains to be seen whether the pathological engagement of control of frontal plane mechanics leads to the emergence of unique neural couplings.
Accordingly we hypothesize that previously highlighted evidence of cross-planar couplings is reflective of a more generalized kinetic constraint across multiple joints in the lower limb. This work seeks to explicitly quantify post-stroke lower limb kinetic outflow by identifying preferences in a volitional torque production during a graded target matching task. 3D modeling has shown that while stability control of the sagittal plane in normal walking can be largely accounted for by the passive mechanics of the limb, active control is required to maintain stability in the frontal plane (Bauby et al. 2000; O’Conner et al. 2009). We further explore this by testing the hypothesis that engaging biomechanical demands in the frontal plane leads to the emergence of aberrant kinetic constraints across multiple lower limb joints that are independent of limb biomechanics. Characterizing the differential effects of abnormal torque patterns and strength impairments may improve clinical treatment of gait dysfunction. Interventions targeting multisegmental abnormalities may facilitate functional improvements in post-stroke gait dysfunction.
Methods
Subjects
A total of 25 participants (Table 1) were recruited for this study including 15 with a single unilateral stroke and 10 unimpaired age-matched control subjects. All subjects gave written informed consent. The study was approved by the Northwestern University Review Board. Impaired subjects presented with right side hemiparesis. A licensed physical therapist scored each subject for a variety of clinical tests (Table 1). All subjects were able to walk 5 meters without assistance and had no history of orthopedic injury or surgery to their lower limbs.
Table 1.
Stroke and control subject information. The clinical assessments were performed by a licensed physical therapist. Abbreviations: H-Hemorrhagic. I –Ischemic. TUG-Time up and go test. LMFM –Lower Motor Fugl-Meyer. Modified Ashworth scores tabulated for knee flexion and ankle dorsiflexion. All subjects had no cognitive deficits and passed a mini – mental examination. Subjects were not excluded due to sensory or proprioceptive impairments. Subjects were excluded if unable to meet the following range of motion requirements: ankle dorsiflexion to 90° , knee flexion to 90 ° , knee extension to 0 ° , hip flexion to 90 ° and hip extension to 10 °.
| Subject | Stroke | Gender | Age | Post (y) | Ashworth Knee |
Ashworth Ankle |
TUG (sec) |
Berg | LMFM |
|---|---|---|---|---|---|---|---|---|---|
| S1 | H | F | 52 | 3 | 0 | 0 | 11.4 | 46/56 | 18/34 |
| S2 | I | F | 58 | 25 | 1/5 | 0 | 11.5 | 53/56 | 21/34 |
| S3 | I | M | 49 | 3 | 0 | 3/5 | 12 | 43/56 | 25/34 |
| S4 | I | M | 56 | 8 | 1/5 | 1/5 | 12 | 55/56 | 26/34 |
| S5 | I | F | 31 | 9 m | 0 | 0 | 11.7 | 51/56 | 18/34 |
| S6 | I | M | 46 | 7 m | 0 | 0 | 8.5 | 54/56 | 30/34 |
| S7 | H | F | 50 | 2 | 0 | 0 | 13.5 | 54/56 | 26/34 |
| S8 | H | M | 53 | 3 | 0 | 0 | 9.8 | 55/56 | 25/34 |
| S9 | I | M | 39 | 2 | 0 | 0 | 13.4 | 46/56 | 27/34 |
| S10 | H | F | 51 | 2 | 0 | 1/5 | 17 | 49/56 | 24/34 |
| S11 | I | M | 46 | 2 | 0 | 0 | 8.2 | 51/56 | 30/34 |
| S12 | I | M | 46 | 3 | 0 | 0 | 20.2 | 52/56 | 25/34 |
| S13 | I | F | 63 | 8 | 0 | 1/5 | 15.3 | 55/56 | 20/34 |
| S14 | I | M | 60 | 16 | 0 | 0 | 30/34 | ||
| S15 | I | F | 34 | 2 | 0 | 0 | 32/34 | ||
| Stroke Mean (SD) | M = 8 | 49 (9) | 5(7) | 25(4) | |||||
| Control Mean (SD) | M = 5 | 45(13) |
Experimental Set-Up
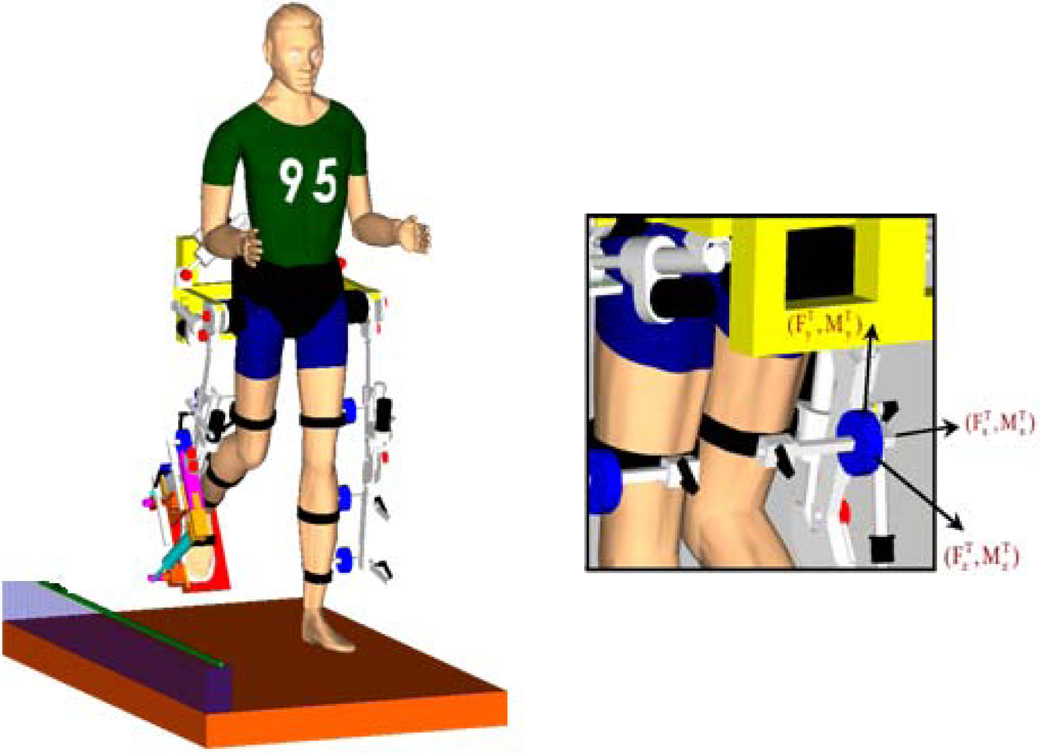
Subjects were secured in a motorized, instrumented exoskeleton (Lokomat; Hocoma, Zurich, Switzerland) and isometrically locked in 2 different gait specific postures. The dimensions of the orthosis were adjusted for each participant to align the orthosis joint centers with those of the subject (Figure 1). The subject’s lower extremities were secured to the orthosis via cuffs instrumented with 4 total six-degree-of freedom (DOF) load cells (JR3, Woodland, CA) to measure the interaction forces and moments of the paretic test limb. The test limb was completely unloaded by the Lokomat such that subjects did not have to actively support the limb during torque production.
