Abstract
The effects of kappa opioid receptors (KOR) on motivated behavior are well established based on studies in male rodents, but relatively little is known about the effects of KOR in females. We examined the effects of KOR activation on conditioned place aversion and social interaction in the California mouse (Peromyscus californicus). Important differences were observed in long-term (place aversion) and short-term (social interaction) effects. Females but not males treated with a 2.5mg/kg dose of U50,488 formed a place aversion, whereas males but not females formed a place aversion at the 10 mg/kg dose. In contrast the short term effects of different doses of U50,488 on social interaction behavior were similar in males and females. Acute injection with 10 mg/kg of U50,488 (but not lower doses) reduced social interaction behavior in both males and females. The effects of U50,488 on phosphorylated extracellular signal regulated kinase (pERK) and p38 MAP kinase were cell type and region specific. Higher doses of U50,488 increased the number of pERK neurons in the ventrolateral bed nucleus of the stria terminals in males but not females, a nucleus implicated in male aggressive behavior. In contrast, both males and females treated with U50,488 had more activated p38 cells in the nucleus accumbens shell. Unexpectedly, cells expressing activated p38 co-expressed Iba-1, a widely used microglia marker. In summary we found strong sex differences in the effects of U50,488 on place aversion whereas the acute effects on U50,488 induced similar behavioral effects in males and females.
Keywords: Kappa opioid receptors, aversion, sex differences, social behavior
Introduction
Activation of KOR induces an aversive state, producing dysphoric like behaviors (Knoll and Carlezon, 2010). Initial work suggested that females might be more sensitive to KOR activation, as clinical studies showed that the analgesic effects of KOR agonists following dental surgery were stronger in women versus men (Gear et al., 1996a, Gear et al., 1996b). Further study has demonstrated that sex differences in the analgesic effects of KOR are dependent on the pain modality assessed (Kavaliers and Innes, 1987, Mogil et al., 2003, Rasakham and Liu-Chen, 2011, Liu et al., 2013). Much less is known about whether the behavioral effects of KOR differ in males and females, although recent reports also suggest that sex differences are context-dependent. Injections of the KOR-specific agonist U50,488 had stronger effects on posture and locomotor behavior in male Guinea pigs, but were more effective at blocking cocaine-induced hyperactivity in females (Wang et al., 2011). Activation of KOR by U50,488 also was more effective at inhibiting intrancranial self-stimulation (which stimulates brain reward systems) in males compared to females (Russell et al., 2013). Suppression of reward-related circuits is thought to contribute to dysphoria, and activation of KOR has been reported to induce dysphoria in humans (Pfeiffer et al., 1986, Walsh et al., 2001) and dysphoric-like states in rodents (Land et al., 2008). In rodents, dysphoric-like states frequently lead to the formation of a conditioned place aversion (Bruchas et al., 2007, Schindler et al., 2012, Chefer et al., 2013). The ability of KOR activation to induce place aversion has not been previously reported in females.
Kappa opioid receptors have also been reported to modulate social behaviors, particularly in the context of social conflict. The KOR antagonist nor-binaltorphimine (nor-BNI) reduced submissive behaviors in male C57Bl6 mice exposed to social defeat stress (McLaughlin et al., 2006) and increased social interaction behavior immediately after exposure to defeat stress (Bruchas et al., 2011). One of the only studies to examine the effects of KOR on social behaviors in females demonstrated that infusions of nor-BNI into the nucleus accumbens (NAc) shell reduced resident-intruder aggression in both male and female prairie voles (Resendez et al., 2012). These effects of KOR on aggressive and submissive behaviors were induced by relatively short term manipulations of KOR function. Other studies have suggested that certain experiences, such as defeat stress, may induce long term neuroplastic changes in the effects of KOR on social behavior. While U50,488 decreased social approach behavior in C57Bl6 mice that had won aggressive encounters, the same treatment increased social interaction in mice exposed to defeat stress for three weeks (Kudryavtseva et al., 2006).
We examined the effects of the selective KOR agonist U50,488 on behavior in female and male California mice (Peromyscus californicus). Unlike other rodents, both male and female California mice are aggressive (Silva et al., 2010), which has allowed for the study of social defeat stress in females (Trainor et al. 2011). We recently observed that the inhibitory effects of dopamine D1 receptors in the NAc shell on social interaction behavior were stronger in females than males (Campi et al., 2014). However, there were no sex differences in D1 receptor mRNA expression. Medium spiny neurons in the NAc that express D1 receptor also express dynorphin (Hara et al., 2006), the primary endogenous ligand for KOR (Chavkin et al., 1982). Based on these data, we hypothesized that female California mice would be more sensitive than males to KOR stimulation. We tested this hypothesis with a dose-response study examining the effects of U50,488 on conditioned place preferences. Although other specific KOR agonists are available, U50,488 is one of the best characterized KOR ligands in studies of place preference. We also examined the effects of U50,488 on social interaction behavior. Finally, we examined the effects of U50,488 on the activation of extracellular signal regulated kinase (ERK) and p38 MAP kinase, as these pathways have been found to mediate the effects of KOR (Belcheva et al., 2005, Bruchas et al., 2006, Potter et al., 2011). We examined core and shell regions of the NAc as previous reports have shown that the NAc is an important site of KOR action. We also examined the bed nucleus of the stria terminalis, which acts as a link between the mesolimbic dopamine and social behavior circuits (O’Connell and Hofmann, 2011, 2012).
Methods
Animals
California mice were bred in the UC Davis laboratory colony. All animals in the study were three month old sexually inexperienced adults. Females were tested at different stages of the estrous cycle as each mouse was tested over the course of one week. Estrous cycle was not continuously monitored because we previously determined that vaginal lavage has strong effects on behavior in California mice (Silva et al., 2010). Mice were individually marked with ear punches and housed 2–3 per cage in same sex groups. Animals were housed in clear polypropylene cages with Sani-chips bedding, environ-dri (Shepherd, Milford, NJ), and cotton nestlets. Harlan Tekland 2016 food and water were provided ad libitum. Mice were maintained on a 16h/light/8hr dark cycle (lights off 15:00 PST). All experiments were approved by the UC Davis Institutional Animal Care and Use Committee. The mice were maintained in accordance with the recommendation of the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Conditioned Place Aversion
The conditioning apparatus consisted of three interconnected standard sized mouse cages (28×17.5×11cm). All three of the cages were visually distinct. The center cage (black and white horizontal stripe background) was connected to a left cage (black and white vertical stripe background) and a right cage (black dots on a white background). Each compartment contained no bedding and the apparatus was cleaned with Quatricide (1:64, Quatricide PV in water, Pharmacal Research Labs, Inc) after each conditioning or testing session. Clear polypropylene lids were used to cover the apparatus during experiments to facilitate video recording.
Place preference testing was conducted over four days (Fig. 1). On day 1 each mouse was given free access to the entire arena for a 30 minute acclimation test (between 0800–1200). Mice were tracked in real time with a visual tracking system (Any-maze, Stoelting). Repeated measures ANOVA showed that on day 1 of testing mice spent significantly more time in the center chamber compared to the side chambers (Table 1, F2,61=15.23, p <0.001). There was also a slight but significant preference for the left chamber compared to the right (p = 0.03), but there were no sex or treatment differences (all p’s > 0.8). For each mouse, initial place biases were corrected for by assigning drug conditioning to the preferred side chamber (McLaughlin et al., 2003, Prus et al., 2009). Although more mice were conditioned in the left chamber (Table 1), there were no systematic differences between males and females or between the different drug treatments. Importantly, conditioning procedures did not change mean chamber preferences (Table 1). This suggests that the effects of conditioning are based on the aversive or rewarding aspects of U50,488 and not due to nonspecific effects on activity or anxiety-like behavior (Tzschentke, 1998). Mice were randomly assigned to be conditioned with either 0, 2.5, 5, or 10 mg/kg of the KOR selective agonist U50,488 (Tocris, Ellisville, MO, USA) dissolved in vehicle consisting of sterile phosphate buffered saline (PBS) with 10% Tween 80 (Fisher Scientific, Fair Lawn, NJ, USA).
