Abstract
BACKGROUND
Invasive cervical cancer is thought to decline in women over 65 years old, the age at which cessation of routine cervical cancer screening is recommended. However, national cervical cancer incidence rates do not account for the high prevalence of hysterectomy in the United States.
METHODS
Using estimates of hysterectomy prevalence from the Behavioral Risk Factor Surveillance System (BRFSS), hysterectomy-corrected age-standardized and age-specific incidence rates of cervical cancer were calculated from the Surveillance, Epidemiology, and End Results (SEER) 18 registry in the United States from 2000 to 2009. Trends in corrected cervical cancer incidence across age were analyzed using Joinpoint regression.
RESULTS
Unlike the relative decline in uncorrected rates, corrected rates continue to increase after age 35–39 (APCCORRECTED = 10.43) but at a slower rate than in 20–34 years (APCCORRECTED = 161.29). The highest corrected incidence was among 65- to 69-year-old women, with a rate of 27.4 cases per 100,000 women as opposed to the highest uncorrected rate of 15.6 cases per 100,000 aged 40 to 44 years. Correction for hysterectomy had the largest impact on older, black women given their high prevalence of hysterectomy.
CONCLUSIONS
Correction for hysterectomy resulted in higher age-specific cervical cancer incidence rates, a shift in the peak incidence to older women, and an increase in the disparity in cervical cancer incidence between black and white women. Given the high and nondeclining rate of cervical cancer in women over the age of 60 to 65 years, when women are eligible to exit screening, risk and screening guidelines for cervical cancer in older women may need to be reconsidered.
Keywords: cervical cancer, incidence, SEER, Behavioral Risk Factor Surveillance System, hysterectomy, disparities, age-specific, human papillomavirus
INTRODUCTION
National survey data suggest that a substantial fraction of women in the United States have undergone a hysterectomy, including the removal of the uterine cervix.1,2 However, national reporting of cervical cancer incidence rates does not remove the proportion of women who have had hysterectomies from the population at risk denominator,3 resulting in underestimation of the burden of cervical cancer. A prior study reported a substantial increase in age-standardized cervical cancer incidence rates after correcting for hysterectomy (73.1% for whites and 93.0% for blacks).2 Although hysterectomy prevalence varies significantly by both race and age, no studies have reported the impact of hysterectomy correction on the age-specific rates of cervical cancer.
We estimated the average annual trends in cervical cancer incidence by age from 2000 to 2009 in the United States before and after correction for age-, race-, year-, and state-specific prevalence of hysterectomy. In addition, corrected and uncorrected age-specific incidence rate estimates are presented for white, black, and women of other and mixed races.
MATERIALS AND METHODS
Data Sources and Database Linkage
Data on the incidence of cervical cancer were collected from the Surveillance, Epidemiology, and End Results (SEER) program of the National Cancer Institute. SEER compiles population-based cancer incidence and survival data from registries throughout the United States. Cervical cancer cases (specifically, site recode ICD-O-3 =cervix uteri, corresponding to C530-C539) were obtained from the SEER18 database, which comprises 18 registries representing approximately 28% of the US population (Connecticut; Hawaii; Iowa; New Mexico; Utah; California excluding San Francisco, San Jose-Monterey, and Los Angeles; Kentucky; Louisiana; New Jersey; San Francisco-Oakland Metropolitan Statistical Area, California; Metropolitan Detroit, Michigan; Seattle (Puget Sound), Washington; Metropolitan Atlanta, Georgia; San Jose-Monterey, California; Los Angeles, California; Alaska Natives; Rural Georgia; and Greater Georgia).4 Case counts (numerator, n) and the population at risk (denominator, p) were selected among women aged 20 years and older and stratified by age in 5-year intervals, year of diagnosis (2000–2009), state of registry (for California and Georgia, which both had more than one registry, data were combined) and race, without distinguishing Hispanic ethnicity (white, black or other/mixed). All data were accessed using SEER*Stat, version 8.0.2.