Figure 1.
Experimental set-up. Instrumented exoskeletal orthosis with four six degree of freedom load cells on the leg attachment. The load cell signals were acquired at a 1000Hz sampling rate and filtered off-line using a 4th order butterworth, low-pass, and zero phase digital filter with a 50-Hz cutoff frequency. The effect of subject-specific limb inertias were accounted for by normalizing the target torques to the subjects MVT. A counterweight system supported up to 30% of the subjects body weight in order to provide functionally relevant and comfortable load bearing levels on the contralateral limb during a relatively long experiment (Cruz & Dhaher 2008).
Protocol
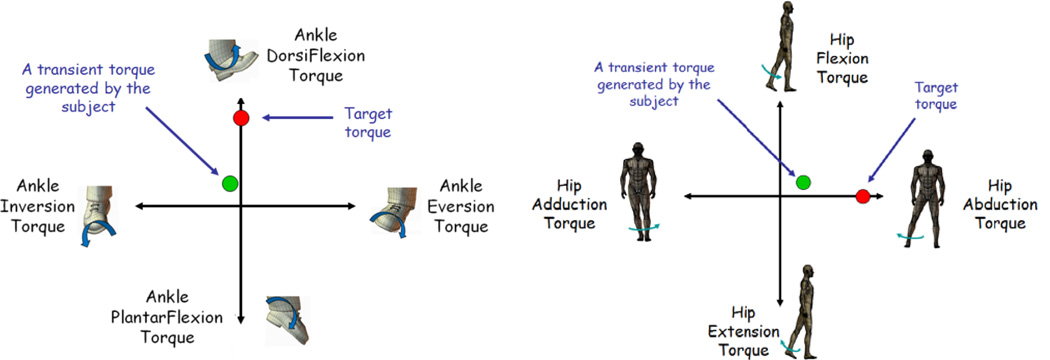
Subjects produced voluntary isometric torques in 4 directions at a primary lower limb joint corresponding to both sagittal plane and frontal plane targets at the hip and ankle. The hip targets are flexion, extension, abduction, and adduction. The ankle targets are dorsiflexion, plantarflexion, eversion, and inversion. Subjects received instantaneous visual feedback of the primary joint torque produced (Figure 2). The torques generated at each joint were calculated from thigh, shank, and ankle load cell signals using static equilibrium equations. While the subject matched target torques at the primary joint, secondary torques produced at the other joints across planes were recorded simultaneously. Specific instruction regarding the kinetic outflow at the secondary joints would have confounded the ability to investigate the intrinsic across-joint coupling. Feedback of secondary joint torque output was not provided.
Figure 2.
Testing paradigm. A volitional torque generation task under isometric conditions with visual feedback. (20% and 40% MVT) In part 1 subjects were given three 30 second opportunities to achieve a MVT torque. The largest MVT achieved was used to calculate the normalized target torque magnitude in part 2. Subjects received visual feedback of the primary joint output torque in the form of the position of a cursor. Normalized target torques are given on pure moment planes. The two primary lower limb joints, hip and ankle, were tested separately on different days. Subjects were given multiple attempts to successfully match the target torque, with each trial lasting up to 15s seconds to prevent fatigue.
The experimental protocol consisted of two parts: In part 1, maximum voluntary torque (MVT) produced at the hip (MVHT), knee (MVKT), and ankle (MVAT) were recorded along the 4 directions in the sagittal and frontal planes. Only knee sagittal plane torques were recorded. In part 2 isometric subjects performed torque target matching at either the hip or the ankle joints for a normalized percentage of MVT. Subjects were presented with targets in randomized order of the 4 directions and were instructed to match primary joint target torques within ±5% of the torque magnitude and hold for a minimum of 200ms for a successful trial.
To investigate the influence of supraspinal drive, two levels of randomized target torque magnitude, 20% and 40% of MVT for the primary joint were tested. To investigate the influence of biomechanics, 2 different gait specific postures were examined. In the toeoff posture (TO) the lower limb was rigidly locked at 15° hip extension, 45° knee flexion, and 90° ankle dorsiflexion (Winter 84). For the midswing posture (MS), the lower limb was rigidly stabilized at 10° hip extension, 65° knee flexion, and 90°ankle dorsiflexion (Winter 84). MVTs were recorded for each target direction for each posture.
Characteristic Kinetic Signature Calculations
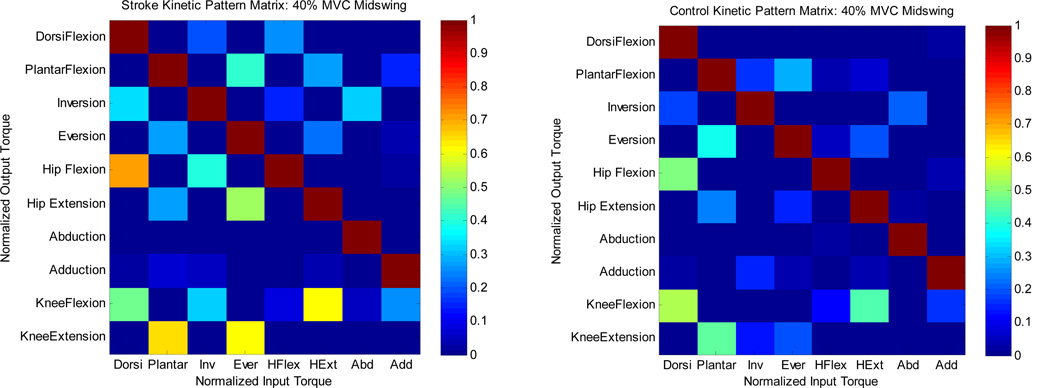
Representative kinetic joint coupling pattern matrices that characterize an aggregate signature of observed primary to secondary joint torque generation patterns were constructed for each subject and averaged over the stroke and control groups separately (Figure 3). Each ith column vector corresponds to the joint torques produced for a given primary volitional input torque at the primary joint. Each i,jth entry represents the corresponding secondary torques at the other joints normalized to MVT. Thus the kinetic coupling matrix represents an array of volitionally matched primary input joint torques to secondary torque coupling profiles across multiple inputs.