Figure 1.

Timeline for place preference experiments and social interaction testing
Table 1.
Cage preferences before and after conditioning.
| cage time (s) | |||
|---|---|---|---|
| Center cage | Left side | Right side | |
|
| |||
| pre-test | 813±43 | 544 ± 35** | 438± 32**,† |
| post-test | 812± 72 | 580± 48** | 408±44**,† |
| number conditioned | 23 | 46 | |
p < 0.01 vs center,
vs. left side p < 0.05
Conditioning sessions took place on Days 2 and 3 based on previous methods (Bolanos et al., 1996). Each day consisted of one training session in the unconditioned chamber (AM) and one training session in the conditioned chamber (PM). First, between 0800–1100 hours each mouse was injected i.p. with vehicle and confined to the unconditioned chamber for 30 min. Subjects were confined to the unconditioned chamber by blocking off the entry way to the center chamber with heavy tape. Between 1200–1500 hr mice were injected i.p. with the assigned dose of U50,488 and confined to the conditioned chamber for 30 min. During all conditioning sessions, mice were observed for abnormal postures and immobility. Normal posture in California mice consists of a huddled position with the tail in the air. California mice treated with i.p. injections of U50,488 demonstrated consistent changes in posture such as extension of the hindlimbs and/or forelimbs, resting the tail on the cage flooring, or lying flat on the cage. These changes in posture were quantified using a Likert rating scale of 0–5, previously used to quantify the effects of KOR agonists on posture in Guinea pigs (Brent and Bot, 1992, Wang et al., 2011). Scores rate the extent to which the forelimbs and/or hind limbs are extended outward (splaying), and whether their bodies are elongated (Table 2). Mice were assigned a score every 5 minutes during each conditioning trial. On Day 4, each mouse was given free access to the entire apparatus for 30 min and was tracked with the video tracking system. The time spent in each cage and the total distance travelled were both recorded.
Table 2.
Abnormal/splaying posture rating scale (based on Wang et al. 2011)
| score | Description |
|---|---|
| 0 | No change in normal body condition |
| 1 | One leg is splayed forward or sideways, moderately elongated |
| 2 | Two legs are splayed, moderately elongated |
| 3 | More than 2 legs are splayed, elongated completely, animal does not move, except head |
| 4 | More than 2 legs are splayed, elongated completely, animal does not move |
| 5 | More than 2 legs are splayed, elongated completely, animal does not move, hind legs splayed backward |
Social Interaction
On Day 5 (Fig. 1) each mouse was tested in the social interaction test in the dark phase (1500–1700) as previously described (Trainor et al., 2013, Greenberg et al., 2014). Each mouse was injected (i.p.) with the same dose of U50,488 used in the afternoon conditioning sessions on Days 2 and 3. Forty-five minutes later subjects were tested during the dark phase of the light cycle under dim red illumination.
The apparatus for social interaction testing consisted of a large open field (89×63×60cm) and a wire cage. There were three phase of the test, 3 minutes each. First, during the open field phase, the mouse was habituated to the empty arena. Next, during the acclimation phase, an empty wire cage (14×17×14.5cm) was placed into the arena at one end. During the social interaction phase an unfamiliar, same-sex sexually inexperienced mouse was placed into the novel cage. Time spent within 8 cm of the wire cage and in two corner zones (8×8cm) opposite the cage was recorded using a video tracking system (Any-maze, Stoelting). Time spent in the center of the arena during the open field phase was recorded as a measure of anxiety-like behavior and distance traveled during the open field phase was used as an estimate of locomotor activity. Mice were anesthetized with isoflurane and euthanized by decapitation 40 minutes after the testing. Brains were immediately collected and were fixed in 5% acrolein in PBS overnight at 4° C. Brains were then transferred to 20% sucrose overnight at 4° C and then frozen and stored at −40° C.
Immunohistochemistry and quantification
Brains were sectioned at 40 μm on a cryostat and stored in cryoprotectant (50% v/v phosphate buffer, 30%w/v sucrose, 1% w/v sucrose, 1% w/v polyvinylpyrrolidone, 30% v/v ethylene glycol) at −20°C. Two different sets of sections were used for immunohistochemical staining for p-ERK and p-p38 based on previously published protocols (Trainor et al., 2010). Sections were washed three times in phosphate buffered saline (PBS) for 5 min, followed by a 10 min wash in 1% sodium borohydride in PBS. Two PBS washes for 5 min were done before blocking in 10% normal goat serum and 0.3% hydrogen peroxide in PBS for 30 min. Sections were washed with PBS twice before being incubated overnight at 4°C in primary pERK (Cat. No. 4376S, Cell Signaling, 1:300) or phosphorylated p38 (p-p38, Cat. No. 4511s, Cell Signaling, 1:500) in 2% normal goat serum and 0.5% Triton X (TX) in PBS. Day 2, sections were washed three times in PBS for 5 min, followed by incubation in secondary biotinylated goat anti-rabbit (Vector Laboratories, Burlingame, CA, 1:500) in 2% normal goat serum and 0.5% TX in PBS for 2 hours. Sections were washed three times in PBS and incubated in avidin-biotin complex (ABC Elite Kit, Vector Laboratories) for 30 minutes. Three PBS washes followed and then sections were developed in nickel enhanced diaminobenzidine (Vector Laboratories) for 90 seconds. Sections were then washed in PBS two times and mounted onto Superfrost Plus microscope slides (Fisher Scientific). Once dry, slides were dehydrated for 1 minute in 100% ethanol followed by 1 minute in Histoclear (National Diagnostics, Atlanta, GA) and coverslipped with Permount (Fisher Scientific).
Specificity for the p-ERK primary antibody in California mice has been previously described (Trainor et al., 2010). To determine specificity of the p-p38 antibody we confirmed that omission of primary antibody resulted in no positive staining (Fig 2B). We also demonstrated that pre-incubation with the immunizing peptide for the p-p38 antibody prevented positive staining (Fig. 2C). To identify the cell types that expressed p-ERK or p-38, we conducted additional double labeling experiments using neuN (to label neurons), GFAP (to label astrocytes), and Iba-1 (to label microglia). Sections were incubated in sodium borohydride and then blocked in 10% normal donkey serum for 30 min. Sections were then double labeled for p-p38 (Cat. No. 4511s, Cell Signaling, 1:500) and mouse antibodies directed at either NeuN (Cat. No. MAB377, Millipore, 1:500) or GFAP (Cat. No. N206A, Neuromab, 1:500). For Iba-1 we used a rabbit antibody Iba-1 (Cat. No. 019-19741, Wako, 1:100). Sections were incubated in primary antibodies overnight at 4° C, washed in PBS, and then incubated in secondary antibodies for 2 h. NeuN and GFAP were visualized using donkey anti-mouse Alexa Fluor 488 (Abcam ab 150105, 1:500). To visualize Iba-1 we first incubated these sections in in goat anti-rabbit fab fragment (Jackson immune Cat No. 111-007-003, 40 mg/ml) for 1 hr. After washing in PBS, these sections were incubated with donkey anti-goat Alexa Fluor 488 (Abcam ab 150129, 1:500) for 2 h. We visualized p-p38 using donkey anti-rabbit Alexa Fluor 555 antibodies (Abcam ab 150074, 1:500) for 2 h. Sections were then washed in PBS, mounted on slides and coverslipped with VectaShield (Vector).
Figure 2.

Immunostaining of phosphorylated p38 MAP kinase in California mouse nucleus accumbens (A). No staining was observed when the primary antibody was omitted (B), or if the primary antibody was preabsorbed with the immunizing peptide (C). Scale bar = 100 μm.