We generated survey weighted estimates of hysterectomy prevalence in the United States from the Behavioral Risk Factor Surveillance System (BRFSS).5 This ongoing household survey, conducted by the Center for Disease Control, collects data from adults aged 18 years or older on health risk behaviors and use of health services by random digit dialing throughout the United States. Hysterectomy status (removal of a woman’s uterus or womb) was collected every other year from 2000 to 2010. Thirteen BRFSS states directly correspond to the states of the SEER18 cancer registries. This analysis is thus restricted to the 13 states with both BRFSS hysterectomy prevalence and corresponding SEER registry data (Alaska, Louisiana, Kentucky, New Jersey, Georgia, Michigan, California, New Mexico, Hawaii, Iowa, Washington, Connecticut, Utah).
Consistent with the SEER18 cervical cancer incidence data, age was categorized into the same 5-year increments from 20 years to ≥85 years and race was categorized into white, black, or other/mixed. Hysterectomy prevalence was further stratified by year of diagnosis. Thus, in order to match SEER’s annual survey, the number of hysterectomies and total women at risk were averaged together from the years bracketing a missing year to give an estimate of annual hysterectomy prevalence. For example, data from 2000 and 2002 were averaged together to estimate data for 2001. Hysterectomy data from Hawaii were not collected in 2004 thus the average of 2002 and 2006 were used to fill-in estimates for 2003–2005. Because data from both SEER18 and BRFSS had the same restriction criteria and extracted data were stratified equivalently on age, state, year, and race, the 2 datasets could be directly merged to create the analytic database for the current project using SAS software, version 9.3.
Statistical Methods
The current analysis is limited to women aged 20 years and older. Age-, race-, year-, and state-specific hysterectomy prevalence estimates were used to correct the age-, race-, year-, and state-specific number of women at risk of cervical cancer by removing from the denominator the proportion of women no longer having a cervix. Specifically, for each strata, the corrected number at risk denominator is pc=p*(1-h), where pc is the population size corrected for hysterectomy, p is the census population, and h is the hysterectomy prevalence. The overall age-standardized rates of cervical cancer for women aged ≥20 years were calculated using the standard 2000 US census population for both the uncorrected denominator and the hysterectomy corrected denominator.6 Next, overall and race-stratified average annual age-specific cervical cancer incidence rates (IR) were calculated using the hysterectomy-corrected number at risk, IRc=(n/pc)* 100000. Corresponding 95% confidence intervals were calculated using the standard Bernoulli equation: . For comparison, uncorrected overall and race stratified incidence rates were also calculated using the same method but with the original, uncorrected denominators. SAS version 9.3 was used for the above computations and the figures were created in STATA version 11.
In addition to descriptive and graphical analysis, trends across age at cancer diagnosis were formally analyzed using log-linear joinpoint regression models, which fit a series of joined, straight lines to the trends in rates of cervical cancer across age. Similar to previous SEER-based studies focused on cancer incidence trends,3,7 this method was implemented using the National Cancer Institute’s Joinpoint Regression Program, version 4.0.1.8,9 Up to 4 change points were allowed in the models and the best model with regards to number and location of joinpoints was chosen using the Bayesian information criteria (BIC) method, which is based on both goodness of fit and a penalty for model complexity. These models estimate the percent change in cervical cancer incidence across 5-year age categories and the ages at which rates statistically change when using the uncorrected and corrected rate data in the population overall and stratified by race.