Figure 3.
Aggregate 3-D kinetic coupling pattern for each group at the midswing posture. Each i,jth entry corresponds to primary to secondary torque generation normalized to MVT.
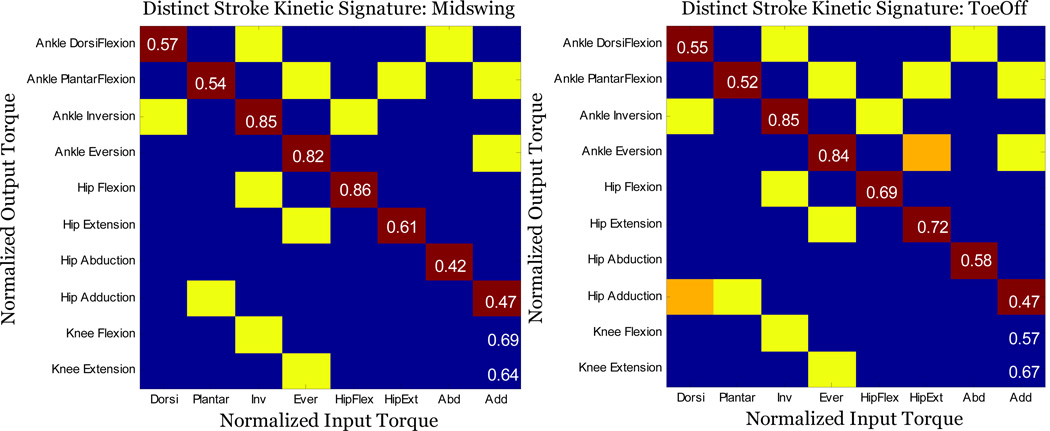
To examine the statistical differences between the kinetic joint coupling pattern matrices in each population, a non-paired t-test was applied to each array cell across the averaged kinetic pattern matrix for each posture. By applying a t-test to the (1,5)th cell, we test the hypothesis of a significant difference between control and stroke groups in the normalized secondary hip flexion output torque generated during an ankle dorsiflexion primary input torque. Each cell of the array represents a separate hypothesis of a significant difference for each primary input torque to secondary torque output parameter. The resulting matrix is referred to as the distinct stroke kinetic signature (Figure 4).
Figure 4.
Between population 3-D kinetic coupling pattern for Left panel) midswing and right panel) toeoff posture. Each yellow highlighted i,jth entry corresponds to statistically significant difference (p < 0.05) in secondary torque production between stroke and control subjects. Diagonal values in the red highlighted squares indicate the ratio of stroke to control MVT for each primary input torque. Orange highlighted squares correspond to significant difference at the toeoff posture only. All features except 2 retain statistical significance across postures. For example the (9, 3) cell of the kinetic signature matrix representing an inversion to knee flexion coupling is significant across both postures.
Principal Component Analysis
Principal component (PC) analysis was used to extract the underlying multijoint kinetic patterns that account for a significant portion of the variance in the torque matrix. We first computed the covariance matrix of the aggregate population torque signature for each condition. The first two eigenvectors of this matrix, rank ordered according to their associated eigenvalues, correspond to the PCs orthogonal directions of maximum variance in the 12 dimensional torque workspace (4 DOF at the ankle and hip, 2 DOF at the knee). The percentage of the total variance accounted for by each PC is represented by the associated eigenvalue. We compare the first 4 PCs of each population’s kinetic signature by computing the vector projection for each condition.
Results
Strength Comparison
For the MS posture, mean MVAT sagittal plane production during dorsiflexion and plantarflexion for the stroke population was significantly smaller than controls (p = 0.0007 & p = 0.002). There was no statistical difference in the mean frontal plane MVAT for inversion and eversion. Mean stroke MVKT production was significantly reduced for knee flexion at TO only (p = 0.0261) and was not significantly different between groups for knee extension for either posture. Mean MS MVHT production for frontal plane hip torque was significantly smaller for both adduction and abduction (p = 0.00365 & p = 0.00127). Sagittal plane MVHT in MS was significantly smaller in the stroke population for hip extension (p = 0.0302) but not for hip flexion. Ratios of the average stroke to control MVT for each primary input torque objective are shown along the diagonal of the kinetic signature matrix (Figure 4).
Distinct Stroke Kinetic Signature
The torque pattern matrix (Figure 3) represents a visual integrated summary of the averaged kinetic outflow patterns for the various tasks in the MS posture at 40% of MVT. Statistically significant differences between the aggregate kinetic signatures for each group can be visualized in a matrix representing the distinct stroke kinetic signature (Figure 4). The yellow highlighted i,jth elements in the matrix represents statistically significant differences in the primary input to secondary torque output cell values between each population (p < 0.05) in the MS posture at 20% MVT.
