ABSTRACT
We evaluated the anti-inflammatory activity of mannanase-hydrolyzed copra meal (MNB), including β-1,4-mannobiose (67.8%), in a dextran sodium sulfate (DSS)-induced porcine model of intestinal inflammation. In the DSS-positive control (POS) and MNB treatment (MCM) groups, DSS was first administered to piglets via intragastric catheter for 5 days, followed by 5 days administration of saline or MCM. A negative control group (NEG) received a saline alternative to DSS and MNB. Inflammation was assessed by clinical signs, morphological and histological measurements, gut permeability and neutrophil infiltration. Local production of TNF-α and IL-6 were analyzed by ELISA, colonic and ileal inflammatory gene expressions were assessed by real time RT-PCR, and CD4+CD25+ cell populations were analyzed by flow cytometry. Crypt elongation and muscle thickness, D-mannitol gut permeation, colonic expression of the inflammatory mediators TNF-α and IL-6 and myeloperoxidase activity were significantly lower in the MCM group than in that of POS group. The mRNA levels of ileal IL-1β, IL-6, IL-17 and TNF-α were significantly lower following MCM treatment than with POS treatment.MNB exerts anti-inflammatory activity in vivo, suggesting that MNB is a novel therapeutic that may provide relief to human and animals suffering from intestinal inflammation.
Keywords: 4-mannobiose, β-1, anti-inflammation, colitis, porcine
Anti-inflammatory agents are very important for livestock, because once inflammation occurs, livestock animals may show decreased productivity and growth [6]. Antibiotics have been used to treat inflammation and to promote growth [6]. However, continued use of dietary antibiotics has led to the development of drug-resistant bacteria [22]. Because of this, Europe banned the use of all antibiotics for promoting growth in 2005 [7], and the FDA has also banned the use of some antibiotics [18].
Inflammatory bowel disease (IBD) in humans and animals is characterized by uncontrolled inflammation of the gastrointestinal tract [27]. It is caused by an imbalance in the gut microbiota and is sometimes called “pathological inflammation” [8]. The changes in gut microbiota, specifically, an increase in pathogenic bacteria and a decrease in health promoting bacteria, such as Bifidobacterium spp. and Lactobacillus spp., play an important role in promoting and maintaining intestinal inflammation in IBD [3].
Local and adaptive immune responses in the inflamed epithelium lead to high concentrations of pro-inflammatory cytokines and chemokines, including interleukin-1β (IL-1β), interleukin-6 (IL-6), interleukin-17 (IL-17), tumor necrosis factor-α (TNF-α) and tissue-damaging reactive oxygen species [13, 15]. Inflammation is further enhanced by the infiltration of luminal bacteria into the damaged tissue. While regulatory T cells (Treg) play a major role in maintaining intestinal homeostasis in the healthy epithelium [13], defects in Treg function have been observed in IBD [4].
Current treatment strategies for the treatment of IBD involve biological drugs, such as antibiotics, as mentioned above, and immunosuppressants that are associated with significant side effects, elevated costs and occasionally poor efficacy [1]; therefore, novel therapies are needed.
Therapeutic IBD candidates are often assessed using animal models of intestinal inflammation, such as the pig model. The gastrointestinal tract of pigs is morphologically and physiologically similar to that of humans [17]. In addition, weaning piglets are susceptible to inflammation by Escherichia coli and other bacteria, followed by mortality [19]. This is a problem faced by the livestock industry.
β-1,4-mannobiose has been shown to increase gut health by increasing IgA production and upregulating the local expression of genes involved in host defense and innate immunity in vitro and in vivo. This can prevent Salmonella enteritidis infection and enhance Salmonella-killing activity [2, 10, 11]. Therefore, it is plausible that mannanase-hydrolyzed copra meal, including β-1,4-mannobiose (MNB), may also have immune-modulating effects during chronic inflammation that reduce overall inflammation. The objective of this research was to examine the anti-inflammatory properties of MNB in a porcine model of colitis.
MATERIALS AND METHODS
Mannanase-hydrolyzed copra meal, including β-1,4-mannobiose (MNB): MNB was derived from copra meal by enzymatic digestion and was provided by Fuji Oil R & D (Osaka, Japan). The supplement contained 67.8% β-1,4-mannobiose (w/w) with the remainder composed of mannose (8.9%), arabinose (0.4%), galactose (2.6%), glucose (5%), fructose (2.3%), sucrose (0.2%), protein (9.7%) and ash (3.1%).