RESULTS
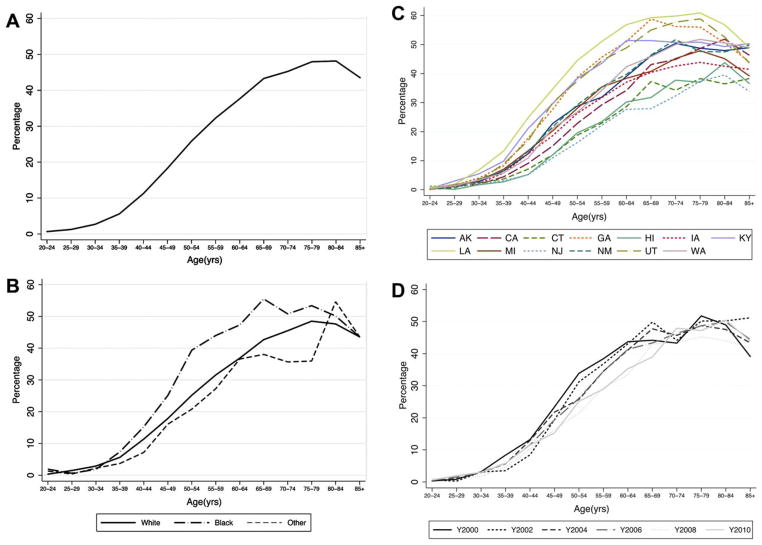
The prevalence of hysterectomy in the total population of women aged 20 and older was 20.1%. Prevalence was highest among women aged 75 to 84 years (Fig. 1A and B). From ages 35 to 75 years, prevalence was highest among black women, then white women, and lowest among other/mixed races, although the general pattern of increasing prevalence with age was similar in all race groups. Hysterectomy prevalence varied by state and was highest at all ages in Louisiana and generally lowest in New Jersey (Fig. 1C and D). Although cumulative hysterectomy prevalence by age 70 was similar across time, the change in slope of hysterectomy prevalence suggests that women in more recent time periods are having hysterectomy at older ages.
Figure 1.
Age-specific prevalence of hysterectomy in the United States from 2000 to 2010 (A) overall (B) by race (C) by state and (D) by year.
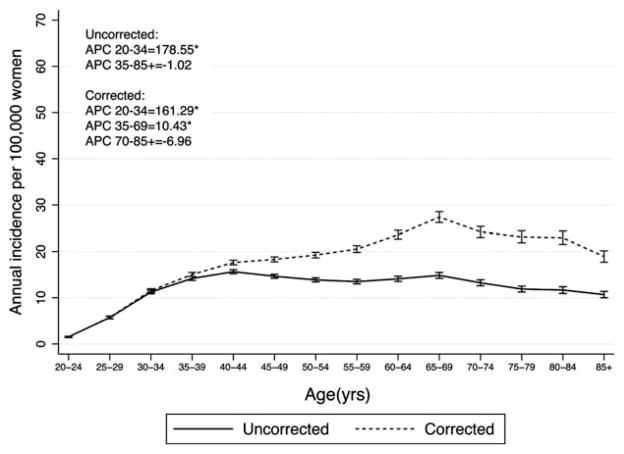
The average annual age-standardized rate of cervical cancer was 11.7 cases per 100,000 (95% confidence interval [CI] =11.5, 11.8). After correction for the prevalence of hysterectomy, the rate was 18.6 per 100,000 (95% CI =18.3, 18.9). Uncorrected incidence rates of cervical cancer increased up to, and plateaued at, age 40 to 44 years (15.6 per 100,000 women; Fig. 2). Corrected data indicate a steady increase in the incidence of cervical cancer up to ages 65–69 (27.4 per 100,000 women), after which the rates decrease slightly (Table 1). Joinpoint regression indicates that the rate of cervical cancer increases significantly across the ages of 20 to 34 years regardless of correction for hysterectomy (APCUNCORRECTED = 178.55, APCCORRECTED = 161.29) (Fig. 2). However, uncorrected rates remain stable from 35 to 85+ years of age (nonsignificant APCUNCORRECTED =−1.02), whereas after correction for hysterectomy rates continue to increase from age 35 to 69 years (APCCORRECTED = 10.43). In fact, the highest corrected incidence was observed among 65- to 69-year-old women, with a rate of 27.4 cases per 100,000 women as opposed to the highest uncorrected rate of 15.6 cases per 100,000 women occurring in 40- to 44-year-old women. The percent change in corrected versus uncorrected cervical cancer incidence increased steadily with age, up to 97% higher incidence after correction for hysterectomy among women aged 80 to 84 years.
Figure 2.
Age-specific incidence and 95% confidence interval of cervical cancer in SEER18 2000–2009. The asterisk indicates that the percent change by age category is significantly different from zero at alpha =0.05.
TABLE 1.