Across Joint Coupling
Cross-planar Coupling: Ankle & Knee Midswing
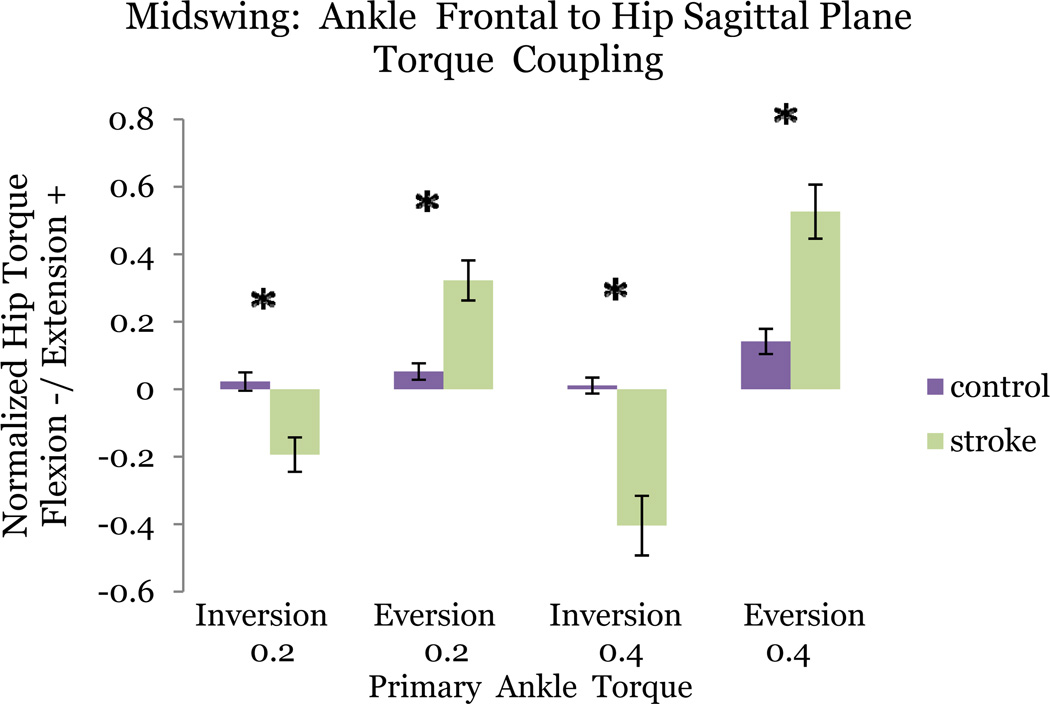
Stroke subjects coupled frontal plane primary ankle torque production with sagittal plane secondary knee torque production. During a primary inversion torque target matching, stroke subjects exerted a significantly larger secondary knee flexion torque than control subjects (p = 0.027). Furthermore stroke subjects exerted a significantly larger secondary knee extension torque during eversion (p = 0.0065). Control subjects did not produce a frontal plane torque statistically different from zero (p > 0.05).
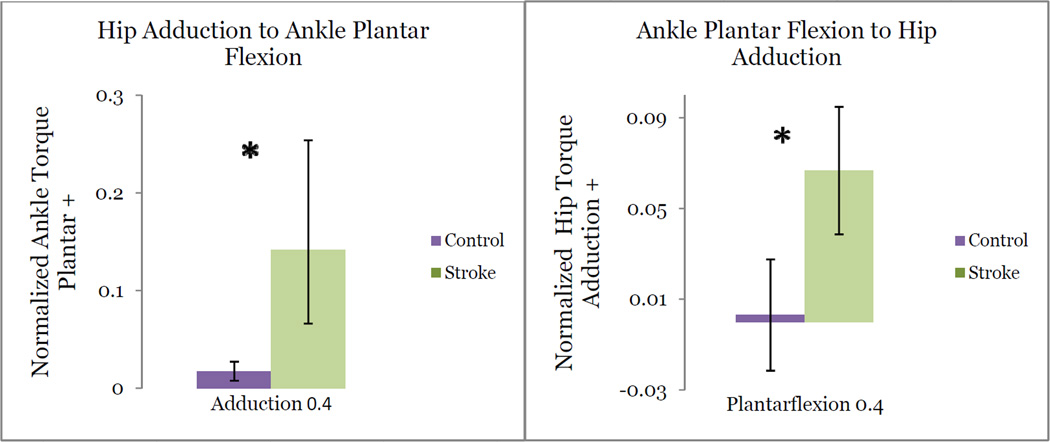
Cross-planar Coupling: Ankle & Hip Midswing
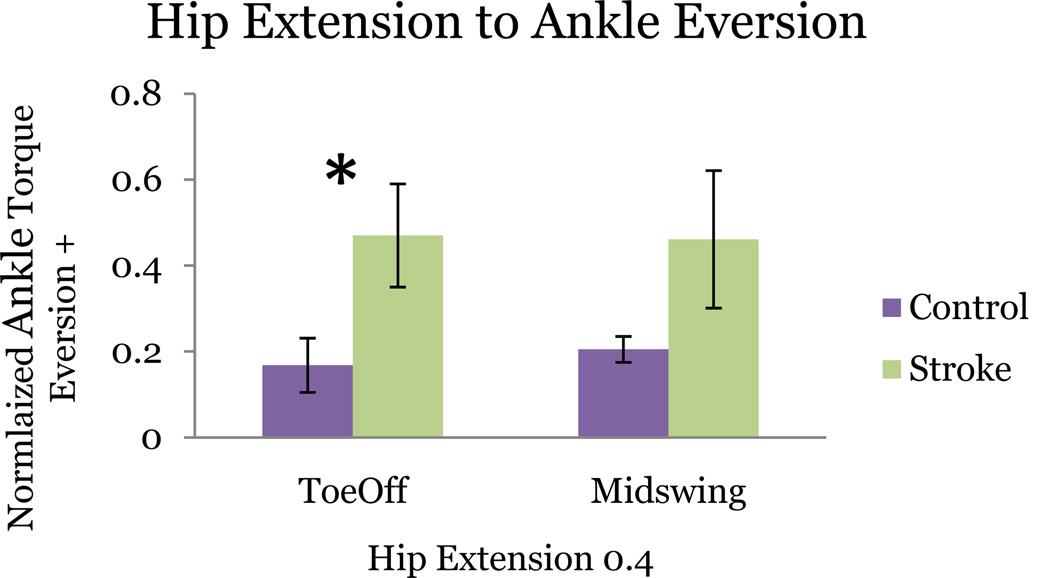
During plantarflexion torque matching, the stroke population produced significantly greater secondary adduction torque (p = 0.046) than control subjects. During adduction, stroke subjects produced statistically greater secondary plantarflexion torque (p =0 .034) Control subjects failed to couple adduction with plantarflexion in any primary joint matching task.
Stroke subjects produced a significantly greater coupling between primary inversion and secondary hip flexion than control subjects (p = 0.0005). In control subjects hip torque production was not statistically different from zero (p > 0.05) during inversion. During primary hip flexion, stroke subjects produced a significantly greater inversion torque (p = 0.0413). Control subjects did not produce a frontal plane torque statistically different from zero (p > 0.05).
Stroke subjects additionally coupled primary eversion torque with secondary hip extension with significant difference between groups (p = 0.0078). Control subjects produced a significantly smaller non-zero hip frontal plane torque. Coupling of hip extension with secondary eversion approached significance (p = 0.0897) in the stroke group (Figure 7).
Figure 7.