Animals and experimental design: Five- to seven-day-old Yorkshire piglets were obtained from the Arkell Swine Research Station (University of Guelph, Guelph, ON, Canada). The piglets were individually housed in an animal facility maintained at 26°C with a 12-hr light/dark cycle. Piglets were fed three times a day with a commercial milk replacement formula (Soweena® Litter Life; Merrick’s Inc., Middleton, WI, U.S.A.) at ad libitum intake volumes. After a two-day acclimatization period, piglets underwent surgery for the placement of an intragastric catheter (Micro-Renathane®; Braintree Scientific Inc., Braintree, MA, U.S.A.). Piglets recovered for 3 days, after which they were randomly assigned to three groups of three animals each. All animal trials were approved by the University of Guelph’s Animal Care Committee and were conducted in accordance with the Canadian Council of Animal Care Guide for the Care and Use of Experimental Animals.
Induction of colitis in vivo and treatment with MNB: The three experimental groups consisted of negative control (NEG), positive control (POS) and MNB treatment (MCM). For five consecutive days, POS and MCM groups were infused with 1.25 g dextran sodium sulfate (DSS)/kg body weight (BW)/day (MW 36,000–50,000; MP Biomedicals, Solon, OH, U.S.A.), which was dissolved in sterile saline. The NEG groups were infused with saline only. The MCM treatment period began concurrently with the DSS administrations and was extended for five additional days for a total of ten days. The MCM group received 50 mg MNB/kg BW/day via intragastric catheter, whereas NEG and POS animals received saline only. The dose of MNB was selected based on a previous report using poultry [11]. Sixteen days after DSS/MCM treatment and sterile saline administration began, all animals were humanely euthanized, and colon tissues were harvested and frozen in liquid nitrogen for future measurement of biochemical parameters.
Growth performance: The body weight of animals in each group was measured on days 1, 6 and 16.
Histological analysis: Immediately after sacrifice, fresh colon tissues were placed into 10% formalin for 24 hr and then transferred into 70% ethanol. Five to six cross and longitudinal tissue sections, approximately 2–3 mm in thickness, were placed into histology cassettes and immersed in 70% ethanol. Tissues were fixed onto slides and stained with hematoxylin and eosin (H & E). Slides were examined using an Olympus BX60 system microscope (Olympus Optical Co., Ltd., Tokyo, Japan) fitted with an Olympus DP71 microscope digital camera (Olympus Optical Co., Ltd.). Crypt depth and muscle thickness were analyzed using Image-Pro Plus software (Media Cybernetics, Rockville, MD, U.S.A.).
In vivo gut permeability analysis: Gut permeability was assessed on the last day of the trial by infusing pigs with 0.6 g/kg BW of D-mannitol and examining the plasma D-mannitol concentration over time. Blood was collected at 0, 35 and 70 min post-infusion, via the suborbital sinus, into heparinized tubes, centrifuged at 800 × g for 5 min to obtain plasma and stored at −20°C until analysis. Plasma was boiled for 5 min and then centrifuged at 16,000 × g for 75 min to minimize background interference from polymer organic compounds. D-mannitol standards and supernatant were combined with β-nicotinamide adenine dinucleotide sodium salt (β-NAD) and D-mannitol dehydrogenase (Megazyme International, Wicklow, Ireland) at final concentrations of 20 µmol/ml and 0.1 unit/ml, respectively. Samples were incubated for 150 min at 40°C, and NADH production was measured spectrophotometrically at 340 nm. D-mannitol concentrations were determined from the standard curve.
Myeloperoxidase assay: Myeloperoxidase (MPO) activity was assessed as a marker of neutrophil infiltration according to the method of Tatzber et al. [23] with minor modifications. All chemicals were obtained from Sigma-Aldrich (St. Louis, MO, U.S.A.). Frozen, finely ground, colon samples were homogenized in 20 mM potassium phosphate buffer, pH 7.4, for 60 sec. The homogenate was then centrifuged at 16,000 × g for 20 min, and the pellets were resuspended in 1 ml of 50 mM potassium phosphate buffer, pH 6.0 containing 0.5% (w/v) hexadecyl-trimethyl-ammonium bromide (HTAB). The samples were subjected to one freeze-thaw cycle followed by sonication for 90 sec and heating at 60°C for 120 min. Samples were centrifuged again, and the resulting supernatants were assayed for MPO activity. MPO standards and samples (10 µl/well) were combined with 200 µl/well of 30% hydrogen peroxide, tetramethylbenzidine and 50 mM potassium phosphate buffer containing 0.5% (w/v) HTAB at a ratio of 1:10:100 in a 96-well microtiter plate. After 15 min, 100 µl/well of 0.5 M H2SO4 was added, and the absorbance was measured at 450 nm using a microplate reader. MPO activity was expressed as mU per g tissue.