Uncorrected and Hysterectomy-Corrected Cervical Cancer Incidence Rate Estimates in the United States and the Percentage Change in Estimates After Correction for Prevalence of Hysterectomy
| Age Group, y |
Overall
|
White
|
Black
|
Other/Mixed
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Uncorrected Rate |
Corrected Rate |
% Change |
Uncorrected Rate |
Corrected Rate |
% Change |
Uncorrected Rate |
Corrected Rate |
% Change |
Uncorrected Rate |
Corrected Rate |
% Change |
|
| 20–24 | 1.5 (1.4, 1.7) | 1.5 (1.4, 1.7) | 0.6 | 1.5 (1.3, 1.7) | 1.5 (1.3, 1.7) | 0.4 | 1.8 (1.4, 2.2) | 1.8 (1.4, 2.2) | 1.6 | 1.3 (0.9, 1.7) | 1.3 (0.9, 1.7) | 1.1 |
| 25–29 | 5.7 (5.4, 6.0) | 5.8 (5.5, 6.0) | 1.1 | 6.2 (5.9, 6.5) | 6.3 (5.9, 6.6) | 1.3 | 5.3 (4.6, 6.0) | 5.3 (4.6, 6.1) | 0.6 | 3.3 (2.7, 3.9) | 3.3 (2.7, 3.9) | 0.2 |
| 30–34 | 11.2 (10.8, 11.6) | 11.5 (11.1, 11.9) | 2.8 | 12.2 (11.7, 12.6) | 12.5 (12.1, 13.0) | 3.1 | 9.7 (8.7, 10.7) | 9.9 (8.8, 10.9) | 1.8 | 7.4 (6.5, 8.3) | 7.6 (6.7, 8.5) | 2.3 |
| 35–39 | 14.2 (13.8, 14.6) | 15.0 (14.6, 15.5) | 6.0 | 15.1 (14.6, 15.6) | 16.0 (15.5, 16.5) | 6.0 | 12.8 (11.7, 13.9) | 13.7 (12.5, 14.9) | 7.1 | 9.9 (8.9, 11.0) | 10.4 (9.3, 11.5) | 4.8 |
| 40–44 | 15.6 (15.2, 16.1) | 17.6 (17.1, 18.1) | 12.7 | 15.8 (15.3, 16.3) | 17.8 (17.3, 18.4) | 12.8 | 16.6 (15.4, 17.9) | 19.3 (17.9, 20.8) | 16.3 | 13.3 (12.0, 14.5) | 14.3 (13.0, 15.6) | 7.8 |
| 45–49 | 14.7 (14.2, 15.1) | 18.3 (17.8, 18.8) | 24.9 | 14.1 (13.7, 14.6) | 17.5 (16.9, 18.1) | 23.7 | 16.7 (15.4, 18.0) | 23.1 (21.3, 24.9) | 38.2 | 16.1 (14.7, 17.5) | 19.3 (17.6, 21.0) | 19.9 |
| 50–54 | 13.9 (13.4, 14.3) | 19.2 (18.6, 19.8) | 38.6 | 13.3 (12.8, 13.8) | 18.2 (17.5, 18.8) | 36.6 | 17.3 (15.9, 18.8) | 29.8 (27.3, 32.3) | 72.1 | 14.5 (13.1, 15.9) | 18.2 (16.4, 19.9) | 25.7 |
| 55–59 | 13.5 (13.0, 14.0) | 20.5 (19.8, 21.3) | 52.1 | 12.7 (12.2, 13.2) | 19.1 (18.3, 19.9) | 50.4 | 18.3 (16.6, 20.0) | 33.1 (30.0, 36.1) | 81.1 | 14.8 (13.2, 16.4) | 20.7 (18.5, 22.9) | 39.8 |
| 60–64 | 14.1 (13.6, 14.7) | 23.6 (22.7, 24.5) | 67.3 | 12.5 (11.9, 13.1) | 20.6 (19.6, 21.6) | 64.4 | 23.2 (20.9, 25.4) | 46.9 (42.4, 51.4) | 102.4 | 17.7 (15.7, 19.7) | 28.5 (25.3, 31.8) | 61.1 |
