Abstract
Standard total vascular exclusion (TVE) of the liver is indicated for resection of tumors involving or adjacent to the vena cava and/or the confluence of the hepatic veins. The duration of liver ischemia can be prolonged by combined portal hypothermic perfusion of the liver (in or ex situ). The use of a venovenous bypass (VVB) during standard TVE maintains stable hemodynamics as well as optimal renal and splanchnic venous drainage. When the hepatic veins can be controlled, TVE preserving the caval flow negates the need for VVB. However this technique remains limited in duration as it is performed under warm ischemia (so-called normothermia) of the liver. To prolong the ischemia time, we have designed a modification of TVE with preservation of the caval flow including the use of temporary porta-caval shunt (PCS) and hypothermic perfusion of the liver. We describe here the first two cases of this new technique. Two patients underwent left hepatectomy extended to segments 5 and 8 (also called extended left hepatectomy) for large centrally located tumors. TVE lasted seventy-two and seventy-nine minutes, respectively. The postoperative course was uneventful and both patients were discharged on day ten and day twenty-five respectively. Both are alive without recurrence at ten and seven months following surgery. Provided the roots of the hepatic veins can be controlled, this technique combines the advantages of standard TVE with in situ hypothermic perfusion and VVB and obviates the need and the subsequent risks of the latter.
Keywords: Total vascular exclusion (TVE), hypothermic perfusion, porta-caval shunt (PCS), venovenous bypass (VVB)
Background
Standard total vascular exclusion (standard TVE) of the liver includes clamping of the portal triad and the vena cava below and above the liver (1). It is indicated for the resection of tumors involving or adjacent to the vena cava and/or to the confluence of the hepatic veins.
With portal hypothermic perfusion, the duration of TVE can be prolonged to up to several hours (2,3). In the vast majority of cases of standard TVE with hypothermic perfusion of the liver, a venovenous bypass (VVB) is installed (usually cavo-porto jugular) to maintain stable hemodynamics and prevent kidneys and splanchnic venous congestion. Some centrally located tumors with intimate contact with larges branches of the hepatic veins but still not invading their roots into the inferior vena cava need TVE to be resected safely. In this situation it is possible to perform the resection under TVE while preserving the caval flow by clamping the portal pedicle and the hepatic veins extra-hepatically (4). This technique obviates both the detrimental hemodynamic effects and the kidneys venous congestion of standard TVE. However, as for the latter, it is limited in duration and it is associated with splanchnic congestion due to portal clamping. We report here the two first cases of a new technique of TVE with a temporary porta-caval shunt (PCS) and in situ portal hypothermic perfusion of the liver. In these cases the use of VVB and its subsequent risks are obviated.
Methods
Case 1
A 72-year-old female underwent a left hepatectomy extended to segments 5 and 8 for a huge (13 cm) hepatocellular carcinoma (HCC) located in segments 4, 5 and 8 of the liver and involving the left and middle hepatic veins below their common root into the vena cava. Tumor and non-tumor liver biopsies confirmed the diagnosis of HCC and normal underlying liver parenchyma, respectively. The liver and kidney function tests were normal. Alpha fetoprotein serum level (AFP) was 1,904 ng/mL. The remnant liver to body weight ratio was 0.88 on computed tomography (CT) volumetry. In addition, CT scan showed the presence of large intra-parenchyma hepatic veins collateral circulation between the middle and the right hepatic vein territory (Figure 1). The TVE was predicted to last potentially longer than 60 minutes and therefore the patient had TVE of the liver with in situ hypothermic portal perfusion with the technique described below.
Figure 1.
