Abstract
The serotonergic system regulates a wide range of behavior, including mood and impulsivity, and its dysregulation has been associated with mood disorders, autism spectrum disorder, and addiction. Diabetes is a risk factor for these conditions. Insulin resistance in the brain is specifically associated with susceptibility to psychostimulant abuse. Here, we examined whether phosphorylation of Akt, a key regulator of the insulin signaling pathway, controls serotonin (5-HT) signaling. To explore how impairment in Akt function regulates 5-HT homeostasis, we used a brain-specific rictor knockout (KO) mouse model of impaired neuronal phosphorylation of Akt at Ser473. Cortical 5-HT1A and 5-HT2A receptor binding was significantly elevated in rictor KO mice. Concomitant with this elevated receptor expression, the 5-HT1A receptor agonist 8-Hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) led to an increased hypothermic response in rictor KO mice. The increased cortical 5-HT1A receptor density was associated with higher 5-HT1A receptor levels on the cortical cell surface. In contrast, rictor KO mice displayed significantly reduced head-twitch response (HTR) to the 5-HT2A/C agonist 2,5-dimethoxy-4-iodoamphetamine (DOI), with evidence of impaired 5-HT2A/C receptor signaling. In vitro, pharmacological inhibition of Akt significantly increased 5-HT1A receptor expression and attenuated DOI-induced 5-HT2A receptor signaling, thereby lending credence to the observed in vivo cross-talk between neuronal Akt signaling and 5-HT receptor regulation. These data reveal that defective central Akt function alters 5-HT signaling as well as 5-HT-associated behaviors, demonstrating a novel role for Akt in maintaining neuronal 5-HT receptor function.
Keywords: serotonin, Akt, cortex, 5HT1A receptor, 5HT2A receptor
1. INTRODUCTION
In the brain, insulin acts through its receptor to stimulate phosphatidylinositol 3-kinase, and downstream protein kinase B (PKB)/Akt isoforms 1 and 2. Akt, a metabolic kinase, is involved in neuronal development, synaptic transmission, and monoamine transporter trafficking and function (Garcia et al., 2005; Robertson et al., 2010; Speed et al., 2011). The mammalian target of rapamycin (mTOR) complex 2 (mTORC2), that comprises the protein rictor, is a protein kinase regulating cell growth and metabolism (Sarbassov et al., 2005) and Akt activity. Akt function is regulated by phosphorylation at 2 key residues: Ser473 by mTORC2 and Thr308 by phosphoinositide-dependant kinase 1. Akt dysregulation has been linked to numerous metabolic diseases including diabetes and obesity, and mental disorders such as schizophrenia (Nicodemus et al., 2010; Thiselton et al., 2008) and drug abuse (Ikeda et al., 2006). As aberrant Akt function has been progressively linked to a growing number of brain disease states, the mechanism of how it contributes to their pathophysiology is under intense study. Recently, it has been proposed that Akt may represent a molecular link between diseases driven by insulin resistance (e.g. diabetes) and disorders associated with central monoaminergic disturbances, including depression, schizophrenia and drug abuse (Daws et al., 2011; Robertson et al., 2010).
The serotonin (5-HT) system mediates a variety of brain functions, including temperature regulation, appetite, mood, libido, and social behaviors (Berger et al., 2009). Perturbations in the serotonergic system are involved in a number of psychiatric disorders, including panic disorder (Nash et al., 2008), anxiety, aggression (Gingrich and Hen, 2001) and most recently, autism spectrum disorder (Veenstra-VanderWeele et al., 2012). The emergence of selective serotonin reuptake inhibitors (SSRIs) as the most prescribed medications in the U.S. has centered attention on the role of the 5-HTsystem in depression and anxiety. Genetic evidence also points to the involvement of the serotonin system in susceptibility to stress and depression (Jans et al., 2007). More than a quarter of the population with diabetes suffers from depression (Lustman and Clouse, 2005). This common comorbidity could reflect an important relationship between dysfunction in the insulin-signaling pathway and altered 5-HT neurotransmission.
We have previously shown that at the neuronal level, insulin signaling regulates monoamine transporter trafficking and that changes in insulin status profoundly affect monoamine homeostasis (Robertson et al., 2010; Speed et al., 2011; Williams et al., 2007). For example, dysregulated brain insulin signaling alters dopamine and norepinephrine homeostasis (Robertson et al., 2010; Speed et al., 2011; Williams et al., 2007) and related behaviors. With respect to its effect on 5-HT regulation, animal models of both diet-induced obesity (DIO) and type 2 diabetes have been shown to increase 5-HT2A receptor density (Park et al., 1999; Sumiyoshi et al., 1997), whereas animal models of food restriction and experimentally-induced type 1 diabetes result in decreased in vivo response to 5-HT1A and 5-HT2A receptor agonists. Thus, the relationship between central insulin signaling and 5-HT neurotransmission is complex. Here, we focused on determining whether genetic impairment of neuronal insulin signaling (i.e. Akt function) affects 5-HT homeostasis, 5-HT receptor signaling, and in vivo response to 5-HT receptor stimulation.
2. MATERIAL AND METHODS
2.1. Generation of mice
All neuronal rictor KO mice were generated and fully backcrossed to the C57BL/6 background as previously described (Siuta et al., 2010). Breeding pairs consisted of animals homozygous for knock-in of loxP sites flanking exon 3 of the rictor gene (Rictor flox/flox) and heterozygous for Nestin-Cre (Nes +/−). Experimental mice (rictor KO) lacked expression of functional rictor due to excision of exon 3 in all neurons (Nes +/−). Control animals (FLOX) contained the loxP sites but did not have Cre (Rictor flox/flox, Nes −/−).
2.2. Drugs
DOI ((±)-2,5-dimethoxy-4-iodoamphet-amine hydrochloride), 8-hydroxy-2-(di-n-propyl-amino)tetralin (8-OH-DPAT), WAY100635, SB269970, WAY100635, fluoxetine, methysergide, ritanserin, and spiperone were purchased from Sigma (St. Louis, MO, USA). The 5-HT2A antagonist MDL 100907 was a gift from Merrell Dow Pharmaceuticals (Kansas City, MO, USA). [3H] citalopram, [3H]-ketanserin, and [3H]-8-OH-DPAT [8-hydroxy-2-(di-n-propylamino)-tetralin] were purchased from PerkinElmer Inc. (Boston, MA, USA). [3H]-mesulergine was purchased from G.E.Healthcare, Inc. (Piscataway, NJ, USA).
2.3. Brain tissue extraction for serotonin content
Tissue extraction and analysis was done by a specific HPLC assay (Vanderbilt Neurochemistry Core) as described previously (Siuta et al., 2010).
2.4. Serotonin receptor and transporter binding
Binding assays for serotonin receptors and serotonin transporter were performed with modification as previously reported (Canal et al., 2010). SERT binding: Homogenized cortical and midbrain membranes were incubated with [3H]citalopram [3.8 nM] in the presence or absence of fluoxetine [10 μM] to measure non-specific and total binding, respectively. 5-HT1A receptor binding: Homogenized cortical membranes were incubated with [3H]8-OH-DPAT [10 nM] in the presence or absence of WAY100635 [10 μM] to measure non-specific and total binding, respectively. Reaction buffer included the 5-HT7 receptor antagonist SB269970 [100 nM]. 5-HT2A receptor binding: Homogenized cortical membranes were incubated with [3H]ketanserin [12 nM]. Non-specific binding was determined using excess methysergide [100 μM]. 5-HT2C receptor binding: Homogenized cortical membranes were incubated with [3H]-mesulergine [10 nM] in the presence or absence of ritanserin [8 μM] to measure non-specific and total binding, respectively. The reaction buffer included the 5-HT1A/2A receptor antagonist spiperone [100 nM] and the 5-HT7 receptor antagonist SB269970 [100 nM]. Single point saturating concentration was chosen based on pilot experiments of complete saturation binding curves in FLOX and rictor KO mice for [3H] citalopram, [3H]ketanserin, [3H]-8-OH-DPAT and [3H]mesulergine binding. For all experiments, specific binding was calculated by subtracting non-specific binding from total binding and expressed as bound ligand (fmol) per mg protein. All experiments were performed in duplicate.
2.5. DOI-induced head twitch response (HTR)
This was conducted as previously described (Veenstra-VanderWeele et al., 2012). Mice received an intraperitoneal (IP) injection of 1.0 mg/kg of 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI, Sigma), a 5HT2A/2C agonist, 30 minutes before testing. After the injection, each mouse was placed in a large glass beaker containing bedding after the injection. A HTR was defined as a clear, rapid, left to right or right to left tic movement of the head of the mouse following DOI treatment. Head twitches were recorded over three 5-minute periods in a quiet room by 3 trained observers blind to the genotype. The mean of the three observers was taken as the HTR. For the studies done with the 5-HT2A antagonist, more than one week later, the same mice were injected with 0.25 mg/kg M-100907 17 minutes prior to the DOI injection. At least one week later, mice were also tested for HTR in response to saline solution alone.