Measurement of TNF-α and IL-6 concentrations in colon tissues: IL-6 and TNF-α in colon tissues were measured using porcine IL-6 and TNF-α Quantikine® ELISA Kits (R & D Systems, Minneapolis, MN, U.S.A.). Frozen colon tissues were weighed and homogenized in ice-cold Hank’s buffered salt solution (HBSS) containing 0.1 mM phenylmethylsulphonyl fluoride (PMSF, Sigma-Aldrich). Homogenates were centrifuged (12,000 × g, 30 min, 4°C), and the supernatants were collected and used in the ELISA in accordance with the manufacturer’s instructions. Colorimetric reactions were measured with a Bio-Rad microplate reader (Model 550; Bio-Rad Laboratories, Hercules, CA, U.S.A.), and cytokine concentration was expressed as picogram (pg) cytokine per gram (g) of tissue.
RNA isolation and analysis of gene expression by real-time RT-PCR: Total RNA was extracted from finely ground, frozen colon tissues using the Aurum™ RNA Mini Kit (Bio-Rad Laboratories). First-strand cDNA synthesis was carried out using the iScript™ cDNA Synthesis Kit (Bio-Rad Laboratories) in accordance with the manufacturer’s instructions. Real-time RT-PCR was carried out using SYBR Green Supermix (Bio-Rad Laboratories) on a MyiQ™ Single Color Time PCR Detection System (Bio-Rad Laboratories) under the following conditions: denaturation 15 sec at 95°C, annealing 15 sec at 56°C and extension 30 sec at 72°C. Porcine primers were designed using Primer3 v.0.4.0 [21] and synthesized by the University of Guelph Laboratory Services Molecular Biology Section (Guelph) (Table 1). Results were expressed as fold changes relative to the negative control (untreated) animals.
Table 1. Sequences of porcine primer pairs used for real-time RT-PCR.
| Gene | Forward primer (5′–3′) | Reverse primer (3′–5′) | Product (base pairs) |
Accession no. |
|---|---|---|---|---|
| β-actin | GGATGCAGAAGGAGATCACG | ATCTGCTGGAAGGTGGACAG | 130 | U07786 |
| IL-1β | CAAAGGCCGCCAAGATATAA | GAAATTCAGGCAGCAACAT | 147 | NM_214055 |
| IL-6 | AAGGTGATGCCACCTCAGAC | TCTGCCAGTACCTCCTTGCT | 151 | M86722 |
| IL-17 | TCATGATCCCACAAAGTCCA | AGTCCATGGTGAGGTGAAGC | 146 | NM_001005729 |
| TNF-α | ATGGATGGGTGGATGAGAAA | TGGAAACTGTTGGGGAGAAG | 151 | X54001 |
Fluorescence activated cell sorting: Whole blood was suspended in red blood cell lysing buffer (Sigma-Aldrich) for 1 min and then diluted with RPMI. The mixture was centrifuged (400 × g, 5 min, 4°C) and washed in RPMI. After centrifugation, the supernatant was discarded, and the pellet was resuspended in RPMI with 5% fetal bovine serum (Cansera, Rexdale, ON, Canada). This cell suspension (50 µl, 107 cells/µl) was then incubated with mouse anti-pig CD25 (AbD Serotec, Oxford, U.K.), goat anti-mouse IgG1:FITC (AbD Serotec), R-Phycoerythrin-conjugated anti-pig CD4a (BD Biosciences, San Diego, CA, U.S.A.) and anti-mouse/rat FoxP3 Alexa Fluor (eBioscience, San Diego, CA, U.S.A.) according to the manufacturers’ instructions. Cells were washed and centrifuged twice in FACS buffer (phosphate buffered saline, pH 7.4, 20 mM glucose and 1% bovine serum albumin, Sigma-Aldrich) before counting on the BD FACScan (BD Biosciences). Each sample was stained for FACS analysis, and 10,000 cells were counted per sample. Stained samples were expressed as percentage of CD4+CD25+ cells.
