ABSTRACT
The objective of this research was to estimate the optimal timing for fertilization to achieve proper embryonic development of in vitro-matured bovine oocytes. First, cumulus-oocyte complexes were subjected to in vitro maturation (IVM) for 14–22 hr. The timing when 50% of oocytes reached metaphase II stage was estimated to be 17.5 hr after IVM start. Next, using oocytes subjected to IVM for 12–30 hr, sperm penetration was examined after 4–18 hr of in vitro fertilization (IVF). A significant negative correlation between IVM duration and the timing when 50% of oocytes were penetrated by sperm after IVF start was observed (P<0.01). Finally, oocytes subjected to 12–30 hr of IVM were inseminated and cultured for 6 days to examine embryonic development. In the group with 22 hr of IVM, the percentages of cleaved embryos and blastocysts were the highest values in all groups. According to the regression equation describing the time from nuclear maturation to sperm penetration (x) and the percentage of blastocysts (y) (y=7.23x − 0.297x2, P<0.01), the blastocyst rate peaked when sperm penetration occurred at 12.2 hr after achieving nuclear maturation. In conclusion, under the present IVM/IVF conditions, it was estimated that oocytes acquired their highest developmental competence at about 30 hr after IVM start, and thus, the optimal IVM duration was calculated to be about 21 hr.
Keywords: bovine oocyte, IVF, IVM, nuclear maturation, sperm penetration
Nuclear and cytoplasmic maturation is essential for oocytes to acquire developmental competence [6, 7]. After the completion of nuclear maturation, oocytes gradually accomplish cytoplasmic maturation and acquire developmental competence. Then, they maintain that competence for a certain period, but eventually undergo deterioration in quality and lose developmental competence, which is called “oocyte aging” [13]. However, the optimal timing for fertilization to achieve proper embryonic development of in vitro-matured bovine oocytes remains unclear, and the developmental competence of in vitro-matured bovine oocytes is lower than that of in vivo-matured oocytes [18]. One of the reasons for the low developmental competence of in vitro-matured bovine oocytes may be that fertilization occurred at a suboptimal timing for oocytes. Therefore, clarification of the optimal timing for fertilization of in vitro-matured bovine oocytes will contribute to improve in vitro production (IVP) efficiency and basic research on oocyte aging.
Many researchers have examined the effect of the duration of in vitro maturation (IVM) on the embryonic development of bovine oocytes [1, 11, 12, 16, 33], but their optimal maturation culture periods for yielding a high blastocyst rate were not consistent (18 to 24 hr). Although it was reported that the timing of fertilization after achieving nuclear maturation affected the blastocyst development of IVM oocytes [4], no studies determined the relationship between the timing of sperm penetration of nuclear-matured oocytes and subsequent embryonic development. The kinetics of meiotic progress during IVM has been shown to be affected by the addition of substances to IVM medium [10, 24], temperature during IVM culture [5], periods of ovary conservation [8] or the size of follicles from which oocytes are retrieved [9]. It was reported that the timing of sperm penetration was influenced by bull variation and conditions of in vitro fertilization (IVF) [19, 26]. There is a possibility that this inconsistency of optimal maturation culture periods is caused by the different timing of nuclear maturation and sperm penetration.
In previous studies, bovine oocytes at about 30 hr after the initiation of IVM were considered as aged or slightly aged oocytes and used to investigate aging-related changes [17, 22, 23]. This was because the oocytes after 30 hr of IVM showed a low developmental rate to blastocysts [17]. However, since it takes several hours for bovine oocytes to be penetrated by sperm after starting IVF [17, 26], studies on the characteristics of aged oocytes should not be based on developmental competence corresponding to the duration of IVM, but instead on the timing of sperm penetration. Exact identification of the changes in the developmental competence based on the timing of sperm penetration may contribute to the establishment of an in vitro model for basic research on oocyte acquisition of developmental competence and oocyte aging.
In the present study, for estimation of the optimal timing of fertilization for achieving proper embryonic development of in vitro-matured bovine oocytes correctly and for clarifying the characteristics of oocytes possessing high developmental competence, we investigated the time of nuclear maturation and sperm penetration during IVM and IVF and also examined the effect of the time of sperm penetration after nuclear maturation on the embryonic development of bovine oocytes.
MATERIALS AND METHODS
All the chemicals and reagents used for this study were purchased from Sigma-Aldrich (St. Louis, MO, U.S.A.), unless otherwise stated.
In vitro maturation and fertilization of bovine oocytes: IVM was performed as previously described [29]. Briefly, bovine cumulus-oocyte complexes (COCs) aspirated from follicles (2 to 8 mm in diameter) of slaughterhouse-derived ovaries were cultured for various periods (12 to 30 hr) under a humidified atmosphere of 5% CO2 in air at 39°C using droplets of IVM medium (about 10 COCs/50 µl). IVM medium was composed of HEPES-buffered TCM-199 (Invitrogen, Grand Island, NY, U.S.A.) supplemented with 10% fetal calf serum (Invitrogen), 0.02 units/ml FSH (from porcine pituitary), 1 µg/ml estradiol-17β, 0.2 mM sodium pyruvate and 50 µg/ml gentamicin sulfate.
