Abstract
Ocular vascular occlusive disorders collectively constitute the most common cause of visual disability. Before a disease can be managed, it is essential to understand its natural history, so as to be able to assess the likely effectiveness of any intervention. I investigated natural history of visual outcome in prospective studies of 386 eyes with non-arteritic anterior ischemic optic neuropathy (NA-AION), 16 eyes with non-arteritic posterior ischemic optic neuropathy, 697 eyes with central retinal vein occlusion (CRVO), 67 eyes with hemi-CRVO (HCRVO), 216 eyes with branch retinal vein occlusion (BRVO), 260 eyes with central retinal artery occlusion (CRAO), 151 eyes with branch retinal artery occlusion (BRAO) and 61 eyes with cilioretinal artery occlusion (CLRAO). My studies have shown that every one of these disorders consists of multiple distinct clinical sub-categories with different visual findings. When an ocular vascular occlusive disorder is caused by giant cell arteritis, which is an ophthalmic emergency, it would be unethical to do a natural history study of visual outcome in them, because in this case early diagnosis and immediate, intensive high-dose steroid therapy is essential to prevent any further visual loss, not only in the involved eye but also in the fellow, normal eye.
In NA-AION in eyes seen ≤2 weeks after the onset, visual acuity (VA) improved in 41% of those with VA 20/70 or worse, and visual field (VF) improved in 26% of those with moderate to severe VF defect. In non-ischemic CRVO eyes with VA 20/70 or worse, VA improved in 47% and in ischemic CRVO in 23%; moderate to severe VF defect improved in 79% in non-ischemic CRVO and in 27% in ischemic CRVO. In HCRVO, overall findings demonstrated that initial VA and VF defect and the final visual outcome were different in non-ischemic from ischemic HCRVO – much better in the former than the latter. In major BRVO, in eyes with initial VA of 20/70 or worse, VA improved in 69%, and moderate to severe VF defect improved in 52%. In macular BRVO with 20/70 or worse initial VA, it improved in 53%, and initial minimal-mild VF defect was stable or improved in 85%. In various types of CRAO there are significant differences in both initial and final VA and VF defects. In CRAO eyes seen within 7 days of onset and initial VA of counting fingers or worse, VA improved in 82% with transient non-arteritic CRAO, 67% with non-arteritic CRAO with cilioretinal artery sparing, 22% with non-arteritic CRAO. Central VF improved in 39% of transient non-arteritic CRAO, 25% of non-arteritic CRAO with cilioretinal artery sparing and 21% of non-arteritic CRAO. Peripheral VF improved in non-arteritic CRAO in 39% and in transient non-arteritic CRAO in 39%. In transient CRAO, finally peripheral VFs were normal in 93%. In non-arteritic CRAO eyes initially 22% had normal peripheral VF and in the rest it improved in 39%. Final VA of 20/40 or better was seen in 89% of permanent BRAO, and in 100% of transient BRAO and non-arteritic CLRAO. In permanent BRAO eyes, among those seen within 7 days of onset, central VF defect improved in 47% and peripheral VF in 52%, and in transient BRAO central and peripheral VFs were normal at follow-up.
My studies showed that AION, CRVO, BRVO, CRAO and BRAO, each consist of multiple distinct clinical sub-categories with different visual outcome. Contrary to the prevalent impression, these studies on the natural history of visual outcome have shown that there is a statistically significant spontaneous visual improvement in each category. The factors which influence the visual outcome in various ocular vascular occlusive disorders are discussed.
Keywords: Branch retinal vein occlusion, Central retinal artery occlusion, Central retinal vein occlusion, Non-arteritic anterior ischemic optic neuropathy
1. Introduction
Ocular vascular occlusive disorders collectively constitute the most common cause of visual disability in the middle-aged and elderly population, although no age is immune. For their management, the most important piece of information required, from the points of view of both patient and ophthalmologist, is the natural history of visual outcome. This is because information on the natural history of a disease is vital to determine if any treatment modality advocated for these diseases is really beneficial or not. The gold standard is to compare the outcome of treatment with the natural history of the disease. The importance of information about natural history of visual outcome is very well illustrated by the following example. A study of optic nerve sheath decompression for treatment of non-arteritic anterior ischemic optic neuropathy claimed to improve visual loss in this disease – “a disorder without any previously effective therapy” (Sergott et al., 1989). It was considered so important that it was published on an expedited basis by the Archives of Ophthalmology and the procedure became widely popular, till a multicenter clinical trial (Ischemic Optic Neuropathy Decompression Trial, 1995.) showed that it was actually harmful; eyes that had the procedure suffered significantly greater (24%) loss of vision than those left alone (12%). This clinical trial also showed that 32.6% of those who had optic nerve sheath decompression had visual improvement compared with 42.7% of the untreated group. This study concluded that this procedure is “not an appropriate treatment for non-arteritic anterior ischemic optic neuropathy”.
As regards ocular vascular occlusive disorders, in spite of a huge volume of literature that has accumulated on their various aspects over almost 150 years, information on the natural history of their visual outcome is scanty, and when available, it is based on retrospective evaluation, usually of a small number of eyes and often from mixed groups of these disorders (see below). Moreover, the information about visual improvement or deterioration in these studies is not only mostly based on visual acuity (VA) alone but also contradictory and confusing.
I investigated the natural history of visual outcome (of both VA and visual fields) in all these disorders, by prospective studies of large cohorts of patients (Table 1), seen in my Ocular Vascular Clinic at the University of Iowa Hospitals and Clinics in Iowa City since 1973.
Table 1.
Total number of eyes in different types of ocular vascular occlusive disorders in my studies about their natural history of visual outcome.
| Major categories | Sub-types | Number of eyes |
|---|---|---|
| Anterior ischemic optic neuropathy (AION) |
Non-arteritic AION | 386 |
| Central retinal vein occlusion (CRVO) |
Non-ischemic CRVO | 588 |
| Ischemic CRVO | 109 | |
| Hemi-central retinal vein occlusion (HCRVO) |
Non-ischemic HCRVO | 57 |
| Ischemic HCRVO | 10 | |
| Branch retinal vein occlusion (BRVO) |
Major BRVO | 144 |
| Macular BRVO | 72 | |
| Central retinal artery occlusion (CRAO) |
Non-arteritic CRAO | 171 |
| Transient CRAO | 41 | |
| CRAO with cilioretinal artery sparing | 35 | |
| Arteritic CRAO | 13 | |
| Branch retinal artery occlusion (BRAO) |
Permanent BRAO | 133 |
| Transient BRAO | 18 | |
| Cilioretinal artery occlusion | 61 |
2. Classification of ocular vascular occlusive disorders
To understand visual outcome in ocular vascular occlusive disorders, it is essential to classify these disorders into appropriate categories to get reliable information. My studies have shown that these disorders need to be classified into the following groups and subgroups, as will become evident from the following discussion.
2.1. Ischemic optic neuropathy
This is of two types: (a) anterior, and (b) posterior ischemic optic neuropathy. Anterior ischemic optic neuropathy is further of two types: (a) arteritic and (b) non-arteritic anterior ischemic optic neuropathy.
2.2. Central retinal vein occlusion (CRVO)
This is of two types: (a) non-ischemic and (b) ischemic.
2.3. Hemi-central retinal vein occlusion (HCRVO)
This, similarly is of two types: (a) non-ischemic and (b) ischemic.
2.4. Branch retinal vein occlusion (BRVO)
This is also of two types: (a) major BRVO and (b) macular BRVO.
2.5. Central retinal artery occlusion (CRAO)
This is of four types: (a) arteritic CRAO due to giant cell arteritis, (b) non-arteritic CRAO, (c) transient CRAO, and (d) CRAO with cilioretinal artery sparing.
2.6. Branch retinal artery occlusion (BRAO)
This is of two types: (a) permanent BRAO, (b) transient BRAO.
2.7. Cilioretinal artery occlusion
In the literature, unfortunately, retinal vein and artery occlusions are not classified as above, and the result is misleading information, because the natural history of visual outcome is different in different types, as discussed below.
3. Visual evaluation in my studies
In all my prospective studies dealing with the natural history of visual outcome in various ocular vascular occlusive disorders, visual evaluation involved recording best corrected VA, using the Snellen VA chart, and visual fields with a Goldmann perimeter (using I-2e, I-4e and V-4e targets) in all patients. Amsler grid chart was also used for evaluating central visual field defects; it provides useful information in the evaluation of visual function when the macula is involved. For example, when perimetry shows no defect but the VA is abnormal, the Amsler grid chart commonly shows metamorphopsia.
VA and visual fields were evaluated initially and at each follow-up visit. A change of at least 3 lines in the Snellen VA chart was considered a significant change, in either direction (i.e. improved or deteriorated), which is equivalent to a logMAR change of at least 0.30. At the same time, change in visual field loss was also examined; a difference in grade of at least 0.5, in either direction, was defined as improvement or deterioration (For grading of visual fields see Hayreh and Zimmerman, 2008).
Importantly, in most other studies, visual outcome was evaluated only from the VA. But it is well-established that VA gives information basically about the function of only the foveal retina and the papillomacular nerve fibers in the optic nerve, and not of the entire retina or the optic nerve. Thus, testing of VA alone does not provide information about the visual status in the entire retina or optic nerve. Currently, visual fields are usually plotted using automated perimetry. Visual field information provided by manual kinetic perimetry (performed with a Goldmann perimeter) is very different from that by automated static threshold perimetry (Humphrey 30-2 or 24-2 SITA). Automated perimetry provides information about peripheral visual fields only up to 24°–30°. Manual kinetic perimetry, by contrast, provides information all the way to about 80°–90° temporally, 70° inferiorly, 60°–70° nasally and 50°–60° superiorly. Thus, a visual field plotted with manual kinetic perimetry gives far more comprehensive information about the peripheral visual field defects, for evaluating visual functional disability. I have discussed at length the clinical significance of central versus peripheral visual field loss in NA-AION (Hayreh and Zimmerman, 2005a). The constant tracking provided by the peripheral visual fields is essential for sensory input to our day-to-day activity, and navigation in the world; the peripheral visual fields are vital for routine activities, except for what requires fine VA. Full information about the peripheral fields is provided only by manual kinetic perimetry performed with a Goldman perimeter; it is most unfortunate that it is rapidly being replaced by automated perimetry.
Thus, VA and visual fields provide very different information about the visual status of an eye, and the two can be totally independent of each other. For example, if an eye has a massive visual field loss but central fixation is spared, the VA is normal in spite of complete loss of one half or more of the visual field in the eye.
4. Natural history of visual outcome in non-arteritic anterior ischemic optic neuropathy
Non-arteritic anterior ischemic optic neuropathy (NA-AION) is one of the most widespread visually disabling diseases in the middle-aged and elderly population, (although no age is immune) (Hayreh et al., 1994a). Information on the natural history of visual outcome is scanty, and when available, it is mostly based on retrospective evaluation. Repka et al. (1983) in 92 eyes with NAION, after a mean follow-up period of five years, found no improvement or deterioration in VA or visual fields. Sawle et al. (1990) in 63 eyes, on mean follow up of 5.3 years, found VA deterioration in 4 and improvement in 7 by more than one line. Arnold and Hepler (1994) in 27 untreated NA-AION eyes, seen within 30 days after onset, found significant worsening in VA in 11% and visual field in 22%; improvement in 24% for VA and 24% for visual field. Of 21 “stable” patients, none worsened for VA and 5% showed late worsening of visual field; 31% showed significant improvement for VA and 32% for visual field.
There is only one reported prospective study on the natural history of visual outcome in NA-AION, which was done as a part of the randomized optic nerve sheath decompression multicenter trial (IONDT) (Ischemic Optic Neuropathy Decompression Trial, 1995, 2000). In this study, 43% of 95 untreated NA-AION patients, seen within 2 weeks of onset, with VA of 20/64 or worse and followed for 6 months, showed VA improvement.
4.1. Our study
Hayreh and Zimmerman (2008) investigated the natural history of visual outcome prospectively in 340 consecutive untreated patients (386 eyes) with NA-AION, seen in my Ocular Vascular Clinic at the University of Iowa Hospitals and Clinics. They were first seen ≤2 weeks after onset. In all patients, changes in VA and visual field defect were assessed from initial visit to optic disc edema resolution, from optic disc edema resolution to 3 months, 9 months, and 2 years after resolution of optic disc edema, and also for the overall follow-up at 3, 6, 12, and 24 months from initial visit.
The findings of this study are discussed at length elsewhere (Hayreh and Zimmerman, 2008). Following is a brief account.
4.1.1. VA and visual fields at initial visit
At the initial visit, in eyes seen ≤2 weeks after the onset of symptoms, 49% had VA of 20/30 or better and 23% had 20/200 or worse; in these eyes, 38% had minimal to mild visual field defect and 43% marked to severe defect.
4.1.2. VA and visual fields during follow-up
Eyes first seen ≤2 weeks after onset, with VA 20/70 or worse, showed spontaneous VA improvement in 41% at 6 months and in 42% at one year after the initial visit; at 6 months, these eyes showed worsening of VA in 19% and none after that. In eyes with initial VA between 20/40 and 20/60, at 6 months 17% showed improvement and 10% deterioration. Two years after the initial visit, in eyes with initial VA of 20/60 or better, there was deterioration in 9%, and in those with initial VA of 20/70 or worse 18% showed deterioration.
In the eyes first seen ≤2 weeks after onset with moderate to severe visual field defect, there was improvement in 26% at 6 months and 27% at one year after the initial visit. Two years after the initial visit, 27% of eyes with initial minimal to mild field defects showed worsening, as did 19% of those with moderate to severe defects.
In this study, patients who were first seen more than 2 weeks (3 weeks to about 10 weeks) after the onset of visual loss and still had optic disc edema, showed both less improvement and less deterioration in VA and visual fields than those first seen within 2 weeks of onset. Obviously, the longer the time interval between onset and first evaluation, the greater the likelihood of permanent visual changes having occurred already, and, hence, the smaller the chance of change in visual status from then on.
I found that the visual change does not always mean consistent improvement or deterioration throughout the entire course of follow-up, but may constitute a changing pattern; for example in some eyes there was improvement/deterioration at one time and vice versa at another time, in the same eye. The findings in this study represent the initial and final visual status only.
Thus, this study showed that VA and visual fields showed improvement or further deterioration mainly up to 6 months after onset, with no significant change after that in untreated eyes.
4.1.3. Association of demographic and systemic conditions with visual outcome
This was examined at 1 year from initial visit in those seen within 2 weeks of onset of NA-AION, with initial VA of 20/40 or worse. After adjusting for the effect of initial VA, VA change at 1 year did not show a significant association with gender (p = 0.44), age at diagnosis (p = 0.35), smoking (p = 0.53), diabetes mellitus (p = 0.35), arterial hypertension (p = 0.38), ischemic heart disease (p = 0.91), hyperlipidemia (p = 0.61), or migraine (p = 0.21). For visual field change at 1 year, except for migraine, where the statistical test suggested a possible association (worsening in 57% with migraine vs. 20% without; p = 0.09), no significant association was observed in the other variables (all p > 0.42).
4.1.4. Comparison between our prospective study and IONDT prospective study
In the IONDT, as well as in most other studies, visual outcome was evaluated only from VA. As mentioned above, VA gives information basically about the function of only the fovea and the papillomacular nerve fibers in the optic nerve, and not of the entire optic nerve. NA-AION may involve the entire optic nerve head or only one part of it; in some cases the papillomacular nerve fibers may not be involved at all, which explains the presence of normal VA in many eyes with NA-AION (see above). Information about the function of the entire optic nerve is provided only by the visual fields. As previously mentioned, VA and visual fields provide very different information about the visual status of an eye, and the two can be totally independent of each other. I have seen eyes with NAAION where only the central 5° to 10° visual field is left, but the VA was 20/15 to 20/20. Therefore, to assess visual outcome in NA-AION, one needs information on both VA and the entire peripheral visual field.
The outcome of a study depends upon its design. IONDT study’s primary objective was to “assess the safety and efficacy of optic nerve decompression surgery compared with careful follow-up alone” in patients with NA-AION. By contrast, the primary objective of our study (Hayreh and Zimmerman, 2008.) was to determine the natural history of visual outcome in NAAION. The study design, inclusion and exclusion criteria and several other parameters differ between the two studies. For example, in the IONDT study, to be eligible for inclusion in the study, the VA must have been 20/64 or worse, age 50 years or older, and duration of symptoms less than 14 days; therefore, it contained a very select group of NA-AION patients. In our study, by contrast, there were no such inclusion/exclusion criteria, because we wanted to determine the natural history of visual outcome in all patients with NA-AION, irrespective of visual acuity, age or duration of visual loss. The IONDT study again, was based only on VA change, but our study was based on change in both VA and visual fields. Thus, there are fundamental differences in the designs of the two studies which explain some of their differences in the results. However, we did compare the VA outcome in the IONDT study with our study, by using only those eyes which had an initial VA of 20/70 or worse, and were seen within 2 weeks of the onset of visual loss, matching the IONDT study. Most interestingly, this comparison showed that, in spite of the age difference between the two studies, the VA outcomes were identical at follow-up of 6 months and 12 months. Both studies showed that VA changed up to 6 months, with no appreciable change after that.
4.1.5. NA-AION and normal VA
Our study (Hayreh and Zimmerman, 2008) showed that about half of the eyes with NA-AION presented with almost normal VA (20/15 to 20/30) at the initial visit – a fact not fully appreciated in the ophthalmic community and sometimes responsible for missing the diagnosis of NA-AION, because of the prevalent belief that eyes with NA-AION cannot have normal VA. Thus, the presence of normal VA does not rule out NA-AION. By contrast, all eyes with classical NAAION do have a visual field loss of variable severity.
4.1.6. Development of amblyopia in first eye with poor VA due to NA-AION
In NA-AION and other ocular vascular disorders studied in my Ocular Vascular Clinic, I have found that sometimes the first eye with poor VA may develop a variable degree of amblyopia, even in middle aged and elderly patients, because the patient may not use that involved eye for central vision when the fellow eye has normal VA (a phenomenon similar to occlusion amblyopia in children). In my studies on NA-AION, I have found that in some patients with bilateral NA-AION, when the second eye developed NA-AION with marked deterioration of VA, then the previously involved eye with comparatively better VA showed spontaneous improvement. This has erroneously been attributed to some treatments (Sergott et al., 1989.).