Images of the left and right side of each brain area were imaged with a Zeiss AxioImager based on a mouse brain atlas and previous descriptions of the California mouse NAc and BNST (Campi et al., 2013). The background for each image was normalized by adjusting the exposure time. The number of immunopositive cells in each brain region was counted in frame of uniform size (0.3 × 0.3 mm) using Image J (NIH, Bethesda, MD) by an observer unaware of treatment assignments. Cell count data are presented as number of positive cells per mm2.
Statistical Analyses
For each afternoon conditioning session, we used splaying ratings to calculate an area under the curve score (Prism Graphpad, La Jolla, CA) to summarize the effect of the injection on posture. Scores for the two conditioning sessions were averaged. Due to the non-normal distribution of these scores, splaying data were analyzed using nonparametric statistics. For place preference tests, we calculated difference scores for the conditioned chamber, unconditioned chamber, and center chamber by subtracting pretest scores (day 1) from posttest scores (day 4). Difference scores were analyzed with two-way ANOVA testing for effects of dose and sex. To test whether place preferences were affected by the location of conditioning, we conducted an additional 3-way ANOVA on these data including sex, dose, and conditioning site (left or right).
Social interaction data were analyzed with two-way ANOVA (sex and dose). For each brain area, we averaged cell counts from the left and right side and analyzed these scores with two-way ANOVA (sex and dose). Spearman rank correlations were used to correlate splaying with place preference or social interaction behavior.
Results
Effects of U50,488 on posture during conditioning
The effects of U50,488 on behavior during conditioned place aversion studies suggest that the short term effects of KOR in males and females are similar, but that there are significant sex differences in the long term effects of KOR. Splaying was never observed during morning conditioning sessions with vehicle (data not shown). During afternoon conditioning sessions, U50,488 induced splaying behavior in both males (Fig. 3, Kruskal-Wallis p < 0.01), and females (Fig. 3, Kruskal-Wallis p < 0.01), and there were no significant sex differences. All three doses of U50,488 induced significant increases in splaying compared to vehicle in both males and females. There was a nonsignificant trend for females to show a greater degree of splaying than males (Mann-Whitney p = 0.05). There were no significant differences in locomotor activity in morning or afternoon conditioning sessions.
Figure 3.
Effects of U50,488 on splaying behavior averaged across the two afternoon conditioning sessions. Posture ratings were taken every 5 minutes during conditioning and used to calculate an area under the curve (AUC) score. * p < 0.05, ** p < 0.01 Mann-Whitney U test vs. saline. n=7–12 per group.
Effects of U50,488 on place preference formation
On day 4, U50,488 induced conditioned place aversions at different doses in males and females (Fig. 4A, F3,61 = 5.14, p < 0.01). Males treated with 10 mg/kg of U50,488 spent significantly less time in the conditioned chamber compared to saline treated males (Fig. 4A). Males treated with 5 mg/kg or 2 mg/kg were not significantly different from saline. In females, a conditioned place aversion was induced by the lowest dose in females (Fig. 4A, p < 0.01 vs. saline). Intriguingly, the highest dose of U50,488 induced a conditioned place preference in females (p < 0.05 vs. saline). On average, females showed a greater increase in time spent in the unconditioned chamber compared to males (Fig. 4B, F3,61=9.1, p < 0.01). In the center chamber, there was a nonsignifcant trend for a sex x stress interaction (Fig. 4C, F3,61=2.59, p 0.06). Females treated with 10 mg/kg spent significantly less time in the center cage compared to saline treated females (p < 0.001). We also ran additional 3-way ANOVA analyses including conditioning location (left or right) as a factor. These analyses confirmed that conditioning location was not a confounding variable. Conditioning location did not explain a significant portion of variance in place preference for the conditioned, unconditioned, or center chamber (all p’s > 0.36). More importantly, the location of conditioning did not alter the effects of sex, dose, sex x dose for any variable we examined.
Figure 4.

Changes in place preferences in the conditioned chamber (A), unconditioned chamber (B) and center chamber (C). * p < 0.05, ** p < 0.01, *** p < 0.001 versus saline; † sex difference p < 0.01. n=7–12 per group
Effects of U50,488 on social interaction behavior
During social interaction testing on day 5, the effects of U50,488 were stronger in the presence of social stimuli. During the social interaction phase, the highest dose of U50,488 reduced social interaction behavior (Fig 5A, F3,61=6.24, p < 0.001) and increased time spent in the corners of the arena opposite the social stimulus (Fig. 5B, F3,61=3.29, p = 0.03). There were no significant sex differences in these variables. There were no significant differences in behavior during the acclimation phase in the absence of a social stimulus (Table 3). There were also no significant differences in locomotor behavior in the open field phase and there were no differences in time spent in the center of the arena (Table 3).
Figure 5.

Effects of U50,488 on time spent in the interaction (A) and corner (B) zones during the social interaction test. ** p < 0.01, *** p < 0.001 vs. vehicle.
Table 3.
Behavior during the acclimation and open field phases of testing immediately before social interaction testing
| Acclimation phase | Open field phase | ||||
|---|---|---|---|---|---|
| interaction zone | corner zone | center zone | distance (m) | ||
|
| |||||
| females | vehicle (n=9) | 108.5±9.8 | 9.2±3.4 | 37.3±4.7 | 22.7±2.8 |
| 2.5mg/kg(n=7) | 118.1±6.6 | 7.1±0.9 | 26.2±4.1 | 21.3±2.9 | |
| 5mg/kg(n=12) | 122.5±7.0 | 5.5±1.2 | 32.6±5.9 | 26.5±1.7 | |
| 10mg/kg(n=6) | 94.6±17.9 | 6.0±2.2 | 33.9±4.4 | 23.3±3.3 | |
| males | vehicle (n=10) | 102.6±8.3 | 3.8±1.0 | 37.0±7.0 | 27.2±2.6 |
| 2.5mg/kg(n=8) | 106.8±11.5 | 6.0±1.6 | 35.7±7.4 | 27.4±4.2 | |
| 5mg/kg(n=9) | 97.6±7.1 | 6.1±1.9 | 25.7±3.7 | 22.4±2.4 | |
| 10mg/kg(n=7) | 94.8±12.1 | 5.6±1.3 | 25.0±2.5 | 27.4±5.8 | |
Correlations between posture, place preferences, and social interaction behavior
Correlational analyses suggest that U50,488 might have different short and long term effects on behavior. Posture scores and social interaction behavior were quantified within 30 minutes of an i.p. injection, and these variables were negatively correlated with each other (Fig. 6A, Spearman rank correlation ρ = −0.50, p < 0.001). In contrast, place preference behavior was assessed in a drug free state approximately 20 hours after the last treatment of U50,488. There was no significant correlation between splaying and place preference in the conditioned chamber (Fig 6B, Spearman rank correlation ρ = −0.04, p = 0.74).
Figure 6.
Correlations between splaying during afternoon conditioning sessions (Days 2 and 3) and social interaction ratio (A, Spearman ρ = −0.5, p < 0.001) and place preference in the conditioned chamber (B, Spearman ρ = −0.04, p = 0.74).
Effects of U50,488 on p-ERK and p-p38 immunoreactivity in the nucleus accumbens and bed nucleus of the stria terminalis
Unexpectedly, p-p38 immunoreactivity was restricted exclusively to cells co-expressing the microglia marker Iba-1 (Fig 7). In the NAc shell, U50,488 treatment increased the number of p-p38 positive cells (Fig. 8A, F3,39=3.44, p < 0.05) but no sex differences or interactions. There were no significant differences in p-p38 positive cells in the NAc core (Fig. 8B). There were no significant differences in the number of p-ERK positive cells in either NAc core or shell (Fig. 8D, 8E). There were no significant correlations between cell counts and behavior in the social interaction test.