2.6. 8-OH-DPAT-induced hypothermia
Body temperature of the mice was measured using implantable programmable temperature transponders (IPTT-300, Bio Medic Data Systems, Inc., Seaford, DE) and probe (DAS-7007 transponder reader system, BMDS). The implantable electronic ID transponders were inserted by means of a needle assembly subcutaneously under brief isoflurane anesthesia at least 24-48 hours prior to conducting the experiment. On the day of the experiment, mice were injected subcutaneously with 2 mg/kg 8-OH-DPAT, as described previously (Veenstra-VanderWeele et al., 2012). Control animals were treated with 0.9% saline in equivalent volumes. The ambient room temperature was recorded for every experiment and ranged between 19.5 −21.5°C. Body temperature measurements were taken every 10 minutes for an hour post-injection, as well as 20 minutes prior to injection. Mice were also monitored at 2 and 4 hours post-injection to ensure a stable return to baseline body temperature.
2.7. Creation of cell lines stably expressing 5-HT1A and 5-HT2A receptors
Cloned human 5-HT1A and 5-HT2A receptors were stably expressed in HEK293 cells and grown under constant selective pressure using 100 μg/mL G418 as previously described (Chang et al., 2009; Cummings et al., 2010).
2.8. Brain slice preparation and biotinylation
This procedure was done as previously described (Siuta et al., 2010) with few modifications. Brain slices were prepared from 6- to 10-week-old mice. The brain was chilled in oxygenated ≈4°C sucrose solution, and while in sucrose solution, 300 μm coronal slices were made using a vibratome (Leica VT1000S). Slices were collected in oxygenated artificial cerebral spinal fluid (ACSF), washed twice with oxygenated 4°C ACSF, and then incubated with 4°C ACSF solution containing 1mg/mL of EZ-Link Sulfo-NHS-SSBiotin (Pierce Chemical; Rockford, IL) for 45min. The reaction was quenched with oxygenated 4°C ACSF containing glycine. Slices were frozen on dry ice and the cortex and hippocampus cut out. These specific brain regions were lysed in 1% Triton buffer and centrifuged at 17,000xg for 30min at 4°C. Biotinylated proteins were then isolated using Streptavidin Agarose Resin (Thermo Scientific) overnight at 4°C with agitation. Biotinylated proteins were eluted in 50 μL of 2X SDS-PAGE sample loading buffer at 95°C and then cooled to room temperature. Total slice lysates and the biotinylated (slice surface) fraction underwent immunodetection for 5-HT1A receptors.
2.9. Immunoblot analysis
Mice were rapidly decapitated for brain removal. Brains were chilled on ice and dissected under a microscope. Tissue was homogenized and lysed, and immunoblotting done as previously described (Siuta et al., 2010). Immunobloting of protein in the HEK-293 cell lines was done as previously described (Garcia et al., 2005). All Akt antibodies were obtained from Cell Signaling. Other antibodies included actin (Sigma), insulin receptor β (Santa Cruz), ERK1/2 (Promega), GSK-3β (Santa Cruz), NaK-ATPase (DBH), and TH (Millipore). Secondary antibodies were obtained from Santa Cruz Biotechnology.
2.10. Statistical analysis
All data are expressed as the mean ± S.E.M. Statistical significance between groups was determined using t-tests or one and two-way ANOVAs followed by post hoc tests when the main effect or interaction was significant at p < 0.05. Statistical analyses were conducted using software from Graph-Pad Prism5®.
3. RESULTS
3.1. Rictor deletion does not alter brain 5-HT content
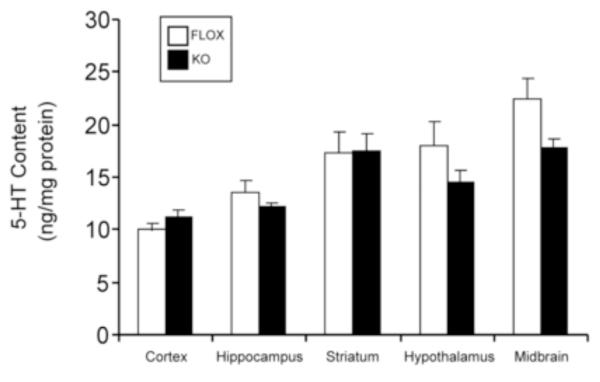
In order to explore how central Akt might regulate 5-HT receptor function and its related behaviors, we used our mouse model of defective neuronal Akt signaling in which Akt phosphorylation at Ser473 is ablated by Cre-LoxP-mediated and therefore, neuronal-specific deletion of the gene encoding the mTORC2 regulatory subunit, rictor (Siuta et al., 2010). Neuronal rictor deletion abolishes Akt Ser473, but not Thr308, phosphorylation within the cortex and substantia nigra/ventral tegmental area in these null (rictor KO) mice (Siuta et al., 2010). We have previously shown that in these rictor KO mice, brain norepinephrine and dopamine tissue content and homeostasis are altered (Siuta et al., 2010). We evaluated brain 5-HT content to determine whether Akt dysfunction leads to imbalance in 5-HT homeostasis. We found no significant changes in 5-HT content in the brain regions examined, including cortex, in the rictor KO mice compared to FLOX mice (Fig. 1). We also examined the turnover rate of 5-HT (5-HIAA/5-HT) and found no significant difference between rictor KO and FLOX mice (data not shown). To determine whether altered central insulin (Akt) signaling might influence peripheral insulin levels, we measured blood insulin levels in both phenotypes. There was no difference in insulin levels between FLOX and rictor KO mice (FLOX: 1.15 ± 0.23 ng/ml vs. KO: 1.07 ± .12 ng/ml; n=8-11, p>0.05 by Student’s t-test).
Figure 1.
5-HT content in brain. Tissue content of 5-HT in different brain regions. HPLC results are the mean ± SEM ng/mg of protein, n=4-15; p>0.05 by Student’s t test comparing FLOX against KO mice.
3.2. [3H]Citalopram binding shows no difference in FLOX vs. rictor KO mice in either cortex or midbrain
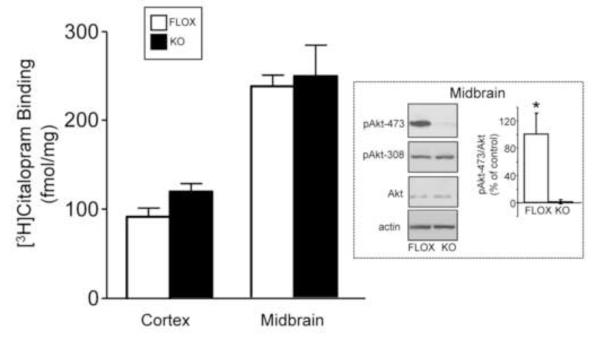
We previously observed changes in cortical norepinephrine transporter membrane expression in our rictor KO mice (Siuta et al., 2010). Here, we sought to evaluate the serotonin transporter (SERT) levels in the rictor KO mice in cortex as well as midbrain by radioligand binding. We first verified that phosphorylation of Akt at Ser473 (but not Thr308) is indeed absent in the midbrain of rictor KO mice (Fig. 2, inset), as has been shown for the cortex (Siuta et al., 2010). With respect to SERT levels, we found no significant difference in [3H]citalopram binding between the FLOX and rictor KO mice, in either cortex or midbrain (Fig. 2). Thus, the impaired insulin signaling of phosphorylation of Akt-473 does not alter central SERT expression.
Figure 2.
Neuronal rictor deletion does not alter SERT expression in cortex and midbrain. Homogenized membranes were incubated with [3H]Citalopram [3.8 nM] for 45 minutes at room temperature. Non-specific binding was determined using unlabeled Fluoxetine [10 μM]. Data are expressed as mean ± SEM, n=3-10; p > 0.05 by Student’s t test. Inset: Neuronal rictor deletion in midbrain ablates pAkt-473, but not pAkt-308 or Akt protein, as demonstrated by immunoblotting. pAkt-473 values in both FLOX and rictor KO midrains were quantified and normalized to Akt. n=5/genotype, *p < 0.05 by Student’s t test.