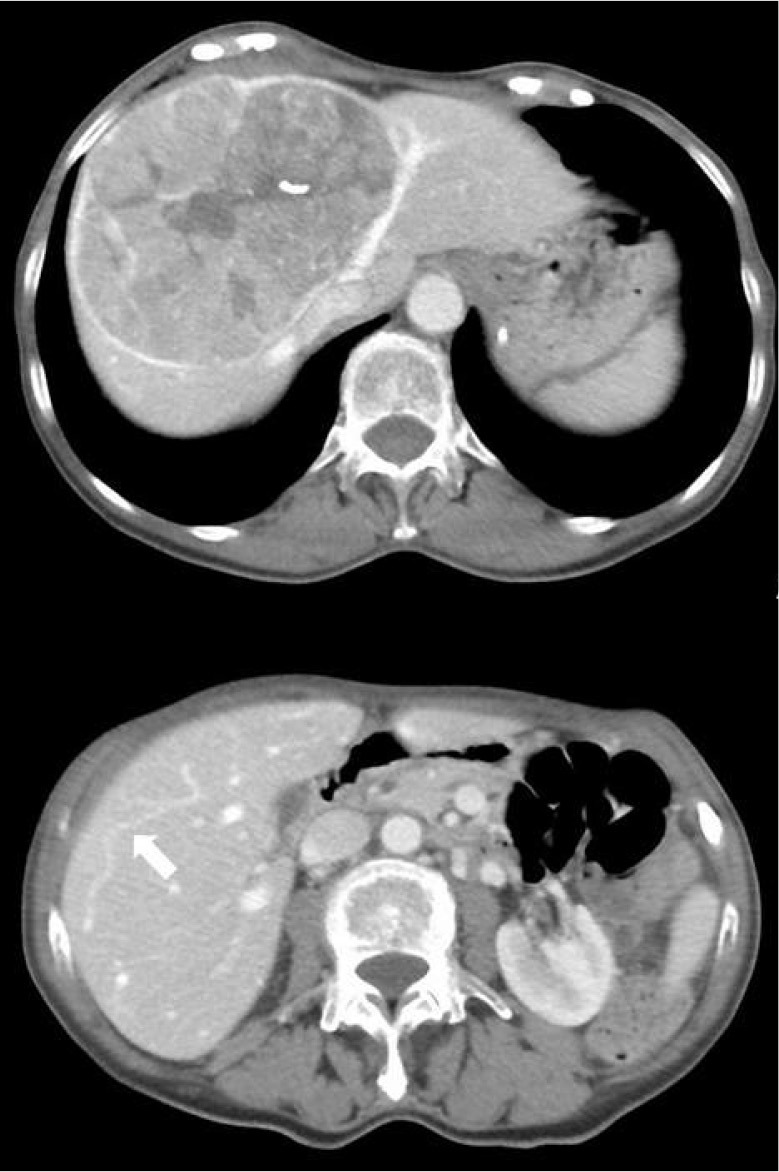
Preoperative CT revealed a huge tumor that involves the left and middle hepatic veins below their common root into the vena cava (case 1), and the presence of a communicating vein (white arrow) between the middle and the right hepatic veins.
Surgery was accomplished through a bilateral subcostal abdominal incision with upper midline extension. The liver attachments and the left branch of the hepatic artery and the portal vein were divided. The proximal stump of the divided left portal vein, sufficiently long in this case, was anastomosed end-to-side to the infra-hepatic vena cava. The common trunk of the left and middle hepatic veins was stapled. All minor hepatic veins as well as the hepatocaval ligament were ligated and divided. The main bile duct, the proper hepatic artery, the right portal vein above the PCS and the right hepatic vein were then clamped. The right portal vein was catheterized above the portal clamp and University of Wisconsin solution chilled at 4 °C was used for hypothermic perfusion of the liver (2 L were perfused). A venotomy was made immediately below the clamp on the right hepatic vein to drain the perfusate.