Similar coupling patterns are preserved across postures even though significance is not achieved in one posture. Example of one of only 2 coupling elements that is significant for toeoff (p=0.023) but not midswing (p=0.089). Starred bars correspond to statistically significant difference in secondary torque production between stroke and control subjects.
Sagittal Plane Couplings: Midswing
Both groups coupled secondary knee extension during primary plantarflexion torque matching with non-significantly different means. Both cohorts exerted a non-significantly different knee flexion torque during dorsiflexion. Between the ankle and hip joints, both populations produced hip flexion during dorsiflexion target matching with no significant difference in means. Both groups also produced secondary hip extension during plantarflexion with no significant difference in the mean secondary torque values. While no statistical significance is found for sagittal plane kinetic patterns, stroke subjects produced larger secondary sagittal plane torques.
Volitional Level
Varying the magnitude of the primary target match torque from 20% to 40% MVT did not change the distinct stroke kinetic signature in the MS posture (Figure 5).. Similar features of cross-planar coupling elements scaled with increasing target magnitude. Each element in the stroke kinetic matrix retained all significantly different secondary torque coupling patterns between groups at each MVT target levels (p < 0.05). Chi-square (χ2) analysis reveals a non-significant effect (p > 0.05) of torque magnitude (20/40% MVT) on secondary hip torque direction during primary ankle eversion (p = 0.31) and inversion (p = 0.14) in MS.
Figure 5.
Coupling patterns independent of primary torque magnitude. Starred bars correspond to statistically significant difference (p < 0.05) in secondary torque production between stroke and control subjects. Two examples of torque coupling patterns that increase with target torque magnitude are shown for the midswing posture: primary ankle inversion coupled with secondary hip flexion, and primary ankle eversion coupled with secondary hip extension.
Toeoff vs. Midswing
The 3-D kinetic coupling patterns calculated for each gait posture were similar. Figure 4 displays an overlaid representation of the stroke kinetic signature for each posture at 40% MVT. All of the elements in the torque pattern matrix for TO remain significantly different from the control group across postures except two: primary hip extension with inversion and primary dorsiflexion with adduction. Even though these two elements are only significant in TO, they retain similar connectivity patterns irrespective of posture (Figure 7).
Discussion
The present study highlights evidence of cross-planar couplings in the post-stroke lower limb that are potentially reflective of a more generalized kinetic constraint across multiple joints. By measuring volitional torque patterns at discrete gait postures, we extracted a characteristic torque pattern signature. This matrix represents a visual integrated summary of the characteristic kinetic outflow patterns for a variety of isometric single joint control objectives. These findings corroborate others that identified abnormal torque coupling in the upper limb (Bourbonnais et al. 1989; Dewald et al. 2001) and lower limb (Cruz et al 2008). Although Neckel et al. 2006 found no evidence for synergistic lower limb torque patterns, only the foot was rigidly fixed with torque measured from a single load cell at the heel allowing subjects to potentially invoke stabilization strategies. While coupling of stereotypical impaired upper limb movements have been analyzed (Beer et al 1999, 2007) along with postural dependence (Ellis et al 2007), multi-joint coordination in the post-stroke lower limb has not yet been systematically evaluated. Here we examine unique cross-planar kinetic coupling features of abnormal multijoint coordination post-stroke and discuss potential implications to hemiparetic gait dysfunction. Elucidating the differential effect of strength and kinetic coupling may provide a useful framework in optimizing rehabilitation strategies.
Coupling Independent of Biomechanics
It may be argued that the observed coupling may arise exclusively from limb mechanics. The moment arms of the muscles spanning two adjacent joints could yield a mechanical coupling signature independent of any neural component. Yet we observe stability in the sign and magnitude of the crossplanar torque coupling features even as the connective tissue moment arms change with posture. These same features retain statistical significance across postures.
Moreover we observe coupling between non-neighboring joints, where the confound of mechanical coupling is eliminated. Significant coupling between the ankle and hip is observed exclusively in stroke. Although anatomically unconnected, ankle frontal plane and hip sagittal plane torque production is tightly coupled (Figure 4). Furthermore if purely biomechanical factors account for the observed coupling, we would expect adduction torques to be exaggerated in MS (Cruz et al. 2008) leading to secondary MS adduction torque bias. Our study fails to observe this feature but instead finds consistent secondary adduction torque bias during frontal plane ankle torque production across postures. Thus mechanical coupling is not sufficient to account for the robustness of the kinetic patterns.
Strength Contributions
It is plausible that strength imbalances in the corresponding musculature can account for the coupled kinetic output observed in the stroke group (Lum et al. 2003). Earlier studies emphasized the contributions of muscle weakness to post-stroke gait dysfunction (Adams et al. 1990; Nadeau et al. 1999). The subjects tested could have entrained a particular pattern of kinematic compensation throughout their recovery period such that certain kinetic patterns become enforced. If so, we would expect the MVT strength ratios for both agonist and antagonist muscles to significantly bias the observed coupling when gait posture is manipulated. Yet while postural changes appropriately scaled the MVT hip strength ratios (Figure 4), it did not elicit new torque coupling patterns. All the elements of the kinetic signature with the exception of only 2 achieve significance across gait postures. Interestingly, we also observe a reciprocal feature of the coupling signature that persists independent of proximal or distal joint control (Figure 6) there are differences in joint strength. Furthermore χ2 analysis confirms no significant effect of torque magnitude on aberrant coupling. These results support the hypothesis that the observed coupling is not associated with a specific pattern of peripheral strength imbalances shared by the tested stroke subjects but more likely due to central neuromechanical constraints
Figure 6.
Coupling patterns independent of proximal or distal joint control. Starred bars correspond to statistically significant difference (p < 0.05) in secondary torque production between stroke and control subjects.
Motor impairments underlying post-stroke gait dysfunction
There are many factors that contribute to pathological stroke gait including exaggerated reflex activity (Zhang et al 2013), peripheral muscle weakness (Hsu et al 2003) and reduced neuromuscular drive (Klein et al 2010). As such it is difficult to speculate a primary source of gait dysfunction. For example reduced dorsiflexion is often the combined result of weak dorsiflexors and spastic plantarflexors (Hesse et al 1996). However only modest improvements in gait speed are observed when spasticity and weakness are treated together (Johnson et al 2004). While proprioceptive deficits may influence gait, functional modulation of knee proprioceptive input during walking did not eliminate aberrant hip frontal plane kinematics (Sulzer 2009). These results suggest that a major source of post-stroke gait dysfunction may not be single joint/muscle pathologies, but impaired coordination.