3.3. 5-HT1A and 5-HT2A receptor binding is increased in rictor KO mice
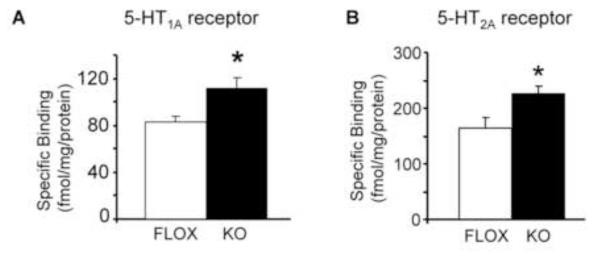
We next examined radioligand binding to 5-HT1A, 5-HT2A, and 5-HT2C receptors. We found a significant increase in radiolabel binding to 5-HT1A and 5-HT2A receptors in the cortex of KO mice compared to the FLOX mice (Fig. 3A,B), and no significant increase for the 5-HT2C receptor binding (data not shown). Elevated expression of these 5-HT receptor subtypes was specific, since expression of the unrelated insulin receptor β (IRβ), as measured by immunoblotting, was unaltered in KO mice compared to FLOX mice (expressed as a % of control: FLOX: 100.2 ± 9.6 % vs. KO: 107.8 ± 18.6 %; n=5-7; p>0.05 by Student’s t test).
Figure 3.
Cortical receptor binding of 5-HT1A and 5-HT2A receptors is significantly increased in KO mice. A. Homogenized membranes were incubated with [3H]mesulergine [10 nM] in the presence or absence of ritanserin [8 mM] to measure non-specific and total binding, respectively. Specific binding was calculated by subtracting non-specific binding from total binding for 5-HT1A receptors and corrected for bound protein (fmol/mg). Reaction buffer included the 5-HT1A/2A receptor antagonist spiperone [100 nM] and the 5-HT7R antagonist SB269970 [100 nM]. B. Homogenized membranes were incubated with [3H]8-OH-DPAT [10 nM] in the presence or absence of WAY100635 [10 μM] to measure non-specific and total binding for 5-HT2A receptor, respectively. Reaction buffer included the 5-HT7 receptor antagonist, SB269970 [100 nM]. Data are expressed as mean ± SEM, n=4-9/genotype, *p<0.05 by Student’s t test.
3.4. Neuronal rictor KO causes increased 5-HT1A receptor membrane expression
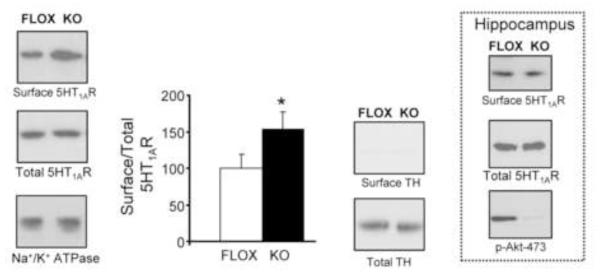
In figure 3, we estimated levels of 5-HT1A receptor by [3H]8-OH-DPAT binding. However, [3H]-8-OHDPAT is not without limitations, since it binds with high affinity only to those 5-HT1A receptors which are G protein-coupled (Emerit et al., 1990; Hall et al., 1985; Mongeau et al., 1992; Nenonene et al., 1994). Moreover, the ligand binding does not completely discriminate between surface and intracellular receptors. Thus, we examined whether the increased 5-HT1A receptor binding observed in KO mice is a consequence of enhanced receptor at the plasma membrane. In brain, protein cell surface redistribution can be assessed by brain slice biotinylation (Matthies et al., 2010; Robertson et al., 2010; Siuta et al., 2010). Using this biotinylation assay for the first time to quantitate 5-HT1A receptor levels, we observed a significant increase of the amount of surface 5-HT1A receptor levels in KO mice in cortical slices (surface) relative to controls (Fig. 4). This increase was not associated with an increase in total 5-HT1A receptor levels. Thus, the increase in functional receptor binding observed in figure 3A is due, largely, to increased receptor expression at the cortical cell surface. Interestingly, there was no significant increase in surface 5-HT1A receptor expression in hippocampal slices of the rictor KO mice (Fig. 4, inset: % of control FLOX: 100 ± 24% vs. KO: 71 ± 19%; n=4; p≥0.3 by Student’s t-test). These results underscore a more prominent role for Akt phosphorylation at Ser473 at cortical serotoninergic pathways.
Figure 4.
Neuronal rictor deletion results in increased cortical 5-HT1A receptor surface density. Levels of surface 5-HT1A receptor are obtained from the biotinylation fraction of cortical slices (surface). The optical density is shown for both FLOX (white bar) and rictor KO mice (black bar), where the surface optical density is normalized to its respective total. Representative immunoblots are shown, including a blot for Na+/K+ ATPase to serve as a plasma membrane/loading control, and tyrosine hydroxylase (TH) which is absent in the biotinylated fractions since it is a cytosolic protein. n=13/genotype, mean +/− S.E.M., *=p < 0.05 by Student’s t test. Inset: Hippocampal slices show no difference in surface 5-HT1A receptor expression between FLOX and rictor KO mice. n=6/genotype, p > 0.05 by Student’s t test.
3.5. Rictor KO mice display hypersensitivity to the 5-HT1A/7 receptor agonist, 8-OH-DPAT
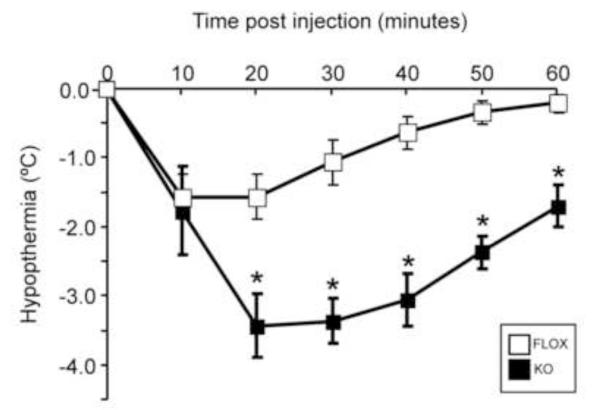
In an effort to explore the in vivo consequences of this increase in cortical 5-HT1A plasma membrane receptor, we conducted a hypothermia test with the 5-HT1A/7 receptor agonist, 8-OH-DPAT. In mice, 8-OH-DPAT-induced hypothermia is thought to be mediated by presynaptic 5-HT1A autoreceptors (Bill et al., 1991; Rusyniak et al., 2007). The dose of 2.0 mg/kg of 8-OH-DPAT was selected based on published data where the administered doses ranged from 0.1-0.25 mg/kg (Heisler et al., 1998; Veenstra-VanderWeele et al., 2012; Wozniak et al., 1991) to 2-5 mg/kg (Abbas et al., 2009; El Yacoubi et al., 2003). As seen in figure 5, we found that in the rictor KO mice, 8-OH-DPAT induced a significant increase in the hypothermic response compared to FLOX mice. Thus, this pronounced increase in presynaptic 5-HT1A receptor signaling/function is likely a consequence of increased 5-HT1A receptor expression (Fig. 3).
Figure 5.
Hypersensitivity to 8-OH-DPAT-induced hypothermia in rictor KO mice. Body temperature of the mice was measured using implantable programmable temperature transponders and scanning them with temperature probe. Mice received a s.c. injection of 2.0 mg/kg of the 5-HT1A/7 receptor agonist 8-OH-DPAT, which mediates a hypothermic response. Body temperature measurements were taken every 10 minutes for an hour post-injection, as well as 20 minutes prior to injection. Data are expressed as mean ± SEM, n=7/genotype; *=p<0.05 by two-way ANOVA, followed by Bonferroni post-hoc test.
3.6. DOI-induced head twitch response is attenuated in KO mice
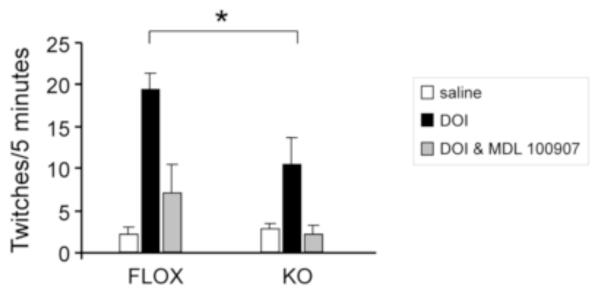
To explore the in vivo consequence of the increase in 5-HT2A binding, we evaluated head twitch response (HTR) induced by the hallucinogen 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI), a 5HT2A/2C agonist. The dose of 1.0 mg/kg of DOI was selected based on a literature review where the doses ranged from 1 mg/kg (Veenstra-VanderWeele et al., 2012) to 5-10 mg/kg (Abbas et al., 2009; Darmani et al., 1991), with 1 mg/kg being a standard dose (Canal et al., 2010). Despite the increase in 5-HT2A binding (Fig. 3B), the KO animals showed significantly decreased head twitches in response to DOI compared to the FLOX mice (Fig. 6). No differences between genotypes were seen after treatment with saline alone or after pre-treatment with the 5-HT2A antagonist M-100907 followed by DOI (Fig. 6).
