ABSTRACT
Subpopulations of peripheral leukocytes and cytokine mRNA expression levels were evaluated in scouring and healthy Holstein calves (age 10 ± 5 days; n=42) treated with a probiotic consisting of Lactobacillus plantarum, Enterococcus faecium and Clostridium butyricum. The calves were assigned to the scouring or healthy group and then subdivided into pathogen-positive treated (n=8), pathogen-positive control (n=8), pathogen-negative treated (n=6), pathogen-negative control (n=6), healthy treated (n=6) and healthy control (n=8) groups. A single dose of the probiotic (3.0 g/100 kg body weight) was given to each calf in the treatment groups for 5 days. Blood samples were collected on the first day of scour occurrence (day 0) and on day 7. In the scouring calves, smaller peripheral leukocyte subpopulations and cytokine mRNA expression levels were noted on day 0. The numbers of CD3+ T cells; CD4+, CD8+ and WC1+ γδ T cell subsets; and CD14+, CD21+ and CD282+ (TLR2) cells were significantly increased in the scouring and healthy treated calves on day 7. Furthermore, interleukin-6, tumor necrosis factor-alpha and interferon-gamma mRNA expression was elevated in the peripheral leukocytes of the scouring and healthy treated calves on day 7. The scouring calves given the probiotic recovered on day 7. A significantly smaller number of peripheral leukocytes and lower cytokine mRNA expression level might be induced by scouring in calves. Repeated probiotic administration might stimulate cellular immunity and encourage recovery from scouring in pre-weaning Holstein calves.
Keywords: cytokine, leukocyte, probiotic, scouring calf, toll-like receptor 2
Calves are born with a naive immune system, and appropriate development of the gastrointestinal tract (GIT) microbiota in the early weeks of life is crucial for a functional immune system [25, 27, 39]. GIT diseases are characterized by acute intestinal inflammation, much of which is thought to be due to inappropriate activation of the immune system in calves [19, 22]. Greater vulnerability to invasive infections and the development of GIT disorders, such as scouring, are associated with the immaturity of the immune system of calves at birth, although the specific mechanism is unclear. Regulation by the immune-stimulatory effects of probiotics may contribute to the treatment of scouring in calves [26]. Probiotics contribute to the homeostasis of the bacterial flora in the GIT, improving animal health and protecting calves against infection [35, 36]. Lactic acid bacteria (LAB) probiotics are known to help with GIT colonization via the competitive exclusion of undesirable microorganisms and the development of a homeostatic gastrointestinal environment in calves [1, 12, 18]. LAB probiotics are also known to have a beneficial impact on intestinal infections and are commonly used to treat calf scouring [19]. More recently, in vivo studies have examined their immune-stimulatory effects in calves [26, 35, 36]. In cases of inadequate initial bacterial colonization, probiotics can be used to achieve a balanced immune response [17]. Therefore, the immune-modulating effect of probiotics is likely more appropriate for young calves, when the intestinal bacterial inhabitants are less well-established. The phagocytic activities of leukocytes have been reported to be increased in the peripheral blood of calves following the administration of a probiotic consisting of Lactobacillus plantarum [18]. A number of systemic immune markers, including CD8+ cells, increased in response to treatment with a probiotic in scouring calves [26]. In adult cattle, the immune-stimulatory effect of a probiotic containing Enterococcus faecium and Saccharomyces cerevisiae has been recognized in feedlot steers and dairy cows [7, 20]. In the intestine, Clostridium butyricum as a probiotic may increase the resistance of the gut to pathogen invasion by inducing the secretion of anti-inflammatory cytokines [11]. The stimuli provided by probiotics are essential for the development of a fully functional and balanced immune system, including the homing of B and T cells to the lamina propria [4]. It has generally been assumed that some probiotic strains predominantly induce cytokine mRNA expression in leukocytes, promote T cell development and augment immune defense to prevent infections, thereby ameliorating inflammatory diseases [7, 20, 33, 34]. Recently, we studied the effects of a probiotic on peripheral leukocytes and their mRNA expression in healthy weaned calves (in press). However, the effect of probiotics on subpopulations of peripheral leukocytes in scouring and healthy pre-weaning calves is unknown. In the present study, the immune-stimulatory effects of a probiotic on peripheral blood mononuclear cells (PBMCs) and their mRNA expression levels were investigated in scouring and healthy pre-weaning Holstein calves.