Figure 7.
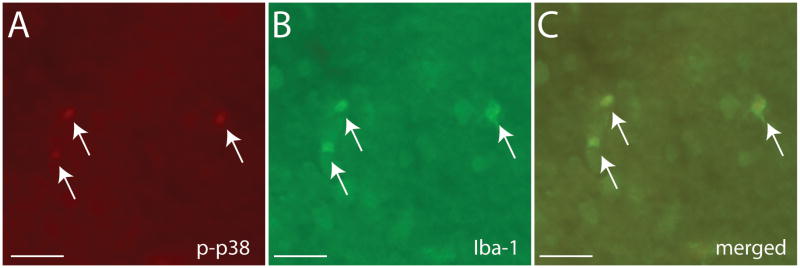
Immunostaining of p-p38 (A) and Iba-1 (B) in California mouse NAc. The merged image (C) demonstrates co-localization of p-p38 and Iba-1 in the same cells. Black indicates double-labeled cells. Scale bar = 100 μm.
Fig. 8.
Cell counts for p38 (A, B) and ERK (D, E) in the NAc shell and core. Representative photomicrographs for phosphorylated p38 (C) and ERK (D) immunostaining. * p < 0.05 vs saline, ** p < 0.01 vs saline, *** p < 0.001 vs saline. n=5–6 per group
In the BNSTam the high dose of U50,488 increased the number of p-ERK positive cells (Fig 9D, F3,39=4.52, p < 0.01). In the BNSTvl, males had more p-ERK positive cells than females (Fig. 9E, F3,39=29.95, p <0.01). Animals treated with U50,488 also had more positive p-ERK cells (F1,39=2.90, p <0.05), but this effect was driven primarily by changes in males. Males but not females treated with the 5 or 10 mg/kg dose had significantly more p-ERK positive cells compared to saline. Males had more p-p38 positive cells than females in both the BNSTam (Fig. 9A, F1,39=6.09, p < 0.05) and the BNSTvl (Fig. 9B, F1,39=7.76, p < 0.1) but there were no effects of U50,488 or interactions. There were no significant correlations between cell counts and behavior in the social interaction test.
Fig. 9.
Cell counts for p38 (A, B) and ERK (D, E) in the BNSTam and BNSTvl. Representative photomicrographs for phosphorylated p38 (C) and ERK (D) immunostaining. * p < 0.05 vs saline, ** p < 0.01 vs saline, *** p < 0.001 vs saline. † sex difference p < 0.05, †† sex difference p < 0.01. n=5–6 per group.
Discussion
This study assessed both long term and short term effects of KOR activation in both males and females. In place preference tests, which measured long term effects of KOR, males and females had different dose-response curves. Females but not males formed place aversions at low level KOR stimulation. In contrast at the highest dose of U50,488 males formed place aversions but females formed place preferences. When the short term effects of KOR stimulation were quantified in the social interaction test, males and females showed more similar behavioral responses. Social stress activates KOR signaling pathways, and in previous studies we observed that the effect of defeat stress on social interaction behavior is stronger in female California mice versus males (Trainor et al., 2011, Trainor et al., 2013, Greenberg et al., 2014). Social withdrawal in females is a long lasting behavioral change, which is consistent with our observation that females were more sensitive to the long term effects of U50,488 in the place preference tests. In contrast, when U50,488 was administered immediately before social interaction tests, KOR activation had similar effects in males and females. Previous studies have demonstrated that KOR agonists can induce rapid activation of ERK via G protein-dependent mechanisms but that long-term effects are more dependent on β-arrestins (Bruchas and Chavkin, 2010, Schmid et al., 2013, Zhou et al., 2013). Together these results suggest that KOR mediated place aversion may be mediated via β-arrestin signaling whereas acute induction of splaying or social withdrawal by KOR activation may more dependent on G protein mediated signaling.
Effects of U50,488 on behavior
The most striking sex difference in the behavioral effects of U50,488 was observed in a test that examined a long-term behavioral change: conditioned place aversion. Similar to a recent report in male rats (Wiley et al., 2009), only the 10 mg/kg dose of U50,488 induced place aversion in male California mice. In contrast, female California mice developed place aversion to 2.5 mg/kg of U50,488 but formed a place preference at 10 mg/kg. Similar biphasic dose-response curves for place aversion have been observed in male rats using either peripheral (Mucha and Herz, 1985, Bechara and Van Der Kooy, 1987) or central (Bals-Kubik et al., 1989, Bals-Kubik et al., 1993) administration of U50,488. Our results suggest that the dose-response curve to the aversive properties of U50,488 is shifted to the left in female California mice. The observation that females treated with 10 mg/kg of U50,488 developed a place preference raises the possibility of nonspecific activation of μ opioid receptors (MOR), which mediates the formation of place preferences by morphine (Sora et al., 2001). However, U50,488 has poor affinity for MOR (Clark and Pasternak, 1988) and we observed increased splaying behavior during conditioning at all doses of U50,488 in both males and females. Intriguingly, several forms of KOR-mediated analgesia in rodents are dependent on NMDA receptors in males but not females (Kavaliers and Choleris, 1997, Kavaliers et al., 1998). Activation of NMDA receptors has important modulatory effects on the formation of mu-opioid dependent place preferences (Tzschentke and Schmidt, 1995, Del Pozo et al., 1996), suggesting that sex differences in KOR-NMDA interactions could impact sex differences in sensitivity to KOR mediated place aversion. Currently, little is known about KOR-NMDA interactions in the context of place preferences.
Splaying was observed shortly after U50,488 administration, and was correlated with behavior in the social interaction test. Indeed, only the highest dose of U50,488 reduced time spent in the interaction zone with a social stimulus and increased time spent in the corner zones opposite the social stimulus. We observed no effects of any dose of U50,488 on distance traveled during the open field phase (conducted 3 min before the social interaction phase), which indicates that reduced social interaction was not due to sedation. Thus, while we observed sex differences in the long term effects of U50,488 in the place preferences tests, we observed no sex differences in the short term effects of U50,488. We originally predicted that U50,488 would have stronger effects on social interaction behavior in females, because KOR is activated by stress (Land et al., 2008) and defeat stress has stronger effects on female social interaction behavior than males (Trainor et al., 2011, Trainor et al., 2013, Greenberg et al., 2014). Thus, the lack of strong sex differences in the social interaction test was unexpected. A key factor may be the time course of KOR activation. In female California mice, social withdrawal behavior is stronger four weeks after defeat compared to 24 hours (Trainor et al., 2011). If KOR mediates effects of defeat stress on social interaction behavior, long term effects of KOR mediated by β-arrestins may be more important.
We observed little evidence for anxiogenic effects of U50,488 during open field testing. Previous studies in male rodents have recorded anxiogenic effects of KOR activation (Privette and Terrian, 1995, Knoll et al., 2007, Wittmann et al., 2009), but all of these studies were conducted during the inactive phase (lights on). Our results are more consistent with a previous study conducted during the active phase (lights off), which reported no effect of prodynorphin deletion on behavior in the zero-maze test of anxiety-like behavior (Bilkei-Gorzo et al., 2008). The behavioral effects of opioids can depend on whether testing is conducted during the active phase or inactive phase (Kavaliers and and Innes, 1987). Overall, these results suggest that the anxiogenic effects of KOR signaling may be stronger during the inactive phase. Splaying was not observed during social interaction testing. The lack of splaying might be due to increased arousal as the mice were tested during the active phase in a novel environment.