Fecal IgA: Feces were collected on days 1, 6, 11 and 16 of the trial and were freeze-dried prior to analysis. IgA was determined using the Pig IgA ELISA Quantitation Set (Bethyl Laboratories Inc., Montgomery, TX, U.S.A.) in accordance with the manufacturer’s instructions. IgA concentration was expressed as pg IgA per mg feces.
Statistical analysis: Statistical analysis was carried out using the GraphPad Software (San Diego, CA, U.S.A.) with comparisons between groups conducted with a one-way analysis of variance (ANOVA) followed by the Tukey-Kramer multiple comparison tests. Groups were considered statistically significant when P<0.05. Results were reported as mean ± SEM.
RESULTS
Growth performance: Table 2 shows the weight changes of NEG, POS and MCM groups. There were no differences in each group on each day.
Table 2. BW changes of MCM treatment, DSS treatment and NEG groups.
| Treatment groups | Days |
|||
|---|---|---|---|---|
| 1 | 6 | 16 | ||
| NEG | 3.36 ± 0.13 | (kg) | 4.40 ± 0.23 | 6.25 ± 0.13 |
| POS | 3.16 ± 0.23 | 4.10 ± 0.14 | 6.46 ± 0.29 | |
| MCM | 3.14 ± 0.04 | 4.26 ± 0.18 | 6.43 ± 0.05 | |
Data are mean ± SEM; n=3; “*” indicates significant difference from NEG; “#” indicates significant difference from POS; P<0.05. NEG, negative control; POS, DSS positive control; MCM, DSS positive with MCM treatment.
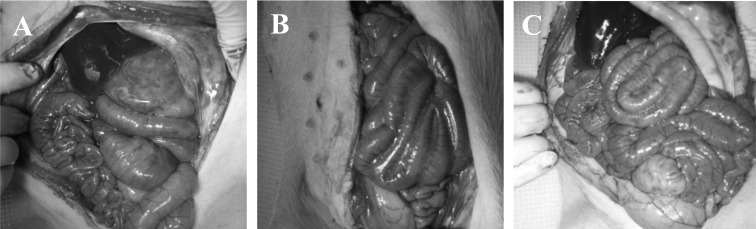
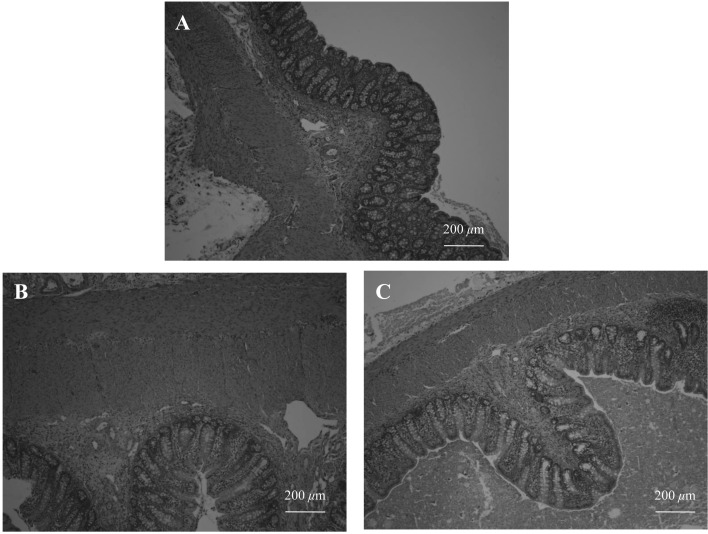
MNB supplementation ameliorates colon morphological, histological and permeability parameters of DSS-induced colitis: Figure 1 shows the intestine of each pig after the anatomy was assessed. Upon sacrifice, gross appearance of the colon in NEG and MCM groups lacked signs of vascularization and thickening of the intestinal wall, as compared to the POS group. Indeed, the POS group showed marked thickening of longitudinal and circular intestinal muscles and architectural distortion of colonic crypts (Fig. 2). Five days after DSS cessation, the POS group had significantly increased colon crypt depth (Table 3, top) and muscle thickness (Table 3, top) compared to the NEG and MCM groups.
Fig. 1.
Effect of MNB on DSS-induced colitis in piglets. Sample necropsy images of the intestines of (A) negative control, (B) positive control, (C) MCM; all piglets are in the ventral position.
Fig. 2.
Histopathological analyses of the colon after 5 days of intragastric DSS infusion and 10 days of MCM supplementation. All photos represent sample colon histological slides stained with H&E. (A) negative control, (B) positive control, (C) MCM. Scale bar: 200 µm.