Correlations between functional activity and motor impairments have met with mixed results. Plantarflexion (Nadeau et al 1999) and knee extension (Bohannon & Andrews 1990) strength have been correlated with gait speed yet poor correlation has been shown between spasticity and gait speed (Lamontagne et al 2000). In our view, such correlations are incomplete as single joint metrics may have limited extrapolation to multiple joint/DOF parameters such as gait velocity. Furthermore they do not address abnormal coupling across joints and planes. Other researchers evaluating the Fugl-Meyer (FM) assessment of lower motor impairment found that discrete voluntary movements as measured by the FM have inadequate predictive ability for clinical walking measures (Bowden et al 2010). While the FM does aggregate parameters for multiple joints, the qualitative assessment of primarily sagittal plane features may limit its prognostic utility. Interestingly, kinetic patterns observed post-stroke during isometric force generation have been identified in dynamic tasks in the upper limb during reaching (Kahn et al 2006) and in the lower limb during gait (Nessler et al 2007).
While this work highlights kinetic coupling resulting from discrete volitional control objectives, it is important to note that walking is a complex interaction of supraspinal and spinal mechanisms. Stepping patterns are argued to be modulated by both peripheral afferent input (af Klint et al 2008) and putative central pattern generators (Dietz & Harekma 2004). Future studies are needed to elucidate how kinetic patterns extracted isometrically relate to rhythmic walking. Correlations between cross-planar couplings and deviations in over ground gait metrics have been evaluated using step wise linear regression (Cruz et al 2009). This analysis revealed that the most significant factors determining gait speed involved the frontal plane: ratio of hip adduction to knee extension torque, maximum hip abduction/adduction torque. Future studies can evaluate the association of frontal plane couplings with paretic gait asymmetry metrics.
The abnormal couplings here may be used to provide a scientific basis for the design of rehabilitation studies targeting gait specific deficiencies. Strategies targeting the hemiparetic upper limb have been shown to reduce synergistic coupling patterns (Ellis et al 2005). Thus it may be possible to impact gait dysfunction by designing training protocols that decouple aberrant kinetic patterns following stroke. Interventions targeting multi-segmental abnormalities may facilitate functional improvement during walking. Muscle synergy analysis (D’Avella et al. 2003) may provide additional insight when comparing isometric and dynamic muscle activation patterns given evidence that paretic muscle modules constrain gait subtasks performance (Allen et al 2013).
Frontal Plane Sensitivity
Dominant torque couplings observed in the stroke group may reflect unique neural constraints on the torque generation workspace. By this interpretation, we expected that the PCs (Shlens 2003) of the associated stroke kinetic signatures will include such frontal plane coupling features. Indeed, while the first 2 PCs of both groups shared covarying sagittal plane kinetic coupling patterns, only the stroke population carried distinct frontal plane coupling features. The eigenvectors associated with the first two PCs of the stroke population included interdependent features associated with ankle eversion and hip adduction production absent in controls. 37 % of the total kinetic signature variance was accounted for by the first stroke PC featuring frontal plane joint coupling as represented by the eigenvalues. In contrast, the first control PC accounted for 22 % of the variance attributed to the common across sagittal plane coupling shared by both populations. This suggests that stroke PCs are uniquely dominated by frontal plane torque coupling.
While quantitative assessments for sagittal plane couplings have been reported for adults with cerebral palsy (Thelen et al 2003), they are lacking in the lower limb post-stroke with the exception of those clinically reported (Brunnstrom 1970). Interestingly, we observe inconclusive evidence of post-stroke sagittal plane couplings such as the ‘flexion synergy’ (hip and knee flexion with ankle dorsiflexion). Although stroke subjects presented with exaggerated sagittal plane coupling patterns relative to controls, they were not statistically significant. For example coupling between dorsiflexion and hip flexion was not significant for neither distal (p = 0.203) nor proximal (p = 0.109) targets. χ2 analysis further reveals a non-significant group effect on sagittal hip torque direction during ankle dorsiflexion (p = 0.235). These results suggest that the kinetic outflow distribution for sagittal plane control objectives are inhomogeneous for each group despite a trend toward exaggerated coupling in stroke.
This analysis provides further evidence of cross-planar couplings that is reflective of a more generalized kinetic constraint across multiple joints in the lower limb. Both gait simulation and experimental studies corroborate the idea that stability control of the ML plane may require a higher degree of active control in neurologically intact subjects (Bauby et al. 2000; O’Conner et al. 2009). Thus increasing the biomechanical demand in the frontal plane in the pathological state may engage these unique torque connectivities we propose. We observed that the relevant torque outflow for gait submovements within the sagittal plane did not explain abnormal paretic kinematic patterns. Stroke subjects retained many within plane torque generation patterns found in controls. Only the frontal plane features were uniquely present in the stroke PC. A functional consequence of the observed abnormal across joint coupling of hip adduction, knee extension, and plantarflexion is a limb extension often observed in hemiparetic gait (Kerrigan et al. 2000). This study supports the idea that coupled cross-planar kinetic outflow between the lower limb joints uniquely emerges during control of frontal plane DOF post-stroke, resulting in a generalized extension of the limb.
Conclusions
This study provides compelling evidence that suggests that torque generation following stoke is characterized by both within joint and across joint abnormalities. The systematic evaluation of the coupled kinetic outflow across planes in the lower limb revealed a robust signature of neural constraints governing an intrinsic impaired coordination. Evaluation of the aggregate kinetic signatures suggests that the DOF under active control post-stroke are neurally constrained especially in frontal plane torque production. PC analysis further elucidated unique differences between the associated covariance patterns of the population kinetic signatures. Features of this impaired coordination may contribute to post-stroke asymmetric gait where the ability to fractionate control of single joints is compromised. The understanding gained from the present study may facilitate a refocusing of rehabilitation paradigms from targeting single joint / sagittal plane impairments to cross-planar multi-joint approaches. Furthermore clinical treatment may benefit from an understanding of peripheral vs. central contributions to kinematic disturbances. Future work will identify the associated variability in the lower limb muscle activation patterns underlying both the isometric kinetic output and the cyclic activation patterns during gait.
Acknowledgments
This work was supported by NIH-NINDS Grant R01NS064084-02and the Falk Foundation of the Rehabilitation Institute of Chicago. The contents are solely the responsibility of the authors and not necessarily represent the official views of the NIH or RIC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All authors were fully involved in the study and preparation of the manuscript. The material within has not been and will not be submitted for publication elsewhere.