MATERIALS AND METHODS
Animals and treatment: The experimental design was approved by the Laboratory Animal Care and Use Committee of Iwate University, Iwate, Japan. Forty-two scouring and healthy pre-weaning Holstein calves (age 10 ± 5 days) housed in two commercial dairy farms were used in the experiment. All calves were examined clinically, and the scouring status of each calf was inspected. The scour condition was watery to muddy yellow, and the appetite of the scouring calves was almost normal. The calves were assigned to treatment and corresponding control groups based on the type of scouring (pathogenic or non-pathogenic) and clinically healthy (non-scouring) status. The treatment groups included scouring pathogen-positive treated (PPT; n=8), scouring pathogen-negative treated (PNT; n=8) and healthy treated (HT; n=6) calves. The corresponding control groups included scouring pathogen-positive control (PPC; n=6) and pathogen-negative control (PNC; n=6) calves, and healthy control (HC; n=8) calves without probiotic treatment were used as the main control group. All calves received colostrum at birth and were fed a milk replacement twice daily.
Treatment of the calves began on the first day of scour occurrence (day 0). After a clinical examination and the collection of blood and stool samples, a probiotic (Miyarisan Pharmaceutical Co., Ltd., Tokyo, Japan) that included L. plantarum strain 220 (9 × 106 colony forming units [CFU]/g), E. faecium strain 26 (9 × 105 CFU/g) and C. butyricum strain Miyari (9 × 104 CFU/g) was administered to each of the calves in the treatment groups once daily at a dose of 3.0 g/100 kg body weight (BW) for 5 days. The probiotic was mixed with a milk replacement and fed to each calf in the morning. The scouring calves were not treated by any other medicine or electrolyte therapy, although all calves had access to fresh water ad libitum.
Blood collection and PBMC isolation: Blood samples were collected on days 0 and 7. A blood specimen (6 ml) was collected in a heparinized tube (BD Vacutainer Systems, Plymouth, U.K.) and used to isolate PBMCs for flow cytometric analysis. Blood (4 ml) was also collected in two EDTA tubes (BD Vacutainer; Becton Dickinson, Franklin Lakes, NJ, U.S.A.) and used for total RNA extraction from peripheral leukocytes and for white blood cell (WBC) counts. The WBC count was determined using an automatic hematology analyzer device equipped with software for bovine samples (pocH-100iV Diff; Sysmex, Kobe, Japan). The percentage of PBMCs was determined using the hemogram method with the Dif-Quick staining protocol. PBMCs were isolated as described by Ohtsuka et al. [27]. To purify PBMCs and to lyse red blood cells, 2 ml of the heparinized blood was mixed with 8 ml of 0.83% ammonium chloride solution. After 5 min at room temperature, the samples were centrifuged (2,000 rpm, 10 min and 4°C). The leukocytes were then purified by washing twice in phosphate-buffered saline (PBS; pH 7.4) followed by centrifugation (2,000 rpm, 5 min and 4°C). After the final wash, the cell pellet was diluted in 500 µl of PBS, and the cells were counted. The cell concentration was then adjusted to 1 × 107/ml in PBS, and the cells were kept at 4°C until they were used for flow cytometry.
Flow cytometry: The primary monoclonal antibodies used in the present study are shown in Table 1. Flow cytometry was performed as described by Ohtsuka et al. [27]. PBMCs were labeled with the primary antibody and incubated for 60 min at 4°C. CD3+ T cells and each of the CD4+ and CD8+ T cell subsets were additionally labeled with a monoclonal antibody against CD45R to mark pan-leukocytes. After incubation, the cells were washed twice to remove unbound antibodies. The labeled cells were then stained with specific purified IgG conjugated to a phycoerythrin (PE) polyclonal secondary antibody (goat anti-mouse IgG1 RPE; AbD Serotec, Kidlington, Oxford, U.K.) or fluorescein isothiocyanate (FITC)-conjugated antibody (goat anti-mouse IgG or IgM FITC; Southern Biotech, Birmingham, AL, U.S.A.) and incubated for 30 min at 4°C. After washing twice, the stained cells were diluted in 500 µl of PBS. Flow cytometry was performed using a FACScan analyzer (Becton Dickinson) equipped with a computer running Cell Quest software (Becton Dickinson).