IVF was conducted using frozen-thawed semen from one Holstein bull according to a procedure described previously [28] with slight modifications. In brief, motile sperm (2 × 106 sperm/ml) separated from thawed semen using a Percoll (GE Healthcare, Buckinghamshire, U.K.) gradient (45 and 90%) were co-incubated with COCs in droplets of IVF medium (about 10 COCs/100 µl). IVF medium was composed of modified Brackett and Oliphant isotonic medium [26] containing 3 mg/ml fatty acid-free BSA, 2.5 mM theophylline, 20 µM penicillamine, 10 µM hypotaurine and 1 µM epinephrine. Co-incubation of COCs and sperm was performed for 4 to 18 hr under 5% CO2, 5% O2 and 90% N2 at 39°C.
Evaluation of the nuclear status of matured and fertilized oocytes: After various periods of IVM or IVF, oocytes were freed from the cumulus cells by vortexing. Denuded oocytes were fixed with ethanol:acetic acid (3:1) and stained with a 1% aceto-orcein solution. Their meiotic stages and fertilization status (sperm penetration and pronucleus formation) were examined under a phase-contrast microscope [14, 15]. Oocytes that reached the metaphase II (M-II) stage were defined as nuclear-matured, and oocytes having enlarged sperm head (s) were defined as penetrated by sperm.
In vitro culture and evaluation of subsequent embryonic development: To determine the developmental competence of oocytes, inseminated oocytes were assigned to in vitro culture (IVC) according to a procedure described previously [27, 29]. In brief, inseminated oocytes were freed from the cumulus cells by vortexing at 18 hr post-insemination (hpi). Cumulus-free oocytes were washed three times and cultured for 6 days under 5% CO2, 5% O2 and 90% N2 at 39°C in droplets of IVC medium (about 30 oocytes/30 µl). IVC medium was a modified synthetic oviduct fluid containing 1 mM glutamine, 12 essential amino acids for basal medium Eagle, 7 non-essential amino acids for minimum essential medium and 10 µg/ml insulin, and further supplemented with 5 mM glycine, 5 mM taurine, 1 mM glucose and 3 mg/ml fatty acid-free BSA. After 44 to 48 and 166 to 169 hpi (2 and 7 days after IVF, respectively), cleavage (developmental stage of cleaved embryos) and development to the blastocyst stage were assessed, respectively. All blastocysts were subjected to counting of the total number of cells by an air-drying method [25].
Experimental design: In experiment 1, to estimate the timing when 50% of oocytes reached the M-II stage, oocytes were subjected to IVM for 14, 16, 17, 18, 19, 20 and 22 hr, and their nuclear statuses were determined. A total of 645 COCs were subjected to IVM culture. After that, the time when 50% of oocytes accomplished nuclear maturation was computed using the percentage values of M-II oocytes at each IVM time point. In brief, the percentage values were modeled using the Gompertz equation, and the time to reach the point of 50% in the modeled Gompertz curve was defined as the time when 50% of oocytes reached the M-II stage. The Gompertz equation can be specified as follows: y=a × exp (− e-b (x − c)) (y=M-II rate, x=duration of IVM, a, b and c=parameters of the equation).
In experiment 2, to investigate the relationship between IVM duration and the timing of fertilization, oocytes after 12, 14, 18, 22, 26 and 30 hr of IVM were assigned for 4, 8, 12 and 18 hr of IVF, and their fertilization statuses were determined. A total of 696 COCs were subjected to IVM and IVF. Then, the timings when 50% of oocytes were penetrated by sperm and formed two pronuclei were estimated using the Gompertz equation, the same as in experiment 1. The relationships between the duration of IVM and the time from IVF start to sperm penetration and pronucleus formation were also analyzed.
In experiment 3, to investigate the timing when M-II oocytes have the highest developmental competence, oocytes subjected to 12, 14, 18, 22, 26 and 30 hr of IVM and 18 hr of IVF were cultured and examined for cleavage and development to the blastocyst stage. A total of 663 oocytes were subjected to IVC. In addition, we estimated the optimal timing for embryonic development of in vitro-matured bovine oocytes by analyzing the results of nuclear maturation, sperm penetration and subsequent embryonic development.
Statistical analysis: Data of nuclear maturation and fertilization subjected to arcsine transformation (Experiments 1 and 2) and of embryonic development (Experiment 3) were analyzed using one-way analysis of variance followed by Tukey-Kramer’s honestly significant different test as a post hoc test. Nonlinear regression analyses for the kinetics of nuclear maturation, sperm penetration and pronucleus formation after the initiation of IVM or IVF (Experiments 1 and 2) were performed by the Gompertz equation. Linear regression analyses were performed for expressing the relationships between the duration of IVM and the time from IVF start to sperm penetration and pronucleus formation (Experiment 2). Quadratic regression analysis to express the relationship between the period from nuclear maturation to sperm penetration and blastocyst rate was performed (Experiment 3). The level of statistical significance was set at P<0.05. Statistical analyses were performed using the software JMP version 10.0.2 (SAS Institute, Cary, NC, U.S.A.) and StatView version 5.0 (Abacus Concepts, Berkeley, CA, U.S.A.).