Figure 6.
DOI-induced head twitch response is attenuated in KO mice. Mice received an i.p. injection of 1.0 mg/kg of DOI (black bars) or saline (white bars) 30 minutes before testing. Head twitches were recorded over three 5-minute periods, which were averaged together. For the studies done with the 5-HT2A receptor antagonist (gray bars), the same mice were injected with 0.25 mg/kg M-100907 17 minutes prior to the DOI injection, and the same procedure for counting head twitches was used. Data are expressed as mean ± SEM, n=5/genotype; *=p<0.05 by two-way ANOVA, followed by Bonferroni post-hoc test.
3.7. Cortical 5-HT2A receptor signaling is impaired in KO mice
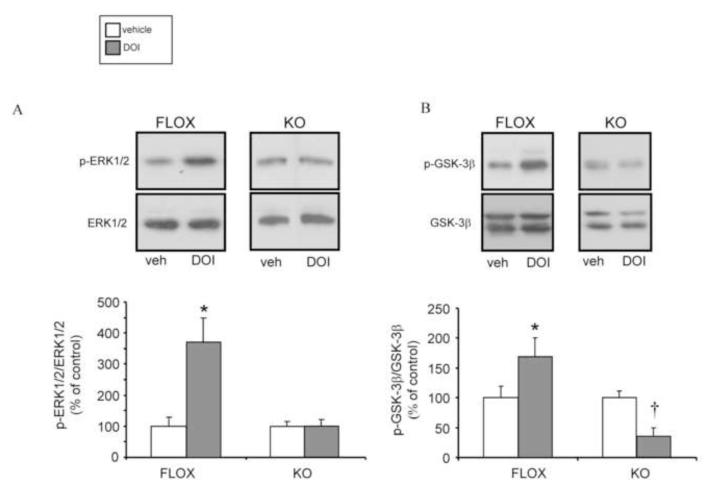
We observed an increase in total cortical 5-HT2A receptors in rictor KO mice associated with a decrease in DOI-induced HTR. Therefore, it is possible that in rictor KO mice, 5-HT2A receptor signaling is impaired. As shown by others, 5-HT2A receptor activation increases phosphorylation of ERK1/2 (Chang et al., 2009; Schmid et al., 2008; Watts, 1996) as well as of GSK-3 (Abbas et al., 2009; Beaulieu et al., 2009), although the latter has been somewhat controversial (Li et al., 2004). The GSK-3β phosphorylation is through activation of Akt. To test ERK1/2 and GSK-3 phosphorylation, the agonist DOI (5 mg/kg, IP) was administered in vivo (Abbas et al., 2009) and cortical tissue was collected for quantification of phosphorylation levels of ERK1/2 and GSK-3β. As expected in FLOX mice, DOI (gray bars) significantly increased p-ERK1/2 (Fig. 7A) and p-GSK-3β (Fig. 7B) with respect to vehicle (white bars). In contrast, there was no DOI-induced increase of cortical p-ERK1/2 (Fig. 7A) or p-GSK-3β (Fig. 7B) in rictor KO mice. Despite the basal levels of p-GSK-3β being comparable in FLOX vs. rictor KO mice, DOI decreased p-GSK-3β significantly to a level below vehicle control (Fig. 7B) in the mutant mice. These data demonstrate that the 5-HT2A receptor has an aberrant signaling response to agonist stimulation in the rictor KO animals.
Figure 7.
Cortical 5-HT2A receptor–mediated ERK1/2 and GSK-3β activation is impaired in rictor KO mice. Mice received an i.p. injection of 5.0 mg/kg of 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI, gray bars), a 5HT2A/2C agonist, or vehicle (white bars) for 15 minutes. Cortical microdissection was performed rapidly thereafter. Representative immunoblots for ERK1/2 (A) and GSK-3β (B) are shown. Quantification was done by normalizing the levels of kinase phosphorylation to the total kinase. Basal active and total levels of ERK1/2 and GSK-3β were the same in FLOX and KO mice. Data are expressed as mean ± SEM, n=6/genotype; *=p<0.05 by one-way ANOVA followed by Dunnett’s post-hoc test in FLOX mice; †= p<0.05 by one-way ANOVA followed by Dunnett’s post-hoc test in KO mice.
3.8. Inhibition of Akt in vitro attenuates 5HT2A receptor-mediated ERK1/2 phosphorylation
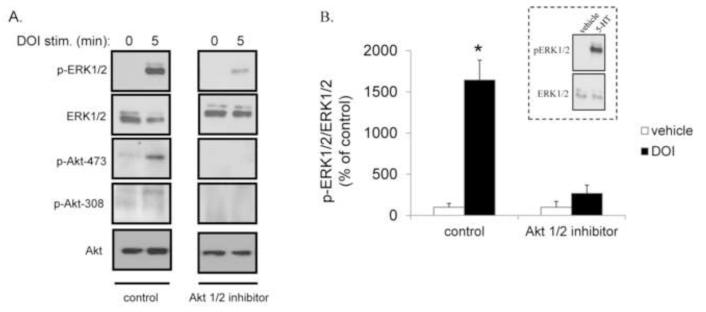
To define whether the decrease in Akt function alone in KO mice is responsible for the impaired agonist-mediated 5-HT2A receptor signaling (Fig. 7), we inhibited Akt pharmacologically in HEK-293 cells stably expressing the 5HT2A receptor. This was achieved with an allosteric Akt inhibitor specific for isoforms 1 and 2 (Akt1/2 inhibitor), which blocks phosphorylation of Akt (Lindsley et al., 2005; Speed et al., 2010). In figure 8A, we show that in cells treated with vehicle, DOI (5 min) induced an increase in p-ERK1/2. This increase was also associated with an increase in Akt phosphorylation (Fig. 8A). In contrast, in cells treated with the Akt1/2 inhibitor, there was a significant decrease in this DOI-mediated effect. The Akt inhibitor was effective in ablating the p-ERK1/2, pAkt-473 and pAkt-308 response, but did not alter the total levels of either ERK1/2 or Akt (Fig. 8A). Quantitation of Akt inhibition on p-ERK1/2 levels is shown in Fig. 8B. (Inset: The cells display a typical response to 5-HT, substantiating the viability of the cell line for 5-HT receptor signaling). These data suggest that Akt is directly influencing 5-HT2A receptor signaling.
Figure 8.
Akt1/2 inhibitor-mediated attenuation of DOI-induced ERK1/2 phosphorylation in HEK-293 cells expressing the 5-HT2A receptor. A. Representative immunoblots of p-ERK1/2, ERK1/2, pAkt-473, pAkt-308 and Akt in HEK-293 cells expressing 5-HT2A receptors. Cells were preincubated with an Akt1/2 inhibitor (5 μM) or vehicle for 60 minutes. DOI (10 μM)-induced ERK1/2 activation for 5 minutes was measured. B. Quantitation of the immunblots, expressed as percent of control (vehicle) stimulation at 5 minutes. Data are expressed as mean ± SEM, n=4, *p<0.05 by Student’s t test. Inset: 5-HT-mediated pERK1/2 activation (10 μM, 5 min) in HEK-293 cells expressing the 5-HT2A receptor.
3.9. Inhibition of Akt in vitro increases 5HT1A receptor expression
We next wanted to understand whether pharmacological inhibition of Akt activity alters 5HT1A receptor expression. HEK-293 cells stably expressing 5HT1A receptors were incubated (16 hr) with the Akt1/2 inhibitor (Lindsley et al., 2005) since 1 hr incubation was ineffective in causing changes in 5HT1A receptor expression (data not shown). Treatment with the Akt1/2 inhibitor (5 μM) caused a significant increase in the amount of detectable 5HT1A receptor in the immunoblots (Fig. 9A,B). These data are consistent with the increased receptor plasma membrane expression observed in the rictor KO cortices (Fig. 4).
Figure 9.
Akt1/2 inhibitor-mediated increase in 5-HT1A receptor expression. HEK-293 cells expressing 5-HT1A receptors were pretreated for 16 hours with the Akt1/2 inhibitor (5 μM) or vehicle. A. Representative immunoblots of 5-HT1A receptor and actin. B. Quantitation of the immunblots, expressed as % of control. Data are expressed as mean ± SEM, n=4, *p<0.05 by Student’s t test.