The liver temperature (3) dropped to a minimum of 17 °C. The transection of the liver was completed with ultrasonic dissector to the left of the right hepatic vein leaving the segments 6 and 7 intact. The liver was then flushed with 500 mL of serum albumin via the portal vein. The portal cannula was removed. Perfusate inflow and outflow incisions were sutured transversally to prevent stenosis with interrupted nonabsorbable sutures and the PCS was divided and closed. The total ischemia time was 72 minutes. The patient received 7 units of packed red blood cells and 5 units of fresh frozen plasma. The weight of the specimen was 964 grams. Histopathological examination showed a huge, well differentiated, encapsulated HCC with macrovascular invasion of the middle hepatic vein and a R0 resection margin. The postoperative course was uneventful and the patient was discharged on postoperative day 10. She is alive and well 10 months after surgery with no evidence of recurrent disease.
Case 2
A 57-year-old male underwent a left hepatectomy extended to segments 5, 8 and 1 for a 8.5 cm intra hepatic cholangiocarcinoma (IHCC) involving the left hepatic duct and the left branch of the portal vein. Large collaterality from the middle and left hepatic veins territory to the right hepatic vein territory was demonstrated on pre-operative CT scan. Tumor and non-tumor liver biopsies confirmed the diagnosis of IHCC and 40% macrovacuolar steatosis of the underlying liver parenchyma.
Liver and kidney function tests were normal as well as tumor markers (i.e., AFP and CA19-9). A preoperative embolization of the left portal vein and of the right anterior portal branch was performed because of the underlying liver steatosis. Following this, the remnant liver to body weight ratio increased from 0.80 to 1.09 on CT scan volumetry. As the duration of TVE was planned to last more than 60 minutes, hypothermic perfusion was performed.
A bilateral subcostal abdominal incision with upper midline extension was performed, as previously described. The left portal vein and the common trunk of left and middle hepatic veins were dissected free. Next, they were clamped and transected, and a side-to-side PCS was performed between the portal trunk and the infrahepatic vena cava. The latter shunt was chosen as the left portal vein was too short. The procedure was then conducted as for the first patient. In that case, a large right inferior hepatic vein was preserved and clamped. The liver temperature dropped to a minimum of 24 °C and the total ischemia time was 79 minutes. Five units of packed red cells were transfused. The postoperative course was uneventful and the patient was discharged on postoperative day 25. Histopathological examination of the specimen confirmed the diagnosis of IHCC and a R0 resection margin. At the time of writing, the patient is alive and well seven months after surgery without any evidence of recurrence.
Results and discussion
With the advance in surgical technique, liver resection under hypothermic perfusion remains rare (<1% of liver resections) and is dedicated to tumours invading the cavo-hepatic junction (and/or associated with intrahepatic hepatic veins collateral circulation) and if vascular resection-reconstruction is required for the remnant liver. The majority of patients with “limited vascular invasion” can nowadays be operated safely with intermittent occlusion of the hepatic pedicle and the most difficult part of the resection can be done under short TVE or isolated occlusion of the infra hepatic vena cava (5). This new technique is very important because resection can be performed safely under hypothermic perfusion without VVB.
Indications
This novel approach should be limited to large, centrally located tumors in contact with large branches of the hepatic veins but not involving their roots into the vena cava particularly when large intrahepatic collaterality between hepatic veins imposes early vascular exclusion. More experience with the presented technique, including right sided hepatectomies, is needed to ascertain its impact on ischemia-reperfusion injury, postoperative morbidity and mortality. For other indications of TVE needing hypothermic perfusion the standard TVE with VVB remains safer (3).
Technical aspects
Other options to operate the type of tumors discussed here could be to start the hepatic transection under intermittent clamping of the hepatic pedicle and apply short standard TVE when approaching the vascular contact (5). We decided to resort to the technique described here as there was, in both cases, a significant risk of bleeding from large interhepatic veins collateral circulation encountered usually from the beginning of the transection. Another approach recently described could have been used (6).
The temporary-portocaval shunt is a straightforward procedure particularly in units specialized in liver transplantation (7). This shunt can be performed in different ways including those described here. Other options include the construction of a temporary mesenterico-caval or spleno-renal shunt.