Open field and social interaction testing were conducted after a third treatment of U50,488, raising the possibility that animals may have developed tolerance with repeated U50,488 treatment (Bhargava et al., 1989). However, previous reports of KOR tolerance require more frequent and higher doses of U50,488 over a longer time frame compared to our study (McLaughlin et al., 2004). For example, in a study of intracranial self-stimulation, multiple doses of 10 mg/kg of U50,488 did not induced tolerance in male or female rats (Russell et al., 2013). Finally, activation of KOR might have different effects on male-female behavioral interactions (as opposed to the same-sex interactions observed in our study). While activation of KOR by U50,488 reduces sexual interest in male rats (Leyton and Stewart, 1992), it enhances copulatory behavior in estradiol-primed female rats (Pfaus and Pfaff, 1992). This suggests that there may be important differences in KOR-sensitive circuits mediating social interaction in same sex individuals versus sexual behavior.
Effects of U50,488 on phosphorylated p38 in the nucleus accumbens
Previous studies have implicated activation of p38 within neurons as a key mechanism mediating the aversive effects of KOR (Bruchas et al., 2007, Bruchas et al., 2011, Lemos et al., 2012). Although U50,488 increased activation of p38 in the NAc and reduced social interaction behavior, these two variables were not significantly correlated. This may be because increases in p-p38 immunoreactivity we observed occurred in microglia not neurons. The p-p38 antibody we used has been used in immunoblot analyses in previous studies, and we confirmed its specificity in California mice. Kappa opioid receptor-microglia interactions can downregulate inflammatory signaling in the brain (Feng et al., 2013), but so far the behavioral implications of these interactions have received little attention.
Microglia are best known for their role in mediating immune responses (Frank et al., 2007), and are often studied in the context of brain injury or ischemia. However, microglia have broader effects on brain function by altering synaptic structure. Microglia can align with apical dendrites, and may even eliminate inactive synapses (Wake et al., 2009, Graeber, 2010). This suggests that microglia may play a role in facilitating neuroadaptive changes that are induced by psychosocial stress (Miczek et al., 2011, Chaudhury et al., 2013). Restraint stress increases microglia proliferation in the brain, a process facilitated by corticosterone (Nair and Bonneau, 2006). Restraint stress increases the number of microglia in the NAc shell, NAc core, dorsal BNST, infralimbic cortex, and hippocampus but not amygdala or paraventricular nucleus (Tynan et al., 2010). Defeat stress also increases microglia activity in frontal cortex (Tanaka et al., 2012). Increased cytokine signaling by microglia has been hypothesized to be an important risk factor for mood disorders such as major depression (Miller et al., 2009). Certain sub-populations of patients with depression have increased levels of inflammatory cytokines (Zorrilla et al., 2001), which in animal models are closely linked to depression-like behaviors such as social withdrawal (Fenn et al., 2013). Our results suggest that these pathways could be regulated by KOR signaling via p38.
We also found that U50,488 increased p38 activation in an anatomically specific fashion. In a previous study we observed that dopamine D1 receptor antagonists infused into the NAc shell increased social interaction behavior in stressed female California mice (Campi et al., 2014). Based on this result we predicted that U50,488 would increase the number of p-p38 cells, because D1 neurons in the NAc coexpress dynorphin (Hara et al., 2006), the primary endogenous ligand for KOR (Chavkin et al., 1982). To some degree our prediction was correct, as U50,488 indeed increased the number of p-p38 positive cells in the NAc shell. However, this effect was not stronger in female than males and we had not expected to see this effect to be mediated by microglia. Our current results indicate that it will be important for future studies to consider whether stress induced activation of KOR signaling in the NAc affects neurons or microglia. Given the role of microglia in synaptic remodeling, an intriguing possibility is that microglia may be more important for mediating the long term effects of KOR signaling.
Effects of U50,488 on phosphorylated ERK in the BNST
Higher doses of U50,488 also increased pERK immunostaining in ventral BNST in males but not females. This raises an interesting question regarding the nature of social interaction and aggressive behavior. In male CD-1 mice both estrogen receptor α (ERα) and c-fos immunostaining in ventral BNST show strong positive correlations with resident-intruder aggression (Trainor et al., 2006). In male California mice, pERK immunostaining in ventral BNST is also positively correlated with male aggressive behaviors (Villalon Landeros and Trainor unpublished data). Furthermore, treatment with KOR antagonists decrease male resident-intruder aggression in prairie voles (Resendez et al., 2012). Together these data suggest that activation of KOR increases activity of the ventral BNST (either directly or indirectly), possibly facilitating aggressive behavior. However, this hypothesis appears to be at odds with our observation that higher doses of U50,488 reduced social approach; a critical first step to engage in aggression. Different neuroendocrine mechanisms govern aggressive behavior in familiar and unfamiliar environments (Fuxjager et al., 2009). This suggests the possibility that the effects of KOR activation may differ in familiar versus unfamiliar environments. Indeed, the behavioral effects of the KOR agonist salvanorin A depend on whether the individual is in a familiar or novel environment (Chartoff et al., 2008). It will be important to test whether there are sex differences in the effects of KOR in familiar versus unfamiliar environments.
Forced swim stress induces KOR-dependent activation of ERK (Bruchas et al., 2008), which has interesting implications for the relationship between stress and aggressive behavior. Interestingly, while defeat stress reliably reduces aggressive behavior (Kudryavtseva et al., 2000, Markham et al., 2010), other forms of stress such as chronic mild stress can increase aggressive behavior (Mineur et al., 2003). This suggests that different forms of stress would have differential effects on KOR signaling networks and that defeat stress would suppress ERK phosphorylation in the ventral BNST. Dynorphin neurons in the CEA have been shown to control GABAergic signaling in the dorsolateral BNST via ERK signaling (Li et al., 2012). It is not clear whether a similar pathway regulates GABAergic transmission in the BNSTvm where we observed U50,488 upregulation of pERK.
Conclusions
Our main finding was that sex differences in the behavioral effects of KOR were more substantial when long term outcomes were assessed while short term behavioral effects of KOR were more similar in males and females. This suggests that there may be stronger sex differences in β-arrestin mediated KOR signaling, which is considered to be the primary mechanism mediating long term effects of KOR. Recent studies in rodents have reported stronger effects of KOR agonists in males versus females, although these studies have focused primarily on short term behavioral effects. In addition to considering short term and long term effects of KOR signaling, it will be important to examine the behavioral effects of KOR agonists in females of different rodent species. Male and female California mice exhibit sex differences in brain anatomy (Campi et al., 2013), immune responses (Klein and Nelson, 1997), and aggressive behavior (Trainor et al., 2011) that are similar to domestic rats and mice. However sex differences in body size (Williams et al., 2013), parental behavior (Bester-Meredith and Marler, 2003) and glucocorticoid levels (Harris et al., 2012) that are commonly observed in domestic rodents are less pronounced or absent in California mice. Observing the effects of KOR in males and females of different species will be necessary to determine whether social organization exerts a consistent effect on KOR action.
Highlights.