| 65–69 | 14.8 (14.2, 15.5) | 27.4 (26.2, 28.6) | 84.4 | 13.5 (12.8, 14.2) | 24.7 (23.4, 25.9) | 82.6 | 23.5 (21.0, 26.0) | 53.0 (47.3, 58.8) | 125.8 | 17.0 (14.8, 19.2) | 28.3 (24.6, 32.0) | 66.3 |
| 70–74 | 13.2 (12.6, 13.9) | 24.2 (23.0, 25.4) | 82.6 | 11.8 (11.2, 12.5) | 21.8 (20.5, 23) | 83.9 | 21.3 (18.6, 24.0) | 44.4 (38.9, 50.0) | 108.7 | 17.7 (15.2, 20.1) | 27.1 (23.3, 30.8) | 53.3 |
| 75–79 | 11.9 (11.2, 12.5) | 23.2 (21.9, 24.4) | 94.9 | 10.1 (9.5, 10.8) | 20.0 (18.8, 21.3) | 98.0 | 24.0 (20.8, 27.2) | 52.2 (45.3, 59.0) | 117.4 | 17.5 (14.7, 20.2) | 26.8 (22.5, 31.0) | 53.2 |
| 80–84 | 11.7 (11.0, 12.4) | 22.9 (21.5, 24.4) | 96.6 | 9.9 (9.2, 10.6) | 19.3 (17.9, 20.6) | 94.6 | 26.5 (22.6, 30.5) | 48.3 (41.1, 55.5) | 82.2 | 17.3 (14.0, 20.6) | 43.2 (34.9, 51.4) | 150.2 |
| ≥85 | 10.7 (10.0, 11.4) | 18.9 (17.7, 20.2) | 76.9 | 8.9 (8.2, 9.5) | 15.6 (14.4, 16.8) | 76.1 | 29.4 (25.0, 33.8) | 57.8 (49.1, 66.4) | 96.5 | 16.0 (12.5, 19.5) | 27.1 (21.1, 33) | 69.0 |
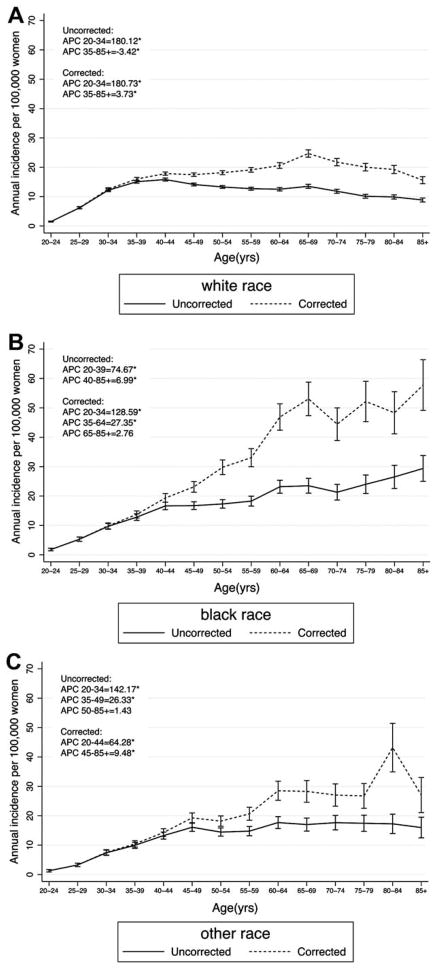
Absolute rates and age-specific patterns of cervical cancer incidence varied by race. Among white women, peak cervical cancer incidence occurred at 65 to 69 years of age (corrected rate 24.7 per 100,000), at which age the corrected rate was 83% greater than uncorrected rates (Table 1). Compared to the rapid increase in incidence with age from 20 to 34 years, uncorrected rates slowly decreased (APCUNCORRECTED =−3.42) whereas corrected rates increased with age from 35–85+ years (APCCORRECTED = 3.73; Fig. 3). Among black women, hysterectomy corrected incidence increased steadily with age up to 53.0 cases per 100,000 women at age 65 to 69 years, which was an increase of 126% compared to the uncorrected rates. The APC for uncorrected data ranged from 74.67 in 20- to 39-year-old women to 6.99 from 40 to ≥85 years. After correction for hysterectomy, the APC was 128.59 from 20 to 34 years, remained high at 27.35 from 35 to 64 years, and then stabilized (nonsignificant APC =2.76) from 65 to 85 years.