Table 1. Monoclonal antibodies used for flow cytometry.
| Item | Target molecule | Specificity | mAb clone | Isotype | Supplier |
|---|---|---|---|---|---|
| CD3 | TCR complex | Pan-T cells | MM1A | IgG1 | VMRD |
| CD4 | CD4 molecule | T-helper cells | CACT183A | IgG1 | VMRD |
| CD8 | CD8β | Cytotoxic T cells | BAT82A | IgG1 | VMRD |
| CD45R | PTPR | Pan-leukocytes | GC6A | IgM | VMRD |
| WC1 | Unknown | γδ T cells | IL-A29 | IgG1 | VMRD |
| CD14 | LPS receptor | Monocytes, macrophages | CAM36A | IgG1 | VMRD |
| CD21 | CR2 | B cells | BAQ15A | IgM | VMRD |
| CD282 | TLR2 | Monocytes | HCA151F | IgG | AbD-Serotec |
mAb, monoclonal antibody; TCR, T cell receptor; PTPR, protein tyrosine phosphatase receptor; LPS, lipopolysaccharide; CR2, complement component receptor 2; TLR2, toll-like receptor 2.
Total RNA extraction from leukocytes and cDNA synthesis: Total RNA was extracted from 1 ml of whole blood leukocytes using the SV Total RNA Isolation System (Promega, Tokyo, Japan). Trace genomic DNA in the crude total RNA samples was removed by incubation with 4–6 U of DNase I per 100 mg of total RNA (Ambion, Austin, TX, U.S.A.) for 30 min at 37°C. The concentration of total RNA was verified on a 1% agarose gel, and the purity was determined using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Rockland, DE, U.S.A.). cDNA was prepared using an oligo (dT) primer and a thermal cycler (MyCycler; Bio-Rad, Tokyo, Japan). cDNA synthesis was performed using the ImProm Reverse Transcription System (Promega). The cDNA was stored at −80°C until it was used for real-time PCR.
Real-time PCR: Real-time PCR was performed using an iQ SYBR Green Supermix Kit (Bio-Rad) as described previously [23]. Specific individual cytokine primer sequences and the internal standard gene used (β-actin) are shown in Table 2. Amplification was performed using a Mini Opticon (Bio-Rad) thermal cycler with the following profile: 1 cycle of 95°C for 2 min followed by 45 cycles of 95°C for 30 sec, and 65 or 59°C for 30 sec and 74°C for 30 sec followed by 74°C for 7 min. A melting curve analysis was performed from 50 to 95°C with readings every 0.5°C (holding for 5 sec). Relative gene expression was calculated using the comparative threshold cycle number (2–ΔCt) method, as described by Livak and Schmitge [23]. The results are summarized as ΔCt values, where ΔCt is the difference in the threshold cycle for the target and β-actin, as an internal control.
Table 2. Primers used for real-time PCR.
| Gene | GenBank acc. no. | Sequence (5′→3′) | Annealing temperature (°C) | Concentration (nM) |
|---|---|---|---|---|
| β-Actin | AY141970 | F: GGCCGAGCGGAAATCG | 65 | 750 |
| R: GCCATCTCCTGCTCGAAGTC | ||||
| IL-6 | NM173923 | F: GTCTTCAAACGAGTGGGTAAAG | 65 | 250 |
| R: TGACCAGAGGAGGGAATGC | ||||
| IL-8 | NM173925 | F: CACTGTGAAAATTCAGAAATCATTGTTA | 59 | 250 |
| R: CTTCACAAATACCTGCACAACCTTC | ||||
| TNF-α | Z48808 | F: CGGTGGTGGGACTCGTATG | 65 | 750 |
| R: GCTGGTTGTCTTCCAGCTTCA | ||||
| IFN-γ | NM174086 | F: TAGGCAAGTCTATGGGATTTC | 59 | 250 |
| R: GCATTCATTACATCATCAAGTG |
Stool analysis: The common pathogens considered essential for calf scouring in the field were examined using stool samples from the calves in the scouring groups. On the first day of scouring, stool samples were obtained from each calf by mechanical stimulation of the anus and preserved in a sterilized tube. Deoxycholate hydrogen sulfide-lactose agar (Elken Chemical Co., Tokyo, Japan) medium was used for the recognition of Salmonella and Escherichia coli according to the methods of Ishihara et al. [15]. The Cryptosporidium parvum test strip BIO K 155 (Bio-X Diagnostics, Jemelle, Belgium) was used according to the manufacturer’s instructions with 0.1 g of stool sample and was evaluated visually. For the detection of coccidia spores, a previously described microscopic method was used [22]. For rotavirus, a specific test strip (SA Scientific, San Antonio, TX, U.S.A.) was used following the product use recommendations. Among the scouring calves diagnosed as pathogen-positive (n=16), the pathogens found were rotavirus (43%), coccidian Eimeria bovis and/or Eimeria zuernii (25%), C. parvum (19%), E. coli (13%) and Salmonella (0%). Those calves with negative stool tests were assigned to the non-pathogenic scouring groups, and clinically healthy calves without scouring were placed in the healthy groups. The scouring status of each calf was inspected every day. Reduced occurrence of scouring and an improvement in clinical status were noted in the probiotic-treated calves on day 7.