4.1.7. Conclusion
In eyes first seen ≤2 weeks after onset, with (i) VA 20/70 or worse, there was improvement in 41% at 6 months, and (ii) with moderate to severe visual field defect, there was improvement in 26% at 6 months. VA and visual fields showed improvement or further deterioration mainly up to 6 months, with no significant change after that. About half of the eyes with NA-AION presented with almost normal VA (20/15 to 20/30) at the initial visit. Thus, the presence of normal visual acuity does not rule out NA-AION. The natural history of visual outcome in NA-AION acts as the gold standard to evaluate the beneficial or detrimental effects of any therapy.
5. Visual outcome in arteritic AION
Arteritic AION is due to giant cell arteritis. Giant cell arteritis is an ophthalmic emergency. It would be unethical to do a natural history study of visual outcome of such a disease, because in this case early diagnosis and immediate, intensive high-dose steroid therapy is essential to prevent any further visual loss, not only in the involved eye but also in the fellow, normal eye. Our studies have shown that in eyes with arteritic AION, when treated with high-dose steroid therapy, only 4% of eyes with visual loss due to giant cell arteritis improved, as judged by both VA and central visual field (by kinetic perimetry and Amsler grid) (Hayreh et al., 2002.). The data also suggest that there is a better (p = 0.065) chance of visual improvement with early diagnosis and immediate start of steroid therapy.
Our study (Hayreh and Zimmerman, 2003a) showed that 4% of the eyes did experience visual deterioration within 5 days after the start of the high dose steroid therapy, and no deterioration happened after that time. It is clear from reports in the literature, as well as our experience, that if a patient is given inadequate steroid therapy or the therapy is tapered off prematurely, visual deterioration can develop at any time. Early, adequate steroid therapy is effective in preventing further visual loss in 96% of giant cell arteritis patients.
Our studies showed no evidence that intravenous megadose steroid therapy was more effective than oral therapy in improving visual outcome or preventing visual deterioration in giant cell arteritis (Hayreh et al., 2002; Hayreh and Zimmerman, 2003a; Hayreh, 2012).
6. Natural history of visual outcome in non-arteritic posterior ischemic optic neuropathy
Compared to non-arteritic anterior ischemic optic neuropathy, this is an uncommon type of ischemic optic neuropathy. The only study dealing with natural history of visual outcome is the one reported by me in 16 eyes (Hayreh, 2004).
6.1. Visual acuity
Among the 16 eyes, there were 12 eyes with visual acuity of 20/70 or worse, and of them 4 improved. Of the 16 eyes, 9 remained stable (20/25–20/25 in 4, and count fingers in 5), and 2 deteriorated (from 20/200 to count fingers).
6.2. Visual fields
On follow-up, the eyes did not show a significant improvement from baseline (p = 0.465).
This shows that in posterior ischemic optic neuropathy, natural history of visual outcome is poor.
7. Natural history of visual outcome in central retinal vein occlusion
The clinical entity of central retinal vein occlusion (CRVO) has been known since 1878 (Michel, 1878) and it is a common, visually disabling disorder; however, there is little definite information in the literature on the natural history of its visual outcome. It is well-established now that CRVO is of two types: non-ischemic and ischemic CRVO (Hayreh, 1965, 1971,1976,1983; Hayreh et al., 1978, 1983,1990), with very different visual outcomes (Hayreh et al., 2011a) and clinical features. The few reports in the literature, which deal with the natural history of visual outcome in CRVO, have some fundamental flaws (Moore, 1924, Zegarra et al., 1979; Quinlan et al., 1990; Chen et al., 1995; Priluck et al., 1980; The Central Vein Occlusion Study Group, 1997; The SCORE Study Research Group, 2009; CRUISE trial 2012; Heier et al., 2014). These include the following: (1) the findings are based on a mixture of the two types, which makes the results unreliable; (2) when CRVO is divided into various types, the criteria used are diverse in nature; (3) all these studies invariably deal only with VA, without any information on the outcome of visual fields; (4) VA testing methods varied; and (5) their criteria of improvement/deterioration in VA vary. Combined information from VA and visual fields provides complete overall information about the visual status.
Hayreh et al. (2011a) conducted a prospective study, in 667 consecutive patients (697 eyes) with CRVO. We evaluated: (1) the natural history of visual outcome (of both VA and visual fields), and (2) the factors that influence the natural history of visual outcome, by differentiating CRVO into ischemic and non-ischemic types using a combination of functional and morphological criteria, discussed at length elsewhere (Hayreh, 1983; Hayreh et al., 1990). At the initial visit, there was non-ischemic CRVO in 588 eyes and ischemic CRVO in 109 eyes.
Results of this study are discussed at length elsewhere (Hayreh et al., 2011a.). Following is a brief account. In determining the natural history of visual outcome in CRVO, it is essential to discuss separately, at length, VA and visual fields in the two types of CRVO and the various factors that influence them.
7.1. Visual findings at initial visit in eyes first seen within 3 months of onset
Initial VA was 20/200 or worse in 99% in ischemic CRVO and in 22% in non-ischemic CRVO. The severity of the initial visual field defect was marked to severe in 44% in ischemic CRVO and only 0.4% in non-ischemic CRVO.
Initial visual field defects in non-ischemic CRVO were minimal or mild in 91%, compared to only 8% ischemic CRVO (p < 0.0001). Central scotoma was the most common type – 41% in ischemic CRVO and 4% in non-ischemic CRVO. With the V4e isopter, 55% of the eyes initially presented with scotoma in ischemic CRVO, compared to only 5% in non-ischemic CRVO (p < 0.0001). The peripheral visual field was normal with the I-2e target in 89% in non-ischemic CRVO compared to only 7% in ischemic CRVO, and with the I-4e, in 99% and 74% respectively. Peripheral visual field defect was present in 3% in non-ischemic CRVO and in 17% with ischemic CRVO – the most frequent defect was a peripheral inferior nasal defect. When there is a cilioretinal artery occlusion associated with non-ischemic CRVO, that can result in centrocecal scotoma or peripheral visual field defect (Hayreh et al., 2008).
This shows that there are significant differences in initial VA and visual field defects between non-ischemic and ischemic CRVO.
7.2. Change in VA on follow-up
It is important to determine VA changes separately when initial VA is only mildly defective (i.e. 20/60 or better) and when it is poor (i.e. 20/70 or worse), because the findings in the two are different, as is evident from the following.
7.2.1. In the eyes with initial VA of 20/60 or better
This was seen in only non-ischemic CRVO eyes. At 3 months of follow-up, 17% showed VA deterioration, and during the 2–5 years follow-up the number was 20%. When VA is almost normal, there cannot be any improvement.
7.2.2. In the eyes with initial VA of 20/70 or worse
Over the follow-up periods, VA improved significantly (p = 0.034) in non-ischemia eyes – at 3 months of follow-up VA improved in 32%, and during the 2–5 years follow-up in 47%. In contrast, a smaller proportion of the eyes with ischemic CRVO (10% and 23%, respectively) improved. Overall, the rate of VA improvement was significantly higher in non-ischemic CRVO than in ischemic CRVO (p = 0.0004).
7.3. Change in visual fields on follow-up
Once again, it is important to determine visual field changes separately when there is minimal to mild initial defect or moderate to severe defect, because the findings in the two are different, as is evident from the following.
7.3.1. In eyes with minimal to mild initial visual field defect
It was primarily the central field defect. In non-ischemic CRVO eyes visual field deteriorated in 17% at 3 months and in 15% during the 2–5 years’ follow-up.
7.3.2. In eyes with moderate to severe initial visual field defect
In non-ischemic CRVO eyes, at 3 months of follow-up, 41% showed improvement and during the 2–5 years of follow-up, 79%. In contrast, in ischemic CRVO it was 15% and 27%, respectively. Overall, the odds ratio of improvement was 5.16 for non-ischemic CRVO relative to ischemic CRVO (p = 0.0006).
This shows that there is a difference in improvement in visual outcome between the two types of CRVO. Changes in visual outcome in our study cannot be compared with other studies because of the limitations discussed above in them and different criteria used for evaluating that.
7.4. Macular edema and visual outcome
The deterioration of VA in CRVO is primarily due to macular edema. Therefore, we investigated VA changes in relation to the presence/absence of macular edema during follow-up.
7.4.1. Macular edema and VA outcome
7.4.1.1. In eyes with initial VA of 20/60 or better
Non-ischemic CRVO eyes seen within 3 months of onset showed a significant association between the presence of macular edema and VA deterioration (p < 0.0001). At the 2-to-5 year follow-up, there was VA deterioration in 39% where macular edema was still present, compared to 15% where macular edema had resolved; in the latter VA deterioration was primarily due to foveal pigmentary change and/or epiretinal membrane (see below).
7.4.1.2. In eyes with initial VA of 20/70 or worse
In non-ischemic CRVO eyes, resolution of macular edema was associated with significant improvement in VA (p < 0.0001). In these eyes, macular edema had resolved in 59% during follow-up and was still present in 26%. In contrast, among the eyes with ischemic CRVO, there was no significant association of presence/absence of macular edema with improvement in VA (p < 0.55). This is because, in ischemic CRVO, ischemia permanently damages the macular retinal ganglion cell, so that VA cannot improve even when macular edema has resolved.
7.4.2. Macular edema and visual field outcome
7.4.2.1. In eyes with minimal to mild initial visual field defect
In non-ischemic CRVO eyes, there is primarily a central visual field defect. At follow-up of 2–5 years, there was visual field deterioration in 32% where macular edema was still present, compared to 9% where macular edema had resolved; in the latter visual field deterioration was primarily due to foveal pigmentary change and/or epiretinal membrane (see below).
7.4.2.2. In eyes with moderate to severe initial visual field defect
In non-ischemic CRVO eyes, visual field improvement was associated with the absence of macular edema (p = 0.037). Overall, in non-ischemic CRVO, visual field improvement was seen in 86% where macular edema had resolved, compared to 50% where macular edema was still present. Among the eyes with ischemic CRVO, there was no significant association between the presence/absence of macular edema and improvement in visual field (p = 0.83), for the reason discussed above.
At the final visit after macular edema had resolved, VA and visual field defect were worst in ischemic CRVO compared to those in non-ischemic CRVO (both p < 0.0001). VA was 20/200 or worse in 85% of ischemic CRVO eyes and in 17% of non-ischemic CRVO. Final visual field defect grade was marked to severe in 50% of eyes with ischemic CRVO compared to only 1% in non-ischemic CRVO. With V4e isopter, scotoma was found in 78% of the eyes with ischemic CRVO compared to only 6% of the eyes with non-ischemic CRVO (p < 0.0001). As mentioned above, this is because retinal ganglion cells are most susceptible to ischemia (Hayreh et al., 2004), and in ischemic CRVO they suffer permanent ischemic damage, particularly in the macular ganglion cells, so that there is little chance of any visual improvement.
These findings show that the changes in visual outcome, as well as the effect of macular edema on the visual outcomes, differ considerably between these two types of CRVO. Thus, a distinction between the two types of CRVO is key to predicting visual outcome in CRVO. It is most unfortunate that this basic fact has been ignored in the vast majority of studies dealing with the visual outcome in CRVO. This is also true of other complications of CRVO – most importantly ocular neovascularization, which is a complication only of ischemic CRVO and does not occur in non-ischemic CRVO, unless the latter is associated with diabetic retinopathy or ocular ischemia (Hayreh et al., 1983; Hayreh and Zimmerman, 2012b). Therefore, in the management of CRVO, the first, crucial step is to determine which type of CRVO one is dealing with. Combining the two types of CRVO into one group is like lumping benign and malignant tumors together in order to predict their outcome.
In almost all studies dealing with visual outcome, VA is used as the criterion, but this provides information only about the function of the macula. CRVO, however, involves the entire retina and not only the macular retina. Visual fields, by contrast, provide information on the function of the entire retina, including the macula. Therefore, it is crucial to evaluate visual outcome by a combination of both the tests in all eyes with CRVO. Moreover, a central scotoma may improve markedly in size and severity on visual field testing, but so long as it involves central fixation, the VA may not improve, giving misleading information on the visual outcome.
In our study, the central visual fields showed a variety of scotomata – the most common being the central scotoma. If CRVO is associated with cilioretinal artery occlusion (Hayreh et al., 2008), then there is usually a centrocecal scotoma. Peripheral visual fields plotted with a Goldmann perimeter remained perfectly normal in all eyes with non-ischemic CRVO throughout the course of follow-up, unless there was some other associated problem, e.g., cilioretinal artery occlusion (Hayreh, 1998; Hayreh et al., 2008). By contrast, in ischemic CRVO, there was always a relative peripheral visual defect with I-2e and/or with I-4e isopters, but with the V-4e isopter it was still normal in the vast majority – even in eyes with VA of counting fingers and in some of those with hand motion. This information about the peripheral visual fields is important for two reasons: (1) for differentiating ischemic from non-ischemic CRVO, as shown by a previous study (Hayreh et al., 1990), and (2) to evaluate the degree of disability caused by the CRVO.
7.5. Effect of foveal pigmentary change and epiretinal membrane on visual outcome
Foveal pigmentary change (Fig. 1) and epiretinal membrane can develop following chronic macular edema. We examined their association with VA deterioration after resolution of macular edema (Hayreh et al., 2011a). In non-ischemic CRVO eyes with initial VA of 20/60 or better, there were 42% that had deterioration in those with pigmentary change, compared to 3% without. Deterioration in VA was present in 45% with epiretinal membrane, compared to 8% without epiretinal membrane. In non-ischemic CRVO eyes that had initial VA of 20/70 or worse, VA improvement was associated with absence of pigmentary change (p = 0.006; 78% in those without vs. 44% in those with pigmentary change), whereas no significant association was seen for epiretinal membrane (p = 0.99). In ischemic CRVO eyes, after resolution of macular edema, there was no significant association of change in VA with foveal pigmentary change or epiretinal membrane (both p > 0.86).
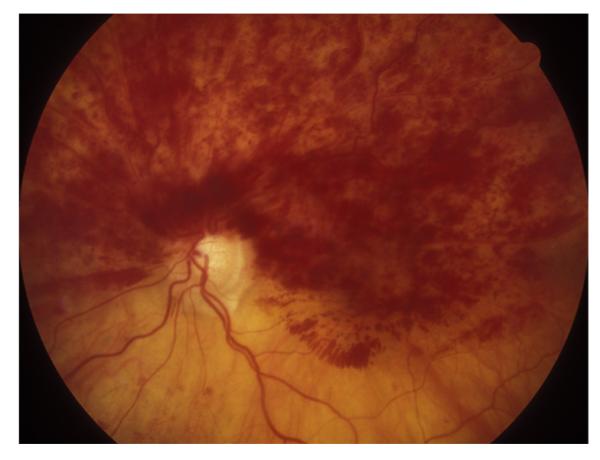
Fig. 1.
Fundus photograph of a resolved non-ischemic CRVO in the right eye. It shows retinociliary collaterals on the optic disc, macular retinal pigmentary degeneration and engorged tortuous retinal veins.
This shows that the development of foveal pigmentary change and epiretinal membrane following chronic macular edema in non-ischemic CRVO adversely influences the visual outcome after resolution of macular edema (foveal pigmentary change: odds ratio 21; p < 0.0001, epiretinal membrane: odds ratio 10; p = 0.0006). This is an important piece of information. However, in ischemic CRVO there was no significant association of change in VA with foveal pigmentary change and epiretinal membrane (both p > 0.86). As discussed above, this is because in ischemic CRVO, macular retinal ganglion cells suffer permanent ischemic damage, so that the presence/absence of foveal pigmentary change and epiretinal membrane makes no difference.
7.6. Effect of retinociliary collaterals on visual outcome
There is a prevalent impression among ophthalmologists that the development of retinociliary collaterals (Fig. 1) has a beneficial effect on the course and visual outcome of CRVO, improving the circulation in the central retinal vein by by-passing the occlusion. Priluck et al. (1980) in a retrospective study of 42 patents with CRVO, aged 40 or younger, stated that the presence of collaterals closely correlated with a favorable prognosis. But Giuffré et al. (1992, 1993) in 94 patients with CRVO, found that the VA of the eyes with CRVO that developed collaterals was not significantly different from the VA of the eyes without collaterals. Quinlan et al. (1990) in a retrospective study of 168 eyes with CRVO also found that the collaterals were not related to any improvement in VA. Garcia-Arumi et al. (2003) in their radial optic neurotomy study, found no significant difference in VA between those with and without these collaterals. Thus, the literature offers firm evidence that the development of retinociliary collaterals does not make any difference in the visual outcome in CRVO.
We investigated the role of retinociliary collaterals in visual outcome (Hayreh et al., 2011a). In our study, in non-ischemic CRVO eyes, retinociliary collaterals developed in 46% with a median time of 15 months; the eyes that developed the collaterals were the eyes with poorer initial vision, with 52% having 20/70 or worse VA. In the eyes that did not develop collaterals, median time to resolution of macular edema was 21 months. In those that did develop collaterals, resolution of macular edema occurred at a median time 40 months from the onset of CRVO, or 29 months after the development of the collaterals. A correlation between VA change (after resolution of macular edema in eyes with initial VA 20/70 or worse) and development of collaterals showed 43% with improved vision, 35% with no change, and 22% with deterioration. In contrast, those that did not develop collaterals had better visual outcome, 76% having improved vision, 18% with no change, and 6% with deterioration (p = 0.015).
In ischemic CRVO eyes, 41% developed retinociliary collaterals with a median time of 13 months. The development of collaterals had no significant effect on the time of resolution of macular edema (p = 0.54). In eyes with initial VA of 20/70 or worse, there was no significant effect of the presence of collaterals on a change in VA (p = 0.81). In eyes with collaterals, 33% had improved VA, 53% no change, and 13% deteriorated, compared to 31%, 46% and 23% respectively in those without collaterals. As discussed above, the primary cause of poor VA and visual fields in ischemic CRVO is ischemic damage to the macular retinal ganglion cells and not so much the macular edema, while in non-ischemic CRVO the primary factor for poor VA and visual fields is macular edema – this distinction is important.
Our study revealed an interesting phenomenon, not previously described: Non-ischemic CRVO (in eyes with initial VA of 20/70 or worse) showed the following:
(1). The eyes that developed collaterals had poorer initial VA than the eyes that did not (52% versus 35%).
(2). Resolution of macular edema took much longer in those with collaterals than in those without (median time 40 months versus 21 months).
(3). After the resolution of macular edema, 35% of eyes that developed collaterals showed no change in VA, 43% improved, and 22% deteriorated. Whereas, in eyes without collaterals, the corresponding numbers were 6%, 76% and 6% respectively.
Eyes with ischemic CRVO had similar findings.