Table 3. Effect of MCM on DSS-induced colitis in piglet groups.
| Treatment |
|||
|---|---|---|---|
| NEG | POS | MCM | |
| Crypt depth and muscle thickness of H&E-stained colonic cross sections | |||
| Crypt Depth, µm | 164 ± 10# | 233 ± 7* | 143 ± 5# |
| Muscle thickness, µm | 270 ± 16# | 422 ± 21* | 276 ± 8# |
| Linear relationships between plasma D-mannitol
concentration in blood and the time post-intragastric infusion of D-mannitol |
|||
| The rate of blood permiability, µmol/min. | 3.33 ± 0.33# | 5.20 ± 0.11* | 2.81 ± 0.14# |
| Correlation efficient, r^2 | 0.84 | 0.98 | 0.83 |
| Colon tissues were assayed for MPO activity. | |||
| MPO activity, mU/mg protein | 23.2 ± 4.8# | 45.4 ± 9.7* | 20.5 ± 4.5# |
Data are mean ± SEM; n=3; “*” indicates significant difference from NEG; “#” indicates significant difference from POS; P<0.05. NEG, negative control; POS, DSS positive control; MCM, DSS positive with MCM treatment.Top row: Crypt depth and muscle thickeness. Middle row: In vivo gastrointestinal permeability. Bottom row: MPO activity in collon tissues.
Gut permeability was assessed using D-mannitol, an inert, non-metabolizable sugar, because increased gastrointestinal absorption is reflected by an increased D-mannitol plasma concentration [24]. In the POS group, the rate of D-mannitol intake was significantly higher (5.20 ± 0.11 µmol mannitol/ml plasma·min) than in the NEG group (3.33 ± 0.33 µmol mannitol/ml plasma·min) and the MCM group (2.81 ± 0.37 µmol mannitol/ml plasma·min; Table 3, middle).
MPO activity was significantly greater in the POS group than in the MCM and NEG groups (Table 3, bottom). Both MPO activity and plasma D-mannitol intake in the MCM group did not differ from those of the NEG group (Table 3, middle and bottom).
MCM treatment affects inflammatory regulators of the adaptive T cell response: To further investigate the effect of MNB administration on inflammation and inflammatory regulators, the expression of key genes involved in the innate immune response, as well as the adaptive T cell response, was examined. POS group pigs showed a marked increase in the TNF-α and IL-6 protein levels and IL-6 gene expression when compared to the untreated NEG group (Table 4). TNF-α mRNA levels were significantly increased in the POS group, but no significant difference existed between NEG and POS groups for IL-1β and IL-17 (Table 4). The MCM group showed significant decreases in the colonic expression of IL-1β, IL-6, IL-17 and TNF-α, as well as in TNF-α protein levels.
Table 4. Protein concentration and gene expression of inflammatory and regulatory mediators in the colon of untreated piglets and DSS-treated piglets that were or not fed MCM.
| Treatment |
|||
|---|---|---|---|
| NEG | POS | MCM | |
| Protein, pg/g tissue | |||
| TNF-α | 291 ± 8# | 478 ± 56* | 413 ± 30* |
| IL-6 | 16 ± 2# | 26 ± 2* | 19 ± 2# |
| Gene Expression, -fold of NEG | |||
| IL-1β | 1.0 ± 0.2 | 0.9 ± 0.4 | 0.2 ± 0.0*# |
| IL-6 | 1.0 ± 0.1# | 4.1 ± 1.4* | 1.1 ± 0.1# |
| IL-17 | 1.0 ± 0.2 | 0.6 ± 0.2 | 0.2 ± 0.0*# |
| TNF-α | 1.0 ± 0.3# | 1.6 ± 0.4 | 0.4 ± 0.0*# |
Data are mean ± SEM. N=3; Means in a row superscripts with “*” differ significantly from NEG and those with “#” differ significantly from POS; P<0.05. NEG, negative control; POS, DSS positive control; MCM, DSS positive with MCM treatment. DSS, dextran sodium sulfate.