RESULTS
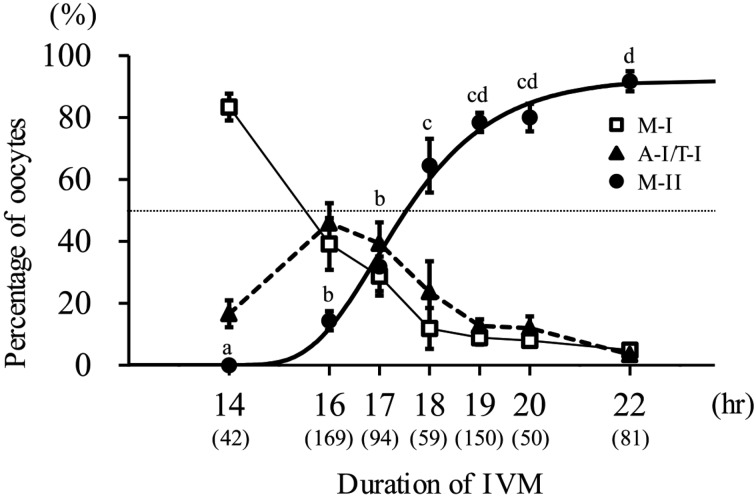
Experiment 1: The data of the nuclear status at each time point of IVM and the Gompertz curve fitted to the M-II rate are shown in Fig. 1. At 14 hr after IVM start, more than 80% of oocytes were at the metaphase I stage. Although no oocyte reached the M-II stage, some oocytes (16.6%) were at the anaphase or telophase I. At 16 hr after IVM start, 14.3% of oocytes reached M-II. At 18 hr after IVM start, more than half of oocytes reached M-II (64.5%). After 19 hr of IVM start, the M-II rate reached a plateau. By the Gompertz equation (r2=0.906), the time when 50% of oocytes reached the M-II stage was estimated to be 17.5 hr after the initiation of IVM.
Fig. 1.
Meiotic progression of bovine oocytes during IVM. Gompertz curve (bold solid line) is fitted to M-II rate. The equation of this curve is y=92.7 × exp (− e− 0.8 (x − 16.9)). Dotted line shows 50%. a,b,c,d Values with different characters differ significantly in the percentage of M-II oocytes (P<0.05). Three to 8 replicates were performed, and 42–169 oocytes were used in each group. Numbers in parentheses show the number of oocytes used. M-I: metaphase I, A-I/T-I: anaphase or telophase I, M-II: metaphase II.
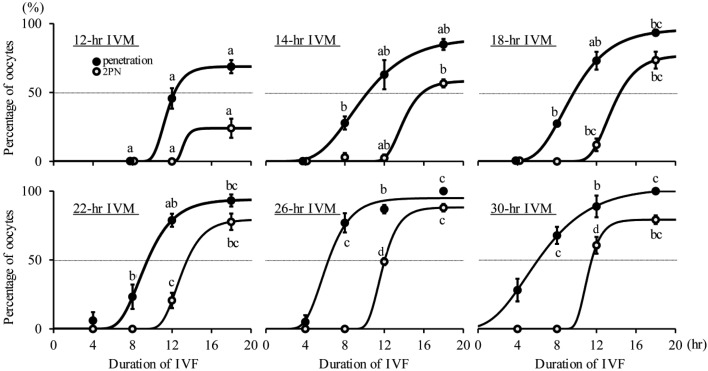
Experiment 2: The data of sperm penetration and pronucleus formation at each time point of IVF in oocytes subjected to different IVM culture periods are shown in Fig. 2. The Gompertz curves fitted to these values are also shown in Fig. 2. At 4 hpi, no oocyte was penetrated by sperm in the groups with 12, 14 and 18 hr of IVM, and some oocytes were penetrated by sperm in the groups with 22, 26 and 30 hr of IVM. At 8 hpi, in the groups with 14 and 18 hr of IVM, some oocytes (27.9 and 27.4%) were penetrated by sperm, and the penetration rate was significantly higher in the groups with 26 and 30 hr of IVM than in the others (P<0.05). Few oocytes showed pronucleus formation in all groups at 8 hpi. At 12 hpi, more than 60% of oocytes showed pronucleus formation in the group with 30 hr of IVM, and the percentages of pronucleus formation in the groups with 26 and 30 hr of IVM were higher than in the others (P<0.05). At 18 hpi, the percentages of sperm penetration and pronucleus formation in the group with 12 hr of IVM were lowest (P<0.05), and those in the groups with 18, 22, 26 and 30 hr of IVM were similar. The proportions of polyspermic oocytes were lower than 10%, regardless of the durations of IVM and IVF (data not shown). The regression equation of the estimated times when 50% of oocytes were penetrated by sperm after IVM culture for 12 to 30 hr was “y = − 0.328 x+ 15.7” (r2=0.905, P<0.01), which indicated that a longer duration of IVM resulted in faster sperm penetration. The regression equation of the estimated times when 50% of oocytes showed pronucleus formation after IVM culture for 14 to 30 hr was “y = − 0.280 x+ 19.6” (r2=0.979, P<0.01). These two regression lines were almost parallel, and the time period from sperm penetration to pronuclear formation was similar regardless of the IVM duration.