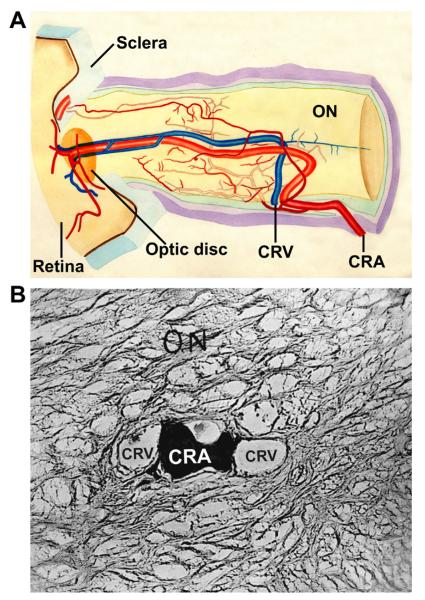
These findings pose an intriguing question about the retinociliary collateral veins in CRVO: why is the presence of cilioretinal collaterals related to poor initial and final visual acuity? Based on my anatomic, basic, experimental and clinical studies, the following seems the most plausible explanation. In all eyes with CRVO, fluorescein fundus angiography shows the presence of retinal blood flow (Fig. 2). Therefore, in CRVO, after the vein is occluded by a thrombus, the eye must develop collaterals to maintain its blood flow, although slower than normal. The collaterals develop from the pre-existing venous tributaries of the central retinal vein situated anterior to the site of occlusion. Within the optic nerve the central retinal vein has multiple prominent tributaries and only a few inconstant ones in the prelaminar region of the optic nerve head (Figs. 3 and 4). The severity of retinopathy in CRVO varies widely, indicating that the severity depends upon the site of occlusion in the central retinal vein and the number of tributaries available anterior to the site of occlusion to develop collaterals, i.e. the farther back the site of occlusion in the optic nerve, the greater the number of tributaries within the optic nerve available to establish collateral circulation, and the milder the retinopathy. Conversely, the closer the site of occlusion to the lamina cribrosa, the fewer tributaries are available in the optic nerve to establish collateral circulation, and the worse the retinopathy. In the latter case, there is far greater stress on the few available tributaries in the prelaminar region (connecting the central retinal vein with the peripapillary choroid), to develop into collaterals than when the site of occlusion is far back in the optic nerve. Therefore, eyes with retinociliary collaterals on the optic disc have a much greater chance of having a severer type of retinopathy, with much worse VA and more marked macular edema than do those without those collaterals, as shown by our study. The severity of retinopathy in turn determines the VA outcome. Therefore, it is not surprising that the higher the prevalence of retinociliary collaterals on the optic disc, the smaller is the chance of visual improvement and the greater the chance of visual deterioration – where there are no retinociliary collaterals, there are plenty of venous tributaries available within the optic nerve and no need to develop collaterals on the disc. On clinical evaluation, there is no way to determine the number and locations of the collaterals that develop within the optic nerve in CRVO. The other factor one has to keep in mind is that in our study 54% of the non-ischemic CRVO eyes did not develop venous tributaries in the prelaminar region. Thus, the prevalence of collaterals on the optic disc does not represent a true incidence of all the collaterals that develop with CRVO, and to judge the prevalence of collaterals simply from the ones seen on the optic disc is highly misleading. Incidentally, the prevalent misconception that the site of occlusion in CRVO is always at the lamina cribrosa is based on histopathology of rare eyes enucleated for painful, uncontrollable neovascular glaucoma due to ischemic CRVO – the worst type of CRVO (Green et al., 1981); that does not apply to the vast majority of the CRVO eyes, where the site of occlusion is within the optic nerve posterior to the lamina cribrosa (Hayreh et al., 1978; Hayreh, 2005).
Fig. 2.
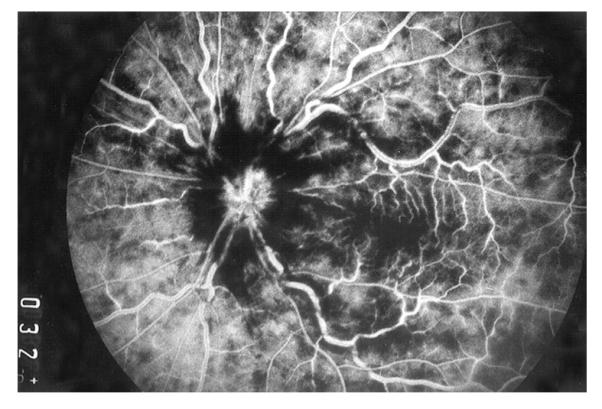
Fluorescein fundus angiogram of left eye, soon after onset of ischemic CRVO, during the arteriovenous phase (32 s after the injection of fluorescein), shows complete filing of the retinal vasculature.
Fig. 3.
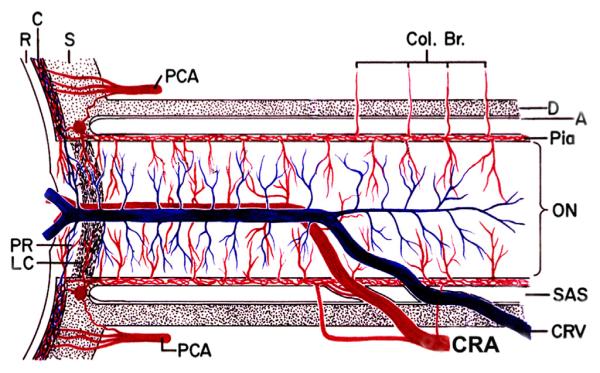
Schematic representation of the blood vessels in the optic nerve. (Modified from Hayreh, 1974a,b; 78: OP240-OP254.) Abbreviations: A = arachnoid; C = choroid; CAR and CRA = central retinal artery; Col. Br. = Collateral branches; CRV = central retinal vein; D = dura; LC = lamina cribrosa; ON = optic nerve; PCA = posterior ciliary artery; PR = prelaminar region; R = retina; S = sclera; SAS = subarachnoid space.
Fig. 4.
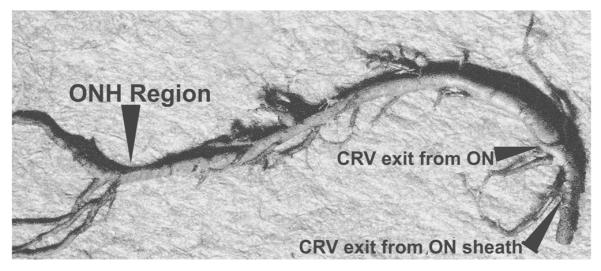
Cast of the central retinal vein shows its entire course from the optic disc to its exit from the optic nerve sheath. Note the presence of a large number of prominent tributaries within the optic nerve and none in the optic nerve head region in this specimen. (Reproduced from Hayreh et al., 2011a, 118: 119–33.) Abbreviations: CRV = Central retinal vein; ON = optic nerve; ONH = optic nerve head.
7.7. Effect of neovascular glaucoma on visual outcome in ischemic CRVO
Neovascular glaucoma is a complication only of ischemic CRVO. The eyes first seen within 3 months of onset, with a follow-up of at least 6 months, developed neovascular glaucoma in 36% in our study (Hayreh et al., 2011a). In eyes with initial VA of 20/70 or worse and neovascular glaucoma there was further deterioration in VA in 44%, compared to 12% deterioration and 32% improvement in those without neovascular glaucoma (p = 0.002). In eyes with moderate to severe initial visual field defect, those with neovascular glaucoma had 76% worsening and 5% improvement of visual field, compared to 38% worsening and 35% improvement in those without neovascular glaucoma (p = 0.008). Thus, the development of neovascular glaucoma has a detrimental effect on the visual outcome.
7.8. Effect of age on VA
Chen et al. (1995) in a study of 59 eyes, found that older patients had a worse visual outcome (p = 0.0029). Glacet-Bernard et al. (1996) in 120 CRVO eyes, found older age was a prognostic factor for poor visual outcome. Similarly, in our study (Hayreh et al., 2011a), no patients aged <45 years with initial VA of 20/60 or better had VA deterioration, and of those with initial VA of 20/70 or worse, VA improved in 80%. In those 45 or older, with initial VA of 20/60 or better, it deteriorated in 15%, and of those with initial VA of 20/70 or worse, it improved in 56%.
7.8.1. Visual outcome in CRVO in young adults
It is important to discuss this topic because conflicting statements have been made. Because of a widespread misconception that CRVO does not occur in young persons, young patients often undergo extensive and unnecessary investigations to find the cause of retinopathy. In our study, 16% of the non-ischemic CRVO patients were under the age of 45 years, and 7% of those with ischemic CRVO were in that age group.
The reports of visual outcome in young individuals vary widely from study to study. Priluck et al. (1980) in a retrospective study of 42 patients with CRVO who were 40 years or younger, concluded that their final visual prognosis could not be predicted by the severity of the venous occlusion at the time of diagnosis. Fong et al. (1992) in a retrospective study of 103 cases of CRVO in young, nondiabetic adults, followed for at least six months, found that 32% had a final VA of 20/200 or worse and 6% no light perception. Giuffré et al. (1992) in a study of 20 CRVO patients aged 40 years or less, found that the visual prognosis of young people with CRVO is often poor. In our study (Hayreh et al., 2011a), however, none of those aged under 45 years, with initial VA of 20/60 or better, had VA deterioration, and, in those with initial VA of 20/70 or worse, it improved in 80%. This shows that the visual prognosis for young people is much better than for those 45 or older.
Thus, increasing age was associated with VA deterioration. Non-ischemic CRVO showed a significant association of age with VA change, with increasing age positively associated with VA deterioration (p = 0.001) in those that presented with 20/60 or better VA, and negatively associated with VA improvement (p = 0.012) in eyes with initial VA of 20/70 or worse.
7.9. Effect of systemic conditions on VA
In eyes with initial VA of 20/60 or better, stroke (p = 0.036) and diabetes mellitus (p = 0.049) showed a significant association with VA deterioration. For those with diabetes mellitus relative to those without, the odds ratio of VA deterioration was 6. There was no significant association between VA deterioration and arterial hypertension (p = 0.14), ischemic heart disease (p = 0.58), or smoking (p = 0.31). In eyes with initial VA of 20/70 or worse, no significant association was seen between VA deterioration and arterial hypertension (p = 0.84), stroke (p = 0.82), ischemic heart disease (p = 0.47), diabetes mellitus (p = 0.58), or smoking (p = 0.24).
7.10. Effect of aspirin and anticoagulants on visual outcome
There is a common practice among ophthalmologists to advise CRVO patients to take aspirin or other antiplatelet drugs, and even to put them on anticoagulants. The genesis of this widespread use of antiplatelet aggregation agents (most commonly aspirin) or anticoagulants for CRVO and other types of retinal vein occlusion comes from the evidence of their proven effectiveness in major systemic venous thrombotic disorders (e.g., deep vein thrombosis); it has been assumed that they must be equally beneficial in various types of retinal vein occlusion.
We investigated systematically the role of antiplatelet aggregating drugs or anticoagulants in CRVO and HCRVO (Hayreh et al., 2011b). In this study there were 478 consecutive patients with non-ischemic CRVO, 89 with ischemic CRVO, and 119 with non-ischemic hemi-CRVO. The findings of this study are discussed at length elsewhere (Hayreh et al., 2011b), and following is a brief account.
In all three types of CRVO, there was a significantly greater severity of retinal hemorrhages among aspirin users than non-users (p < 0.001). Initial VA and visual fields were significantly worse for aspirin users than non-users in non-ischemic CRVO and hemi-CRVO, but did not differ for ischemic CRVO. Among the patients with non-ischemic CRVO that initially presented with 20/60 or better VA, aspirin use resulted in a significant deterioration in VA. The odds ratio of VA deterioration, for aspirin users relative to non-users, after adjusting for age, diabetes, ischemic heart disease, and hypertension, was 2.24. Of those whose macular edema resolved, over-all cumulative VA outcome also suggested a higher percentage with deterioration among aspirin users, with the odds ratio for deterioration of 3.62 for aspirin users relative to non-users. For the non-ischemic CRVO patients that had 20/70 or worse VA at their initial visit, after resolution of macular edema, improvement in VA was less likely for aspirin users than non-users. Aspirin use did not have a significant effect on time to resolution of macular edema (p = 0.632) in eyes with non-ischemic CRVO.
For changes in visual field defect among the non-ischemic CRVO eyes that initially presented with minimal to mild defect, significant differences in the proportion with visual field worsening due to aspirin use was seen at the 6 and 9 months follow-up (p = 0.003 and p = 0.019, respectively), but was not observed at the later follow-up (15 months p = 0.540; 2–5 years p = 0.724). In those where macular edema had resolved, over-all cumulative visual field outcome did not show a significant effect of aspirin use on visual field deterioration. Among the non-ischemic CRVO eyes with moderate to severe initial visual field defect, there was no significant difference between aspirin users and non-users with respect to improvement in visual field.
Among those with non-ischemic HCRVO and those with ischemic CRVO, there was no significant effect of aspirin use in changes in VA and visual field.
Thus, the findings of this study showed that aspirin or other antiplatelet aggregating agents or anticoagulants adversely influence the visual outcome in patients with CRVO and hemi-CRVO, without any evidence of protective or beneficial effect.
7.11. Conclusion
The visual outcomes of the two types of CRVO are totally different; the outcome is good in non-ischemic CRVO and poor in ischemic CRVO. Therefore, a differentiation of CRVO into non-ischemic and ischemic types is crucial in determining visual outcome. The most reliable tests for differentiation of CRVO into its two types during the initial acute phase are functional (visual acuity, visual fields, relative afferent pupillary defect and electroretinography); morphological tests (fluorescein fundus angiography and ophthalmoscopy) are much less reliable (Hayreh et al., 1990). Visual outcome is also adversely influenced to some extent by several other factors, including increasing age, cerebrovascular disease, diabetes mellitus and use of aspirin and anticoagulants; also in non-ischemic CRVO by development of foveal pigmentation and epiretinal membrane, and in ischemic CRVO by neovascular glaucoma.
8. Natural history of visual outcome in hemi-central retinal vein occlusion
In 1980, I first described the clinical entity “hemi-central retinal vein occlusion” (HCRVO) (Hayreh and Hayreh, 1980). During embryonic life, there are two trunks of the central retinal vein lying on either side of the central retinal artery (Mann, 1969) (Figs. 5 and 6); one of the two usually disappears before birth, leaving the central retinal vein as a single trunk. However, both trunks persist during postnatal life as a congenital abnormality in 20% of eyes (Chopdar, 1982, 1984, 1986). Occlusion of one of those two trunks in the optic nerve results in the development of HCRVO, which may involve the inferior half (Figs. 7 and 8), superior half (Figs. 9 and 10), or superior temporal (Fig. 11) or inferior temporal (Fig. 12) sector of the retina. When branch retinal vein occlusion involves half of the retina (also called “hemispheric retinal vein occlusion”) (Sanborn and Magargal 1984; Gómez-Ulla de Irazazabal et al., 1986), it is sometimes confused with HCRVO, and involvement of one sector of the retina by HCRVO (Figs. 11 and 12) has been misdiagnosed as branch retinal vein occlusion. Some ophthalmologists tend to consider “hemispheric retinal vein occlusion” and HCRVO as the same disease or use the terms interchangeably. However, the two are very different entities pathogenetically and clinically (Hayreh and Hayreh, 1980), since HCRVO is a variant of CRVO, and therefore it is of ischemic and non-ischemic types.
Fig. 5.
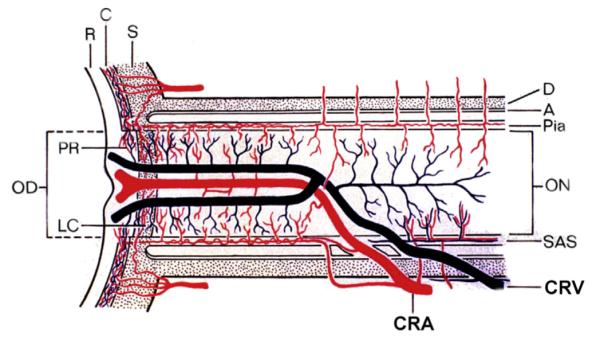
Schematic representation of two trunks of central retinal vein in the optic nerve. Abbreviations: A = arachnoid; C = choroid; CRA = central artery of retina; CRV = central retinal vein; D = dural sheath; LC = lamina cribrosa; OD = optic disc; ON = optic nerve; PR = prelaminar region; SAS = subarachnoid space; R = retina; S = sclera; P = pia mater. (Reproduced from Hayreh and Hayreh, 1980; 98: 1600–1609.)
Fig. 6.
Diagrammatic reconstruction from serial sections (10-μm thick) of anterior part of optic nerve. (A) It shows intraneural course of central retinal vessels. Note duplicate trunks the central retinal vein anteriorly. (B) One of the transverse sections of the specimen shows, in the center of the optic nerve, two trunks of the central retinal vein, with the central retinal artery (filled with Prussian blue) interposed between the two veins. CRA = central retinal artery; CRV = central retinal vein; ON, optic nerve. (Reproduced from Hayreh SS. Master of surgery thesis. Panjab University, India 1958.)
Fig. 7.
Right eye of a 67-year-old man with non-ischemic HCRVO involving lower half of retina. (A) Fundus photograph during the acute phase. (Two arrows show 2 trunks of central retinal vein). (B, C) Fluorescein fundus angiograms during: (B) during arteriovenous phase shows delayed filling of the occluded vein, and (C) late phase. (D) After resolution of retinopathy shows macular pigmentary degeneration and venous collaterals on the optic disc. (A, B and C reproduced from Hayreh and Hayreh, 1980; 98: 1600–1609.)
Fig. 8.
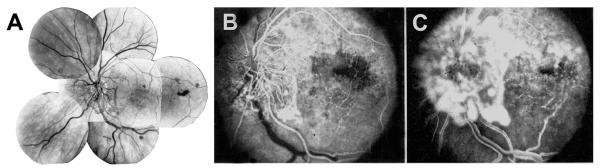
Left eye of a 67-year-old man with ischemic HCRVO involving lower half of retina, 18 months after the onset. (A) Composite fundus photograph shows optic disc and retinal neovascularization. (B) Fluorescein fundus angiogram during early arteriovenous phase, and (C) during the venous phase shows retinal capillary non-perfusion in the involved retina and fluorescein leak from the neovascularization. (Reproduced from Hayreh and Hayreh, 1980; 98: 1600–1609.)