Low doses of kappa opioid receptor agonist induced place aversion in females but not males
Acute effects of kappa agonist on social interaction behavior are similar in males and females
Kappa agonist induces activation of p38 map kinase in microglia in the brain
Acknowledgments
The authors thank C. J. Clayton for animal care. This work supported by NIH R01 MH097714 to BCT.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bals-Kubik R, Albeitner A, Herz A, Shippenberg TS. Neuroanatomical sties mediating the motivational effects of opioids as mapped by the conditioned place preference paradigm in rats. J Pharmacol Exp Ther. 1993;264:489–495. [PubMed] [Google Scholar]
- Bals-Kubik R, Herz A, Shippenberg TS. Evidence that the aversive effects of opioid antagonists and κ-agonists are centrally mediated. Psychopharmacology. 1989;98:203–206. doi: 10.1007/BF00444692. [DOI] [PubMed] [Google Scholar]
- Bechara A, Van Der Kooy D. Kappa receptors mediate the peripheral aversive effects of opiates. Pharm Biochem Behav. 1987;28:227–233. doi: 10.1016/0091-3057(87)90219-x. [DOI] [PubMed] [Google Scholar]
- Belcheva MM, Clark AL, Haas PD, Serna JS, Hahn JW, Kiss A, Coscia CJ. mu and kappa opioid receptors activate ERK/MAPK via different protein kinase C isoforms and secondary messengers in astrocytes. Journal of Biological Chemistry. 2005;280:27662–27669. doi: 10.1074/jbc.M502593200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bester-Meredith JK, Marler CA. The association between male offspring aggression and paternal and maternal behavior of Peromyscus mice. Ethology. 2003;109:797–808. [Google Scholar]
- Bhargava HN, Gulati A, Ramarao P. Effect of chronic administration of U50,488H on tolerance to its pharmacological actions and on multiple opioid receptors in rat brain regions and spinal cord. J Pharmacol Exp Ther. 1989;251:21–26. [PubMed] [Google Scholar]
- Bilkei-Gorzo A, Racz I, Michel K, Mauer D, Zimmer A, Klingmuller D, Zimmer A. Control of hormonal stress reactivity by the endogenous opioid system. Psychoneuroendocrinology. 2008;33:425–436. doi: 10.1016/j.psyneuen.2007.12.010. [DOI] [PubMed] [Google Scholar]
- Bolanos CA, Garmsen GM, Clair MA, McDougall SA. Effects of the k-opioid receptor agonist U-50,488 on morphine-induced place preference conditioning in the developming rat. Eur J Pharmacol. 1996;317:1–8. doi: 10.1016/s0014-2999(96)00698-x. [DOI] [PubMed] [Google Scholar]
- Brent PJ, Bot G. Morphine, D-Pen2, D-Pen5 enkephalin and U50,488H differentially affect the locomotor activity and behaviours induced by quinpirole in guinea-pigs. Psychopharmacology (Berl) 1992;107:581–590. doi: 10.1007/BF02245274. [DOI] [PubMed] [Google Scholar]
- Bruchas M, Chavkin C. Kinase cascades and ligand-directed signaling at the kappa opioid receptor. Psychopharmacology. 2010;210:137–147. doi: 10.1007/s00213-010-1806-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, Aita M, Xu M, Barot SK, Li S, Chavkin C. Stress-induced p38 mitogen-activated protein kinase activation mediates kappa-opioid-dependent dysphoria. Journal of Neuroscience. 2007;27:11614–11623. doi: 10.1523/JNEUROSCI.3769-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Macey TA, Lowe JD, Chavkin C. Kappa opioid receptor activation of p38 MAPK is GRK3-and arrestin-dependent in neurons and astrocytes. Journal of Biological Chemistry. 2006;281:18081–18089. doi: 10.1074/jbc.M513640200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Schindler AG, Shankar H, Messinger DI, Miyatake M, Land BB, Lemos JC, Hagan CE, Neumaier JF, Quintana A, Palmiter RD, Chavkin C. Selective p38α MAPK Deletion in Serotonergic Neurons Produces Stress Resilience in Models of Depression and Addiction. Neuron. 2011;71:498–511. doi: 10.1016/j.neuron.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Xu M, Chavkin C. Repeated swim stress induced kappa opioid-mediated activation of extracellular signal-regulated kinase 1/2. Neuroreport. 2008;19:1417–1422. doi: 10.1097/WNR.0b013e32830dd655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campi KL, Greenberg GD, Kapoor A, Ziegler TE, Trainor BC. Sex differences in effects of dopamine D1 receptors on social withdrawal. Neuropharmacology. 2014;77:208–216. doi: 10.1016/j.neuropharm.2013.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campi KL, Jameson C, Trainor BC. Sexual dimorphism in the brain of the monogamous California mouse (Peromyscus californicus) Brain Behav Evol. 2013;81:236–249. doi: 10.1159/000353260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartoff EH, Potter D, Damez-Werno D, Cohen BM, Carlezon WA., Jr Exposure to the selective k-opioid receptor agonist salvinorin A modulates the behavioral and molecular effects of coaine in rats. Neuropsychopharmacology. 2008;33:2676–2687. doi: 10.1038/sj.npp.1301659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW, Ferguson D, Tsai HC, Pomeranz L, Christoffel DJ, Nectow AR, Ekstrand M, Domingos A, Mazei-Robison MS, Mouzon E, Lobo MK, Neve RL, Friedman JM, Russo SJ, Deisseroth K, Nestler EJ, Han MH. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature. 2013;493:532–536. doi: 10.1038/nature11713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavkin C, James IF, Goldstein A. Dynorphin is a specific endogenous ligand of the kappa opioid receptor. Science. 1982;215:413–415. doi: 10.1126/science.6120570. [DOI] [PubMed] [Google Scholar]
- Chefer VI, Backman CM, Gigante ED, Shippenberg TS. Kappa Opioid Receptors on Dopaminergic Neurons Are Necessary for Kappa-Mediated Place Aversion. Neuropsychopharmacology. 2013 doi: 10.1038/npp.2013.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JA, Pasternak GW. U50,488: A kappa-selective agent with poor affinity for mu1 opiate binding sites. Neuropharmacology. 1988;27:331–332. doi: 10.1016/0028-3908(88)90052-4. [DOI] [PubMed] [Google Scholar]
- Del Pozo E, Barrios M, Baeyens JM. The NMDA receptor antagonist dizocilpine (MK-801) stereoselectively inhibits morphine-induced place preference conditioning in mice. Psychopharmacology. 1996;125:209–213. doi: 10.1007/BF02247330. [DOI] [PubMed] [Google Scholar]
- Feng X, Wu C-Y, Burton FH, Loh HH, Wei L-N. β-arrestin protects neurons by mediating endogenous opioid arrest of inflammatory microglia. Cell Death Differ. 2013 doi: 10.1038/cdd.2013.152. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenn AM, Gensel JC, Huang Y, Popovich PG, Lifshitz J, Godbout JP. Immune activation promotes depression 1 month after diffuse brain injury: a role for primed microglia. Biological Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.10.014. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Baratta MV, Sprunger DB, Watkins LR, Maier SF. Microglia serve as a neuroimmune substrate for stress-induced potentiation of CNS pro-inflammatory cytokine responses. Brain Beahvior and Immunity. 2007;21:47–59. doi: 10.1016/j.bbi.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Fuxjager MJ, Mast G, Becker EA, Marler CA. The ‘home advantage’ is necessary for a full winner effect and changes in post-encounter testosterone. Hormones and Behavior. 2009;56:214–219. doi: 10.1016/j.yhbeh.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Gear RW, Gordon NC, Heller PH, Paul S, Miaskowski C, Levine JD. Gender difference in analgesic response to the kappa-opioid pentazocine. Neurosci Lett. 1996a;205:207–209. doi: 10.1016/0304-3940(96)12402-2. [DOI] [PubMed] [Google Scholar]
- Gear RW, Miaskowski C, Gordon NC, Paul SM, Heller PH, Levine JD. Kappa-opioids produce significantly greater analgesia in women than in men. Nat Medicine. 1996b;2:1248–1250. doi: 10.1038/nm1196-1248. [DOI] [PubMed] [Google Scholar]