Fig. 2.
The effects of duration of IVM on sperm penetration and pronucleus (PN) formation after IVF. Gompertz curves (bold solid line) were fitted to the data of sperm penetration and PN formation. The equations of the curves for the percentage of oocytes penetrated were y=68.8 × exp (− e− 0.990 (x − 11.1)) (r2=0.871), 89.6 × exp (− e− 0.306 (x − 8.55)) (r2=0.877), 96.0 × exp (− e− 0.382 (x − 8.59)) (r2=0.964), 93.9 × exp (− e− 0.519 (x − 8.64)) (r2=0.934), 94.9 × exp (− e− 0.615 (x − 5.62)) (r2=0.946) and 1.02 × 102 × exp (− e− 0.281 (x − 4.88)) (r2=0.874) for 12, 14, 18, 22, 26 and 30 hr of IVM, respectively. The equations of the curves for the percentage of oocytes with 2PN were y=24.1 × exp (− e− 2.24 (x − 13.0)) (r2=0.706), 58.4 × exp (− e− 0.775 (x − 13.5)) (r2=0.970), 77.1 × exp (− e− 0.608 (x − 13.0)) (r2=0.950) 79.4 × exp (− e− 0.693 (x − 12.4)) (r2=0.949), 88.2 × exp (− e− 0.912 (x − 11.4)) (r2=0.991) and 79.3 × exp (− e− 1.12 (x − 10.8)) (r2=0.966) for 12, 14, 18, 22, 26 and 30 hr of IVM, respectively. Dotted line shows 50%. a,b,c,d Values with different characters differ significantly in the percentage of sperm penetration and PN formation at each time post-insemination (P<0.05). Each group had 2-4 replicates and 21-46 oocytes.
Experiment 3: The data of embryonic development in oocytes subjected to different IVM durations are shown in Table 1. At 2 days after IVF, the cleavage rate (≥2-cell) in the group with 12 hr of IVM was lower than that in the group with 22 hr of IVM (P<0.05). The proportion of cleaved oocytes that developed to ≥5 cells (beyond the third cell cycle) was significantly higher in the groups with 22 and 26 hr of IVM than in that with 14 hr (P<0.05) and tended to be higher in the group with 22 hr of IVM than in that with 12 hr (P=0.09). At 7 days after IVF, the percentage of blastocysts based on inseminated oocytes in the group with 22 hr of IVM was higher than those in the groups with 12 and 30 hr (P<0.05). The total number of cells in blastocysts was higher in the group with 22 hr of IVM than in the groups with 12 and 18 hr of IVM (P<0.05).
Table 1. The effects of the timing of IVF start after IVM (duration of IVM) on embryo development.
| Timing of IVF start after IVM (duration of IVM: hr) |
No. of oocytes (replicates) |
Cleavage and developmental stage at 2 days after IVF start |
Blastocyst development at 7 days after IVF start |
||||
|---|---|---|---|---|---|---|---|
| %≥2 cellst /oocytes |
%≥3 cellst /cleaved |
%≥5 cellst /cleaved |
% blastocystst /oocytes |
% blastocystst /cleaved |
Total cell no.t in blastocyst (n) |
||
| 12 | 104 (4) | 67.2 ± 4.8a) | 73.2 ± 6.7 | 9.4 ± 12.5a,b) | 32.8 ± 7.8a) | 49.2 ± 13.1 | 131.5 ± 54.9a) (34) |
| 14 | 106 (4) | 77.4 ± 2.5a,b) | 69.5 ± 8.2 | 6.0 ± 7.4a) | 35.6 ± 7.3a,b) | 46.1 ± 10.2 | 153.3 ± 62.6a,b) (33) |
| 18 | 109 (4) | 78.9 ± 3.0a,b) | 70.2 ± 20.3 | 17.2 ± 11.2a,b) | 38.4 ± 5.9a,b) | 48.6 ± 7.2 | 133.5 ± 61.2a) (42) |
| 22 | 136 (5) | 80.5 ± 6.0b) | 78.5 ± 9.9 | 28.2 ± 7.6b) | 50.0 ± 9.5b) | 62.6 ± 14.0 | 173.3 ± 75.6b) (68) |
| 26 | 108 (4) | 78.7 ± 5.5a,b) | 73.1 ± 6.9 | 27.9 ± 5.2b) | 42.7 ± 9.1a,b) | 54.7 ± 12.7 | 147.8 ± 72.4a,b) (46) |
| 30 | 100 (4) | 71.1 ± 9.9a,b) | 69.8 ± 6.1 | 13.8 ± 9.4a,b) | 29.0 ± 4.4a) | 40.8 ± 2.6 | 156.1 ± 58.3a,b) (29) |
a, b) Different superscripts within the same column differ significantly (P<0.05). Data are presented as means ± SD.