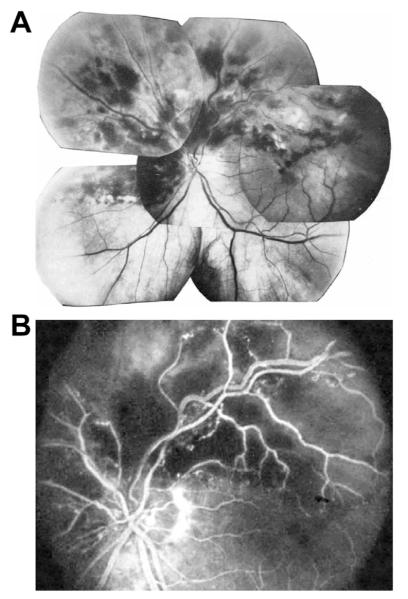
Fig. 9.
Left eye of an 83-year woman with non-ischemic HCRVO, involving upper half of the retina and entire macular region.
Fig. 10.
Left eye of a 68-year-old man with ischemic HCRVO of 3½ months’ duration, involving upper half of retina, with iris neovascularization, and neovascular glaucoma. Two weeks later, optic disc neovascularization developed. (A) Composite fundus photograph. (B) Fluorescein fundus angiogram during arteriovenous phase shows retinal capillary non-perfusion in the involved retina. (Reproduced from Hayreh and Hayreh, 1980, 98: 1600–1609.)
Fig. 11.
Right eye of a 39-year-old man with non-ischemic HCRVO involving superior temporal sector only.
Fig. 12.
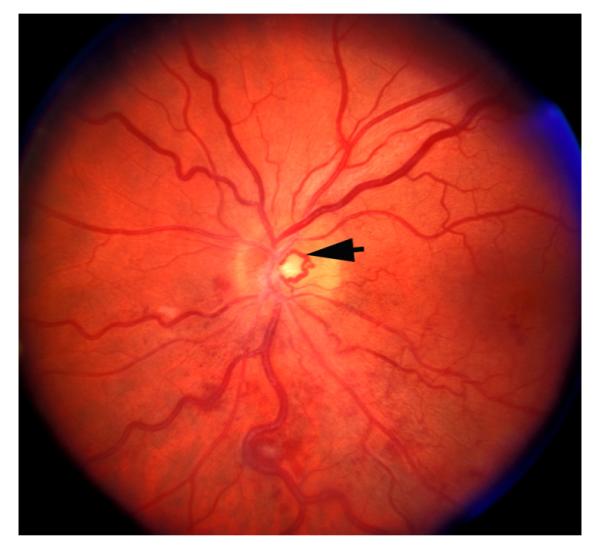
Left eye of a 66-year old man with non-ischemic HCRVO involving inferior temporal region only, 5 months after the onset, and shows venous collateral on the optic disc connecting the two trunks of the central retinal vein (arrow).
Previously, two studies (Chopdar, 1982; Parodi et al., 1992) have reported the visual outcome in HCVRO without any intervention. In one (Chopdar, 1982), there were 11 eyes – 36% non-ischemic and 64% ischemic. Because there was no information about the initial VA in 5 of 11 eyes, the visual findings in this study are unreliable. In the other study (Parodi et al., 1992), there were 26 eyes, 77% non-ischemic and 23% ischemic. In the non-ischemic HCRVO, mean initial and final visual acuities were 20/200 and 20/50 respectively and in the ischemic 20/200 and 10/200 respectively. The visual outcome recorded in these studies was based entirely on the VA alone. As discussed above, visual fields provide very different information about the visual outcome compared to VA.
8.1. Our study
Hayreh and Zimmerman (2012a) investigated the natural history of visual outcome in 67 consecutive eyes with HCRVO. The findings of that study are discussed in detail elsewhere (Hayreh and Zimmerman, 2012a), and following is a brief account.
There were 57 non-ischemic and 10 ischemic HCRVO eyes. In the 57 eyes with non-ischemic HCRVO, 56% involved the inferior half alone or a part of the adjacent retina also, 39% involved the superior half alone or a part of the adjacent retina also, and 5% only the superior temporal sector. In the ten eyes with ischemic HCRVO, the superior half alone or that plus a part of the adjacent retina was involved in 5, the inferior half alone or with a part of the adjacent retina also in 4, and only the inferior temporal segment sector in 1 eye. As in CRVO (see above), differentiation of non-ischemic HCRVO from ischemic HCRVO is essential to evaluate initial and final VA and visual fields outcome.
8.1.1. Visual acuity findings
VA depends entirely on the involvement of the foveal zone, which is a part of the temporal retina. Therefore, VA is affected in HCRVO whenever it involves the lower half or upper half of the retina or only a superior or inferior temporal sector.
In non-ischemic HCRVO, the initial VA when it involved the superior temporal retina was 20/30 or better in 58%, 20/60 or better in 83% and 20/200 or worse in 4%, while when it involved the inferior temporal retina it was 45%, 66% and 13% respectively. After resolution of macular edema, in non-ischemic HCRVO, of those with 20/60 or better initial VA, 12% with inferior temporal involvement and none of those with superior temporal involvement had worse VA. For the eyes with 20/70 or worse initial VA, improvement was seen in 57% with inferior temporal involvement; there was no change in the 1 eye with superior temporal involvement.
In ischemic HCRVO, the initial VA when it involved the superior temporal retina was 20/40–20/60 in 60% and 20/200 or worse in 20%, while when it involved the inferior temporal it was 20% and 80% respectively. In eyes with ischemic HCRVO at the 9–15 month follow-up, of the 4 eyes that presented with 20/60 or better VA, 1 eye had worsened, and 3 eyes showed no change. Of the 5 eyes that had 20/70 or worse initial VA, 3 improved, and the other 2 showed no change.
Overall, after macular edema had resolved, only 6% of the eyes with initial VA of 20/60 or better showed deterioration and 4 of 8 eyes with initial VA of 20/70 or worse showed improvement.
8.1.2. Visual field findings
In non-ischemic HCRVO, the severity of initial visual field loss was minimal to mild in 95% when HCRVO involved the superior half of the retina, and in 97% when the inferior half of the retina was involved. Moderate visual field defect was seen in 5% and 3% respectively. After resolution of macular edema, of those with minimal to mild visual field defect, worsening was found in only 8% with the inferior half involved, and none of those with the superior half involved. There were a very few eyes with moderate visual field defect.
In ischemic HCRVO, the severity of initial visual field loss was minimal to mild in 40% when HCRVO involved the superior half of the retina, and in 66% when the inferior half of the retina was involved; and it was moderate in 60% and 33% respectively. At the 9–15 month follow-up, of the 4 eyes with minimal to mild initial visual field defect, 2 worsened and the other 2 showed no change. Of the 4 eyes with moderate to severe visual field defect, 2 showed improvement, and the other 2 had no change.
Comparing VA and visual field at initial visit in the eyes with non-ischemic HCRVO showed no significant difference among the groups based on sector involvement.
These findings demonstrate that initial VA and visual fields and the final visual outcome are different in the two types of HCRVO – much better in the non-ischemic than ischemic HCRVO.
8.2. Effect of foveal pigmentary change and epiretinal membrane on visual outcome
In the non-ischemic HCRVO eyes, foveal pigmentation developed in 16% (Fig. 7D) and epiretinal membrane in 9%. These changes result in VA deterioration.
8.3. Effect of venous collaterals on visual outcome
In HCRVO, venous collaterals develop on the optic disc, connecting the two trunks of the central retinal vein (Figs. 12 and 13). These collaterals are of a totally different type from those seen in CRVO (Fig. 1). In the non-ischemic HCRVO eyes, collaterals developed on the optic disc in 58%. There was no significant (p = 0.56) association between development of collaterals and change in visual outcome in either non-ischemic or ischemic HCRVO.
Fig. 13.
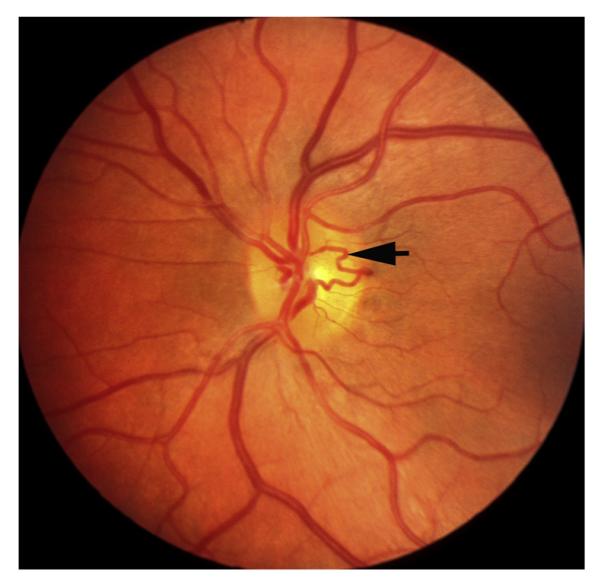
Left eye of a 48-year woman with non-ischemic HCRVO involving inferior half of the retina 14 months after the onset shows venous collateral on the optic disc connecting the two trunks of the central retinal vein (arrow).
8.4. Effect of systemic conditions on VA
There was no significant association between age (p > 0.89) or arterial hypertension (p > 0.43) and change in VA.
8.5. Neovascularization in HCRVO
We investigated the development of ocular neovascularization in HCRVO (Hayreh and Zimmerman, 2012b). As in CRVO, this is a complication of only ischemic HCRVO (Fig. 8). Within 12 months after the onset of ischemic HCRVO, the most prevalent type of neovascularization was retinal neovascularization, with a cumulative probability of 29%, and less often iris neovascularization (in 12%) and disc neovascularization (in 12%). Angle neovascularization was found in 10% and neovascular glaucoma in 5% within 6 months of onset. None of the eyes with non-ischemic HCRVO developed any neovascularization. The development of ocular neovascularization has a detrimental effect on visual outcome.
8.6. Conclusion
In non-ischemic HCRVO, overall, on resolution of macular edema, only 6% eyes with initial VA of 20/60 or better showed deterioration and 50% with initial VA of 20/70 or worse showed improvement. Out of the eyes that presented with minimal to mild visual field defect only 5% showed deterioration. This suggests a good prognosis in the natural history of visual outcome for non-ischemic hemi-CRVO; however, that is not the case with ischemic HCRVO.
9. Natural history of visual outcome in branch retinal vein occlusion
Branch retinal vein occlusion (BRVO) has been known since 1896 (Oeller, 1896); however, there has been conflicting information on its visual outcome. In the available data on the natural history of visual outcome in BRVO, the criterion of VA improvement and study designs vary widely among different studies, so that it is impossible to compare their findings. Rogers et al. (2010) reviewed the natural history of visual outcome in BRVO in articles published in the English language up to 2010, and concluded that VA generally improved in eyes with BRVO without intervention, although clinically significant improvement beyond 20/40 was uncommon. In the available studies, visual outcome was evaluated by doing VA and there is little information about the visual field changes.
9.1. Our study
My studies have shown that BRVO actually consists of two distinct clinical entities: major BRVO and macular BRVO (Hayreh et al., 1983, 1994b). Therefore, we (Hayreh and Zimmerman, 2014a) investigated the natural history of visual outcome separately for major and macular BRVO in 216 consecutive untreated eyes (144 eyes with major BRVO and 72 eyes with macular BRVO). The findings are discussed in detail elsewhere (Hayreh and Zimmerman, 2014a), and the following is a brief account.
Major BRVO involved the retina in superior temporal region in 65%, inferior temporal in 31% and nasal retina in 3.5%. Macular BRVO involved the superior in 81% and inferior macular regions in 19%.
9.1.1. Visual acuity
The VA is affected only in temporal BRVO. Initially in temporal BRVO, VA was 20/60 or better in 51% and 20/70 or worse in 49%. In temporal BRVO, there was no difference (p = 0.555) in VA among superior and inferior temporal BRVO. In macular BRVO, VA was 20/60 or better in 74% and 20/70 or worse in 26%, with no difference (p = 0.119) in VA among superior and inferior macular BRVO.
In major BRVO eyes with initial VA of 20/70 or worse, median VA of 20/200 at the initial visit improved to 20/50 at 15 months. Overall, in eyes with initial VA of 20/60 or better, 75% had improved or stable VA, and in eyes with initial VA of 20/70 or worse 69% had improved VA. In macular BRVO, VA was stable or improved in 87% of eyes with 20/60 or better initial VA, and improved in 53% of eyes with 20/70 or worse initial VA.
The median time to macular edema resolution was 21 months in major BRVO and 18 months in macular BRVO. When the effect of macular edema resolution on VA was considered, in major BRVO VA improved in 76% of those with resolved edema and in 50% with unresolved macular edema, at last follow-up. In macular BRVO, with initial VA of 20/70 or worse, VA after macular edema resolved improved in 58%. There were only 3 macular BRVO eyes with initial VA of 20/70 or worse where macular edema did not resolve, all of which had VA of 20/40 at last follow-up; VA was stable or improved in 87% of eyes with 20/60 or better initial VA in macular BRVO.
This shows that VA does not improve to the same extent in eyes with macular BRVO as in the major BRVO.
9.1.2. Visual fields
Initially in the region of the temporal BRVO, minimal to mild defect was seen in 72% and moderate in 26%, and there was no difference (p = 0.190) between superior and inferior temporal BRVO. Initially in the macular BRVO, minimal to mild defect was seen in 98%, with no difference (p = 0.907) between superior and inferior macular BRVO.
On follow-up, in temporal BRVO, visual field defect improved or remained stable in 68% of eyes with minimal-mild initial defect, and improved in 52% of eyes with moderate to severe initial defect. In macular BRVO, visual field defect remained stable or improved in 85% of eyes with minimal-mild initial defect.
The Amsler grid chart always showed a defect when there was no central defect on kinetic perimetry. In 20 eyes with macular and 7 with major BRVO, when perimetry showed no defect but the VA was abnormal, it commonly showed metamorphopsia. Thus, the Amsler grid chart provides useful information in evaluation of visual function when the macula is involved.
9.1.3. Effect of state of foveal capillary arcade on fluorescein fundus angiography
Clemett et al. (1973) found that at one year, in eyes with initial VA of 20/60 or worse, 67% of those with an intact foveal capillary arcade had final VA of 20/40 or better, while those with a broken arcade showed no improvement. Our study also evaluated the association of capillary foveal arcade (intact or broken) with improvement in VA after macular edema had resolved in eyes with initial VA of 20/70 or worse. Of these eyes, with capillary foveal arcade intact, VA improved in 81% and in those with broken arcade 58% (p = 0.082).
Finkelstein (1992), showed that eyes with capillary obliteration in the macular region had a greater frequency of improvement (91%) in VA than those without capillary obliteration (29%). In our study, there was no significant association of VA improvement with severity of capillary obliteration in the macular region (p = 0.740). VA improved in 65% of eyes with extensive obliteration and in 69% of those with no capillary obliteration.
9.1.4. Association of age, systemic conditions and smoking with change in VA
This was examined in eyes with initial VA of 20/70 or worse with improvement in VA after macular edema had resolved. Of these factors, only age showed a significant association with VA improvement; those that improved were significantly younger than those with no improvement (p = 0.002).
9.1.5. Resolution of BRVO
The median time to resolution of BRVO, based on Kaplan–Meier analysis, was 4 years for major BRVO, and 1.5 years for macular BRVO. Criteria for resolution of BRVO retinopathy were: resolution of both macular edema and retinal hemorrhages.
The findings from previous studies on the natural history of visual outcome cannot be compared with those of our study, because of the different study designs, for example:
(a) In our study, visual outcome was stratified by initial VA, and we used the currently accepted criterion, a change of at least 3 lines (0.3 logMAR) in the Snellen VA chart, for a significant change. The “Branch vein occlusion study group” (1984), by contrast, defined 2 lines in the Snellen VA chart as a significant change. In more recent studies, an EDTRS letter score has been used to evaluate change in VA.
(b) Our studies (Hayreh et al., 1983,1994b), have shown that major and macular BRVOs are two distinct clinical entities. This is shown by the comparison of various parameters above between the two types of BRVO, and the different initial visual status and final visual outcomes of the two types. Previous studies did not make that distinction.
(c) Also, in previous studies, visual outcome measurement was based on VA only, with little information about visual fields. But VA provides information only about the function of the foveal region, not of the entire retina, whereas the visual field provides information about the entire retina. Major BRVO involves not only the fovea but also the entire sector of the retina up to the periphery drained by the occluded vein. Thus, for complete evaluation of visual loss in BRVO, information about the visual field loss is essential.
Although a majority of BRVO eyes had variable amounts of VA improvement without treatment, there was a lack of improvement in some eyes, which may be due to the same factors as those seen in ischemic central retinal vein occlusion (Hayreh et al., 2011a), since most BRVO cases (particularly major BRVO) are ischemic in nature. These factors may include: (1) Ischemic damage to macular retinal ganglion cells, which are most vulnerable to ischemic damage (Hayreh et al., 2004). (2) Pigmentary degeneration and/or epiretinal membrane may develop in the foveal region secondary to prolonged macular edema.
9.1.6. Conclusions
Major and macular BRVOs are two distinct clinical entities, and initial visual status and final visual outcome in the two types are quite different. Overall, on resolution of macular edema, our study suggests that in both major and macular BRVO, VA and visual fields improved to a variable degree in majority of eyes without any treatment; but in eyes with macular BRVO, VA does not improve to the same extent as in the major BRVO.
10. Natural history of visual outcome in central retinal artery occlusion
Central retinal artery occlusion (CRAO) has been a well-known clinical entity for almost 150 years (Von Graefe, 1859). A voluminous literature has accumulated on it. Its clinical findings are classical and its diagnosis is easy. A whole host of treatment modalities have been advocated and tried for recovery of visual function, and enthusiastic success has been claimed for treatment after treatment, but none has stood the test of the time. Therefore, the first essential to evaluate beneficial effect of a treatment on the visual outcome in CRAO is to obtain information about the natural history of visual outcome without any treatment. There is little scientifically valid information on this topic, drawn from systematic detailed study. This has resulted in claims that visual improvement is due to treatment, when it may very well simply represent the natural history of the disease. For example, claim of a 66% success rate for visual improvement with local fibrinolysis using tPA in CRAO (Richard et al., 1999) was found to represent simply natural history (Hayreh, 1999).
We (Hayreh and Zimmerman, 2005b) conducted a prospective study on natural history of visual outcome in CRAO with this in view, and also to test the general belief that there is little chance of spontaneous visual improvement in CRAO. The findings are discussed in detail elsewhere (Hayreh and Zimmerman, 2005b); a brief account follows.
10.1. Classification of CRAO
Our studies showed that CRAO is of four distinct types (Hayreh and Zimmerman, 2005b, 2007). Initial and final visual outcomes are different in each of them (see below). This classification is essential to characterize and differentiate the visual outcomes among these four CRAO types.