During TVE special attention must be paid to the assurance that the liver is completely excluded. An unknown patency of an accessory hepatic vein during hypothermic perfusion could cause cold perfusion to the heart of the patient with consequent cardioplegy. All minor hepatic veins should be divided and any inferior large hepatic vein to be preserved should be clamped during the liver hypothermic perfusion.
Advantages and drawbacks of this technique with reference to resection under other types of vascular occlusion without hypothermic perfusion
One of the major advantages of this new technique (schematized in Figures 2 and 3) as compared to the conventional techniques is to combine the advantages of standard TVE with in situ hypothermic perfusion of the liver with those of VVB (3). This achieves stable hemodynamics and optimal venous drainage of the kidneys via the preservation of the caval flow; and prevents splanchnic congestion via the PCS while obviating the specific risks of VVB. The latter are significant and include bleeding from vascular injury, air embolism, hemomediastinum, hypotension, atrial fibrillation, seromas or lymphoceles, wound infections and nerve injuries (8,9). In addition, extracoporeal circulation might favor tumor cell dissemination and jeopardize the oncologic outcome of these patients (10).
Figure 2.
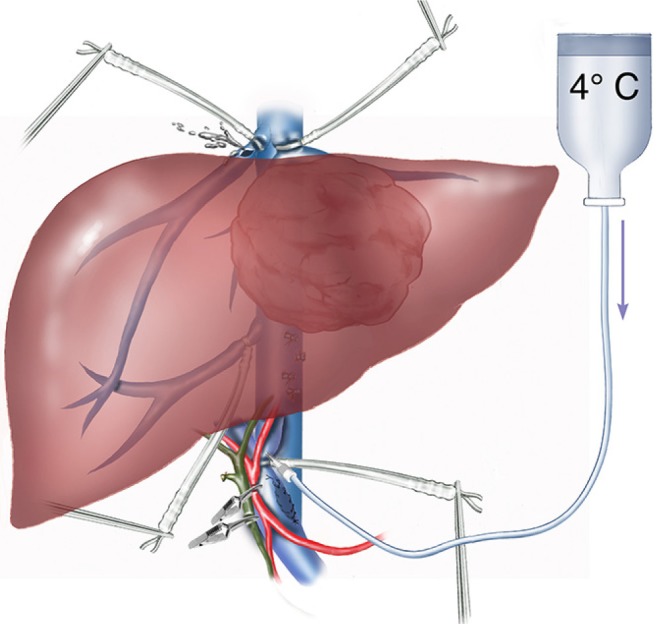
Schematisation of extended left hepatectomy. Vascular exclusion preserving the caval flow, temporary porta-caval shunt (PCS), in situ portal hypothermic perfusion.
Figure 3.
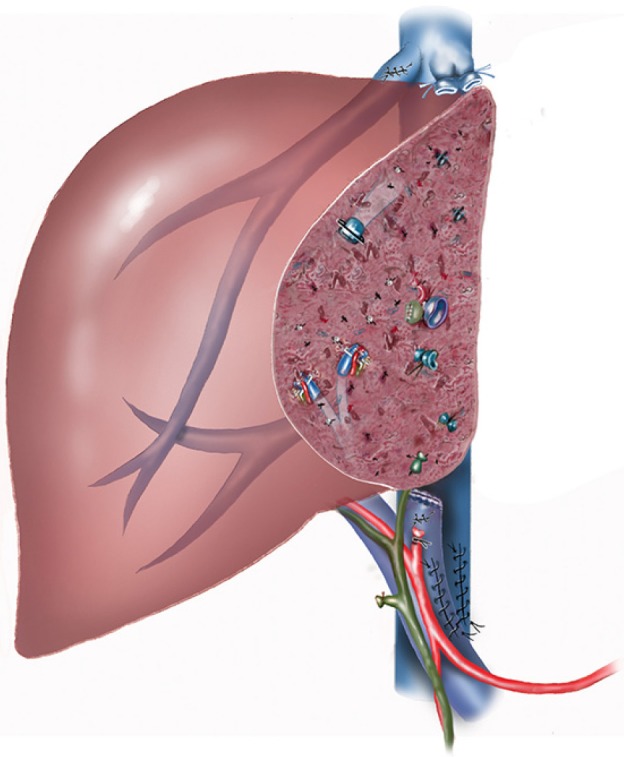
Extended left hepatectomy completed. The porta-caval shunt (PCS) has been divided. The holes in the portal vein and the hepatic vein for perfusate inflow and outflow have been sutured.