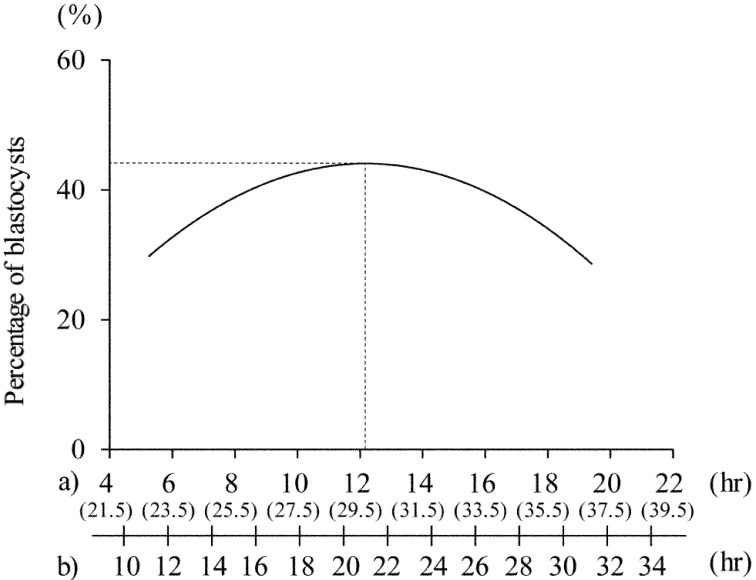
The correlation between the period from nuclear maturation to sperm penetration and blastocyst rate based on inseminated oocytes is shown in Fig. 3. The developmental rate to the blastocyst stage (based on inseminated oocytes: 44%) peaked when sperm penetration occurred at 12.2 hr after achieving nuclear maturation (i.e., 29.7 hr after the initiation of IVM), and the optimal IVM duration was estimated to be about 21 hr.
Fig. 3.
The regression curves describing the relationship between the time from nuclear maturation to sperm penetration and the percentage of blastocysts. Time from initiation of IVM to sperm penetration and duration of IVM corresponding to time from nuclear maturation to sperm penetration are described on the x-axis. a) Time from nuclear maturation to sperm penetration is shown, and the time from initiation of IVM to sperm penetration is also shown in parentheses. b) Duration of IVM culture. Regression equation was y=7.23x − 0.297x2 (r2=0.963, P<0.01). Dotted lines show the time from nuclear maturation to sperm penetration and blastocyst rate when the developmental rate to the blastocyst stage reached its maximum value.
DISCUSSION
From the estimation in the present study, bovine oocytes matured for about 21 hr before IVF have the highest rate of development to the blastocyst stage. This is in agreement with the results of previous reports [1, 11]. In the present study, 50% of oocytes reached the M-II stage after 17.5 hr of IVM culture. This meiotic progression of oocytes is in agreement with the finding of a previous report in which a similar IVM system was employed [21].
The present study demonstrated that the time of sperm penetration was affected by IVM duration: a longer duration of IVM resulted in faster sperm penetration (interval from IVF start to sperm penetration: y= −0.328x + 15.7). In our IVF system [25, 26], sperm capacitation mainly depended on the glycosaminoglycans (GAGs) produced by cumulus cells [3, 20], and the production of GAGs from cumulus cells increased during IVM culture [3]. Therefore, in our IVF conditions, sperm may have difficulty penetrating oocytes after short-term IVM culture, but easily penetrate them after long-term IVM culture. Our results also showed that the time period from sperm penetration to pronuclear formation was similar regardless of the IVM duration. On the other hand, a previous report indicated that M-II oocytes recovered after 16 hr of IVM culture and immediately subjected to IVF required a longer period for pronuclear formation than those cultured for another 8 hr before being subjected to IVF [4]. This inconsistency may be due to the delay of sperm penetration after short-term IVM culture in the present study.
Our results of the time from nuclear maturation (50% M-II at 17.5 hr) to sperm penetration and blastocyst development indicated that oocytes acquired the highest developmental competence at around 12 hr after achieving nuclear maturation (30 hr after the initiation of IVM culture, Fig. 3). To our knowledge, the present study is the first to estimate the optimal timing for fertilization to achieve proper embryonic development of in vitro-matured bovine oocytes based on the times of nuclear maturation and sperm penetration. This estimated time may be comparable to a previous report by Dominko and First (1997) [4]. They indicated that oocytes extruded a first polar body after 16 hr of IVM required another 8 hr of culture to acquire high developmental capacity. In their IVF system, the presence of a sperm head in the cytoplasm was first observed at 5 hpi, regardless of the time of M-II or the time of insemination. Therefore, the time of sperm penetration is estimated to be about 13 hr after achieving nuclear maturation (29 hr after the initiation of IVM culture), and our findings support their results.