10.1.1. Type 1. Non-arteritic (NA) CRAO
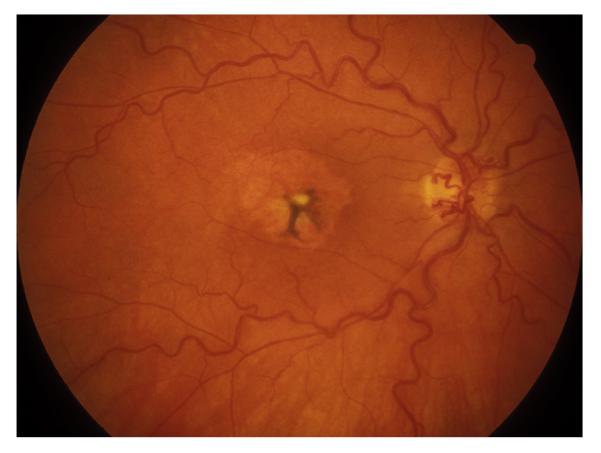
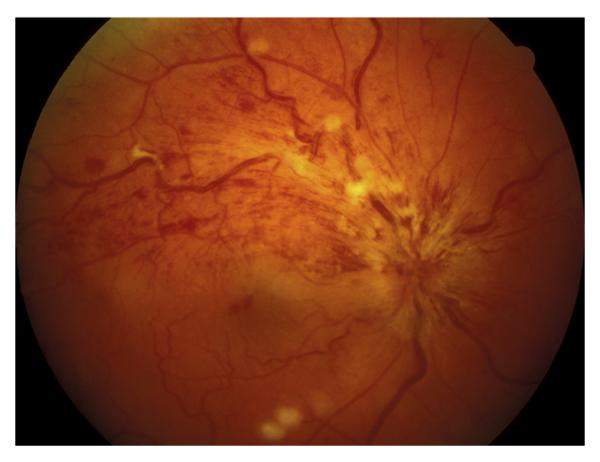
This includes eyes with the classical clinical picture of permanent CRAO with retinal infarction, cherry red spot, and attenuated retinal vessels (Fig. 14), and absent or poor residual retinal circulation on fluorescein angiography, and no evidence of giant cell arteritis.
Fig. 14.
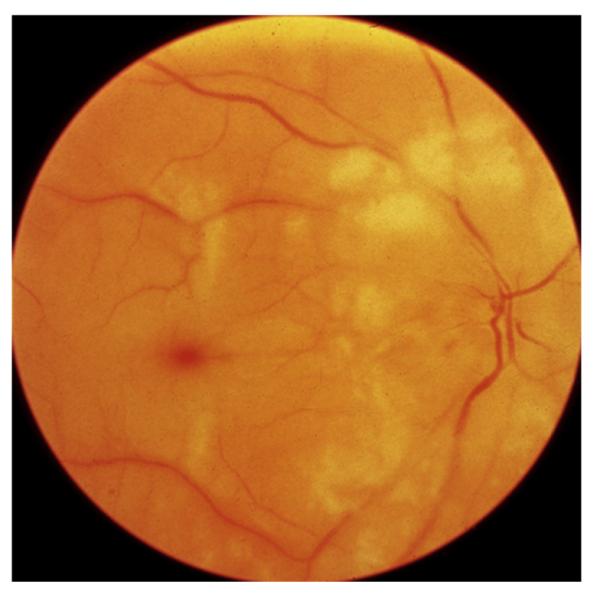
Fundus photograph of the right eye with CRAO. It shows retinal infarction, cherry red spot, optic disc edema and narrow retinal arterioles.
10.1.2. Type 2. NA-CRAO with cilioretinal artery sparing
The presence and size of a patent cilioretinal artery in permanent NA-CRAO can have a marked influence on the visual outcome and retinal circulation (Figs. 15 and 16); it is imperative not to lump these eyes with the Type 1 above.
Fig. 15.
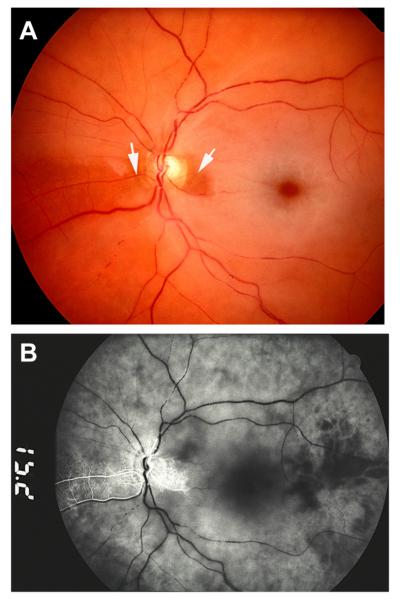
Left eye of a 77-year old woman with CRAO and cilioretinal artery sparing. (A) Fundus photograph shows central retinal macular ischemic opacity, cherry red spot and box-carring (cattle trucking). Arrows indicate two patent cilioretinal arteries. (B) Fluorescein fundus angiogram shows filling of the cilioretinal arteries and the choroid, but no filling of the central retinal artery circulation 15.2 s after injection of the dye.
Fig. 16.
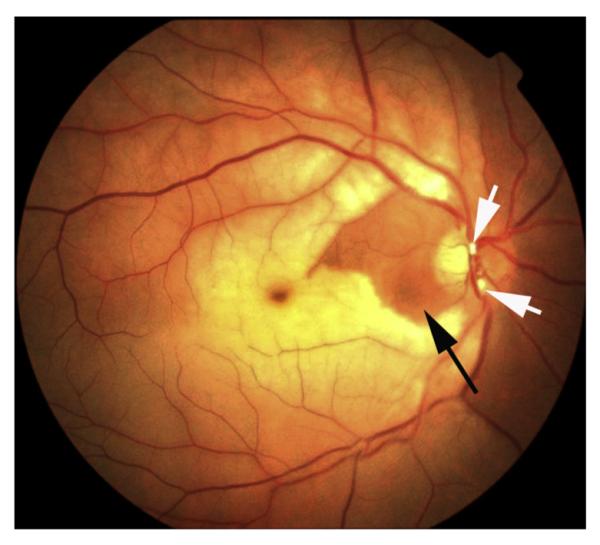
Right eye of a 35-year old man with CRAO and cilioretinal artery sparing. There are two emboli (white arrows) situated in two branches of the central retinal artery on the optic disc. Black arrow indicates the area supplied by the patent cilioretinal artery. Reproduced by courtesy of Dr. Patel from India.
10.1.3. Type 3. Arteritic CRAO
In this type, giant cell arteritis is the cause of development of permanent CRAO. My studies on giant cell arteritis have shown that these eyes almost invariably have associated arteritic anterior ischemic optic neuropathy (Hayreh, 1974a; Hayreh et al., 1998). This is because in some cases the central retinal artery and posterior ciliary artery arise by a common stem from the ophthalmic artery (Fig. 17). In them if giant cell arteritis involves the common stem, that results in occlusion of both central retinal artery and posterior ciliary artery. Therefore, the visual loss is the result of acute ischemia, not only of the retina but also of the optic nerve head. Clinically, these eyes have the classical fundus findings of CRAO with or without optic disc edema, but, most importantly, on fluorescein angiography there is evidence of a posterior ciliary artery occlusion in addition to CRAO (Fig. 18). Without fluorescein fundus angiography, the presence of a posterior ciliary artery occlusion and the diagnosis of giant cell arteritis may be missed, resulting in the tragedy of bilateral blindness that could have been prevented by immediate institution of high dose steroid therapy. Since giant cell arteritis is an ophthalmic emergency, it would be unethical to do a natural history study of visual outcome in arteritic CRAO.
Fig. 17.
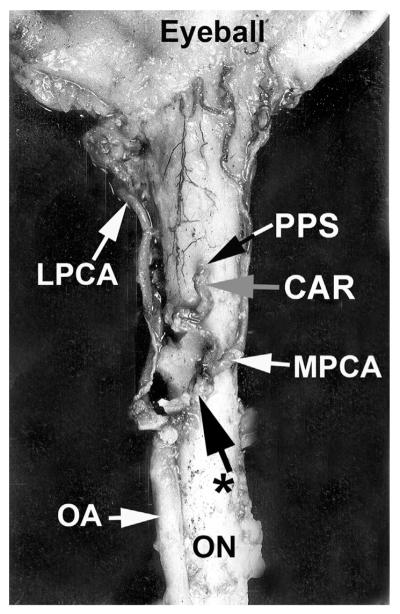
An example of a common trunk of origin of central retinal artery and posterior ciliary artery (PCA) from the ophthalmic artery, as seen from below. Reproduced from Hayreh, 2009; 28: 34–62. CAR = Central retinal artery, LPCA = Lateral PCA, MPCA = Medial PCA, OA = Ophthalmic artery, ON = Optic nerve. PPS = Point of penetration into the sheath by CAR, * = Common trunk of origin of CRA and medial PCA.
Fig. 18.
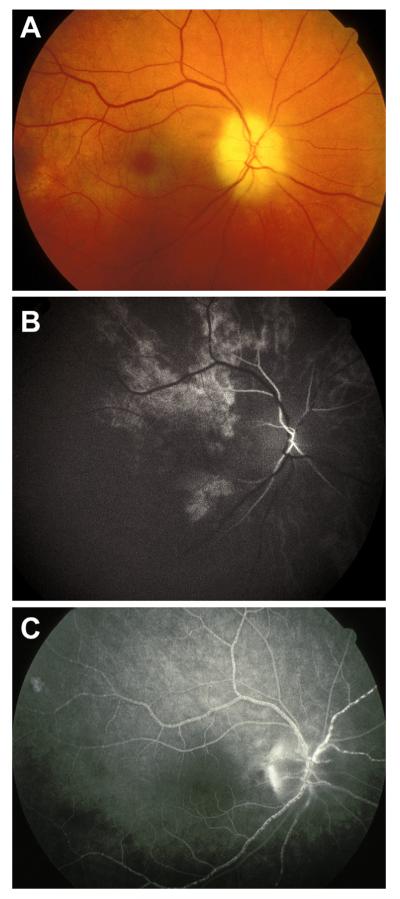
Right eye of a 69-year old man with giant cell arteritis, with arteritic anterior ischemic optic neuropathy and CRAO. (A) Fundus photograph shows chalky-white optic disc edema, some mild box-carring (cattle-trucking) in the retinal vessels and cherry red spot with perifoveolar ischemic retinal opacity. (B) Fluorescein fundus angiogram during the transit of the dye shows late and slow filling of the central retinal artery and of a few patches of the choroid. (C) Fluorescein fundus angiogram during the late phase shows box-carring (cattle-trucking) in the retinal vessels, with almost no disc staining, partial filling of the choroid.
10.1.4. Type 4. Transient NA-CRAO
The visual outcome in this type of CRAO can be totally different from any of the other types, depending upon the duration of transient CRAO, which may vary from several minutes to many hours. Its diagnosis is based on a history of marked sudden visual loss (not amaurosis fugax) and fundus findings of CRAO but normal retinal circulation on initial fluorescein angiography. Figs. 19 and 20 represent two variations in transient CRAO findings.
Fig. 19.
Right eye of a 69-year old man; one day old transient CRAO. (A) Fundus photograph shows central macular retinal infarct with cherry red spot and normal retinal vessels. (B) Fluorescein fundus angiogram that day during the retinal arteriovenous phase shows normal retinal vascular bed filling, with poor filling of the central foveal retinal capillaries. (C) Visual fields plotted with a Goldmann perimeter, show normal peripheral field with I-4e, island field with I-2e, and a large absolute central scotoma.
Fig. 20.
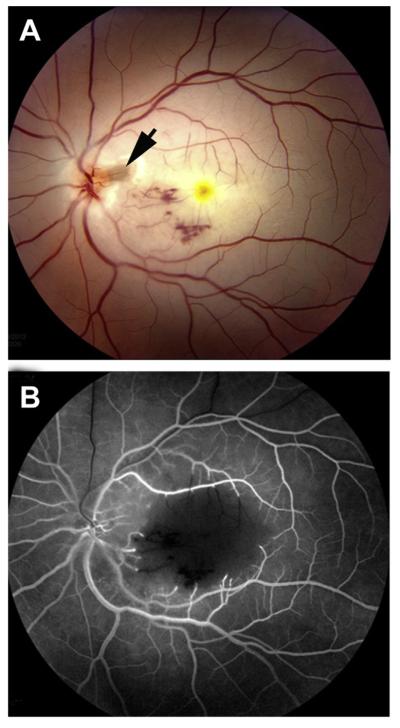
Left eye of a 30-year old man with transient CRAO. (A) Fundus photograph shows ischemic retinal opacity in the macular region, with a few punctate hemorrhages, normal retinal vessels, and tiny patent cilioretinal artery (Arrow). (B) Fluorescein fundus angiogram 30 s after injection of the dye shows filling of the retinal vessels but no filling of the retinal vessels in the central macular region.
10.2. Our study
The prospective study by Hayreh and Zimmerman (2005b) was based on 244 patients (260 eyes) with CRAO, seen consecutively.
10.2.1. Visual acuity
10.2.1.1. Initial VA in eyes seen within 7 days of onset
At the initial visit, 10.8% had VA of 20/40 or better and 74% counting fingers (CF) or worse. The initial VA differed significantly among the 4 types of CRAO (p < 0.0001); it was 20/40 or better in 38% of transient NA-CRAO, in 20.0% of NA-CRAO with cilioretinal artery sparing and in none of the NA-CRAO and arteritic CRAO, while the corresponding findings for CF or worse vision were 38%, 60%, 93.2% and 75% respectively.
10.2.1.2. VA outcome on follow-up
Of the eyes that presented with CF or worse initial VA, 37% of those seen within 7 days of onset showed an improvement in VA. In contrast, only 5% of those seen 8–30 days after onset and 9% of those seen more than 30 days after onset had any improvement in visual acuity (p < 0.0001). This indicated that CRAO with CF or worse initial VA eyes, seen within 7 days after onset, have the best potential for improved VA. Of those seen within 7 days of onset, VA outcome differed among the four types of CRAO in the eyes with initial visual acuity of CF or worse. VA improved in 82% with the transient NA-CRAO, 67% with NA-CRAO with cilioretinal artery sparing, and 22% with NA-CRAO (p < 0.001). Arteritic CRAO is caused by giant cell arteritis, which, as discussed above, is an ophthalmic emergency. This requires immediate treatment with high-dose corticosteroid therapy. Therefore, it is unethical to do a natural history study of visual outcome in it. Moreover, these eyes suffer from not only arteritic CRAO but also arteritic AION (Hayreh, 1974a; Hayreh et al., 1998).
Thus, our study showed that it is essential to classify NA-CRAO into its four types to evaluate the visual outcome in NA-CRAO realistically – this has never been done in previous studies.
10.2.2. Visual fields
As discussed above, VA essentially represents the function of the foveal region and not the rest of the retina. By contrast, visual fields plotted with a Goldmann perimeter provide information about the function of the entire retina. Ours was the first detailed, systematic study to evaluate visual fields in a large number of CRAO eyes. It provided invaluable information on visual function.
10.2.2.1. Initial visual fields in eyes seen within 7 days of onset
In these eyes, at the initial visit, in the central 30° visual field, central scotoma was the most common defect, with paracentral scotoma the next. Eyes with NA-CRAO with cilioretinal artery sparing showed an intact central island field corresponding to the area of the retina supplied by the patent cilioretinal artery. There was no scotoma in 38.5% of the eyes with transient NA-CRAO and in 6% in NA-CRAO with cilioretinal artery sparing.
Generalized constriction of the peripheral fields was more common than other types of field defect in eyes with NA-CRAO with cilioretinal artery sparing (32%) and also in those with transient NA-CRAO (17%). By contrast, 52% of NA-CRAO eyes had only a peripheral island residual field, most frequently located in the temporal region (42%). Most interestingly, the peripheral visual field was normal in 63% of the eyes with transient CRAO and 22% of those with NA-CRAO.
10.2.2.2. Visual field outcome on follow-up
This was evaluated in eyes that had a follow-up of at least 30 days, since it was felt highly unlikely that any visual field change could be expected thereafter. In all types of CRAO, both central and peripheral visual fields in a variable number of eyes showed improvement. Central visual field improved in 39% of transient NA-CRAO, 25% of NA-CRAO with cilioretinal artery sparing and 21% with NA-CRAO. Peripheral fields improved in NA-CRAO (in 39%) and in transient NA-CRAO (in 39%). Interestingly, in transient NA-CRAO with initial field defects, the central and peripheral visual fields recovered to normal in 26% and 30% respectively. The clinical importance of intact peripheral visual field is discussed above. Since 63% of the eyes with transient CRAO initially had normal peripheral field and in the rest it improved to normal in 30%; this means that it was finally normal in 93%. NA-CRAO eyes initially had normal peripheral visual field in 22% and in the rest it improved in 39%.
Thus, our natural history study showed that, contrary to the prevailing impression, spontaneous improvement in both VA and visual fields does occur in the first few days after the onset of CRAO, the extent of improvement depending very much upon the type of CRAO. There is an almost universal impression that CRAO causes generalized loss of vision. As discussed above, central scotoma is the most common visual field defect. However, it is not generally realized that CRAO can produce central scotoma only, with fairly normal peripheral visual fields (Fig. 19C). The mechanism of the selective development of the central scotoma without any peripheral visual field defect is as follows. It is well known that the macular region has more than one layer of retinal ganglion cells, unlike the rest of the retina, and it is the thickest part of the retina – maximal thickness being close to the foveola. In our experimental (Hayreh and Weingeist, 1980a; Hayreh et al., 2004) and previous clinical (Hayreh, 1976) studies and also in this clinical CRAO study, we have found that in CRAO ischemic retinal whitish opacity and swelling is essentially located in the macular region – maximum in the perifoveolar region (Figs. 14, 15A and 16, 19A, 20A). In view of this, there are two mechanisms for the development of central scotoma. (1) Permanent ischemic damage to retinal ganglion cells in the central macular region results in central scotoma. (2) In eyes with transient CRAO, when there is restoration of circulation in the central retinal artery, the retinal capillaries in the central, thickest part of the macular region may not re-fill (Fig. 20B) because of compression by the surrounding swollen superficial retinal tissue, resulting in the “no re-flow phenomenon” (Hayreh and Weingeist, 1980b), and consequently in permanent ganglion cell death in the non-perfused retina; the area of central retinal capillary non-filling may vary from eye to eye, depending upon the severity of retinal swelling in the macula region. This results in the variable size of the permanent central scotoma. Oxygen supply and nutrition from the choroidal vascular bed to the thinner peripheral retina help in its much longer survival, and the maintenance of peripheral visual fields.