Conflict of Interest:
None.
References
- Adams RW, Gandevia SC, Skuse NF. The distribution of muscle weakness in upper motoneuron lesion affecting the lower limb. Brain. 1990;113(Pt5):p1459–p1476. doi: 10.1093/brain/113.5.1459. [DOI] [PubMed] [Google Scholar]
- Allen JL, Kautz SA, Neptune RR. The influence of merged muscle excitation modules on post-stroke hemiparetic walking performance. Clin Biomech. 2013;28(6):697–704. doi: 10.1016/j.clinbiomech.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauby CE, Kuo AD. Active control of lateral balance in human walking. J Biomech. 2000;33:1433–1440. doi: 10.1016/s0021-9290(00)00101-9. [DOI] [PubMed] [Google Scholar]
- Beer RF, Given JD, Dewald JP. Task-dependent weakness at the elbow in patients with hemiparesis. Arch Phys Med Rehabil. 1999;80(7):766–772. doi: 10.1016/s0003-9993(99)90225-3. [DOI] [PubMed] [Google Scholar]
- Beer RF, Ellis MD, Holubar BG, Dewald JP. Impact of gravity loading on post-stroke reaching and its relationship to weakness. Muscle Nerve. 2007;36(2):242–250. doi: 10.1002/mus.20817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohannon RW, Andrews AW. Correlation of knee extensor muscle torque and spasticity with gait speed in patients with stroke. Arch Phys Med Rehabil. 1990;71(5):330–333. Erratum in: Arch Phys Med Rehabil. 71(7): 464. [PubMed] [Google Scholar]
- Bourbonnais D, VandenNoven S, Carey KM, Rymer WZ. Abnormal spatial patterns of elbow muscle activation in hemiparetic human subjects. Brain. 1989;112(Pt 1):85–102. doi: 10.1093/brain/112.1.85. [DOI] [PubMed] [Google Scholar]
- Bowden MG, Clark DJ, Kautz SA. Evaluation of abnormal synergy patterns poststroke: relationship of the Fugl-Meyer Assessment to hemiparetic locomotion. Neurorehabil Neural Repair. 2010;24(4):328–337. doi: 10.1177/1545968309343215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunnstrom S. A Neurophysiological Approach. New York: Harper & Row; 1970. Movement Therapy in Hemiplegia. [Google Scholar]
- Clark DJ, Ting LH, Zajac FE, Neputne RR, Kautz SA. Merging of Healthy Motor Modules Predicts Reduced Locomotor Performance and muscle coordination complexity post-stroke. J Neurophys. 2010;103:844–857. doi: 10.1152/jn.00825.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz TH, Dhaher Y. Evidence of lower limb torque synergies after stroke: an isometric study. Stroke. 2008;39(1):139–147. doi: 10.1161/STROKEAHA.107.492413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz TH, Lewek MD, Dhaher YY. Biomechanical impairments and gait adaptations post-stroke: multi-factorial associations. J Biomech. 2009;42(11):1673–1677. doi: 10.1016/j.jbiomech.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Avella A, Bizzi E. Combinations of muscle synergies in the construction of natural motor behavior. Nat. Neurosci. 2003;6:300–308. doi: 10.1038/nn1010. [DOI] [PubMed] [Google Scholar]
- Daly JJ, Roenig KL, Butler KM, Gansen JL. Response of sagittal plane gait kinematics to weight supported treadmill training and functional neuromuscular stimulation following stroke. J Rehabil Res Dev. 2004;41(6):807–820. doi: 10.1682/jrrd.2003.08.0120. [DOI] [PubMed] [Google Scholar]
- Dewald JP, Beer RF. Abnormal joint torque patterns in the paretic upper limb of subjects with hemiparesis. Muscle Nerve. 2001;24(2):273–283. doi: 10.1002/1097-4598(200102)24:2<273::aid-mus130>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Dietz V, Harkema SJ. Locomotor activity in spinal cord-injured persons. J Appl Physiol. 2004;96(5):1954–1960. doi: 10.1152/japplphysiol.00942.2003. [DOI] [PubMed] [Google Scholar]
- Dietz V, Quintern J, Berger W. Electrophysiological studies of gait in spasticity and rigidity. Evidence that altered mechanical properties of muscle contribute to hypertonia. Brain. 1981;104(3):431–449. doi: 10.1093/brain/104.3.431. [DOI] [PubMed] [Google Scholar]
- Ellis MD, Holubar BG, Acosta AM, Beer RF, Dewald JP. Modifiability of abnormal isometric elbow and shoulder joint torque coupling after stroke. Muscle Nerve. 2005;32(2):170–178. doi: 10.1002/mus.20343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis MD, Acosta AM, Yao J, Dewald JP. Position-depenent torque coupling and associated muscle activation in the hemiparetic upper extremity. Exp Brain Res. 2007;176(4):594–602. doi: 10.1007/s00221-006-0637-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley JM, Perreault EJ, Dhaher YY. Stretch reflex coupling between the hip and knee: implications for impaired gait following stroke. Exp. Brain. Res. 2008;188(4):529–540. doi: 10.1007/s00221-008-1383-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg SR, Anderson FC. Muscles that influence knee flexion velocity in double support: implications for stiff-knee gait. J Biomech. 2004;37(8):1189–1196. doi: 10.1016/j.jbiomech.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Hesse S, Krajnik J, Luecke D, Jahnke MT, Gregoric M, Mauritz KH. Ankle muscle activity before and after botulinum toxin therapy for lower limb extensor spasticity in chronic hemiparetic patients. Stroke. 1996;27(3):455–460. doi: 10.1161/01.str.27.3.455. [DOI] [PubMed] [Google Scholar]
- Higginson JS, Zajac FE, Neptune RR, Kautz SA, Delp SL. Muscle contributions to support during gait in an individual with post-stroke hemiparesis. J Biomech. 2006;39(10):1769–1777. doi: 10.1016/j.jbiomech.2005.05.032. [DOI] [PubMed] [Google Scholar]
- Hsu AL, Tang PF, Jan MH. Analysis of impairments influencing gait velocity and asymmetry of hemiplegic patients after mild to moderate stroke. Arch Phys Med Rehabil. 2003;84(8):1185–1193. doi: 10.1016/s0003-9993(03)00030-3. [DOI] [PubMed] [Google Scholar]