In the present study, the cleavage rate, the blastocyst rate based on inseminated oocytes and the total cell number in blastocysts were lower in the group with 12 hr of IVM than in the group with 22 hr. This result may reflect lower sperm penetration rate at 18 hpi in the group with 12 hr of IVM than that in the group with 22 hr. Another possible explanation for this result is inadequate cytoplasmic maturation of oocytes. As described above, under our IVF system, the timing of sperm penetration depended on the function of cumulus cells. To clarify the time of oocyte acquisition of developmental competence, we should develop an IVF system that can allow sperm to penetrate oocytes whenever we want.
Although the percentages of sperm penetration at 18 hpi and cleavage in groups with 22 and 30 hr of IVM were similar, the developmental competence to the blastocyst stage of the group with 30 hr of IVM was significantly lower than that of the group with 22 hr. It has been reported that mitochondria can play important roles in the production of ATP for fertilization and pre-implantation embryo development and act as stores of intracellular calcium and proapoptotic factors [7, 32]. Tarazona et al. (2006) [30] suggested the important role of ATP produced by mitochondria for the embryonic genome activation (EGA) process. In bovine oocytes, minor and major EGAs were observed at the 1- to 4-cell and 8- to 16-cell stages and were found to be responsible for successful subsequent embryonic development [2]. Therefore, it is speculated that the low percentages of cleavage and blastocyst development are caused by the failure of the necessary EGA process in aged oocytes. In a future study, we should examine EGAs, the mitochondrial activity and ATP content of oocytes derived from different culture durations of IVM.
The present study showed that oocytes penetrated by sperm at around 30 hr after the initiation of IVM culture had the highest developmental competence. In previous studies, bovine oocytes at about 30 hr after IVM were considered as aged or slightly aged oocytes and used to reveal aging-related changes [17, 22]. However, after 30 hr of IVM, degradation of the microfilament-rich domain overlying the spindle in bovine oocytes was not observed, which had been observed in porcine aged oocytes [22]. Moreover, maturation-promoting factor (MPF) activity was similar to that in bovine oocytes matured for 24 and 32 hr [17], although a decrease in activity of MPF in bovine oocytes matured for 40 hr was observed [31]. Because aged oocytes were defined as those deteriorated in quality and lost developmental competence [13], from our result and previous results, we recommend that bovine oocytes at 30 hr after the initiation of IVM should not be considered as aged oocytes, but considered as fully matured ones.
In conclusion, in the present culture system, bovine oocytes acquire their highest developmental competence at around 12 hr after achieving nuclear maturation (i.e., around 30 hr after the initiation of IVM culture). To achieve stable IVP of bovine embryos, it is recommended to use the IVF system that permits oocytes to be penetrated by sperm around this timing. In addition, in the case of conducting basic studies on oocyte acquisition and loss of developmental competence by using the present culture system, bovine oocytes at 30 hr after the initiation of IVM should be considered as fully matured oocytes, not as aged ones.
Acknowledgments
Part of this study was supported by Grant-in-Aid for Scientific Research (No. 21580339) from the Japan Society for the Promotion of Science to Y. Takahashi.