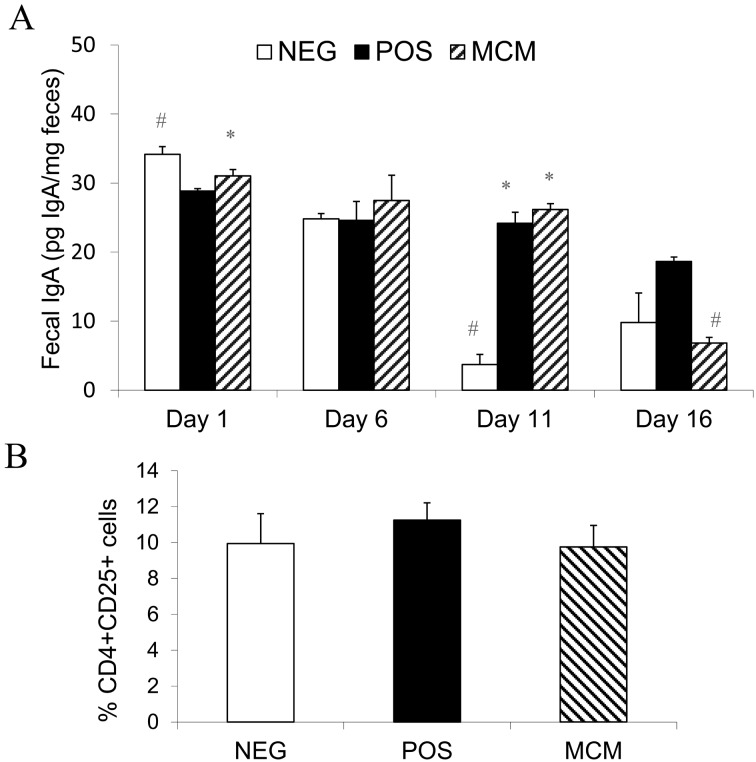
Effect of MNB on fecal IgA levels: On the day of 1, fecal IgA concentrations in the NEG group was significantly higher than in the POS group and did not differ between the NEG and MCM groups, but after surgery and recovery, fecal IgA levels were not statistically significant among all groups on day 6 (Fig. 3A). On Day 11, POS and MCM animals had significantly elevated IgA compared to NEG, but at the end of the trial, on day 16, fecal IgA in the MCM group was significantly lower than that of the POS group.
Fig. 3.
Effect of MCM on (A) fecal IgA concentrations and (B) plasma CD4+CD25+ cell populations. Data are mean ± SEM; n=3; “*” indicates significant difference from NEG; “#” indicates significant difference from POS; P<0.05.
CD4+CD25+ cell population: In our study, no significant difference was found between the groups’ CD4+CD25+ cell populations in the peripheral blood (Fig. 3B).
DISCUSSION
Intestinal inflammation causes digestive tract distress, weight loss and gastrointestinal permeability in both humans and DSS-induced animals [20, 25]. Furthermore, DSS-induced colitis commonly results in crypt damage, ulceration and erosion of the intestinal mucosa and a mass infiltration of immune cells into the inflamed colon [16]. Once DSS administration ends, the colon undergoes a recovery and repair process characterized by elongation of the crypts [26]. Elongated crypts and a thicker muscle layer were pronounced in the POS group, and indeed, histological measurements confirm these alterations. MCM piglets had a similar crypt depth and muscle thickness as those of NEG group, indicating a protective effect in the colon. Similar rates of gut permeability and neutrophil infiltration in the MCM and NEG groups also suggest that an intact epithelium is maintained in pigs fed MNB supplementation, despite the tissue-erosive effect of DSS.
DSS-induced permeability has also been attributed to pro-inflammatory cytokines, such as TNF-α and INF-γ, which interfere with the composition of tight junction proteins, resulting in a damaged mucosal epithelium [5]. MNB treatment significantly lessened TNF-α protein and gene expression concentrations (Table 3), suggesting a probable mechanism by which gut barrier integrity was restored, leading to reduced intestinal permeability. This improvement in gut homeostasis was supported by the significantly lowered neutrophil infiltration in the NEG and MCM groups compared to POS controls, as assessed by myeloperoxidase activity (Table 3, bottom).
When IL-6 levels are reduced, the FoxP3+ Treg pathway is preferentially induced, rather than the Th17 pathway [14]. In our study, however, no significant difference was found among the groups’ CD4+CD25+ cell populations in the peripheral blood (Fig 3B), suggesting that MNB plays a more prominent role in the reduction of pro-inflammatory mediators.
Prebiotics have been shown to improve intestinal morphology and gut health [12] and to modulate innate immune responses in the gut [9]. MNB has been demonstrated to prevent bacterial infections in chickens by increasing IgA production. DSS-induced damage exposes luminal gut colonies and content to the underlying intestinal substructure [16], so increased IgA levels may play a role in preventing infection and inflammation. In our study, we observed a decrease in IgA concentrations after 16 days, although MNB was only administered for 10 consecutive days. Agunos et al. [2] fed chickens on a MNB-supplemented diet for 14 days and observed a peak in cecal IgA levels 14 days after infection with Salmonella enteritidis. This suggested that IgA responses may be better approximated at a later time post-DSS administration.