Statistical analysis: The number of CD-positive or -negative cells was calculated from the percentages of leukocytes gated by flow cytometry and total PBMCs. The values are expressed as means ± standard error (SE). GraphPad Prism ver.5.01 software (La Jolla, CA, U.S.A.) was used for the statistical calculations and one-way ANOVA, and the Kruskal-Wallis test was subsequently used to evaluate differences among the groups. A P-value<0.05 was considered significant.
RESULTS
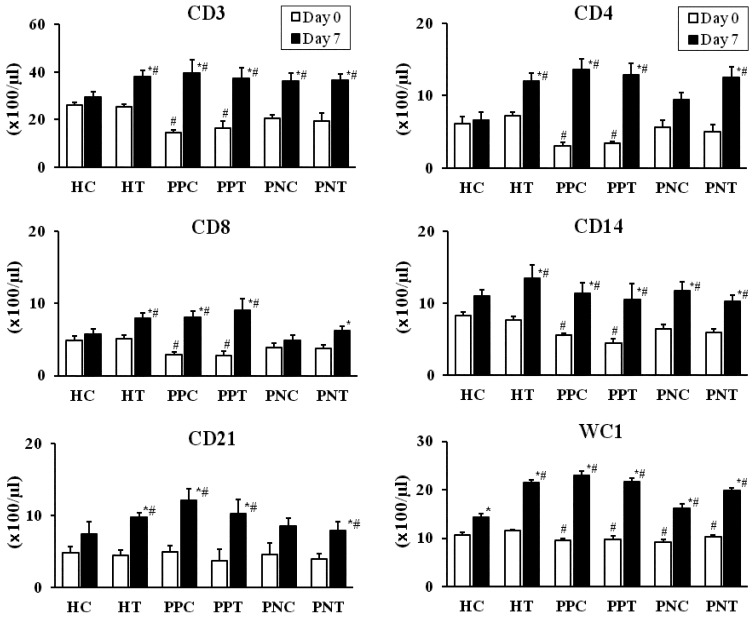
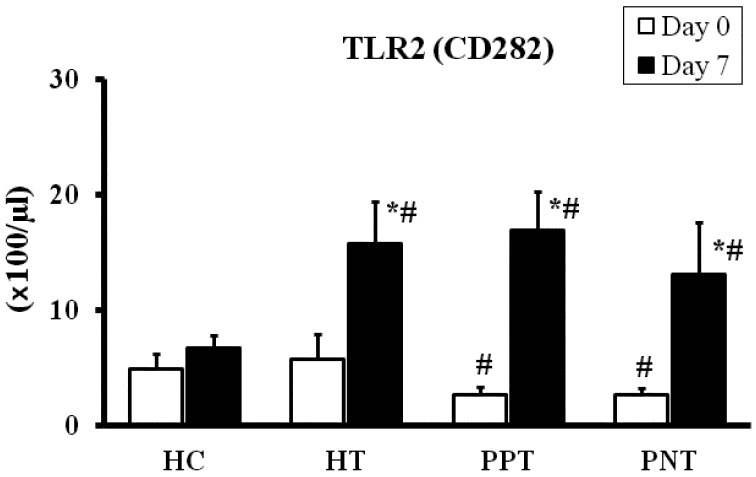
Subpopulations of peripheral leukocytes: Compared with the values of the HC and HT groups on day 0, a significantly lower (P<0.05) number of CD3+ T cells; CD4+, CD8+ and WC1+ γδ T cell subsets; and CD14+ cells were noted in the peripheral blood of scouring PPT and PPC calves. On day 0, the numbers of CD3+ T cells; the CD4+, CD8+ and WC1+ γδ T cells subsets; and CD14+ cells were lower (P<0.05) in the PPT and PPC groups than in the other groups. The number of CD21+ cells was similar among the scouring and healthy groups on day 0 (Fig. 1). Compared to the HC group, CD3+ T cells; the CD4+, CD8+ and WC1+ γδ T cell subsets; and CD14+ and CD21+ cells were increased (P<0.05) in the scouring PPT, PNT and PPC groups on day 7. However, the numbers of CD8+ and CD21+ cells were unchanged in the scouring PNC group on day 7. The values among the scouring PPT, PNT, PPC and PNC groups were not significantly different on day 7. Moreover, compared with the values on day 0 and those of the HC group, an increased (P<0.05) number of CD3+ T cells; the CD4+, CD8+ and WC1+ γδ T cell subsets; and CD14+ and CD21+ cells were noted in the HT group on day 7. Compared with the value on day 0, WC1+ γδ T cells were increased (P<0.05) in the HC group on day 7. Likewise, compared with the values on day 0, PBMCs were increased (P<0.05) in the scouring PPT, PNT and HT groups on day 7. A discrete analysis of the data indicated that the numbers of CD45R+ and CD45R− T cells were increased (P<0.05) in the proportion of CD3+ (CD3+D45R+/CD45R−) T cells, CD4+ (CD4+CD45R+/CD45R−) and CD8+ (CD8+CD45R+/CD45R−) T cell subsets in