4. DISCUSSION
The global obesity epidemic correlates with a staggering increase in diabetes. In 2000, the WHO estimated that 2.2% of the total global population was diabetic and predicted a two-fold increase to 4.4% by 2030 (Wild et al., 2004). Notably, a key aspect of type 2 diabetes is its comorbidity with neuropsychiatric disorders including depression, schizophrenia, anxiety, and drug addiction (Niswender et al., 2011), as well as gestational diabetes contributing to offspring risk of neurodevelopmental disorders including autism and attention deficit hyperactivity disorder (Krakowiak et al., 2012; Lyall et al., 2012; Nomura et al., 2012). These brain disorders have been linked, at least in part, with dysregulated 5-HT neurocircuitry (Daws et al., 2011). The association between mental and metabolic disorders (e.g. diabetes) has been well established, but the underlying mechanism(s) for this link, which probably include genetic, environmental as well as behavioral factors, remain under investigation. Recently, we have shown in rodents that an obesogenic diet that promotes diabetes reduces central insulin signaling and impairs brain Akt function (Speed et al., 2011). Thus, considering the role played by Akt in diabetes and obesity as well as the role of 5-HT in neuropsychiatric disorders, we sought to investigate a possible role of Akt in controlling neuronal 5-HT signaling and homeostasis.
To probe the specific impact of Akt signaling dysfunction on the 5-HT system, we used neuronal deletion of the rictor protein, thereby ablating Akt phosphorylation at Ser473. The resulting rictor KO mice do not show any changes in the levels of 5-HT in any of the brain regions examined (Fig. 1). Furthermore, there were no significant changes in the turnover rate of brain 5-HT. Since promoter gene polymorphisms in the SERT have been described to be associated with type 2 diabetes, eating disorders, and obesity (Lan et al., 2009; Toperoff et al., 2012; Zhao et al., 2012), we examined the levels of SERT in our FLOX and rictor KO mice. They were not significantly different between the genotypes in either the midbrain or the cortex (Fig. 3).
In contrast to normal 5-HT levels and SERT binding, we show that 5-HT1A and 5-HT2A receptor binding are significantly elevated in the rictor KO mice (Fig. 3). The changes in cortical receptor expression are specific since the levels of 5-HT2C receptor and IRβ (data not shown) were unchanged. Experimentally-induced diabetes, which induces impairments in Akt function in brain (Robertson et al., 2010), has been shown to increase cortical 5-HT2A receptor density (Sumiyoshi et al., 1997). Moreover, diet-induced obesity has been linked to an increase in 5-HT1A and 5-HT2A receptor density in rats (Park et al., 1999). There is also evidence that diabetes leads to increased cortical 5-HT1A receptor levels in humans (Price et al., 2002). The mechanism for this observed increased receptor density is not readily apparent. In theory, exposure to decreased levels of cortical dopamine in these rictor KO mice (Siuta et al., 2010) could alter receptor expression, since dopamine has been shown to be a ligand for 5-HT1A and 5-HT2A receptors (Bhattacharyya et al., 2006; Oz et al., 2003), and causes internalization and recycling of the 5-HT2A receptor (Bhattacharyya et al., 2006). Taken together, our data suggest that neuronal Akt-473 phosphorylation can influence cortical 5-HT receptor expression.
It is noteworthy that SERT levels remain unaltered in the face of these changes in 5-HT receptor levels, a finding which does not preclude, however, the possibility of SERT/5-HT interactions. Studies of transporter/receptor interactions have increased considerably over the last decade (such as D1 dopamine and NMDA receptors mediating GABA transporter function in rat striatum (Schoffelmeer et al., 2000), as well as glycine transporter activity in rat hippocampus, and DAT and D2 dopamine receptors (Bertolino et al., 2009) or cannabinoid receptors (Schulze et al., 2012), as well as NET with the neurokinin-1 receptor (Arapulisamy et al., 2013)). Yet, those of specifically SERT/5-HT receptors are emerging slowly. Functional interactions between SERT and 5-HT receptors have been demonstrated in pulmonary arteries (Morecroft et al., 2005). Indirect interactions between the two have been studied in SERT-deficient mice, where the consequences of 5-HT receptor activation stem from increased availability of 5-HT in the SERT KO background (Fox et al., 2010). Centrally, SERT interactions with other G protein-coupled receptors, such as the A3 adenosine receptors in mouse midbrain serotonergic neurons and heterologous expression systems (Zhu et al., 2011), where SERT physically complexes with and functionally regulates the receptor. In humans, the interaction of SERT and striatal D2/D3 dopamine receptors has recently be hypothesized to contribute to the mechanism of harm avoidance in eating disorders (Bailer et al., 2013). In the future, therefore, it will be interesting to see whether central, direct SERT and 5-HT receptors interact not only physiologically, but also pathophysiologically as in models of diabetes or insulin signaling impairment.
The genetic neuronal deletion of RICTOR also leads to profound increase in cortical 5-HT1A receptor ligand binding. This increase appears to not be due to augmented synthesis of the receptor since total receptor expression of cortical 5-HT1A receptor is unaltered in KO mice (Fig. 4). The cortical slice biotinylation experiments reveal more receptor at the cell surface available for ligand binding, an effect that could be due to altered trafficking dynamics such as a slower rate of internalization of the 5-HT1A receptor. The increased cell surface receptor expression could explain the increased cortical radioligand binding observed in rictor KO mice. This is because [3H]-8-OH-DPAT binds with high affinity only to those 5-HT1A receptors which are G protein-coupled (Emerit et al., 1990; Hall et al., 1985; Mongeau et al., 1992; Nenonene et al., 1994). We find that ablation of Akt-473 phosphorylation alters the cortical distribution of the 5-HT1A receptor in the rictor KO mice, but not that in the hippocampus (Fig. 4). The reason for this increase in cell surface 5-HT1A receptor in the cortex is not readily obvious. Since central serotonergic imbalance or drugs that function centrally on serotonin levels such as SSRIs have been linked to hyperglycemia (Nonogaki et al., 1998; Yamada et al., 1999), insulin resistance (Chen et al., 2012) and diabetes (Lam and Heisler, 2007), our observed 5-HT1A receptor increase might represent a compensatory strategy to overcome the manifestations of the disease. In support of this hypothesis is the finding that SERT KO mice exhibit an attenuated insulin-induced increase of pAKT (Chen et al., 2012). Furthermore, a recent study showed that insulin-resistance rendered normal serotonin-dependent phosphorylation of Akt in the hypothalamus nonfunctional (Papazoglou et al., 2012). Thus, our data underscores the importance of an intact central insulin-serotonergic signaling axis under physiologic conditions.
The 5-HT1A receptor has important roles in behavior and physiology, including thermoregulation (Bert et al., 2006; Ramboz et al., 1998; Richardson-Jones et al., 2010; Richardson-Jones et al., 2011). We show that the increase in 5-HT1A surface expression is associated with a greater hypothermic response to 8-OH-DPAT in rictor KO mice (Fig. 5). These data are consistent with a transgenic mouse model of 5-HT1A receptor overexpression in the cortex that shows enhanced 8-OH-DPAT-induced hypothermia (Bert et al., 2006). Thus, our data suggests that dysregulated Akt phosphorylation disrupts cortical 5-HT receptor expression and function with significant physiological consequences.
Although expression of the 5-HT2A receptor is significantly increased in rictor KO mice, it is functionally impaired. Rictor KO mice fail to show the expected DOI-induced kinase activation (Schmid et al., 2008) after in vivo administration of DOI (Fig. 7), as evidenced by the lack of ERK1/2 as well as GSK-3β phosphorylation. Unfortunately, slice biotinylation studies of 5-HT2A receptor expression on the cell membrane are difficult due to the dearth of reliable antibodies. 5-HT2A receptors, mainly found in cortical layers II, III, V and VI, are the main site of action of LSD-like hallucinogens (Nichols, 2004). The functional consequence of this lack of signaling is the blunted HTR in the rictor KO mice (Fig. 6). These findings are in line with previous work in which experimentally-induced diabetes significantly reduced the DOI-mediated HTR (Li and France, 2008; Miyata et al., 2004), an alteration which could be restored by insulin replacement (Li and France, 2008). Together, these data suggest that the diminished behavioral response in the rictor KO mice is mediated by impaired 5-HT2A receptor signaling.
In order to mechanistically probe whether ablation of Akt phosphorylation at Ser473 itself in the rictor KO mice contributes to altered 5-HT receptor signaling, we assessed the consequences of Akt inhibition in a heterologous expression system. Here, we show that pretreatment with an Akt1/2 inhibitor significantly diminished DOI-induced 5-HT2A receptor-mediated ERK1/2 activation (Fig. 8). In mouse cortex, previous intriguing studies identify β-arrestin, Src and Akt as critical for the HTR to the agonist 5-HT (Gonzalez-Maeso et al., 2007; Schmid and Bohn, 2010) but not N-methyltryptamines or, presumably, DOI (Schmid et al., 2008). To our knowledge, our data are the first to demonstrate dependence of 5-HT2A receptor signaling on Akt phosphorylation, specifically at Ser473. While 5-HT-mediated 5-HT2A receptor signaling and behavior (HTR) are β-arrestin- and Akt-dependent, DOI-mediated 5-HT2A receptor signaling (Schmid et al., 2008) and behavior (Schmid and Bohn, 2010) are not. Thus, it is conceivable that in our studies, DOI could also partially be acting via the β-arrestin/Akt-dependent pathway, thereby explaining our observations of significant (albeit not complete) reduction of the HTR in rictor KO mice in response to DOI. We could hypothesize, then, that rictor KO mice are not able to signal (Fig. 7) or elicit HTR (Fig. 6) in response to 5-HT2A receptor activation because Akt is not functioning. Furthermore, if β-arrestin is also not functioning (by virtue of not forming the complex with Akt and Src (Schmid and Bohn, 2010)), this mechanism might explain why we observe more cortical 5-HT2A receptor on the cell surface, since β-arrestin has been reported to mediate many 5-HT induced downstream events at 5-HT2A receptors, including internalization (Abbas and Roth, 2008).