REFERENCES
- 1.Agung B., Otoi T., Wongsrikeao P., Taniguchi M., Shimizu R., Watari H., Nagai T.2006. Effect of maturation culture period of oocytes on the sex ratio of in vitro fertilized bovine embryos. J. Reprod. Dev. 52: 123–127. doi: 10.1262/jrd.17055 [DOI] [PubMed] [Google Scholar]
- 2.Badr H., Bongioni G., Abdoon A. S., Kandil O., Puglisi R.2007. Gene expression in the in vitro-produced preimplantation bovine embryos. Zygote 15: 355–367. doi: 10.1017/S0967199407004315 [DOI] [PubMed] [Google Scholar]
- 3.Chen L., Wert S. E., Hendrix E. M., Russell P. T., Cannon M., Larsen W. J.1990. Hyaluronic acid synthesis and gap junction endocytosis are necessary for normal expansion of the cumulus mass. Mol. Reprod. Dev. 26: 236–247. doi: 10.1002/mrd.1080260307 [DOI] [PubMed] [Google Scholar]
- 4.Dominko T., First N. L.1997. Timing of meiotic progression in bovine oocytes and its effect on early embryo development. Mol. Reprod. Dev. 47: 456–467. doi: [DOI] [PubMed] [Google Scholar]
- 5.Edwards J. L., Saxton A. M., Lawrence J. L., Payton R. R., Dunlap J. R.2005. Exposure to a Physiologically Relevant Elevated Temperature Hastens In vitro Maturation in Bovine Oocytes. J. Dairy Sci. 88: 4326–4333. doi: 10.3168/jds.S0022-0302(05)73119-2 [DOI] [PubMed] [Google Scholar]
- 6.Eppig J. J.1996. Coordination of nuclear and cytoplasmic oocyte maturation in eutherian mammals. Reprod. Fertil. Dev. 8: 485–489. doi: 10.1071/RD9960485 [DOI] [PubMed] [Google Scholar]
- 7.Ferreira E. M., Vireque A. A., Adona P. R., Meirelles F. V., Ferriani R. A., Navarro P. A.2009. Cytoplasmic maturation of bovine oocytes: structural and biochemical modifications and acquisition of developmental competence. Theriogenology 71: 836–848. doi: 10.1016/j.theriogenology.2008.10.023 [DOI] [PubMed] [Google Scholar]
- 8.Iwata H., Ohota M., Hashimoto S., Nagai Y.2003. Free oxygen radicals are generated at the time of aspiration of oocytes from ovaries that have been stored for a long time. Zygote 11: 1–5. doi: 10.1017/S0967199403001011 [DOI] [PubMed] [Google Scholar]
- 9.Iwata H., Hashimoto S., Ohota M., Kimura K., Shibano K., Miyake M.2004. Effects of follicle size and electrolytes and glucose in maturation medium on nuclear maturation and developmental competence of bovine oocytes. Reproduction 127: 159–164. doi: 10.1530/rep.1.00084 [DOI] [PubMed] [Google Scholar]
- 10.Izadyar F., Colenbrander B., Bevers M. M.1996. In vitro maturation of bovine oocytes in the presence of growth hormone accelerates nuclear maturation and promotes subsequent embryonic development. Mol. Reprod. Dev. 45: 372–377. doi: [DOI] [PubMed] [Google Scholar]
- 11.Long C. R., Damiani P., Pinto-Correia C., MacLean R. A., Duby R. T., Robl J. M.1994. Morphology and subsequent development in culture of bovine oocytes matured in vitro under various conditions of fertilization. J. Reprod. Fertil. 102: 361–369. doi: 10.1530/jrf.0.1020361 [DOI] [PubMed] [Google Scholar]
- 12.Merton J. S., de Roos A. P., Mullaart E., de Ruigh L., Kaal L., Vos P. L., Dieleman S. J.2003. Factors affecting oocyte quality and quantity in commercial application of embryo technologies in the cattle breeding industry. Theriogenology 59: 651–674. doi: 10.1016/S0093-691X(02)01246-3 [DOI] [PubMed] [Google Scholar]
- 13.Miao Y.L., Kikuchi K., Sun Q.Y., Schatten H.2009. Oocyte aging: cellular and molecular changes, developmental potential and reversal possibility. Hum. Reprod. Update 15: 573–585. doi: 10.1093/humupd/dmp014 [DOI] [PubMed] [Google Scholar]
- 14.Nagano M., Katagiri S., Takahashi Y.2006. ATP content and maturational/developmental ability of bovine oocytes with various cytoplasmic morphologies. Zygote 14: 299–304. doi: 10.1017/S0967199406003807 [DOI] [PubMed] [Google Scholar]
- 15.Nagano M., Katagiri S., Takahashi Y.2006. Relationship between bovine oocyte morphology and in vitro developmental potential. Zygote 14: 53–61. doi: 10.1017/S0967199406003510 [DOI] [PubMed] [Google Scholar]
- 16.Park Y. S., Kim S. S., Kim J. M., Park H. D., Byun M. D.2005. The effects of duration of in vitro maturation of bovine oocytes on subsequent development, quality and transfer of embryos. Theriogenology 64: 123–134. doi: 10.1016/j.theriogenology.2004.11.012 [DOI] [PubMed] [Google Scholar]
- 17.Rispoli L. A., Lawrence J. L., Payton R. R., Saxton A. M., Schrock G. E., Schrick F. N., Middlebrooks B. W., Dunlap J. R., Parrish J. J., Edwards J. L.2011. Disparate consequences of heat stress exposure during meiotic maturation: embryo development after chemical activation vs fertilization of bovine oocytes. Reproduction 142: 831–843. doi: 10.1530/REP-11-0032 [DOI] [PubMed] [Google Scholar]