Although mannobiose has been shown to possess a number of immunomodulatory effects, both in vivo and in vitro, to our knowledge, this is the first report describing the use of MNB to treat colitis. In the present study, the administration of MNB significantly reduced the expression of inflammatory mediators in vivo in a porcine model of experimental inflammation. MNB supplementation exerted inhibitory effects on histological measurements and gut permeability and on the innate T helper pro-inflammatory pathways in the colon. These results indicate that MNB is novel and therapeutic and may provide relief to human and diseased animal suffering from intestinal inflammation.
REFERENCES
- 1.Abraham C., Cho J. H.2009. Inflammatory bowel disease. N. Engl. J. Med. 361: 2066–2078. doi: 10.1056/NEJMra0804647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agunos A., Ibuki M., Yokomizo F., Mine Y.2007. Effect of dietary β 1–4 mannobiose in the prevention of Salmonella enteritidis infection in broilers. Br. Poult. Sci. 48: 331–341. doi: 10.1080/00071660701370442 [DOI] [PubMed] [Google Scholar]
- 3.Andoh A., Fujiyama Y.2006. Therapeutic approaches targeting intestinal microflora in inflammatory bowel disease. World J. Gastroenterol. 12: 4452–4460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown S. J., Mayer L.2007. The immune response in inflammatory bowel disease. Am. J. Gastroenterol. 102: 2058–2069. doi: 10.1111/j.1572-0241.2007.01343.x [DOI] [PubMed] [Google Scholar]
- 5.Bruewer M., Luegering A., Kucharzik T., Parkos C. A., Madara J. L., Hopkins A. A., Nusrat A.2003. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J. Immunol. 171: 6164–6172 [DOI] [PubMed] [Google Scholar]
- 6.Connor E. E., Baldwin V. I., Blanton J. R. Jr., Johnson S. E., Poulos S., Welsh T. H. Jr.2012. Growth and development symposium: Understanding and mitigating the impacts of inflammation on animal growth and development. J. Anim. Sci. 90: 1436–1437. doi: 10.2527/jas.2011-5234 [DOI] [PubMed] [Google Scholar]
- 7.Dibner J. J., Richards J. D.2005. Antibiotic growth promoters in agriculture: History and mode of action. Poult. Sci. 84: 634–643. doi: 10.1093/ps/84.4.634 [DOI] [PubMed] [Google Scholar]
- 8.Frank D. N., St Amand A. L., Felfman R. A., Boedeker E. C., Harpaz N., Pace N. R.2007. Molecular-phlyogenetics characterization of microbial community imbalances in human inflammatory bowel disease. Proc. Natl. Acad. Sci. U.S.A. 104: 13780–13785. doi: 10.1073/pnas.0706625104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gómez-Verduzco G., Cortes-Cuevas A., Lopez-Coello C., Avila-Gonzalez E., Nava G. M.2009. Dietary supplementation of mannan-oligosaccharide enhances neonatal immune responses in chickens during natural exposure to Eimeria spp. Acta Vet. Scand. 51: 11–17. doi: 10.1186/1751-0147-51-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ibuki M., Kovacs-Nolan J., Fukui K., Kanatani H., Mine Y.2011. β 1–4 mannobiose enhances Salmonella-killing activity and activates innate immune responses in chicken macrophages. Vet. Immunol. Immunopathol. 139: 289–295. doi: 10.1016/j.vetimm.2010.10.011 [DOI] [PubMed] [Google Scholar]
- 11.Ibuki M., Kovacs-Nolan J., Fukui K., Kanatani H., Mine Y.2010. Analysis of gut immune-modulating activity of beta-1,4-mannobiose using microarray and real-time reverse transcription polymerase chain reaction. Poult. Sci. 89: 1894–1904. doi: 10.3382/ps.2010-00791 [DOI] [PubMed] [Google Scholar]
- 12.Iji P. A., Saki A. A., Tivey D. R.2001. Intestinal structure and function of broiler chickens on diets supplemented with a mannan oligosaccharide. J. Sci. Food Agric. 81: 1186–1192. doi: 10.1002/jsfa.925 [DOI] [Google Scholar]