- Graeber MB. Changing face of microglia. Science. 2010;330:783–788. doi: 10.1126/science.1190929. [DOI] [PubMed] [Google Scholar]
- Greenberg GD, Laman-Maharg A, Campi KL, Voigt H, Orr VN, Simion C, Sweeney CA, Trainor BC. Sex differences in stress-induced social withdrawal: role of brain derived neurotrophic factor in the bed nucleus of the stria terminalis. Front Behav Neurosci. 2014;7:223. doi: 10.3389/fnbeh.2013.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara Y, Yakovleva T, Bakalkin G, Pickel VM. Dopamine D1 receptors have subcellular distributions conducive to interactions with prodynorphin in the rat nucleus accumbens shell. Synapse. 2006;60:1–19. doi: 10.1002/syn.20273. [DOI] [PubMed] [Google Scholar]
- Harris BN, Saltzman W, De Jong TR, Milnes MR. Hypothalamic-pituitary-adrenal (HPA) axis function in the California mouse (Peromyscus californicus): changes in baseline activity, reactivity, and fecal excretion of glucocorticoids aross the diurnal cycle. Gen Comp Endocrinol. 2012;179:436–450. doi: 10.1016/j.ygcen.2012.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavaliers M, Innes D. Stress-induced opiod analgesia and activity in deer mice: sex and population differences. Brain Research. 1987;425:49–56. doi: 10.1016/0006-8993(87)90482-3. [DOI] [PubMed] [Google Scholar]
- Kavaliers M, Choleris E. Sex differences in N-methyl-D-aspartate involvement in kappa opioid and non-opioid predator-induced analgesia in mice. Brain Res. 1997;768:30–36. doi: 10.1016/s0006-8993(97)00569-6. [DOI] [PubMed] [Google Scholar]
- Kavaliers M, Colwell DD, Choleris E. Sex differences in opioid and N-methyl-D-aspartate mediated non-opioid biting fly exposure induced analgesia in deer mice. Pain. 1998;77:163–171. doi: 10.1016/S0304-3959(98)00092-X. [DOI] [PubMed] [Google Scholar]
- Kavaliers M, Innes DGL. Sex and day-night differences in opiate-induced responses of insular wild deer mice, Peromyscus maniculatus triangularis. Pharmacology Biochemistry & Behavior. 1987;27:477–482. doi: 10.1016/0091-3057(87)90351-0. [DOI] [PubMed] [Google Scholar]
- Klein SL, Nelson RJ. Sex differences in immunocompetence differ between two Peromyscus species. Am J Physiol. 1997;273:R655–R660. doi: 10.1152/ajpregu.1997.273.2.R655. [DOI] [PubMed] [Google Scholar]
- Knoll AT, Carlezon WA., Jr Dynorphin, stress, and depression. Brain Res. 2010;1314:56–73. doi: 10.1016/j.brainres.2009.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll AT, Meloni EG, Thomas JB, Carroll FI, Carlezon WA., Jr Anxiolytic-like effects of κ-opioid receptor antagonists in models of unlearned and learned fear in rats. J Pharmacol Exp Ther. 2007;323:838–845. doi: 10.1124/jpet.107.127415. [DOI] [PubMed] [Google Scholar]
- Kudryavtseva NN, Bondar NP, Alekseyenko OV. Behavioral correlates of learned aggression in male mice. Agg Behav. 2000;26:386–400. [Google Scholar]
- Kudryavtseva NN, Gerrits MAFM, Avgustinovich DF, Tenditnik MV, Van Ree JM. Anxiety and ethanol consumption in victorious and defeated mice; effect of κ-opioid receptor activation. Eur Neuropsychopharmacol. 2006;16:504–511. doi: 10.1016/j.euroneuro.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, Chavkin C. The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:407–414. doi: 10.1523/JNEUROSCI.4458-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos JC, Roth CA, Messinger DI, Gill HK, Phillips PEM, Chavkin C. Repeated stress dysregulates k-opioid receptor signaling in the dorsal raphe through a p38a MAPK-dependent mechanism. J Neurosci. 2012;32:12325–12336. doi: 10.1523/JNEUROSCI.2053-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyton M, Stewart J. The stimulation of central k opioid receptors decreases male sexual behavior and locomotor activity. Brain Res. 1992;594:56–74. doi: 10.1016/0006-8993(92)91029-e. [DOI] [PubMed] [Google Scholar]
- Li C, Pleil KE, Stamatakis AM, Busan S, Vong L, Lowell BB, Stuber GD, Kash TL. Presynaptic inhibition of gamma-aminobutytic acid release in the bed nucleus of the stria terminalis by kappa opioid receptor signaling. Biol Psychiatry. 2012;71:725–732. doi: 10.1016/j.biopsych.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N-J, Schnell S, Wessendorf MW, Gintzler AR. Sex, Pain, and Opioids: Interdependent Influences of Sex and Pain Modality on Dynorphin-Mediated Antinociception in Rats. Journal of Pharmacology and Experimental Therapeutics. 2013;344:522–530. doi: 10.1124/jpet.112.199851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham CM, Taylor SL, Huhman KL. Role of amygdala and hippocampus in the neural circuit subserving conditioned defeat in Syrian hamsters. Learn Mem. 2010;17:109–116. doi: 10.1101/lm.1633710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JP, Li S, Valdez J, Chavkin TA, Chavkin C. Social defeat stress-induced behavioral responses are mediated by the endogenous kappa opioid system. Neuropsychopharmacology. 2006;31:1241–1248. doi: 10.1038/sj.npp.1300872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JP, Marton-Popovici M, Chavkin C. Kappa opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses. J Neurosci. 2003;23:5674–5683. doi: 10.1523/JNEUROSCI.23-13-05674.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JP, Myers LC, Zarek PE, Caron MG, Lefkowitz RJ, Czyzyk TA, Pintar JE, Chavkin C. Prolonged Kappa Opioid Receptor Phosphorylation Mediated by G-protein Receptor Kinase Underlies Sustained Analgesic Tolerance. J Biol Chem. 2004;279:1810–1818. doi: 10.1074/jbc.M305796200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek KA, Nikulina EM, Shimamoto A, Covington HE., III Escalated or suppressed cocaine reward, tegmental BDNF, and accumbal dopamine caused by episodic versus continuous social stress in rats. J Neurosci. 2011;31:9848–9857. doi: 10.1523/JNEUROSCI.0637-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineur YS, Prasol DJ, Belzung C, Crusio WE. Agonistic behavior and unpredictable chronic mild stress in mice. Behavior Genetics. 2003;33:513–519. doi: 10.1023/a:1025770616068. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Wilson SG, Chesler EJ, Rankin AL, Nemmani KV, Lariviere WR, Groce MK, Wallace MR, Kaplan L, Staud R, Ness TJ, Glover TL, Stankova M, Mayorov A, Hruby VJ, Grisel JE, Fillingim RB. The melanocortin-1 receptor gene mediates female-specific mechanisms of analgesia in mice and humans. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:4867–4872. doi: 10.1073/pnas.0730053100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucha RF, Herz A. Motivational properties of kappa and mu opioid receptor agonists studied with place and taste conditioning. Psychopharmacology. 1985;86:274–280. doi: 10.1007/BF00432213. [DOI] [PubMed] [Google Scholar]
- Nair A, Bonneau RH. Stress-induced elevation of glucocorticoids increases microglia proliferation through NMDA receptor activation. J Neuroimmunol. 2006;171:72–85. doi: 10.1016/j.jneuroim.2005.09.012. [DOI] [PubMed] [Google Scholar]
- O’Connell LA, Hofmann HA. The vertebrate mesolimbic reward system and social behavior network: a comparative synthesis. J Comp Neurol. 2011:3599–3639. doi: 10.1002/cne.22735. [DOI] [PubMed] [Google Scholar]
- O’Connell LA, Hofmann HA. Evolution of a vertebrate social decision-making network. Science. 2012;336:1154–1157. doi: 10.1126/science.1218889. [DOI] [PubMed] [Google Scholar]
- Pfaus JG, Pfaff DW. μ-, δ- and κ-opioid receptor agonists selectively modulate sexual behaviors in the female rat: differential dependence on progesterone. Horm Behav. 1992;26:457–473. doi: 10.1016/0018-506x(92)90014-m. [DOI] [PubMed] [Google Scholar]