- 18.Rizos D., Ward F., Duffy P., Boland M. P., Lonergan P.2002. Consequences of bovine oocyte maturation, fertilization or early embryo development in vitro versus in vivo: implications for blastocyst yield and blastocyst quality. Mol. Reprod. Dev. 61: 234–248. doi: 10.1002/mrd.1153 [DOI] [PubMed] [Google Scholar]
- 19.Saeki K., Nagao Y., Hoshi M., Nagai M.1995. Effects of heparin, sperm concentration and bull variation on in vitro fertilization of bovine oocytes in a protein-free medium. Theriogenology 43: 751–759. doi: 10.1016/0093-691X(95)00017-3 [DOI] [PubMed] [Google Scholar]
- 20.Salustri A., Yanagishita M., Underhill C. B., Laurent T. C., Hascall V. C.1992. Localization and synthesis of hyaluronic acid in the cumulus cells and mural granulosa cells of the preovulatory follicle. Dev. Biol. 151: 541–551. doi: 10.1016/0012-1606(92)90192-J [DOI] [PubMed] [Google Scholar]
- 21.Sirard M. A., Florman H. M., Leibfried-Rutledge M. L., Barnes F. L., Sims M. L., First N. L.1989. Timing of nuclear progression and protein synthesis necessary for meiotic maturation of bovine oocytes. Biol. Reprod. 40: 1257–1263. doi: 10.1095/biolreprod40.6.1257 [DOI] [PubMed] [Google Scholar]
- 22.Somfai T., Kikuchi K., Kaneda M., Akagi S., Watanabe S., Mizutani E., Haraguchi S., Dang-Nguyen T. Q., Inaba Y., Geshi M., Nagai T.2011. Cytoskeletal abnormalities in relation with meiotic competence and ageing in porcine and bovine oocytes during in vitro maturation. Anat. Histol. Embryol. 40: 335–344. doi: 10.1111/j.1439-0264.2011.01079.x [DOI] [PubMed] [Google Scholar]
- 23.Sugimura S., Matoba S., Hashiyada Y., Aikawa Y., Ohtake M., Matsuda H., Kobayashi S., Konishi K., Imai K.2012. Oxidative phosphorylation-linked respiration in individual bovine oocyte. J. Reprod. Dev. 58: 636–641. doi: 10.1262/jrd.2012-082 [DOI] [PubMed] [Google Scholar]
- 24.Süss U., Wuthrich K., Stranzinger G.1988. Chromosome configurations and time sequence of the first meiotic division in bovine oocytes matured in vitro. Biol. Reprod. 38: 871–880. doi: 10.1095/biolreprod38.4.871 [DOI] [PubMed] [Google Scholar]
- 25.Takahashi Y., First N. L.1992. In vitro development of bovine one-cell embryos: Influence of glucose, lactate, pyruvate, amino acids and vitamins. Theriogenology 37: 963–978. doi: 10.1016/0093-691X(92)90096-A [DOI] [PubMed] [Google Scholar]
- 26.Takahashi Y., First N. L.1993. In vitro fertilization of bovine oocytes in the presence of theophylline. Anim. Reprod. Sci. 34: 1–18. doi: 10.1016/0378-4320(93)90045-S [DOI] [Google Scholar]
- 27.Takahashi Y., Kanagawa H.1998. Effects of glutamine, glycine and taurine on the development of in vitro fertilized bovine zygotes in a chemically defined medium. J. Vet. Med. Sci. 60: 433–437. doi: 10.1292/jvms.60.433 [DOI] [PubMed] [Google Scholar]
- 28.Takahashi Y., Kanagawa H.1998. Effect of oxygen concentration in the gas atmosphere during in vitro insemination of bovine oocytes on the subsequent embryonic development in vitro. J. Vet. Med. Sci. 60: 365–367. doi: 10.1292/jvms.60.365 [DOI] [PubMed] [Google Scholar]
- 29.Takahashi Y., Hishinuma M., Matsui M., Tanaka H., Kanagawa H.1996. Development of in vitro matured/fertilized bovine embryos in a chemically defined medium: influence of oxygen concentration in the gas atmosphere. J. Vet. Med. Sci. 58: 897–902. doi: 10.1292/jvms.58.897 [DOI] [PubMed] [Google Scholar]
- 30.Tarazona A. M., Rodriguez J. I., Restrepo L. F., Olivera-Angel M.2006. Mitochondrial activity, distribution and segregation in bovine oocytes and in embryos produced in vitro. Reprod. Domest. Anim. 41: 5–11. doi: 10.1111/j.1439-0531.2006.00615.x [DOI] [PubMed] [Google Scholar]
- 31.Tian X. C., Lonergan P., Jeong B. S., Evans A. C., Yang X.2002. Association of MPF, MAPK, and nuclear progression dynamics during activation of young and aged bovine oocytes. Mol. Reprod. Dev. 62: 132–138. doi: 10.1002/mrd.10072 [DOI] [PubMed] [Google Scholar]
- 32.Wang L. Y., Wang D. H., Zou X. Y., Xu C. M.2009. Mitochondrial functions on oocytes and preimplantation embryos. J. Zhejiang Univ. Sci. B 10: 483–492. doi: 10.1631/jzus.B0820379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ward F., Enright B., Rizos D., Boland M., Lonergan P.2002. Optimization of in vitro bovine embryo production: effect of duration of maturation, length of gamete co-incubation, sperm concentration and sire. Theriogenology 57: 2105–2117. doi: 10.1016/S0093-691X(02)00696-9 [DOI] [PubMed] [Google Scholar]