Figure 3.
Age-specific incidence and 95% confidence interval of cervical cancer in SEER18 2000–2009 among (A) white, (B) black and (C) other or mixed race individuals. The asterisk indicates that the percent change by age category is significantly different from zero at alpha =0.05.
DISCUSSION
Complete hysterectomy will effectively confer protection against future cervical cancer risk, analogous to the use of bilateral oophorectomy and mastectomy for prevention of ovarian and breast cancers, respectively. However, many reports of invasive cervical cancer (ICC) rates are based on a total census population, leaving the women with hysterectomy in the population at risk denominator. Using data from a large national survey of US women, we found that prevalence of hysterectomy was high, increased with increasing age, and varied substantially by race and state. Thus, failure to remove these women from the population at risk denominator when calculating ICC rates will result in substantial bias. In fact, we found that removing the proportion of women with hysterectomy from the population at risk denominator for ICC rates in 13 SEER states resulted in higher age-standardized and age-specific cervical cancer incidence rates, a shift in the peak incidence to older women, and an increase in the disparity in cervical cancer incidence between black and white women in the United States.
In the United States, cervical cancer screening guidelines recommend cessation of routine screening at age 65, depending on recent screening history or history of cervical intraepithelial neoplasia (CIN).10 The quality of the evidence guiding these recommendations was judged to be moderate to low, based largely on mathematical models,11 and ultimately guided by expert opinion regarding the balance of benefits and harms of screening at increasing ages. It was argued in this most recent guideline that the low rates of ICC in older women and the difficulty in screening postmenopausal women would result in detection of relatively few cases of high-grade CIN (CIN2+) and thus prevention of few additional invasive cervical cancers. It will be important to reconsider this viewpoint in light of our findings that rates of ICC in women over age 65 years are >80% higher than previously reported, peaking at 27.4/100,000 in the 65–69 year age group. Although recognizing the difficulties in screening after menopause, recent data from the United States show a clear protective effect of screening in older women,12 suggesting that the benefit to screening is retained at older ages. In fact, recent audits of organized cervical cancer screening in several European countries have reported a higher than expected cervical cancer incidence among women past the age of screening cessation.13,14
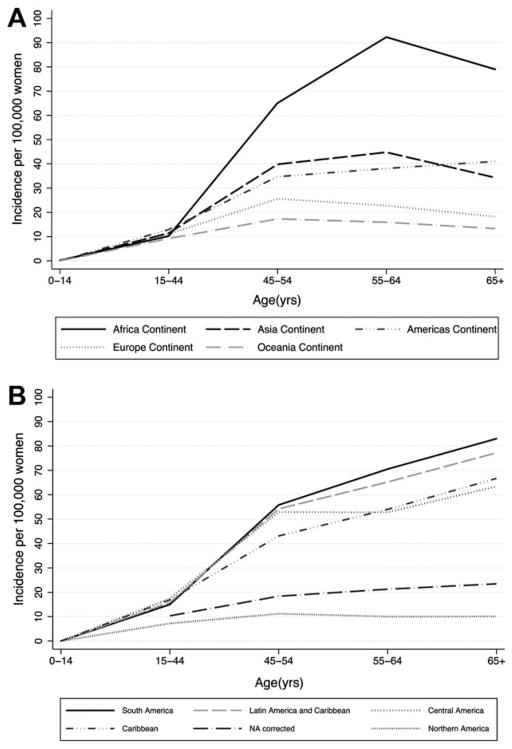
The plateau in ICC rates near the age of menopause that is observed in North America and Europe has been used to support a hypothesis that cervical cancer is “fueled” by estrogen. However, caution is warranted when extrapolating age-specific rates of disease to understanding the natural history of disease in an aging population. Our data suggest that the decline in cervical cancer incidence in the United States at older ages appears to be an artifact resulting from, at least in part, failure to correct for hysterectomy prevalence. Age-specific cervical cancer rates by world region also show an increase in incidence up through age 64 in Africa, a more stable increase after age 54 in the Americas, and a decline after age 54 in Asia, Oceania, and Europe15 (Fig. 4). Careful examination of the data from the Americas shows a continuous increase in cervical cancer incidence well past age 65 years in all regions except North America, where effective screening has prevented a substantial fraction of cervical cancers.16 Thus, these data may also contribute new information relevant to the potential role of aging in the natural history of cervical cancer.