We also demonstrated that inhibition of Akt function stimulates an increase in 5-HT1A receptor density (Fig. 9). This increase is comparable to that observed in the cortexes of the rictor KO mice. No previous studies have demonstrated an effect of Akt on 5-HT1A expression or trafficking.
CONCLUSIONS
Our data pose that inhibition of phosphorylation of Akt is, at least in part, responsible for the upregulation and potential uncoupling of two 5-HT receptor subtypes, both in vivo and in vitro. Together, they suggest that the cross-talk between these two signaling pathways (insulin and 5-HT receptors) could contribute to the comorbidity of diseases driven by insulin resistance (e.g. diabetes) and disorders associated to central serotonergic disturbances, including depression and drug abuse.
HIGHLIGHTS.
Neuronal insulin regulates central monoamine homeostasis via down-stream kinase Akt.
Neuronal ablation of p-Akt at Ser473 increases cortical 5-HT1A and -2A receptors.
Elimination of p-Akt at Ser473 changes 5-HT receptor subtype signaling and behaviors.
We identified a novel link between Akt dysfunction (diabetes) and mental illness.
Acknowledgements
This work was supported by National Institutes of Health Grants P50 MH078028-Pilot Project (C.S.), MH063162 (J.A.S.), MH081066 (J.V.), and DK085712 (A.G. and K.D.N.). We thank Amanda Poe for assistance in genotyping and maintaining the mouse colonies. We are very grateful to the Conte Center Bioanalytical Core, specifically to Brett Begely, for his technical skills with the 5-HT receptor and transporter binding studies.
Abbreviations
- 5-HT
serotonin
- Akt
protein kinase B
- DOI
(±)-2,5-dimethoxy-4-iodoamphetamine hydrochloride
- ERK1/2
extracellular signal-regulated protein kinases 1 and 2
- GSK-3β
glycogen synthase kinase-3beta
- KO
knock out
- 8-OH-DPAT
[8-hydroxy-2-(di-n-propylamino)tetralin]
- HTR
head-twitch response
- SERT
serotonin transporter
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abbas A, Roth BL. Arresting serotonin. Proc Natl Acad Sci U S A. 2008;105:831–832. doi: 10.1073/pnas.0711335105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbas AI, Yadav PN, Yao WD, Arbuckle MI, Grant SG, Caron MG, Roth BL. PSD-95 is essential for hallucinogen and atypical antipsychotic drug actions at serotonin receptors. J Neurosci. 2009;29:7124–7136. doi: 10.1523/JNEUROSCI.1090-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arapulisamy O, Mannangatti P, Jayanthi LD. Regulated Norepinephrine Transporter Interaction with the Neurokinin-1 Receptor Establishes Transporter Subcellular Localization. J Biol Chem. 2013 doi: 10.1074/jbc.M113.472878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailer UF, Frank GK, Price JC, Meltzer CC, Becker C, Mathis CA, Wagner A, Barbarich-Marsteller NC, Bloss CS, Putnam K, Schork NJ, Gamst A, Kaye WH. Interaction between serotonin transporter and dopamine D2/D3 receptor radioligand measures is associated with harm avoidant symptoms in anorexia and bulimia nervosa. Psychiatry Res. 2013;211:160–168. doi: 10.1016/j.pscychresns.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR, Caron MG. Akt/GSK3 signaling in the action of psychotropic drugs. Annu Rev Pharmacol Toxicol. 2009;49:327–347. doi: 10.1146/annurev.pharmtox.011008.145634. [DOI] [PubMed] [Google Scholar]
- Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annu Rev Med. 2009;60:355–366. doi: 10.1146/annurev.med.60.042307.110802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bert B, Fink H, Hortnagl H, Veh RW, Davies B, Theuring F, Kusserow H. Mice over-expressing the 5-HT(1A) receptor in cortex and dentate gyrus display exaggerated locomotor and hypothermic response to 8-OH-DPAT. Behav Brain Res. 2006;167:328–341. doi: 10.1016/j.bbr.2005.09.020. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Fazio L, Di Giorgio A, Blasi G, Romano R, Taurisano P, Caforio G, Sinibaldi L, Ursini G, Popolizio T, Tirotta E, Papp A, Dallapiccola B, Borrelli E, Sadee W. Genetically determined interaction between the dopamine transporter and the D2 receptor on prefronto-striatal activity and volume in humans. J Neurosci. 2009;29:1224–1234. doi: 10.1523/JNEUROSCI.4858-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S, Raote I, Bhattacharya A, Miledi R, Panicker MM. Activation, internalization, and recycling of the serotonin 2A receptor by dopamine. Proc Natl Acad Sci U S A. 2006;103:15248–15253. doi: 10.1073/pnas.0606578103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bill DJ, Knight M, Forster EA, Fletcher A. Direct evidence for an important species difference in the mechanism of 8-OH-DPAT-induced hypothermia. Br J Pharmacol. 1991;103:1857–1864. doi: 10.1111/j.1476-5381.1991.tb12342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canal CE, Olaghere da Silva UB, Gresch PJ, Watt EE, Sanders-Bush E, Airey DC. The serotonin 2C receptor potently modulates the head-twitch response in mice induced by a phenethylamine hallucinogen. Psychopharmacology (Berl) 2010;209:163–174. doi: 10.1007/s00213-010-1784-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CW, Poteet E, Schetz JA, Gumus ZH, Weinstein H. Towards a quantitative representation of the cell signaling mechanisms of hallucinogens: measurement and mathematical modeling of 5-HT1A and 5-HT2A receptor-mediated ERK1/2 activation. Neuropharmacology. 2009;56(Suppl 1):213–225. doi: 10.1016/j.neuropharm.2008.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Margolis KJ, Gershon MD, Schwartz GJ, Sze JY. Reduced serotonin reuptake transporter (SERT) function causes insulin resistance and hepatic steatosis independent of food intake. PLoS One. 2012;7:e32511. doi: 10.1371/journal.pone.0032511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings DF, Canseco DC, Sheth P, Johnson JE, Schetz JA. Synthesis and structure-affinity relationships of novel small molecule natural product derivatives capable of discriminating between serotonin 5-HT1A, 5-HT2A, 5-HT2C receptor subtypes. Bioorg Med Chem. 2010;18:4783–4792. doi: 10.1016/j.bmc.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darmani NA, Martin BR, Pandey U, Glennon RA. Inhibition of 5-HT2 receptor-mediated head-twitch response by cocaine via indirect stimulation of adrenergic alpha 2 and serotonergic 5-HT1A receptors. Pharmacol Biochem Behav. 1991;38:353–357. doi: 10.1016/0091-3057(91)90290-i. [DOI] [PubMed] [Google Scholar]
- Daws LC, Avison MJ, Robertson SD, Niswender KD, Galli A, Saunders C. Insulin signaling and addiction. Neuropharmacology. 2011;61:1123–1128. doi: 10.1016/j.neuropharm.2011.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Yacoubi M, Bouali S, Popa D, Naudon L, Leroux-Nicollet I, Hamon M, Costentin J, Adrien J, Vaugeois JM. Behavioral, neurochemical, and electrophysiological characterization of a genetic mouse model of depression. Proc Natl Acad Sci U S A. 2003;100:6227–6232. doi: 10.1073/pnas.1034823100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerit MB, el Mestikawy S, Gozlan H, Rouot B, Hamon M. Physical evidence of the coupling of solubilized 5-HT1A binding sites with G regulatory proteins. Biochem Pharmacol. 1990;39:7–18. doi: 10.1016/0006-2952(90)90642-x. [DOI] [PubMed] [Google Scholar]
- Fox MA, Stein AR, French HT, Murphy DL. Functional interactions between 5-HT2A and presynaptic 5-HT1A receptor-based responses in mice genetically deficient in the serotonin 5-HT transporter (SERT) Br J Pharmacol. 2010;159:879–887. doi: 10.1111/j.1476-5381.2009.00578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia BG, Wei Y, Moron JA, Lin RZ, Javitch JA, Galli A. Akt is essential for insulin modulation of amphetamine-induced human dopamine transporter cell-surface redistribution. Mol Pharmacol. 2005;68:102–109. doi: 10.1124/mol.104.009092. [DOI] [PubMed] [Google Scholar]