10.3. Factors influencing the visual outcome in CRAO
My experimental and clinical studies on CRAO have shown that these factors include:
10.3.1. Length of CRAO
This is by far the most important factor. Our experimental study of CRAO in elderly, atherosclerotic and hypertensive rhesus monkeys (similar to most patients with CRAO) showed that the retina suffers no detectable damage with CRAO of up to 97 min but, after that, the longer the CRAO, the more extensive the irreversible damage (Hayreh et al., 2004). The study suggested that CRAO lasting for about 240 min results in massive, irreversible retinal damage (Hayreh and Jonas, 2000; Hayreh et al., 2004.)
10.3.2. Residual retinal circulation in CRAO
It is important to consider the following two issues.
10.3.2.1. Is it true that occlusion of the central retinal artery is almost always incomplete in CRAO eyes with residual retinal circulation?
The subject is discussed at length elsewhere (Hayreh, 2005). Our experimental (Hayreh et al., 2004; Hayreh, 1969, 1970, 1971; Hayreh et al., 1978, 1980; Hayreh and Weingeist, 1980a; Juarez et al., 1986a,b; Petrig et al., 1999; Kwon et al., 2005) and clinical (Hayreh, 1971, 1974b; Hayreh and Podhajsky, 1982; Mizener et al., 1997; Hayreh and Zimmerman, 2005b) studies on CRAO have shown that although the central retinal artery is completely occluded in CRAO, fluorescein fundus angiography in these eyes initially almost always shows a highly variable amount of residual retinal circulation, with sluggish filling of the retinal vasculature (Figs. 18B,C, 21). The mechanism of this residual retinal circulation with complete occlusion of the central retinal artery is discussed at length elsewhere (Hayreh, 1971; Petrig et al., 1999). It has erroneously led to a widespread belief that the artery is usually only partially occluded (David et al., 1967; Brown and Magargal, 1982), which, in turn, is used as a justification for the visual improvement claimed with many advocated therapies, even when instituted many hours or even days after the onset of CRAO (Richard et al., 1999).
Fig. 21.
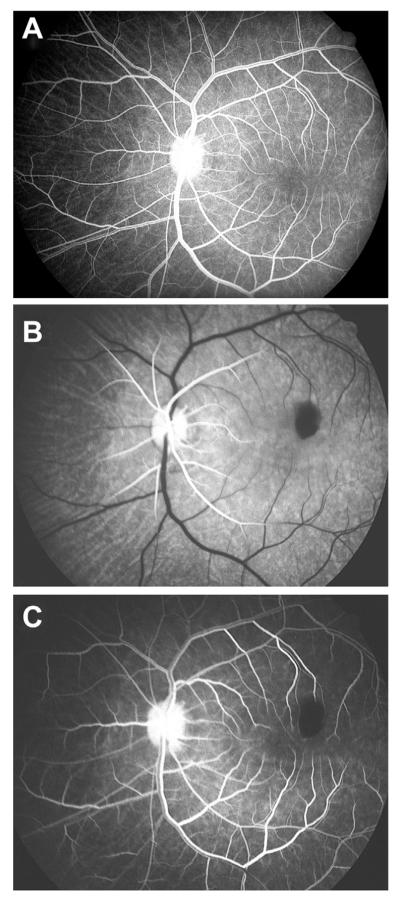
Fluorescein fundus angiograms of the left eye of a rhesus monkey, (A) before and (B,C) immediately after experimental cutting of the central retinal artery at its site of entry into the optic nerve. (A) Before cutting the artery, normal angiogram 14 s after injection of the dye, shows normal retinal vascular filling during the late arteriovenous phase. (B) After cutting the artery, angiogram 14 s after injection of the dye, shows start of filling of the retinal arterioles only up to a short distance away the disc. (C) After cutting the artery, angiogram 52 s after the injection of the dye, showing retinal vascular filling during the late arteriovenous phase, corresponding to the phase seen in (A) above. Reproduced from Hayreh, 2005; 24: 493–519.
10.3.2.2. Is it possible to obtain substantial recovery of vision in eyes with even a minimal residual retinal circulation after CRAO?
Watson (1969) very well reflected the prevailing view that if there is even minimal retinal circulation after CRAO, it is possible to obtain a considerable recovery of vision in these eyes. To place this concept in its proper perspective, I specially investigated this subject in 101 eyes of rhesus monkeys in two experimental CRAO studies (Hayreh and Weingeist, 1980a,b; Hayreh et al., 2004). That clearly showed that there was no correlation between the residual retinal circulation and recovery of visual function, as judged by electrophysiologic and morphologic studies.
10.3.3. Site of occlusion in the central retinal artery in CRAO
It is universally believed that the site of occlusion in CRAO is invariably at the level of the lamina cribrosa (as revealed by histopathologic studies of enucleated eyes). Embolism is the most common cause of CRAO. A detailed anatomical study of 100 human central retinal arteries showed that the narrowest lumen of the artery is where it pierces the dura mater of the optic nerve sheath (Fig. 22) [Hayreh 1958, Singh (Hayreh) and Dass 1960a]. Therefore, in cases of CRAO due to embolism, the chances of an embolus getting impacted at this site are much higher than at any other site in the artery. When the site of occlusion is in the dural sheath, multiple anastomoses by all the pial and intraneural collaterals of the central retinal artery (Fig. 3), distal to the occlusion site are left intact [Hayreh 1958, Singh (Hayreh) and Dass 1960b], and they play a major role in determining the amount of residual retinal circulation (Hayreh, 1971; Petrig et al., 1999). As discussed elsewhere, angiography provides very useful information about the site of occlusion (Hayreh and Zimmerman, 2005b).
Fig. 22.
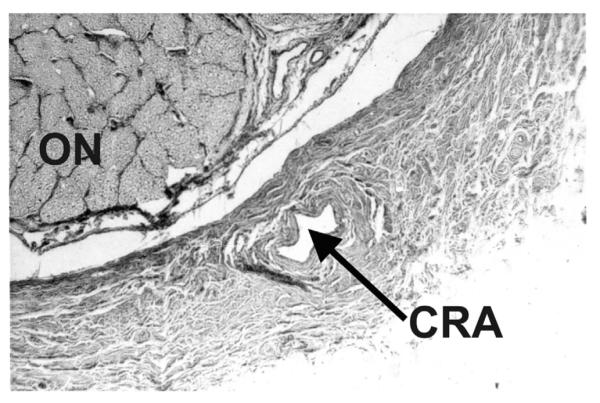
Transverse section of a human optic nerve at the level of entry of the central retinal artery into the optic nerve sheath. It shows the central retinal artery lying in the dural sheath. CRA = central retinal artery; ON = Optic nerve. (Reproduced from Hayreh SS. Master of surgery thesis. Panjab University, India 1958.)
10.3.4. Presence and the area of supply by a patent cilioretinal artery in CRAO
This can have a major impact on the visual outcome, as shown above. It depends upon the size and area supplied by the patent cilioretinal artery.
10.3.5. Causes of occlusion in CRAO
It is generally believed that the CRAO is always either embolic or thrombotic in origin. This and our previous (Hayreh and Podhajsky, 1982; Hayreh and Zimmerman, 2005b) studies on CRAO have shown that embolism is far more common than thrombosis. Very rarely, vasculitis or trauma can also cause CRAO. As discussed above, giant cell arteritis is an important and a well-known cause.
10.3.6. Fall of perfusion pressure below the critical level in the retinal vascular bed causing CRAO
Our study has revealed that several factors which produce a fall of perfusion pressure below the critical level in the retinal vascular bed can cause CRAO – usually transient CRAO. The mechanism for this is discussed at length elsewhere (Hayreh and Zimmerman, 2005b).
In conclusion, all these factors play important roles in the visual outcome in CRAO and require evaluation.
10.4. Conclusion
Classification of CRAO into 4 types is crucial for understanding the differences in its visual outcome. Significant improvement in VA and visual fields can occur without treatment and is determined by several factors. Visual field information is essential to evaluate visual disability in CRAO.
11. Natural history of visual outcome in branch retinal artery occlusion
Branch retinal artery occlusion (BRAO) is a common ocular vascular occlusive disorder. It is due to occlusion of a branch of the central retinal artery. In BRAO, as in other ocular vascular occlusive disorders (and indeed, in any disease) it is necessary to know the natural history in order to assess the effectiveness of any therapy. On this topic, I found only three studies with large enough cohorts of patients, published in peer review journals. They were those of Hayreh and Podhajsky (1982) in 44 eyes, Yuzurihara and Iijima (2004) in 30 and Mason et al. (2008) in 52. Yuzurihara and Iijima (2004), in a retrospective study of 30 eyes with BRAO, reported a final VA of 20/40 or better in 80% and worse than 20/200 in 3%. They concluded, “visual acuity in patients with BRAO is far better both at presentation and at the final visit”. Mason et al. (2008), in a retrospective study of 52 eyes with BRAO, reported that 60% of all eyes had final VA of 20/40 or better. They stated that 89% of the eyes with initial VA of 20/40 or better retained it, and 25% of those with VA of 20/50 or worse improved to 20/40 or better. They concluded: “Visual prognosis after BRAO seems to be correlated to presenting VA.”
Hayreh et al. (2009) prospectively evaluated the visual outcome (both VA and visual fields) in a cohort of 199 consecutive patients (212 eyes) with various types of BRAO. The following is a brief account of those findings (Hayreh et al., 2009).
11.1. Classification of branch retinal artery occlusion
In the literature, BRAO has been considered a single clinical entity as regards evaluation of visual outcome and management, and that has resulted in confusion and controversy. My studies have shown that BRAO is not one clinical entity, but it comprises the following 2 distinct clinical entities: (a) permanent BRAO, and (b) transient BRAO.
Therefore, to get valid information on the natural history of visual outcome in BRAO, one has to subdivide BRAO into these two categories.
11.2. Our study
In the study of Hayreh et al. (2009), there were 133 eyes with permanent BRAO and 18 transient BRAO.
11.2.1. Permanent BRAO
11.2.1.1. Visual acuity
Of the 133 eyes with permanent BRAO, among those seen within 7 days of onset, VA was 20/40 or better at the initial visit in 74% of eyes; in 64% of those with superior temporal BRAO and in 85% of those with inferior temporal BRAO. Those whose initial visit was more than 1 week after onset showed similar VA.
On follow-up of those seen within 7 days of onset, VA improved in 79% of the eyes with VA worse than 20/40. Improvement was seen in 3 of 4 (75%) eyes with inferior temporal BRAO, in 7 of 9 (78%) with superior temporal BRAO and 1 (of 1) with superior temporal + inferior temporal BRAO. In eyes seen within 7 days of onset, VA worsened in only 3% of the eyes with 20/40 or better VA. Final VA of 20/40 or better was seen in 89%.
11.2.1.2. Visual fields
Of those seen within 7 days of onset, 7% had no scotoma, 20% had central scotoma, and 13% had inferior central altitudinal defect; and there was no peripheral defect in 15% of them, with peripheral defect in 29% in the inferior nasal sector and 24% in the superior nasal sector.
On follow-up of those seen within 7 days of onset, central visual field defect improved in 47% while only 6% got worse, and peripheral visual field defect improved in 52% and got worse in 3%.
11.2.2. Transient BRAO
Of the 18 eyes with transient BRAO, 94% initially had VA of 20/40 or better and one (6%) worse than 20/40, which improved to 20/30, and the rest maintained vision of 20/40 or better on follow-up. Central and peripheral visual fields remained normal during the entire follow-up period.
Mason et al. (2008), in a retrospective study of 52 eyes with BRAO, concluded: “Visual prognosis after BRAO seems to be correlated to presenting VA.” Though we also reported that a high proportion of those that presented with VA of 20/40 or better retained 20/40 or better all along, as in the Mason et al. (2008) study, we differed in the outcome for those that had initial visual acuity of worse than 20/40; we found a significantly greater proportion of these eyes improving to 20/40 or better than their study (64% vs. 25%; p = 0.037). Thus, our study does not support the conclusion by Mason et al. (2008) that “Visual prognosis after BRAO seems to be correlated to presenting VA.”.
We also found in our study that initial VA was worse than 20/40 in 1 eye with transient BRAO, but at follow-up that improved to 20/40 or better. Final VA was 20/40 or better in 100% of transient BRAO.
The finding of an excellent final VA in patients with BRAO has important clinical implications, casting doubt on claims made from time to time about the beneficial effects on VA of one treatment modality or another. For example, it has been claimed that YAG laser embolysis/embolectomy in BRAO resulted in VA improvement (Mason et al., 2007; Opremcak et al., 2008). As discussed elsewhere (Hayreh, 2008), there were flaws in the study by Opremcak et al. (2008) which invalidate their claims; moreover, the procedure resulted in complications in 47%, including vitreous/subhyaloid hemorrhages. Similarly, Garcia-Arumi et al. (2006) claimed that in 4 of 6 eyes with temporal BRAO, “surgical embolus removal” resulted in VA improvement; but as discussed elsewhere (Hayreh, 2007) in these cases the claimed VA improvement simply represented natural history – in addition to which the procedure had complications, including development of vitreous hemorrhage. The same applies to claims of visual acuity improvement with local fibrinolysis (Richard et al., 1999; Hayreh, 1999). Of the various treatments proposed so far, in other words, none has achieved a visual outcome any better than the natural history, and all are associated with serious complications.
11.3. Factors influencing the visual outcome in BRAO
As discussed above concerning CRAO, our experimental studies on CRAO (Hayreh and Jonas, 2000; Hayreh et al., 2004), in elderly, atherosclerotic, hypertensive rhesus monkeys showed that the retina suffers no detectable damage with CRAO of 97 min but above that level, the longer the acute retinal ischemia, the more extensive and irreversible the damage, so that ischemia lasting for about 240 min results in massive, irreversible retinal damage. The retina can recover/improve function only so long as it is not irreversibly damaged by acute ischemia. Almost all of advocated modes of treatments claiming VA improvement have been performed far longer than 4 h after the onset, which indicates that their results simply reflected natural history.
My studies (Hayreh, 2005) have shown that there are two other reasons for VA improvement in BRAO without any treatment.
When the junction between the normal and infarcted retina passes through the fovea, as is the case in the majority of temporal BRAO eyes (Fig. 23) and also in CLRAO (Fig. 25), the VA may suddenly deteriorate initially, but marked spontaneous VA improvement can occur within several days or weeks, from 20/200 or worse even to normal.
Fig. 23.
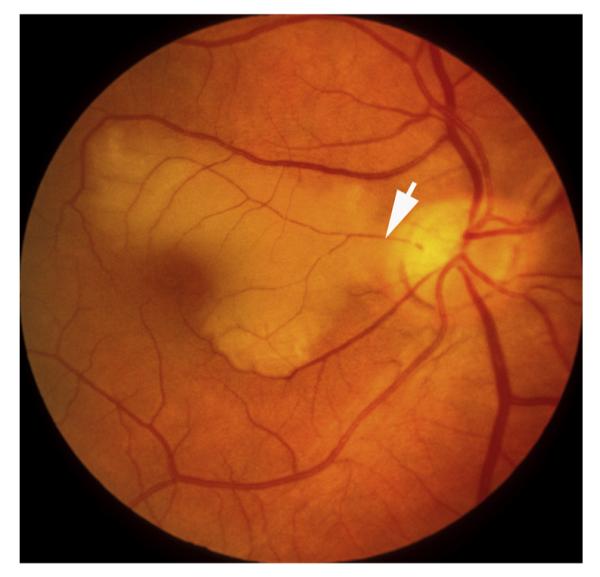
Right fundus photograph of a 71-year old woman with cilioretinal artery occlusion (Arrow).
Fig. 25.
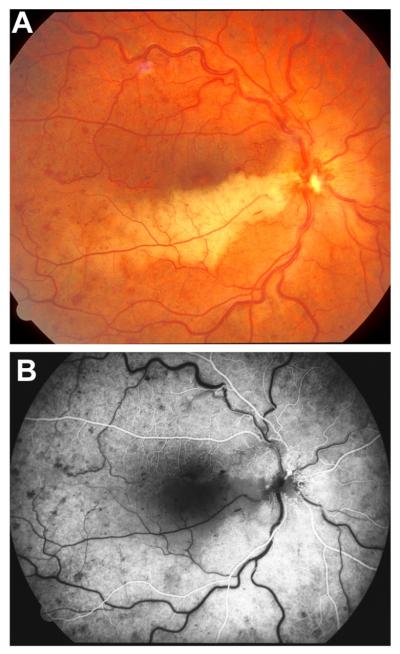
Right eye of a 32-year old man with cilioretinal artery occlusion and non-ischemic central retinal vein occlusion. (A) Fundus photograph shows retinal infarct in the region of the occluded cilioretinal artery, with engorged retinal veins, sparse retinal hemorrhages and retinociliary collaterals on the optic disc. (Reproduced from Hayreh et al., 2008; 28: 581–594.) (B) Fluorescein fundus angiogram during the retinal arterial phase shows non-filling of the retina supplied by the occluded cilioretinal artery.
VA improvement may also be due to the patient learning to fixate eccentrically.
11.4. Conclusion
These findings show that visual acuity of 20/40 or better is seen initially in 74% of permanent BRAO and 94% of transient BRAO; and finally on follow-up, in 89% and 100% respectively. Advocates of various treatments in BRAO often see this spontaneous improvement as the beneficial result of their therapy (Garcia-Arumi et al., 2006; Opremcak et al., 2008.).
12. Natural history of visual outcome in cilioretinal artery occlusion
12.1. Classification of cilioretinal artery occlusion (CLRAO)
Cilioretinal artery is a branch of the posterior ciliary artery, arising either directly from the posterior ciliary artery or from the choroid (Hayreh, 1963). Cilioretinal retinal artery occlusion (CLRAO) etiologically is of three distinct types: (i) non-arteritic CLRAO alone (Fig. 23), (ii) arteritic CLRAO associated with giant cell arteritis (Fig. 24) (Hayreh, 1974a, 1990; Hayreh et al., 1998; Hayreh and Zimmerman, 2003b), and (iii) CLRAO associated with central retinal vein occlusion (CRVO)/hemi-CRVO (Fig. 25) (Hayreh et al., 2008). Therefore, to get valid information on the natural history of visual outcome in CLRAO, one has to subdivide CLRAO into these 3 categories.
Fig. 24.