Figure 4.
Age-specific ICC incidence (rates per 100,000 per year) from WHO/ICO (A) by world region and (B) by region of the Americas, with the SEER-based hysterectomy-corrected rates present for comparison, referred to as “NA corrected.”15,16
Although corrected rates were higher than uncorrected rates for all races at all ages, we found a higher than expected incidence among black women and a lower than expected incidence among women of other/mixed races compared to white women at nearly all ages after correcting for hysterectomy. The disparity between rates of cervical cancer in black and white women increased after correcting for hysterectomy, with an 89% higher rate of ICC in black than white women using age-standardized corrected rates (62% higher rate in blacks than whites using uncorrected data). Although declines in ICC rates in black US women over the past 30 years have been observed and there is no difference in screening rates between black (77.8%) and white (77.7%) women, disparities in incidence and survival rates remain and may be even greater than previously recognized, especially among older women.17,18
Using annual population-based estimates of hysterectomy prevalence, collected by asking a nationally representative sample of women in the BRFSS whether they have “ever had a hysterectomy,” there are important limitations that must be noted. First, we cannot distinguish the small number of women per year who had a hysterectomy because of cervical cancer and thus should not have been removed from the denominator at risk. However, the number of women diagnosed with cervical cancer each year is far fewer than the number reporting a hysterectomy so this likely had only a negligible over-estimating effect on the corrected estimates. Also, due to the use of self-report, hysterectomy data are subject to recall bias, which may differ by age and race, and may at least partially account for the relatively higher prevalence in older and African American women. Finally, BRFSS data did not distinguish between a full hysterectomy compared to a partial hysterectomy where the cervix is left intact and women remain at risk of developing ICC. However, it was previously estimated that <2% of all hysterectomies in the United States left the cervix intact.2 Therefore, this limitation likely had little effect on calculated rate estimates, which can be interpreted as ICC rates assuming all women reporting a hysterectomy were no longer at risk. Despite the limitations of using these existing data sources, this analysis provides corrected, nationally representative estimates of the average annual incidence of cervical cancer over a 10-year period.
In conclusion, failing to remove women who are not at risk of developing invasive cervical cancer due to hysterectomy not only underestimates the true incidence of cervical cancer but it results in misleading race and age-specific comparisons. The incidence of cervical cancer among all women with an intact cervix does not decline after menopause in the United States, but in fact continues to increase through at least age 69 years, an effect which is particularly pronounced in African American women. The current recommendations on age for cessation of routine cervical cancer screening might be re-evaluated in light of these new results. The higher rates of cervical cancer after correction for hysterectomy highlights the fact that although a large proportion of cervical cancer has been prevented through early detection and treatment, it remains a significant problem and further emphasizes the need for broad uptake of prophylactic HPV vaccination in the United States.3,19
Acknowledgments
FUNDING SOURCES
This work was supported by the US National Cancer Institute (R01 CA123467), the Institutional Research Cancer Epidemiology Fellowship funded by the National Cancer Institute (T32 CA0009314), and the Career Development Award for Bridging Interdisciplinary Research Careers in Women’s Health (K12 HD043489-12).
Footnotes
CONFLICT OF INTEREST DISCLOSURE
The authors made no disclosure.