- Gingrich JA, Hen R. Dissecting the role of the serotonin system in neuropsychiatric disorders using knockout mice. Psychopharmacology (Berl) 2001;155:1–10. doi: 10.1007/s002130000573. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R, Lira A, Bradley-Moore M, Ge Y, Zhou Q, Sealfon SC, Gingrich JA. Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron. 2007;53:439–452. doi: 10.1016/j.neuron.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Hall MD, el Mestikawy S, Emerit MB, Pichat L, Hamon M, Gozlan H. [3H]8-hydroxy-2-(di-n-propylamino)tetralin binding to pre- and postsynaptic 5-hydroxytryptamine sites in various regions of the rat brain. J Neurochem. 1985;44:1685–1696. doi: 10.1111/j.1471-4159.1985.tb07155.x. [DOI] [PubMed] [Google Scholar]
- Heisler LK, Chu HM, Brennan TJ, Danao JA, Bajwa P, Parsons LH, Tecott LH. Elevated anxiety and antidepressant-like responses in serotonin 5-HT1A receptor mutant mice. Proc Natl Acad Sci U S A. 1998;95:15049–15054. doi: 10.1073/pnas.95.25.15049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda M, Iwata N, Suzuki T, Kitajima T, Yamanouchi Y, Kinoshiya Y, Sekine Y, Iyo M, Harano M, Komiyama T, Yamada M, Sora I, Ujike H, Inada T, Ozaki N. Positive association of AKT1 haplotype to Japanese methamphetamine use disorder. Int J Neuropsychopharmacol. 2006;9:77–81. doi: 10.1017/S1461145705005481. [DOI] [PubMed] [Google Scholar]
- Jans LA, Riedel WJ, Markus CR, Blokland A. Serotonergic vulnerability and depression: assumptions, experimental evidence and implications. Mol Psychiatry. 2007;12:522–543. doi: 10.1038/sj.mp.4001920. [DOI] [PubMed] [Google Scholar]
- Krakowiak P, Walker CK, Bremer AA, Baker AS, Ozonoff S, Hansen RL, Hertz-Picciotto I. Maternal metabolic conditions and risk for autism and other neurodevelopmental disorders. Pediatrics. 2012;129:e1121–1128. doi: 10.1542/peds.2011-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam DD, Heisler LK. Serotonin and energy balance: molecular mechanisms and implications for type 2 diabetes. Expert Rev Mol Med. 2007;9:1–24. doi: 10.1017/S1462399407000245. [DOI] [PubMed] [Google Scholar]
- Lan MY, Chang YY, Chen WH, Kao YF, Lin HS, Liu JS. Serotonin transporter gene promoter polymorphism is associated with body mass index and obesity in non-elderly stroke patients. J Endocrinol Invest. 2009;32:119–122. doi: 10.1007/BF03345699. [DOI] [PubMed] [Google Scholar]
- Li JX, France CP. Food restriction and streptozotocin treatment decrease 5-HT1A and 5-HT2A receptor-mediated behavioral effects in rats. Behav Pharmacol. 2008;19:292–297. doi: 10.1097/FBP.0b013e328308f1d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zhu W, Roh MS, Friedman AB, Rosborough K, Jope RS. In vivo regulation of glycogen synthase kinase-3beta (GSK3beta) by serotonergic activity in mouse brain. Neuropsychopharmacology. 2004;29:1426–1431. doi: 10.1038/sj.npp.1300439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley CW, Zhao Z, Leister WH, Robinson RG, Barnett SF, Defeo-Jones D, Jones RE, Hartman GD, Huff JR, Huber HE, Duggan ME. Allosteric Akt (PKB) inhibitors: discovery and SAR of isozyme selective inhibitors. Bioorg Med Chem Lett. 2005;15:761–764. doi: 10.1016/j.bmcl.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Lustman PJ, Clouse RE. Depression in diabetic patients: the relationship between mood and glycemic control. J Diabetes Complications. 2005;19:113–122. doi: 10.1016/j.jdiacomp.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Lyall K, Pauls DL, Spiegelman D, Ascherio A, Santangelo SL. Pregnancy complications and obstetric suboptimality in association with autism spectrum disorders in children of the Nurses’ Health Study II. Autism Res. 2012;5:21–30. doi: 10.1002/aur.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthies HJ, Moore JL, Saunders C, Matthies DS, Lapierre LA, Goldenring JR, Blakely RD, Galli A. Rab11 supports amphetamine-stimulated norepinephrine transporter trafficking. J Neurosci. 2010;30:7863–7877. doi: 10.1523/JNEUROSCI.4574-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata S, Hirano S, Kamei J. Diabetes inhibits the DOI-induced head-twitch response in mice. Psychopharmacology (Berl) 2004;177:224–229. doi: 10.1007/s00213-004-1942-3. [DOI] [PubMed] [Google Scholar]
- Mongeau R, Welner SA, Quirion R, Suranyi-Cadotte BE. Further evidence for differential affinity states of the serotonin1A receptor in rat hippocampus. Brain Res. 1992;590:229–238. doi: 10.1016/0006-8993(92)91100-s. [DOI] [PubMed] [Google Scholar]
- Morecroft I, Loughlin L, Nilsen M, Colston J, Dempsie Y, Sheward J, Harmar A, MacLean MR. Functional interactions between 5-hydroxytryptamine receptors and the serotonin transporter in pulmonary arteries. J Pharmacol Exp Ther. 2005;313:539–548. doi: 10.1124/jpet.104.081182. [DOI] [PubMed] [Google Scholar]
- Nash JR, Sargent PA, Rabiner EA, Hood SD, Argyropoulos SV, Potokar JP, Grasby PM, Nutt DJ. Serotonin 5-HT1A receptor binding in people with panic disorder: positron emission tomography study. Br J Psychiatry. 2008;193:229–234. doi: 10.1192/bjp.bp.107.041186. [DOI] [PubMed] [Google Scholar]
- Nenonene EK, Radja F, Carli M, Grondin L, Reader TA. Heterogeneity of cortical and hippocampal 5-HT1A receptors: a reappraisal of homogenate binding with 8-[3H]hydroxydipropylaminotetralin. J Neurochem. 1994;62:1822–1834. doi: 10.1046/j.1471-4159.1994.62051822.x. [DOI] [PubMed] [Google Scholar]
- Nichols DE. Hallucinogens. Pharmacol Ther. 2004;101:131–181. doi: 10.1016/j.pharmthera.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Nicodemus KK, Law AJ, Radulescu E, Luna A, Kolachana B, Vakkalanka R, Rujescu D, Giegling I, Straub RE, McGee K, Gold B, Dean M, Muglia P, Callicott JH, Tan HY, Weinberger DR. Biological validation of increased schizophrenia risk with NRG1, ERBB4, and AKT1 epistasis via functional neuroimaging in healthy controls. Arch Gen Psychiatry. 2010;67:991–1001. doi: 10.1001/archgenpsychiatry.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswender KD, Daws LC, Avison MJ, Galli A. Insulin regulation of monoamine signaling: pathway to obesity. Neuropsychopharmacology. 2011;36:359–360. doi: 10.1038/npp.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura Y, Marks DJ, Grossman B, Yoon M, Loudon H, Stone J, Halperin JM. Exposure to gestational diabetes mellitus and low socioeconomic status: effects on neurocognitive development and risk of attention-deficit/hyperactivity disorder in offspring. Arch Pediatr Adolesc Med. 2012;166:337–343. doi: 10.1001/archpediatrics.2011.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonogaki K, Strack AM, Dallman MF, Tecott LH. Leptin-independent hyperphagia and type 2 diabetes in mice with a mutated serotonin 5-HT2C receptor gene. Nat Med. 1998;4:1152–1156. doi: 10.1038/2647. [DOI] [PubMed] [Google Scholar]
- Oz M, Zhang L, Rotondo A, Sun H, Morales M. Direct activation by dopamine of recombinant human 5-HT1A receptors: comparison with human 5-HT2C and 5-HT3 receptors. Synapse. 2003;50:303–313. doi: 10.1002/syn.10273. [DOI] [PubMed] [Google Scholar]
- Papazoglou I, Berthou F, Vicaire N, Rouch C, Markaki EM, Bailbe D, Portha B, Taouis M, Gerozissis K. Hypothalamic serotonin-insulin signaling cross-talk and alterations in a type 2 diabetic model. Mol Cell Endocrinol. 2012;350:136–144. doi: 10.1016/j.mce.2011.12.007. [DOI] [PubMed] [Google Scholar]