- Pfeiffer A, Brantl V, Herz A, Emrich HM. Psychotomimesis mediated by κ opiate receptors. Science. 1986;233:774–776. doi: 10.1126/science.3016896. [DOI] [PubMed] [Google Scholar]
- Potter DN, Damez-Werno D, Carlezon WA, Jr, Cohen BM, Chartoff EH. Repeated exposure to the k-opioid receptor agonist salvinorin a modulates extracellular signal-regulated kinase and reward sensitivity. Biol Psychiatry. 2011;70:744–753. doi: 10.1016/j.biopsych.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Privette TH, Terrian DM. Kappa opioid agonists produce anxiolytic-like behavior on the elevated plus-maze. Psychopharmacology. 1995;118:444–450. doi: 10.1007/BF02245945. [DOI] [PubMed] [Google Scholar]
- Prus AJ, James JR, Rosencrans JA. Conditioned place preference. In: Buccafusco JJ, editor. Methods of behavior analysis in neuroscience. Boca Raton, FL: CRC Press; 2009. [Google Scholar]
- Rasakham K, Liu-Chen L-Y. Sex differences in kappa opioid pharmacology. Life Sci. 2011;88:2–16. doi: 10.1016/j.lfs.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resendez SL, Kuhnmuench M, Krzywosinski T, Aragona BJ. κ-opioid receptor within the nucleus accumbens shell mediate pair bond maintenance. J Neurosci. 2012;32:6771–6784. doi: 10.1523/JNEUROSCI.5779-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell SE, Rachlin AB, Smith KL, Muschamp JW, Berry L, Zhao Z, Chartoff EH. Sex difference in sensitivity to the depressive-like effects of the kappa opioid receptor agonist U-50488 in rats. Biol Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.07.042. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler AG, Messinger DI, Smith JS, Shankar H, Gustin RM, Schattauer SS, Lemos JC, Chavkin NW, Hagan CE, Neumaier JF, Chavkin C. Stress produces aversion and potentiates cocaine reward by releasing endogenous dynorphins in the ventral striatum to locally stimulate serotonin reuptake. J Neurosci. 2012;32:17582–17596. doi: 10.1523/JNEUROSCI.3220-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid CL, Streicher JM, Groer CE, Munro TA, Zhou L, Bohn LM. Functional selectivity of 6′-guanidinonaltrindole (6′-GNTI) at κ-opioid receptors in striatal neurons. J Biol Chem. 2013;288:22387–22398. doi: 10.1074/jbc.M113.476234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AL, Fry WH, Sweeney C, Trainor BC. Effects of photoperiod and experience on aggressive behavior in female California mice. Behav Brain Res. 2010;208:528–534. doi: 10.1016/j.bbr.2009.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sora I, Elmer G, Funada M, Pieper J, Li X-F, Hall FS, Uhl GR. μ opiate receptor gene dose effects on different morphine actions: evidence for differential in vivo μ receptor reserve. Neuropsychopharmacology. 2001;25:41–54. doi: 10.1016/S0893-133X(00)00252-9. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Furuyashiki T, Kitaoka S, Senzai Y, Imoto Y, Segi-Nishida E, Deguchi Y, Breyer RM, Breyer MD, Narumiya S. Prostaglandin E2-Mediated Attenuation of Mesocortical Dopaminergic Pathway Is Critical for Susceptibility to Repeated Social Defeat Stress in Mice. The Journal of Neuroscience. 2012;32:4319–4329. doi: 10.1523/JNEUROSCI.5952-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor BC, Crean KK, Fry WHD, Sweeney C. Activation of extracellular signal-regulated kinases in social behavior circuits during resident-intruder aggression tests. Neuroscience. 2010;165:325–336. doi: 10.1016/j.neuroscience.2009.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor BC, Greiwe KM, Nelson RJ. Individual differences in estrogen receptor α in select brain nuclei are associated with individual differences in aggression. Horm Behav. 2006;50:338–345. doi: 10.1016/j.yhbeh.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor BC, Pride MC, Villalon Landeros R, Knoblauch NW, Takahashi EY, Silva AL, Crean KK. Sex differences in social interaction behavior following social defeat stress in the monogamous California mouse (Peromyscus californicus) PLOS One. 2011;6:e17405. doi: 10.1371/journal.pone.0017405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor BC, Takahashi EY, Campi KL, Florez SA, Greenberg GD, Laman-Maharg A, Laredo SA, Orr VN, Silva AL, Steinman MQ. Sex differences in stress-induced social withdrawal: independence from adult gonadal hormones and inhibition of female phenotype by corncob bedding. Horm Behav. 2013;63:543–550. doi: 10.1016/j.yhbeh.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tynan RJ, Naicker S, Hinwood M, Nalivaiko E, Buller KM, Pow DV, Day TA, Walker FR. Chronic stress alterrs the density and morphology of microglia in a subset of stress-responsive brain regions. Brain Beahvior and Immunity. 2010;24:1058–1068. doi: 10.1016/j.bbi.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference paradigm: a comprehenzive review of drug effects, recent progress and new issues. Prog Neurobiol. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM, Schmidt WJ. N-Methyl-D-aspartic acid receptor antagonists block morphine-induced conditioned place preference in rats. Neurosci Lett. 1995;193:37–40. doi: 10.1016/0304-3940(95)11662-g. [DOI] [PubMed] [Google Scholar]
- Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses In vivo and determine the fate of ischemic terminals. J Neurosci. 2009;29:3974–3980. doi: 10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh SL, Geter-Douglas B, Strain EC, Bigelow GE. Endoline and butorphanol: evaluation of k-agonists on cocaine pharmacodynamics and cocaine self-administration in humans. J Pharmacol Exp Ther. 2001;299:147–158. [PubMed] [Google Scholar]
- Wang YJ, Rasakham K, Huang P, Chudnovskaya D, Cowan A, Liu-Chen LY. Sex Difference in kappa-Opioid Receptor (KOPR)-Mediated Behaviors, Brain Region KOPR Level and KOPR-Mediated Guanosine 5 ′-O-(3-[S-35]Thiotriphosphate) Binding in the Guinea Pig. Journal of Pharmacology and Experimental Therapeutics. 2011;339:438–450. doi: 10.1124/jpet.111.183905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley MD, Poveromo LB, Antapasis J, Herrera CM, Bolaños Guzmán CA. k-opioid system regulates the long-lasting behavioral adaptations induced by early-life exposure to methylphenidate. Neuropsychopharmacology. 2009;34:1339–1350. doi: 10.1038/npp.2008.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SA, Jasarevic E, Vandas GM, Warzak DA, Geary DC, Ellersieck MR, Roberts RM, Rosenfeld CS. Effects of Developmental Bisphenol A Exposure on Reproductive-Related Behaviors in California Mice (Peromyscus californicus): A Monogamous Animal Model. PloS One. 2013;8:e55698. doi: 10.1371/journal.pone.0055698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann W, Schunk E, Rosskothen I, Gaburro S, Singewald N, Herzog H, Schwarzer C. Prodyndophin-derived peptides are critical modulators of anxiety and regulate neurochemistry and corticosterone. Neuropsychopharmacology. 2009;34:775–785. doi: 10.1038/npp.2008.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Lovell KM, Frankowski KJ, Slauson SR, Phillips AG, Streicher JM, Stahl E, Schmid CL, Hodder P, Madoux F, Cameron MD, Prisinzano TE, Aube J, Bohn LM. Development of functionally selective small molecule agonists at kappa opioid receptors. J Biol Chem. 2013;288:36703–36716. doi: 10.1074/jbc.M113.504381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla EP, Luborsky L, McKay JR, Rosenthal R, Houldin A, Tax A, McCorkle R, Seligman DA, Schmidt K. The relationship of depression and stressors to immunological assays: a meta-analytic review. Brain Beahvior and Immunity. 2001;15:199–226. doi: 10.1006/brbi.2000.0597. [DOI] [PubMed] [Google Scholar]