References
- 1.Merrill RM. Hysterectomy surveillance in the United States, 1997 through 2005. Med Sci Monit. 2008;14:CR24–CR31. [PubMed] [Google Scholar]
- 2.Merrill RM. Impact of hysterectomy and bilateral oophorectomy on race-specific rates of corpus, cervical, and ovarian cancers in the United States. Ann Epidemiol. 2006;16:880–887. doi: 10.1016/j.annepidem.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Simard EP, Dorell C, et al. Annual Report to the Nation on the Status of Cancer, 1975–2009, featuring the burden and trends in human papillomavirus (HPV)-associated cancers and HPV vaccination coverage levels. J Natl Cancer Inst. 2013;105:175–201. doi: 10.1093/jnci/djs491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 18 Regs Research Data +Hurricane Katrina Impacted Louisiana Cases, Nov 2011 Sub(1973–2009 varying)-Linked to County Attributes - Total U.S., 1969–2010 Counties. Surveillance, Epidemiology, and End Results (SEER) Program, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2012, based on the November 2011 submission. Accessed February 22, 2013.
- 5.Behavioral Risk Factor Surveillance System Survey Data 2000, 2002, 2004, 2006, 2008, 2010. US Department of Health and Human Services, Centers for Disease Control and Prevention; [Accessed February 22, 2013]. [Google Scholar]
- 6.Selvin S. Statistical Analysis of Epidemiologic Data. 3. New York, NY: Oxford University Press; 2004. [Google Scholar]
- 7.Simard EP, Ward EM, Siegel R, Jemal A. Cancers with increasing incidence trends in the United States: 1999 through 2008. CA Cancer J Clin. 2012;62:118–128. doi: 10.3322/caac.20141. [DOI] [PubMed] [Google Scholar]
- 8.National Cancer Institute. Surveillance Research. [Accessed February 22, 2013];Joinpoint Regression Program. http://surveillance.cancer.gov/joinpoint/
- 9.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 10.Saslow D, Solomon D, Lawson HW, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62:147–172. doi: 10.3322/caac.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kulasingam SL, Havrilesky L, Ghebre R, Myers ER. Evidence Syntheses, No 86s. Rockville, MD: Agency for Healthcare Research and Quality; 2011. Screening for Cervical Cancer: A Decision Analysis for the U.S. Preventive Services Task Force. [PubMed] [Google Scholar]
- 12.Kamineni A, Weinmann S, Shy KK, Glass AG, Weiss NS. Efficacy of screening in preventing cervical cancer among older women. Cancer Causes Control. 2013;24:1653–1660. doi: 10.1007/s10552-013-0239-4. [DOI] [PubMed] [Google Scholar]
- 13.Pettersson BF, Hellman K, Vaziri R, Andersson S, Hellstrom AC. Cervical cancer in the screening era: who fell victim in spite of successful screening programs? J Gynecol Oncol. 2011;22:76–82. doi: 10.3802/jgo.2011.22.2.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rebolj M, van Ballegooijen M, Lynge E, et al. Incidence of cervical cancer after several negative smear results by age 50: prospective observational study. BMJ. 2009;338:b1354. doi: 10.1136/bmj.b1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization, WHO/ICO HPV Information Centre. [Accessed June 19, 2013];Human Papillomavirus and Related Cancers in World. Summary Report. 2009 www.hpvcentre.net.
- 16.World Health Organization. WHO/ICO HPV Information Centre. [Accessed June 19, 2013];Human Papillomavirus and Related Cancers in Americas. Summary Report. 2009 www.hpvcentre.net.
- 17.Simard EP, Naishadham D, Saslow D, Jemal A. Age-specific trends in black-white disparities in cervical cancer incidence in the United States: 1975–2009. Gynecol Oncol. 2012;127:611–615. doi: 10.1016/j.ygyno.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 18.DeSantis C, Naishadham D, Jemal A. Cancer statistics for African Americans, 2013. CA Cancer J Clin. 2013;63:155–166. doi: 10.3322/caac.21173. [DOI] [PubMed] [Google Scholar]
- 19.Laz TH, Rahman M, Berenson AB. Human papillomavirus vaccine uptake among 18- to 26-year-old women in the United States: National Health Interview Survey, 2010. Cancer. 2013;119:1386–1392. doi: 10.1002/cncr.27894. [DOI] [PMC free article] [PubMed] [Google Scholar]