- Park S, Harrold JA, Widdowson PS, Williams G. Increased binding at 5-HT(1A), 5-HT(1B), and 5-HT(2A) receptors and 5-HT transporters in diet-induced obese rats. Brain Res. 1999;847:90–97. doi: 10.1016/s0006-8993(99)02055-7. [DOI] [PubMed] [Google Scholar]
- Price JC, Kelley DE, Ryan CM, Meltzer CC, Drevets WC, Mathis CA, Mazumdar S, Reynolds CF., 3rd Evidence of increased serotonin-1A receptor binding in type 2 diabetes: a positron emission tomography study. Brain Res. 2002;927:97–103. doi: 10.1016/s0006-8993(01)03297-8. [DOI] [PubMed] [Google Scholar]
- Ramboz S, Oosting R, Amara DA, Kung HF, Blier P, Mendelsohn M, Mann JJ, Brunner D, Hen R. Serotonin receptor 1A knockout: an animal model of anxiety-related disorder. Proc Natl Acad Sci U S A. 1998;95:14476–14481. doi: 10.1073/pnas.95.24.14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson-Jones JW, Craige CP, Guiard BP, Stephen A, Metzger KL, Kung HF, Gardier AM, Dranovsky A, David DJ, Beck SG, Hen R, Leonardo ED. 5-HT1A autoreceptor levels determine vulnerability to stress and response to antidepressants. Neuron. 2010;65:40–52. doi: 10.1016/j.neuron.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson-Jones JW, Craige CP, Nguyen TH, Kung HF, Gardier AM, Dranovsky A, David DJ, Guiard BP, Beck SG, Hen R, Leonardo ED. Serotonin-1A autoreceptors are necessary and sufficient for the normal formation of circuits underlying innate anxiety. J Neurosci. 2011;31:6008–6018. doi: 10.1523/JNEUROSCI.5836-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson SD, Matthies HJ, Owens WA, Sathananthan V, Christianson NS, Kennedy JP, Lindsley CW, Daws LC, Galli A. Insulin reveals Akt signaling as a novel regulator of norepinephrine transporter trafficking and norepinephrine homeostasis. J Neurosci. 2010;30:11305–11316. doi: 10.1523/JNEUROSCI.0126-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusyniak DE, Zaretskaia MV, Zaretsky DV, DiMicco JA. 3,4-Methylenedioxymethamphetamine- and 8-hydroxy-2-di-n-propylamino-tetralin-induced hypothermia: role and location of 5-hydroxytryptamine 1A receptors. J Pharmacol Exp Ther. 2007;323:477–487. doi: 10.1124/jpet.107.126169. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and Regulation of Akt/PKB by the Rictor-mTOR Complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- Schmid CL, Bohn LM. Serotonin, but not N-methyltryptamines, activates the serotonin 2A receptor via a ss-arrestin2/Src/Akt signaling complex in vivo. J Neurosci. 2010;30:13513–13524. doi: 10.1523/JNEUROSCI.1665-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid CL, Raehal KM, Bohn LM. Agonist-directed signaling of the serotonin 2A receptor depends on beta-arrestin-2 interactions in vivo. Proc Natl Acad Sci U S A. 2008;105:1079–1084. doi: 10.1073/pnas.0708862105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoffelmeer AN, Vanderschuren LJ, De Vries TJ, Hogenboom F, Wardeh G, Mulder AH. Synergistically interacting dopamine D1 and NMDA receptors mediate nonvesicular transporter-dependent GABA release from rat striatal medium spiny neurons. J Neurosci. 2000;20:3496–3503. doi: 10.1523/JNEUROSCI.20-09-03496.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze DR, Carroll FI, McMahon LR. Interactions between dopamine transporter and cannabinoid receptor ligands in rhesus monkeys. Psychopharmacology (Berl) 2012;222:425–438. doi: 10.1007/s00213-012-2661-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siuta MA, Robertson SD, Kocalis H, Saunders C, Gresch PJ, Khatri V, Shiota C, Kennedy JP, Lindsley CW, Daws LC, Polley DB, Veenstra-Vanderweele J, Stanwood GD, Magnuson MA, Niswender KD, Galli A. Dysregulation of the Norepinephrine Transporter Sustains Cortical Hypodopaminergia and Schizophrenia-Like Behaviors in Neuronal Rictor Null Mice. PLoS Biol. 2010;8:e1000393. doi: 10.1371/journal.pbio.1000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speed N, Saunders C, Davis AR, Owens WA, Matthies HJ, Saadat S, Kennedy JP, Vaughan RA, Neve RL, Lindsley CW, Russo SJ, Daws LC, Niswender KD, Galli A. Impaired striatal Akt signaling disrupts dopamine homeostasis and increases feeding. PLoS One. 2011;6:e25169. doi: 10.1371/journal.pone.0025169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speed NR, Matthies HJG, Kennedy JP, Vaughan RA, Javitch JA, Russo SJ, Lindsley CW, Niswender KD, Galli A. Akt-Dependent and Isoform-Specific Regulation of Dopamine Transporter Cell Surface Expression. American Chemical Society. 2010;1:476–481. doi: 10.1021/cn100031t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumiyoshi T, Ichikawa J, Meltzer HY. The effect of streptozotocin-induced diabetes on dopamine2, serotonin1A and serotonin2A receptors in the rat brain. Neuropsychopharmacology. 1997;16:183–190. doi: 10.1016/S0893-133X(96)00185-6. [DOI] [PubMed] [Google Scholar]
- Thiselton DL, Vladimirov VI, Kuo PH, McClay J, Wormley B, Fanous A, O’Neill FA, Walsh D, Van den Oord EJ, Kendler KS, Riley BP. AKT1 is associated with schizophrenia across multiple symptom dimensions in the Irish study of high density schizophrenia families. Biol Psychiatry. 2008;63:449–457. doi: 10.1016/j.biopsych.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toperoff G, Aran D, Kark JD, Rosenberg M, Dubnikov T, Nissan B, Wainstein J, Friedlander Y, Levy-Lahad E, Glaser B, Hellman A. Genome-wide survey reveals predisposing diabetes type 2-related DNA methylation variations in human peripheral blood. Hum Mol Genet. 2012;21:371–383. doi: 10.1093/hmg/ddr472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenstra-VanderWeele J, Muller CL, Iwamoto H, Sauer JE, Owens WA, Shah CR, Cohen J, Mannangatti P, Jessen T, Thompson BJ, Ye R, Kerr TM, Carneiro AM, Crawley JN, Sanders-Bush E, McMahon DG, Ramamoorthy S, Daws LC, Sutcliffe JS, Blakely RD. Autism gene variant causes hyperserotonemia, serotonin receptor hypersensitivity, social impairment and repetitive behavior. Proc Natl Acad Sci U S A. 2012;109:5469–5474. doi: 10.1073/pnas.1112345109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts SW. Serotonin activates the mitogen-activated protein kinase pathway in vascular smooth muscle: use of the mitogen-activated protein kinase kinase inhibitor PD098059. J Pharmacol Exp Ther. 1996;279:1541–1550. [PubMed] [Google Scholar]
- Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- Williams JM, Owens WA, Turner GH, Saunders C, Dipace C, Blakely RD, France CP, Gore JC, Daws LC, Avison MJ, Galli A. Hypoinsulinemia regulates amphetamine-induced reverse transport of dopamine. PLoS Biol. 2007;5:e274. doi: 10.1371/journal.pbio.0050274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak KM, Durcan MJ, Linnoila M. Is NAN-190 an effective antagonist of the hypothermia and hyperglycemia induced by the 5-HT1A receptor agonist, 8-OH-DPAT? Eur J Pharmacol. 1991;193:253–256. doi: 10.1016/0014-2999(91)90044-q. [DOI] [PubMed] [Google Scholar]
- Yamada J, Sugimoto Y, Inoue K. Selective serotonin reuptake inhibitors fluoxetine and fluvoxamine induce hyperglycemia by different mechanisms. Eur J Pharmacol. 1999;382:211–215. doi: 10.1016/s0014-2999(99)00593-2. [DOI] [PubMed] [Google Scholar]
- Zhao J, Goldberg J, Vaccarino V. Promoter methylation of serotonin transporter gene is associated with obesity measures: a monozygotic twin study. Int J Obes (Lond) 2012;37:140–145. doi: 10.1038/ijo.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu CB, Lindler KM, Campbell NG, Sutcliffe JS, Hewlett WA, Blakely RD. Colocalization and regulated physical association of presynaptic serotonin transporters with A(3) adenosine receptors. Mol Pharmacol. 2011;80:458–465. doi: 10.1124/mol.111.071399. [DOI] [PMC free article] [PubMed] [Google Scholar]