- 13.Izcue A., Coombes J. L., Powrie F.2006. Regulatory T cells suppress systemic and mucosal immune activation to control intestinal inflammation. Immunol. Rev. 212: 256–271. doi: 10.1111/j.0105-2896.2006.00423.x [DOI] [PubMed] [Google Scholar]
- 14.Kim J. M., Rudensky A.2006. The role of the transcription factor Foxp3 in the development of regulatory T cells. Immunol. Rev. 212: 86–98. doi: 10.1111/j.0105-2896.2006.00426.x [DOI] [PubMed] [Google Scholar]
- 15.Kruidenier L., Verspaget H. W.2002. Review article: oxidative stress as a pathogenic factor in inflammatory bowel disease–radicals or ridiculous? Aliment. Pharmacol. Ther. 16: 1997–2015. doi: 10.1046/j.1365-2036.2002.01378.x [DOI] [PubMed] [Google Scholar]
- 16.Kwon K. H., Murakami A., Tanaka T., Ohigashi H.2005. Dietary rutin, but not its aglycone quercetin, ameliorates dextran sulfate sodium-induced experimental colitis in mice: attenuation of pro-inflammatory gene expression. Biochem. Pharmacol. 69: 395–406. doi: 10.1016/j.bcp.2004.10.015 [DOI] [PubMed] [Google Scholar]
- 17.Miller E. R., Ullrey D. E.1987. The pig as a model for human nutrition. Annu. Rev. Nutr. 7: 361–382. doi: 10.1146/annurev.nu.07.070187.002045 [DOI] [PubMed] [Google Scholar]
- 18.Ohshima H.2006. International situation related to drug resistance bacteria. Proceeding of Japanese Society of Antimicrobials for Animal 28: 2–6 in Japanese). [Google Scholar]
- 19.Osek J.1999. Prevalence of virulence factors of Escherichia coli strains isolated from diarrheic and healthy piglets after weaning. Vet. Microbiol. 68: 209–217. doi: 10.1016/S0378-1135(99)00109-1 [DOI] [PubMed] [Google Scholar]
- 20.Poritz L. S., Garver K. I., Green C., Fitzpatrick L., Ruggiero F., Koltun W. A.2007. Loss of the tight junction protein ZO-1 in dextran sulfate sodium induced colitis. J. Surg. Res. 140: 12–19. doi: 10.1016/j.jss.2006.07.050 [DOI] [PubMed] [Google Scholar]
- 21.Roze S., Skaletsky H. J.2000. Primer3 on the WWW for general users and for biologist programmers–Bioinformatics methods and protocols, pp. 365–386. In: Methods in Molecular Biology (Krawetz, S. and Misener, S. eds.), Humana Press, Totowa. [DOI] [PubMed]
- 22.Sørum H., Sunde M.2001. Resistance to antibiotics in the normal flora of animals. Vet. Res. 32: 227–241. doi: 10.1051/vetres:2001121 [DOI] [PubMed] [Google Scholar]
- 23.Tatzber F., Griebenow S., Wonisch W., Winkler R.2003. Dual method for the determination of peroxidase activity and total peroxides-iodide leads to a significant increase of peroxidase activity in human sera. Anal. Biochem. 316: 147–153. doi: 10.1016/S0003-2697(02)00652-8 [DOI] [PubMed] [Google Scholar]
- 24.Thymann T., Burrin D. G., Tappenden K. A., Bjornvad C. R., Jensen S. K., Sangild P. T.2006. Formula-feeding reduces lactose digestive capacity in neonatal pigs. Br. J. Nutr. 95: 1075–1081. doi: 10.1079/BJN20061743 [DOI] [PubMed] [Google Scholar]
- 25.Welcker K., Martin A., Kölle P., Siebeck M., Gross M.2004. Increased intestinal permeability in patients with inflammatory bowel disease. Eur. J. Med. Res. 9: 456–460 [PubMed] [Google Scholar]
- 26.Williams K. L., Fuller C. R., Dieleman L. A., DaCosta C. M., Haldeman K. M., Sartor R. B., Lund P. K.2001. Enhanced survival and mucosal repair after dextran sodium sulfate-induced colitis in transgenic mice that overexpress growth hormone. Gastroenterology 120: 925–937. doi: 10.1053/gast.2001.22470 [DOI] [PubMed] [Google Scholar]
- 27.Xavier R. J., Podolsky D. K.2007. Unravelling the pathogenesis of inflammatory bowel disease. Nature 448: 427–434. doi: 10.1038/nature06005 [DOI] [PubMed] [Google Scholar]