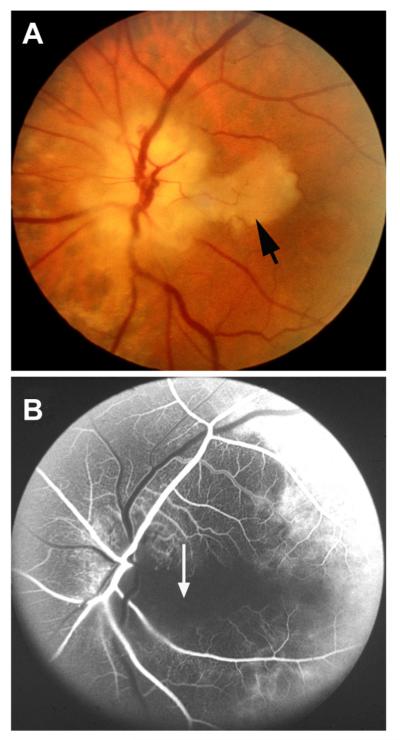
Left eye with arteritic anterior ischemic optic neuropathy and cilioretinal artery occlusion in 63-year woman with giant cell arteritis. (A) Fundus photograph shows chalky-white optic disc edema and a patch of retinal opacity in the distribution of the cilioretinal artery occlusion (Arrow). (B) Fluorescein fundus angiogram shows normal filling of the central retinal artery and of the choroid supplied by the lateral posterior ciliary artery, but no filling of the choroid and optic disc supplied by the medial posterior ciliary artery as well as of the cilioretinal artery (arrow). (Reproduced from Hayreh, 1981; 38: 675–678.)
12.2. Our study
In the study of Hayreh et al. (2009), there were 11 eyes with non-arteritic CLRAO, 12 with arteritic CLRAO and 38 with CRVO/hemi-CRVO.
12.2.1. Non-arteritic CLRAO alone
Of the 11 eyes in this category, initial VA in 3 eyes was worse than 20/40, and all 3 improved to better than 20/40 during follow-up. All the 11 eyes had a central visual defect at initial visit, (6 eyes with centrocecal scotoma, 3 with central scotoma and 2 other types of defects). Peripheral visual field defects were seen in 3 eyes with cilioretinal arteries of large size. Of the 9 eyes with follow-up, the central field improved in 4 eyes, worsened in 1, and remained stable in 4. Of the 3 eyes with peripheral field defect, only 1 had follow-up and remained stable. The 8 eyes with normal peripheral field, all remained normal during the follow-up period.
Final VA was 20/40 or better in 100% of non-arteritic CLRAO. This is similar to the number reported by Brown et al. (1983) in 10 eyes with non-arteritic CLRAO alone, of which 90% achieved 20/40 or better vision at final follow-up.
12.2.2. Arteritic CLRAO associated with giant cell arteritis
This is an extremely important clinical entity. Giant cell arteritis is a prime ophthalmic emergency. Early diagnosis and prompt treatment with intensive corticosteroid therapy can prevent any further visual loss. The diagnosis of CLRAO with giant cell arteritis is easy. The presence of a combination of chalky white optic disc edema (due to arteritic AION), retinal infarct in the region of the occluded cilioretinal artery (due to arteritic CLRAO) and the presence of posterior ciliary artery occlusion on fluorescein fundus angiography (Fig. 24) are diagnostic of this type of CLRAO (Hayreh 1974a; Hayreh and Zimmerman, 2003b).
CLRAO in giant cell arteritis has been erroneously diagnosed as “branch retinal artery occlusion” (Fineman et al., 1996), but the so-called “branch retinal arteries” are in fact arterioles, and giant cell arteritis is a disease of the medium-sized and large arteries and not of the arterioles (Hayreh et al., 1998; Hayreh and Zimmerman, 2003b). I have seen patients with CLRAO occlusion diagnosed by ophthalmologists as ordinary BRAO and left untreated, resulting in catastrophic visual loss in both eyes, which could have been prevented, if the possibility of giant cell arteritis as one of its causes had been borne in mind. The presence of posterior ciliary artery occlusion on fluorescein fundus angiography provides the correct information.
There were 12 eyes in this category in our study, with temporal artery biopsy confirmed giant cell arteritis. Three eyes had episodes of amaurosis before permanent visual loss. Of the 12 eyes, 10 had associated arteritic anterior ischemic optic neuropathy (Fig. 24A), one arteritic posterior ischemic optic neuropathy and in only one eye CLRAO was not associated with ischemic optic neuropathy. Initial deterioration of VA was primarily due to associated arteritic anterior/posterior ischemic optic neuropathy in all but one where it was 20/70. In the remaining 11 eyes, initial visual acuity was 20/20 in 1, 20/25 in 2, 20/40 in 1, counting fingers in 2, hand motion in 1, light perception in 2 and no light perception in 2. As discussed above, it is unethical to do a natural history study in arteritic CLRAO because of its association with giant cell arteritis.
12.2.3. CLRAO associated with CRVO/hemi-CRVO
A detailed account of the results of this group of CLRAO eyes is given elsewhere (Hayreh et al., 2008). In summary, of the 38 eyes, 30 had non-ischemic CRVO (Fig. 25), 5 ischemic CRVO and 3 non-ischemic hemi-CRVO. At least one third of the patients gave a definite history of episode(s) of transient visual blurring before the onset of constant blurred vision. The initial deterioration of VA in all the three groups was due to either the CLRAO involving the foveal region or macular edema caused by CRVO or hemi-CRVO; therefore, VA data are not necessarily related to CLRAO in this type of CLRAO. By contrast, central visual field defects were usually due to CLRAO, and of the 38 eyes, 16 had centrocecal scotoma, 7 central scotoma, 5 paracentral scotoma, 5 inferior nasal defect and 5 had other types.
During follow-up, VA improved markedly in eyes with associated non-ischemic CRVO (p < 0.001) and non-ischemic hemi-CRVO, but deteriorated in those associated with ischemic CRVO (primarily due to the ischemic CRVO). Like the VA, visual field improvement was common in the non-ischemic CRVO group, except in eyes where an area of retina had suffered irreversible ischemic damage. Of the 30 eyes with non-ischemic CRVO, central visual fields improved in 21, remained stable in 4 and worsened in 2, with no data in the remaining 3 eyes; in non-ischemic hemi-CRVO, the central field improved in 2 of the 3 eyes, and remained stable in one.
12.3. Factors influencing the visual outcome in CLRAO
These are the same as discussed above in BRAO.
12.4. Conclusion
The findings of our study show that VA of 20/40 or better is seen initially in 73% of non-arteritic CLRAO, and finally, on follow-up in 100%. This indicates that natural history of visual outcome in nonarteritic CLRAO is very good. The effectiveness of various treatment modalities for visual outcome has to be judged against this background.
13. Choroidal ischemia
This is seen extremely rarely. My experimental studies in rhesus monkeys showed it is due to occlusion of the posterior ciliary artery (Hayreh and Baines, 1972a,b; Hayreh and Chopdar, 1982). Since posterior ciliary artery also supplies the optic nerve head, it is almost always associated with anterior ischemic optic neuropathy. Thus, in choroidal ischemia the visual loss is primarily due to anterior ischemic optic neuropathy.
14. Amaurosis fugax in ocular vascular occlusive disorders
Before the onset of visual loss in many of the ocular vascular occlusive disorders, episodes of transient visual blurring may occur. There is little information in the literature about the prevalence of amaurosis fugax in various ocular vascular occlusive disorders, other than in giant cell arteritis with visual loss and ocular ischemic syndrome.
I systematically investigated prevalence of amaurosis fugax separately in each of these disorders in my Ocular Vascular Clinic at the University of Iowa Hospitals and Clinics. A detailed account of this is reported elsewhere (Hayreh and Zimmerman, 2014b), and following is a brief summary.
The prevalence of amaurosis fugax was 12% in CRAO, 14% in BRAO, 15% in ocular ischemic syndrome, 5% in CRVO, 38% in CRVO with cilioretinal artery occlusion, 13% in HCRVO, 0.35% in BRVO and 2.54% in NA-AION. In giant cell arteritis, 32% of patients with ocular involvement had a history of amaurosis fugax or 26.5% of the involved eyes. There was no difference in the nature of amaurosis fugax between the various ocular vascular occlusive disorders, except in BRAO, where transient visual loss or blurring was located only in the area of supply by the occluded retinal artery. The time lapse between development of amaurosis fugax and the occurrence of visual loss in the various disorders varied widely, anywhere from few hours to several days. There was no definite pattern at all.
14.1. Pathogeneses of amaurosis fugax in various ocular vascular occlusive disorders
Little information exists in the literature on this topic. Based on my basic, experimental and clinical studies on all these ocular vascular occlusive disorders, I have discussed this at length elsewhere (Hayreh and Zimmerman, 2014b). Amaurosis fugax in CRAO, BRAO and NA-AION is mostly due to transient embolism. In other conditions, other mechanisms are involved (Hayreh and Zimmerman, 2014b).
14.2. Conclusion
The prevalence and pathogenesis of amaurosis fugax in various ocular vascular occlusive disorders varies widely. It may be the presenting symptom in these disorders, and that always requires urgent evaluation. For example, in giant cell arteritis amaurosis fugax is an ominous sign of impending visual loss, and requires immediate and aggressive treatment with high-dose corticosteroid therapy to prevent visual loss.
15. Conclusions and future directions
Ocular vascular occlusive disorders collectively constitute the most common cause of visual disability. Knowledge of the natural history of visual outcome is the primary essential, because information on the natural history of a disease is vital to determine if any treatment modality advocated for these diseases is really beneficial or not. The gold standard is to compare the outcome of treatment with the natural history of the disease.
In the literature, information on the natural history of visual outcome in various ocular vascular occlusive disorders is scant, and when available, it is based on retrospective evaluation, usually of a small number of eyes and often from mixed groups of these disorders. Moreover, the information about visual improvement or deterioration in these studies is mostly based on visual acuity alone, and is also contradictory. As discussed above, my prospective studies have shown that AION, CRVO, BRVO, CRAO and BRAO each consists of multiple distinct clinical categories with different visual outcome. The prevalent impression among ophthalmologists is that there is not significant visual improvement in these disorders. My studies on the natural history of visual outcome have shown that there is a significant improvement in the majority. For information on natural history of visual outcome in each to be scientifically valid, each disorder needs further classification. Furthermore, visual fields provide important information which is not provided by the visual acuity alone. Using these classifications, and testing both visual acuity and visual fields, gives us comprehensive information about the natural history of visual outcome in these disorders, and their management.
Acknowledgments
I am grateful to many persons who have contributed in one way or another to the various studies on ocular vascular occlusive disorders over the years. The extraordinary biostatistical expertise of Professor Bridget Zimmerman has been crucial in statistical data analysis. Mrs. Patricia Podhajsky’s help with data management for all the clinical studies has been critical. I am grateful to Mrs. Patricia Duffel and Ms. Georgiane Perret for their invaluable help with bibliography, and to my wife Shelagh for her help with the manuscript.
Footnotes
Supported by grants EY-1151 and 1576 from the National Institutes of Health, and in part by unrestricted grant from Research to Prevent Blindness, Inc., New York.
Conflict of interest The author has no conflict of interest.
References
- Arnold AC, Hepler RS. Natural history of nonarteritic anterior ischemic optic neuropathy. J. Neuroophthalmol. 1994;14:66–69. [PubMed] [Google Scholar]
- Brown GC, Magargal LE. Central retinal artery obstruction and visual acuity. Ophthalmology. 1982;89:14–19. doi: 10.1016/s0161-6420(82)34853-8. [DOI] [PubMed] [Google Scholar]
- Brown GC, Moffat K, Cruess A, Magargal LE, Goldberg RE. Cilioretinal artery obstruction. Retina. 1983;3:182–187. doi: 10.1097/00006982-198300330-00007. [DOI] [PubMed] [Google Scholar]
- Chen JC, Klein ML, Watzke RC, Handelman IL, Robertson JF. Natural course of perfused central retinal vein occlusion. Can. J. Ophthalmol. 1995;30:21–24. [PubMed] [Google Scholar]
- Chopdar A. Hemi-central retinal vein occlusion. Pathogenesis, clinical features, natural history and incidence of dual trunk central retinal vein. Trans. Ophthalmol. Soc. U. K. 1982;102:241–248. [PubMed] [Google Scholar]
- Chopdar A. Dual trunk central retinal vein incidence in clinical practice. Arch. Ophthalmol. 1984;102:85–87. doi: 10.1001/archopht.1984.01040030069038. [DOI] [PubMed] [Google Scholar]
- Chopdar A. Hemispheric retinal vein occlusion or hemicentral retinal vein occlusion. Arch. Ophthalmol. 1986;104:1128–1130. doi: 10.1001/archopht.1986.01050200034028. [DOI] [PubMed] [Google Scholar]
- Clemett RS, Kohner EM, Hamilton AM. The visual prognosis in retinal branch vein occlusion. Trans. Ophthalmol. Soc. U. K. 1973;93:523–535. [PubMed] [Google Scholar]
- David N, Norton EWD, Gass JD, Beauchamp J. Fluorescein angiography in central retinal artery occlusion. Arch. Ophthalmol. 1967;77:619–629. doi: 10.1001/archopht.1967.00980020621010. [DOI] [PubMed] [Google Scholar]
- Fineman MS, Savino PJ, Federman JL, Eagle RC. Branch retinal artery occlusion as the initial sign of giant cell arteritis. Am. J. Ophthalmol. 1996;122:428–430. doi: 10.1016/s0002-9394(14)72073-2. [DOI] [PubMed] [Google Scholar]
- Finkelstein D. Ischemic macular edema. Recognition and favorable natural history in branch vein occlusion. Arch. Ophthalmol. 1992;110:1427–1434. doi: 10.1001/archopht.1992.01080220089028. [DOI] [PubMed] [Google Scholar]
- Fong AC, Schatz H, McDonald HR, Burton TC, Maberley AL, Joffe L, Zegarra H, Nadel AJ, Johnson RN. Central retinal vein occlusion in young adults (papillophlebitis) Retina. 1992;12:3–11. doi: 10.1097/00006982-199212010-00002. [DOI] [PubMed] [Google Scholar]
- Garcia-Arumi J, Boixadera A, Martinez-Castillo V, Castillo R, Dou A, Corcostegui B. Chorioretinal anastomosis after radial optic neurotomy for central retinal vein occlusion. Arch. Ophthalmol. 2003;121:1385–1391. doi: 10.1001/archopht.121.10.1385. [DOI] [PubMed] [Google Scholar]
- Garcia-Arumi J, Martinez-Castillo V, Boixadera A, Fonollosa A, Corcostegui B. Surgical embolus removal in retinal artery occlusion. Br. J. Ophthalmol. 2006;90:1252–1255. doi: 10.1136/bjo.2006.097642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuffré G, Palumbo C, Randazzo-Papa G. Optociliary veins and central retinal vein occlusion. Br. J. Ophthalmol. 1993;77:774–777. doi: 10.1136/bjo.77.12.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuffré G, Randazzo-Papa G, Palumbo C. Central retinal vein occlusion in young people. Doc. Ophthalmol. 1992;80:127–132. doi: 10.1007/BF00161238. [DOI] [PubMed] [Google Scholar]
- Glacet-Bernard A, Coscas G, Chabanel A, Zourdani A, Lelong F, Samama MM. Prognostic factors for retinal vein occlusion: prospective study of 175 cases. Ophthalmology. 1996;103:551–560. doi: 10.1016/s0161-6420(96)30653-2. [DOI] [PubMed] [Google Scholar]
- Gómez-Ulla de Irazazabal FJ, Cadarso Suarez L, Orduña, Domingo E. Hemispheric retinal branch vein occlusions. Ophthalmologica. 1986;193:14–22. doi: 10.1159/000309673. [DOI] [PubMed] [Google Scholar]
- Green WR, Chan CC, Hutchins GM, Terry JM. Central vein occlusion: a prospective histological study of 29 eyes in 28 cases. Trans. Am. Ophthalmol. Soc. 1981;79:371–421. [PMC free article] [PubMed] [Google Scholar]
- Hayreh SS. A study of the central artery of the retina in human beings (Thesis for the Master of Surgery) Panjab University; India: 1958. [Google Scholar]
- Hayreh SS. The cilio-retinal arteries. Br. J. Ophthalmol. 1963;47:71–89. doi: 10.1136/bjo.47.2.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayreh SS. Occlusion of the central retinal vessels. Br. J. Ophthalmol. 1965;49:626–645. doi: 10.1136/bjo.49.12.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayreh SS. Blood supply of the optic nerve head and its role in optic atrophy, glaucoma and oedema of the optic disc. Br. J. Ophthalmol. 1969;53:72l–748. doi: 10.1136/bjo.53.11.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayreh SS. Pathogenesis of visual field defects – role of the ciliary circulation. Br. J. Ophthalmol. 1970;54:289–311. doi: 10.1136/bjo.54.5.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayreh SS. Pathogenesis of occlusion of the central retinal vessels. Am. J. Ophthalmol. 1971;72:998–1011. doi: 10.1016/0002-9394(71)91706-5. [DOI] [PubMed] [Google Scholar]
- Hayreh SS. Anterior ischaemic optic neuropathy II. Fundus on ophthalmoscopy and fluorescein angiography. Br. J. Ophthalmol. 1974a;58:964–980. doi: 10.1136/bjo.58.12.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayreh SS. Central retinal vascular occlusion: morphology and pathogenesis. In: Brancato R, Pratesi F, editors. Ocular Angiology. Centro Minerva Medica; Rome: 1974b. pp. 15–40. [Google Scholar]