One of the major disadvantage of this technique is an increased risk of postoperative mortality and morbidity, as shown by the fact that these patients had been transfused of 7 and 5 units of blood, respectively.
Conclusions
When the indication for surgery is for tumors not invading or not strictly involving the hepatic vein confluence or the vena cava and with an expected TVE time ≥60 minutes, we propose an alternative technique to TVE with caval clamping, hypothermic perfusion and VVB.
Our new technique, successfully employed in two patients, consists in a TVE preserving the caval flow with in situ hypothermic perfusion with an end-to-side or a side-to-side temporary PCS obviating the need for extracorporeal VVB and its specific risks.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Huguet C, Nordlinger B, Galopin JJ, et al. Normothermic hepatic vascular exclusion for extensive hepatectomy. Surg Gynecol Obstet 1978;147:689-93 [PubMed] [Google Scholar]
- 2.Pichlmayr R, Grosse H, Hauss J, et al. Technique and preliminary results of extracorporeal liver surgery (bench procedure) and of surgery on the in situ perfused liver. Br J Surg 1990;77:21-6 [DOI] [PubMed] [Google Scholar]
- 3.Azoulay D, Eshkenazy R, Andreani P, et al. In situ hypothermic perfusion of the liver versus standard total vascular exclusion for complex liver resection. Ann Surg 2005;241:277-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elias D, Lasser P, Debaene B, et al. Intermittent vascular exclusion of the liver (without vena cava clamping) during major hepatectomy. Br J Surg 1995;82:1535-9 [DOI] [PubMed] [Google Scholar]
- 5.Berney T, Mentha G, Morel P.Total vascular exclusion of the liver for the resection of lesions in contact with the vena cava or the hepatic veins. Br J Surg 1998;85:485-8 [DOI] [PubMed] [Google Scholar]
- 6.Reiniers MJ, van Golen RF, Heger M, et al. In situ hypothermic perfusion with retrograde outflow during right hemihepatectomy: first experiences with a new technique. J Am Coll Surg 2014;218:e7-16 [DOI] [PubMed] [Google Scholar]
- 7.Tzakis AG, Reyes J, Nour B, et al. Temporary end to side portacaval shunt in orthotopic hepatic transplantation in humans. Surg Gynecol Obstet 1993;176:180-2 [PMC free article] [PubMed] [Google Scholar]
- 8.Budd JM, Isaac JL, Bennett J, et al. Morbidity and mortality associated with large-bore percutaneous venovenous bypass cannulation for 312 orthotopic liver transplantations. Liver Transpl 2001;7:359-62 [DOI] [PubMed] [Google Scholar]
- 9.Sakai T, Planinsic RM, Hilmi IA, et al. Complications associated with percutaneous placement of venous return cannula for venovenous bypass in adult orthotopic liver transplantation. Liver Transpl 2007;13:961-5 [DOI] [PubMed] [Google Scholar]
- 10.Kauffmann M, Krüger T, Aebert H.Surgery on extracorporeal circulation in early and advanced non-small cell lung cancer. Thorac Cardiovasc Surg 2013;61:103-8 [DOI] [PubMed] [Google Scholar]