- Hunter BV, Thelen DG, Dhaher YY. A three-dimensional biomechanical evaluation of quadriceps and hamstrings function using electrical stimulation. IEEE Trans on Neural Systems and Rehabilitation Engineering. 2009;17(2):167–175. doi: 10.1109/TNSRE.2009.2014235. [DOI] [PubMed] [Google Scholar]
- Johnson CA, Burridge JH, Strike PW, Wood DE, Swain ID. The effect of combined use of botulinum toxin type A and functional electric stimulation in the treatment of spastic drop foot after stroke: a preliminary investigation. Arch Phys Med Rehabil. 2004;85(6):902–909. doi: 10.1016/j.apmr.2003.08.081. [DOI] [PubMed] [Google Scholar]
- Jonkers I, Delp S, Patten C. Capacity to increase walking speed is limited by impaired hip and ankle power generation in lower functioning persons post-stroke. Gait Posture. 2009;29(1):129–137. doi: 10.1016/j.gaitpost.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn LE, Lum PS, Rymer WZ, Reinkensmeyer DJ. Robot-assisted movement training for the stroke-impaired arm: Does it matter what the robot does? J. Rehabil. Res. Dev. 2006;43(5):619–630. doi: 10.1682/jrrd.2005.03.0056. [DOI] [PubMed] [Google Scholar]
- Kerrigan DC, Frates EP, Rogan S, Riley PO. Hip hiking and circumduction: quantitative definitions. Am J Phys Med Rehabil. 2001;79(3):247–252. doi: 10.1097/00002060-200005000-00006. [DOI] [PubMed] [Google Scholar]
- Kerrigan DC, Gronley Stiff-legged gait in spastic paresis. A study of quadriecps and hamstring muscle activity. Am J Phys Med Rehabil. 1991;70(6):294–300. [PubMed] [Google Scholar]
- Klein CS, Brooks D, Richardson D, McIlroy WE, Bayley MT. Voluntary activation failure contributes more to plantar flexor weakness than antagonist coactivation and muscle atrophy in chronic stroke survivors. J Appl Physiol. 2010;109(5):1337–1346. doi: 10.1152/japplphysiol.00804.2009. [DOI] [PubMed] [Google Scholar]
- af Klint R, Nielsen JB, Cole J, Sinkjaer T, Grey MJ. Within-step modulation of leg muscle activity by afferent feedback in human walking. J Physiol. 2008;586(Pt 19):4643–4648. doi: 10.1113/jphysiol.2008.155002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamontagne A, Malouin F, Richards CL. Contribution of passive stiffness to ankle plantarflexor moment during gait after stroke. Arch Phys Med Rehabil. 2000;81(3):351–358. doi: 10.1016/s0003-9993(00)90083-2. [DOI] [PubMed] [Google Scholar]
- Lum PS, Burgar CG, Shor PC. Evidence for strength imbalances as a significant contributor to abnormal synergies in hemiparetic subjects. Muscle Nerve. 2003;27(2):211–221. doi: 10.1002/mus.10305. [DOI] [PubMed] [Google Scholar]
- MacKinnon CD, Winter DA. Control of whole body balance in the frontal plane during human walking. J Biomech. 1993;26(6):633–644. doi: 10.1016/0021-9290(93)90027-c. [DOI] [PubMed] [Google Scholar]
- Nadeau S, Gravel D, Arsenault AB, Bourbonnais D. Plantarflexor weakness as a limiting factor of gait speed in stroke subjects and the compensating role of hip flexors. Clin Biomech. 1999;14(2):125–135. doi: 10.1016/s0268-0033(98)00062-x. [DOI] [PubMed] [Google Scholar]
- Neckel ND, Pelliccio M, Nichols D, Hidler J. Quantification of functional weakness and abnormal synergy patterns in the lower limb of individuals chronic stroke. J. Neuroengineering Rehabil. 2006;3(1):17. doi: 10.1186/1743-0003-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nessler JA, Winsean L, Dhaher Y. Synergistic moments at the hip and knee joints are altered in post-stroke hemiplegic gait. Proceedings of the ASME Summer Bioenginnering conference; Keystone, Colorado USA. June 20–24.2007. [Google Scholar]
- O’Conner SM, Kuo AD. Direction-Dependent Control of Balance During Walking and Standing. J Neurophysiol. 2009;102:1411–1419. doi: 10.1152/jn.00131.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandy MG, Lin YC, Kim HJ. Muscle coordination of medio-lateral balance in normal waling. J Biomech. 2010;43:2055–2064. doi: 10.1016/j.jbiomech.2010.04.010. [DOI] [PubMed] [Google Scholar]
- Piazza SJ, Delp SL. The influence of muscles on knee flexion during the swing phase of gait. J Biomech. 1996;29(6):723–733. doi: 10.1016/0021-9290(95)00144-1. [DOI] [PubMed] [Google Scholar]
- Rogers LM, Brown DA, Gruben KG. Foot force direction control during leg pushes against fixed and moving pedals in persons post-stroke. Gait Posture. 2004;19(1):58–68. doi: 10.1016/s0966-6362(03)00009-2. [DOI] [PubMed] [Google Scholar]
- Shlens J. A tutorial on Principal component analysis: derivation, discussion, and singular value decomposition. 2003 [Google Scholar]
- Sulzer JS, Gordon KE, Dhaher YY, Peshkin MA, Patton JL. Preswing knee flexion assistance is coupled with hip abduction in people with stiff-knee gait after stroke. Stroke. 2009;41(8):1709–1714. doi: 10.1161/STROKEAHA.110.586917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung DH, Bang HJ. Motor branch block of the rectus femoris: its effectiveness in stiff-legged gait in spastic paresis. Arch Phys Med Rehabil. 2000;81(7):910–915. doi: 10.1053/apmr.2000.5615. [DOI] [PubMed] [Google Scholar]
- Thelen DD, Riewald SA, Asakawa DS, Sanger TD, Delp SL. Abnormal coupling of knee and hip moments during maximal exertions in persons with cerebral palsy. Muscle Nerve. 2003;27(4):486–493. doi: 10.1002/mus.10357. [DOI] [PubMed] [Google Scholar]
- Winter AD. Kinematic and Kinetic patterns In human gait: Variability and compensating effects. 1984;3(1–2):p56–p58. [Google Scholar]
- Zhang LQ, Chung SG, Ren Y, Liu L, Roth EJ, Rymer WZ. Simultaneous characterizations of reflex and nonreflex dynamic and static changes in spastic hemiparesis. J Neurophysiol. 2013;110(2):418–430. doi: 10.1152/jn.00573.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]