- Hayreh SS. So-called “central retinal vein occlusion”: 1. Pathogenesis, terminology, clinical features. Ophthalmologica. 1976;172:1–13. doi: 10.1159/000307579. [DOI] [PubMed] [Google Scholar]
- Hayreh SS. Anterior ischemic optic neuropathy. Arch Neurol. 1981;38:675–678. doi: 10.1001/archneur.1981.00510110035002. [DOI] [PubMed] [Google Scholar]
- Hayreh SS. Classification of central retinal vein occlusion. Ophthalmology. 1983;90:458–474. doi: 10.1016/s0161-6420(83)34530-9. [DOI] [PubMed] [Google Scholar]
- Hayreh SS. Anterior ischaemic optic neuropathy: differentiation of arteritic from non-arteritic type and its management. Eye. 1990;4:25–41. doi: 10.1038/eye.1990.4. [DOI] [PubMed] [Google Scholar]
- Hayreh SS. Central retinal vein occlusion. Ophthalmol. Clin. North Am. 1998;11:559–590. [Google Scholar]
- Hayreh SS. Retinal arterial occlusion with LIF using rTPA. Ophthalmology. 1999;106:1236–1238. 2044. doi: 10.1016/S0161-6420(99)10101-5. [DOI] [PubMed] [Google Scholar]
- Hayreh SS. Posterior ischaemic optic neuropathy: clinical features, pathogenesis and management. Eye. 2004;18:1188–1206. doi: 10.1038/sj.eye.6701562. [DOI] [PubMed] [Google Scholar]
- Hayreh SS. Prevalent misconceptions about acute retinal vascular occlusive disorders. Prog. Retin. Eye Res. 2005;24:493–519. doi: 10.1016/j.preteyeres.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Hayreh SS. Surgical embolus removal in retinal artery occlusion. Br. J. Ophthalmol. 2007;91:1096–1097. [PMC free article] [PubMed] [Google Scholar]
- Hayreh SS. Restoration of retinal blood flow via translumenal Nd:YAG embolysis/embolectomy (TYL/E) for central and branch retinal artery occlusion. Retina. 2008;28:1369–1372. doi: 10.1097/IAE.0b013e3181846640. [DOI] [PubMed] [Google Scholar]
- Hayreh SS. Ischemic optic neuropathy. Prog Retin Eye Res. 2009;28:34–62. doi: 10.1016/j.preteyeres.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Hayreh SS. Treatment of acute visual loss in giant cell arteritis: should we prescribe high-dose intravenous steroids or just oral steroids? J. Neuroophthamol. 2012;32:278–287. doi: 10.1097/WNO.0b013e3182688218. [DOI] [PubMed] [Google Scholar]
- Hayreh SS, Baines JAB. Occlusion of the posterior ciliary artery II. Chorio-retinal lesions. Br. J. Ophthalmol. 1972a;56:736–753. doi: 10.1136/bjo.56.10.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayreh SS, Baines JAB. Occlusion of the posterior ciliary artery III. Effects on the optic nerve head. Br. J. Ophthalmol. 1972b;56:754–764. doi: 10.1136/bjo.56.10.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayreh SS, Chopdar A. Occlusion of the posterior ciliary artery V. Protective influenceof simultaneous vortex vein occlusion. Arch. Ophthalmol. 1982;100:1481–1491. doi: 10.1001/archopht.1982.01030040459019. [DOI] [PubMed] [Google Scholar]
- Hayreh SS, Fraterrigo L, Jonas J. Central retinal vein occlusion associated with cilioretinal artery occlusion. Retina. 2008;28:581–594. doi: 10.1097/IAE.0b013e31815ec29b. [DOI] [PubMed] [Google Scholar]
- Hayreh SS, Hayreh MS. Hemi-central retinal vein occlusion. Pathogenesis, clinical features, and natural history. Arch. Ophthalmol. 1980;98:1600–1609. doi: 10.1001/archopht.1980.01020040452011. [DOI] [PubMed] [Google Scholar]
- Hayreh SS, Jonas JB. Optic disk and retinal nerve fiber layer damage after transient central retinal artery occlusion: an experimental study in rhesus monkeys. Am. J. Ophthalmol. 2000;129:786–795. doi: 10.1016/s0002-9394(00)00384-6. [DOI] [PubMed] [Google Scholar]
- Hayreh SS, Joos KM, Podhajsky PA, Long CR. Systemic diseases associated with non-arteritic anterior ischemic optic neuropathy. Am. J. Ophthalmol. 1994a;118:766–780. doi: 10.1016/s0002-9394(14)72557-7. [DOI] [PubMed] [Google Scholar]
- Hayreh SS, Klugman MR, Beri M, Kimura AE, Podhajsky P. Differentiation of ischemic from non-ischemic central retinal vein occlusion during the early acute phase. Graefes Arch. Clin. Exp. Ophthalmol. 1990;228:201–217. doi: 10.1007/BF00920022. [DOI] [PubMed] [Google Scholar]
- Hayreh SS, Kolder HE, Weingeist TA. Central retinal artery occlusion and retinal tolerance time. Ophthalmology. 1980;87:75–78. doi: 10.1016/s0161-6420(80)35283-4. [DOI] [PubMed] [Google Scholar]
- Hayreh SS, Podhajsky P. Ocular neovascularization with retinal vascular occlusion II. Occurrence in central and branch retinal artery occlusion. Arch. Ophthalmol. 1982;100:1585–1596. doi: 10.1001/archopht.1982.01030040563002. [DOI] [PubMed] [Google Scholar]
- Hayreh SS, Podhajsky PA, Zimmerman B. Ocular manifestations of giant cell arteritis. Am. J. Ophthalmol. 1998;125:509–520. doi: 10.1016/s0002-9394(99)80192-5. [DOI] [PubMed] [Google Scholar]
- Hayreh SS, Podhajsky PA, Zimmerman MB. Branch retinal artery occlusion: natural history of visual outcome. Ophthalmology. 2009;116:1188–1194. doi: 10.1016/j.ophtha.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayreh SS, Podhajsky PA, Zimmerman MB. Natural history of visual outcome in central retinal vein occlusion. Ophthalmology. 2011a;118:119–133. doi: 10.1016/j.ophtha.2010.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayreh SS, Podhajsky PA, Zimmerman MB. Central and hemi-central retinal vein occlusion: role of anti-platelet aggregation agents and anticoagulant. Ophthalmology. 2011b;118:1603–1611. doi: 10.1016/j.ophtha.2011.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayreh SS, Rojas P, Podhajsky P, Montague P, Woolson RF. Ocular neovascularization with retinal vascular occlusion III. Incidence of ocular neovascularization with retinal vein occlusion. Ophthalmology. 1983;90:488–506. doi: 10.1016/s0161-6420(83)34542-5. [DOI] [PubMed] [Google Scholar]
- Hayreh SS, van Heuven WAJ, Hayreh MS. Experimental retinal vascular occlusion I. Pathogenesis of central retinal vein occlusion. Arch. Ophthalmol. 1978;96:311–323. doi: 10.1001/archopht.1978.03910050179015. [DOI] [PubMed] [Google Scholar]
- Hayreh SS, Weingeist TA. Experimental occlusion of the central artery of the retina l. Ophthalmoscopic and fluorescein fundus angiographic studies. Br. J. Ophthalmol. 1980a;64:896–912. doi: 10.1136/bjo.64.12.896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayreh SS, Weingeist TA. Experimental occlusion of the central artery of the retina IV. Retinal tolerance time to acute ischaemia. Br. J. Ophthalmol. 1980b;64:818–825. doi: 10.1136/bjo.64.11.818. (See corrected bibliography and text corrections published in Br. J. Ophthalmol. 1981) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayreh SS, Zimmerman B. Visual deterioration in giant cell arteritis patients while on high doses of corticosteroid therapy. Ophthalmology. 2003a;110:1204–1215. doi: 10.1016/S0161-6420(03)00228-8. [DOI] [PubMed] [Google Scholar]
- Hayreh SS, Zimmerman B. Management of giant cell arteritis. Our 27-year clinical study: new light on old controversies. Ophthalmologica. 2003b;217:239–259. doi: 10.1159/000070631. [DOI] [PubMed] [Google Scholar]
- Hayreh SS, Zimmerman B. Visual field abnormalities in nonarteritic anterior ischemic optic neuropathy: their pattern and prevalence at initial presentation. Arch. Ophthalmol. 2005a;123:1554–1562. doi: 10.1001/archopht.123.11.1554. [DOI] [PubMed] [Google Scholar]
- Hayreh SS, Zimmerman B. Central retinal artery occlusion: visual outcome. Am. J. Ophthalmol. 2005b;140:376–391. doi: 10.1016/j.ajo.2005.03.038. [DOI] [PubMed] [Google Scholar]
- Hayreh SS, Zimmerman MB. Fundus changes in central retinal artery occlusion. Retina. 2007;27:276–289. doi: 10.1097/01.iae.0000238095.97104.9b. [DOI] [PubMed] [Google Scholar]
- Hayreh SS, Zimmerman MB. Non-arteritic anterior ischemic optic neuropathy: natural history of visual outcome. Ophthalmology. 2008;115:298–305. doi: 10.1016/j.ophtha.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayreh SS, Zimmerman MB. Hemi-central retinal vein occlusion: natural history of visual outcome. Retina. 2012a;32:68–76. doi: 10.1097/IAE.0b013e31821801f5. [DOI] [PubMed] [Google Scholar]
- Hayreh SS, Zimmerman MB. Ocular neovascularization associated with central and hemi-central retinal vein occlusion. Retina. 2012b;32:1553–1565. doi: 10.1097/IAE.0b013e318246912c. [DOI] [PubMed] [Google Scholar]
- Hayreh SS, Zimmerman MB. Branch retinal vein occlusion: natural history of visual outcome. JAMA Ophthalmol. 2014a;132:3–22. doi: 10.1001/jamaophthalmol.2013.5515. [DOI] [PubMed] [Google Scholar]
- Hayreh SS, Zimmerman MB. Amaurosis fugax in ocular vascular occlusive disorders: prevalence and pathogeneses. Retina. 2014b;34:115–122. doi: 10.1097/IAE.0b013e31829234b5. [DOI] [PubMed] [Google Scholar]
- Hayreh SS, Zimmerman B, Kardon RH. Visual improvement with corticosteroid therapy in giant cell arteritis: report of a large study and review of literature. Acta Ophthalmol. Scand. 2002;80:355–367. doi: 10.1034/j.1600-0420.2002.800403.x. [DOI] [PubMed] [Google Scholar]
- Hayreh SS, Zimmerman MB, Kimura A, Sanon A. Central retinal artery occlusion. Retinal survival time. Exp. Eye Res. 2004;78:723–736. doi: 10.1016/s0014-4835(03)00214-8. [DOI] [PubMed] [Google Scholar]
- Hayreh SS, Zimmerman MB, Podhajsky P. Incidence of various types of retinal vein occlusion and their recurrence and demographic characteristics. Am. J. Ophthalmol. 1994b;117:429–441. doi: 10.1016/s0002-9394(14)70001-7. [DOI] [PubMed] [Google Scholar]
- Heier JS, Clark WL, Boyer DS, Brown DM, Vitti R, Berliner AJ, Kazmi H, Ma Y, Stemper B, Zeitz O, Sandbrink R, Haller JA. Intravitreal Aflibercept injection for macular edema due to Central retinal vein occlusion: two-year results from the COPERNICUS study. Ophthalmology. 2014 Mar 26;:S0161–6420. doi: 10.1016/j.ophtha.2014.01.027. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Ischemic Optic Neuropathy Decompression Trial Research Group Ischemic optic neuropathy decompression trial: twenty-four-month update. Arch. Ophthalmol. 2000;118:793–797. [PubMed] [Google Scholar]
- Ischemic Optic Neuropathy Decompression Trial Research Group Optic nerve decompression surgery for nonarteritic anterior ischemic optic neuropathy (NAION) is not effective and may be harmful. J. Am. Med. Assoc. 1995;273:625–632. [PubMed] [Google Scholar]
- Juarez CP, Tso MOM, van Heuven WAJ, Hayreh MS, Hayreh SS. Experimental retinal vascular occlusion II. A clinico-pathologic correlative study of simultaneous occlusion of central retinal vein and artery. Int. Ophthalmol. 1986a;9:77–87. doi: 10.1007/BF00159836. [DOI] [PubMed] [Google Scholar]
- Juarez CP, Tso MOM, van Heuven WAJ, Hayreh MS, Hayreh SS. Experimental retinal vascular occlusion III. An ultrastructural study of simultaneous occlusion of central retinal vein and artery. Int. Ophthalmol. 1986b;9:89–101. doi: 10.1007/BF00159837. [DOI] [PubMed] [Google Scholar]
- Kwon YH, Rickman DW, Baruah S, Zimmerman MB, Kim C-S, Boldt HC, Russell SR, Hayreh SS. Vitreous and retinal amino acid concentrations in experimental central retinal artery occlusion in the primate. Eye. 2005;9:455–463. doi: 10.1038/sj.eye.6701546. [DOI] [PubMed] [Google Scholar]
- Mann I. The Development of the Eye. Grune & Stratton Inc.; New York: 1969. p. 228. [Google Scholar]
- Mason JO, Nixon PA, Albert MA. Trans-luminal nd: YAG laser embolysis for branch retinal artery occlusion. Retina. 2007;27:573–577. doi: 10.1097/IAE.0b013e3180546c4d. [DOI] [PubMed] [Google Scholar]
- Mason JO, Shah AA, Vail RS, Nixon PA, Ready EL, Kimble JA. Branch retinal artery occlusion: visual prognosis. Am. J. Ophthalmol. 2008;46:455–457. doi: 10.1016/j.ajo.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Michel J. Ueber die anatomischen Ursachen von Veranderungen des Augenhintergrundes bei einigen Allgemeinerkrankungen. Dtsch. Arch. Klin. Med. 1878;22:339–345. [Google Scholar]
- Mizener JB, Podhajsky P, Hayreh SS. Ocular ischemic syndrome. Ophthalmology. 1997;104:859–864. doi: 10.1016/s0161-6420(97)30221-8. [DOI] [PubMed] [Google Scholar]
- Moore RF. Retinal venous thrombosis. A clinical study of sixty-two cases followed over many years. Br. J. Ophthalmol. 1924;2(Suppl.):4. [Google Scholar]
- Oeller J. Atlas der Ophtlzaimoscopie Wiesbaden. C. Tabs XIII. 1896-1899.
- Opremcak E, Rehmar AJ, Ridenour CD, Borkowski LM, Kelley JK. Restoration of retinal blood flow via traslumenal Nd:YAG embolysis/embolectomy (TYL/E) for central and branch retinal artery occlusion. Retina. 2008;28:226–235. doi: 10.1097/IAE.0b013e31814b1d6e. [DOI] [PubMed] [Google Scholar]
- Parodi MB, Moretti G, Ravalico G. Hemicentral and hemispheric retinal vein occlusions. Metab. Pediatr. Syst. Ophthalmol. 1992;15:64–67. [PubMed] [Google Scholar]
- Petrig BL, Riva CE, Hayreh SS. Laser Doppler flowmetry and optic nerve head blood flow. Am. J. Ophthalmol. 1999;127:413–425. doi: 10.1016/s0002-9394(98)00437-1. [DOI] [PubMed] [Google Scholar]
- Priluck IA, Robertson DM, Hollenhorst RW. Long-term follow-up of occlusion of the central retinal vein in young adults. Am. J. Ophthalmol. 1980;90:190e–202. doi: 10.1016/s0002-9394(14)74853-6. [DOI] [PubMed] [Google Scholar]
- Quinlan PM, Elman MJ, Bhatt AK, Mardesich P, Enger C. The natural course of central retinal vein occlusion. Am. J. Ophthalmol. 1990;110:118–123. doi: 10.1016/s0002-9394(14)76979-x. [DOI] [PubMed] [Google Scholar]
- Repka MX, Savino PJ, Schatz NJ, Sergott RC. Clinical profile and long-term implications of anterior ischemic optic neuropathy. Am. J. Ophthalmol. 1983;96:478–483. doi: 10.1016/s0002-9394(14)77911-5. [DOI] [PubMed] [Google Scholar]
- Richard G, Lerche RC, Knospe V, Zeumer H. Treatment of retinal arterial occlusion with local fibrinolysis using recombinant tissue plasminogen activator. Ophthalmology. 1999;106:768–773. doi: 10.1016/S0161-6420(99)90165-3. [DOI] [PubMed] [Google Scholar]
- Rogers SL, McIntosh RL, Lim L, Mitchell P, Cheung N, Kowalski JW, Nguyen HP, Wang JJ, Wong TY. Natural history of branch retinal vein occlusion: an evidence-based systematic review. Ophthalmology. 2010;117:1094–1101. doi: 10.1016/j.ophtha.2010.01.058. [DOI] [PubMed] [Google Scholar]
- Sanborn GE, Magargal LE. Characteristics of the hemispheric retinal vein occlusion. Ophthalmology. 1984;91:1616–1626. doi: 10.1016/s0161-6420(84)34111-2. [DOI] [PubMed] [Google Scholar]
- Sawle GV, James CB, Russell RW. The natural history of non-arteritic anterior ischaemic optic neuropathy. J. Neurol. Neurosurg. Psychiatry. 1990;53:830–833. doi: 10.1136/jnnp.53.10.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergott RC, Cohen MS, Bosley TM, Savino PJ. Optic nerve decompression may improve the progressive form of nonarteritic ischemic optic neuropathy. Arch. Ophthalmol. 1989;107:1743–1754. doi: 10.1001/archopht.1989.01070020825022. [DOI] [PubMed] [Google Scholar]
- Singh (Hayreh) S., Dass R. The central artery of the retina I. Origin and course. Br. J. Ophthalmol. 1960a;44:193–212. doi: 10.1136/bjo.44.4.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh (Hayreh) S., Dass R. The central artery of the retina II. Distribution and anastomoses. Br. J. Ophthalmol. 1960b;44:280–299. doi: 10.1136/bjo.44.5.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Branch Vein Occlusion Study Group Argon laser photocoagulation for macular edema in branch retinal vein occlusion. Am. J. Ophthalmol. 1984;98:271–282. doi: 10.1016/0002-9394(84)90316-7. [DOI] [PubMed] [Google Scholar]
- The Central Vein Occlusion Study Group Natural history and clinical management of central retinal vein occlusion. Arch. Ophthalmol. 1997;115:486–491. doi: 10.1001/archopht.1997.01100150488006. [DOI] [PubMed] [Google Scholar]
- The SCORE Study Research Group A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with observation to treat vision loss associated with macular edema secondary to central retinal vein occlusion. Arch. Ophthalmol. 2009;127:1101–1114. doi: 10.1001/archophthalmol.2009.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma R, Bressler NM, Suñer I, Lee P, Dolan CM, Ward J, Colman S, Rubio RG, BRAVO and CRUISE Study Groups Improved vision-related function after ranibizumab for macular edema after retinal vein occlusion: results from the BRAVO and CRUISE trials. Ophthalmology. 2012;119:2108–2118. doi: 10.1016/j.ophtha.2012.05.017. [DOI] [PubMed] [Google Scholar]
- Von Graefe A. Über Embolie der Arteria centralis retinae als Ursache plötzlicher Erblindung. Graefes Arch. Ophthalmol. 1859;5(1)):136–185. [Google Scholar]
- Watson PG. The treatment of acute retinal arterial occlusion. In: Cant JS, editor. The William MacKenzie Centenary Symposium on the Ocular Circulation in Health and Disease (1968, Glasgow); Kimpton, London. 1969. pp. 234–245. [Google Scholar]
- Yuzurihara D, Iijima H. Visual outcome in central retinal and branch retinal artery occlusion. Jpn. J. Ophthalmol. 2004;48:490–492. doi: 10.1007/s10384-004-0102-y. [DOI] [PubMed] [Google Scholar]
- Zegarra H, Gutman FA, Conforto J. The natural course of central retinal vein occlusion. Ophthalmology. 1979;86:1931–1942. doi: 10.1016/s0161-6420(79)35327-1. [DOI] [PubMed] [Google Scholar]