the scouring PPT, PNT, PPC and HT groups on day 7, compared to the values on day 0 in the same group or the HC group. However, the values were increased (P<0.05) only in the number of CD3+ (CD3+CD45R+) T cells and CD4+ (CD4+CD45R+) T cell subset in the scouring PNC group on day 7. The values were not significantly different among the scouring PPT, PNT, PPC and PNC groups (Table 3A and 3B). Peripheral CD282+ [toll-like receptor 2 (TLR2)] monocytes were evaluated only in the scouring PPT, PNT, HT and HC groups. Similar to that of the T cell subpopulations, a significantly lower (P<0.05) number of peripheral TLR2+ monocytes were noted in the scouring calves on day 0. However, the number of TLR2+ monocytes was increased (P<0.05) in the scouring PPT, PNT and HT groups on day 7, compared with the values on day 0 and that of the HC group (Fig. 2).
Fig. 1.
The numbers of CD3, CD4, CD8, CD14, CD21 and WC1 cells in the peripheral blood of the scouring PPT (n=8), PPC (n=8), PNT (n=6), PNC (n=6), HT (n=6) and HC (n=8) groups. Calves in the treated groups were given 3.0 g/100 kg BW of the probiotic once daily for 5 days, and calves without probiotic treatment served as corresponding controls. The values represent the mean ± SE. * Compared to day 0 in the same group. # Compared to the HC group (P<0.05). The first day of scouring occurrence and probiotic treatment was designated as day 0.
Table 3A. Numbers of peripheral blood mononuclear cells (PBMCs) and CD45R− /CD45R+ T cells in the scouring probiotic-treated and scouring control calves.
| PPCa) |
PPTb) |
PNCc) |
PNTd) |
|||||
|---|---|---|---|---|---|---|---|---|
| Day 0 | Day 7 | Day 0 | Day 7 | Day 0 | Day 7 | Day 0 | Day 7 | |
| PBMCs | 4,231 ± 430 | 5,658 ± 952 | 3,822 ± 313 | 5,947 ± 554* | 4,427 ± 495 | 5,126 ± 425 | 4,140 ± 422 | 5,610 ± 393* |
| CD3+CD45R− | 1,088 ± 103 | 2,656 ± 565* | 1,064 ± 85 | 2,342 ± 282* | 1,185 ± 79 | 2,558 ± 401* | 1,375 ± 127 | 2,228 ± 268* |
| CD3+CD45R+ | 361 ± 28 | 1,305 ± 288* | 564 ± 27 | 1,394 ± 150* | 678 ± 85 | 968 ± 160* | 552 ± 60 | 1,419 ± 131*# |
| CD4+CD45R− | 133 ± 18 | 377 ± 53* | 73 ± 14 | 320 ± 47* | 111 ± 17 | 287 ± 75* | 155 ± 41 | 205 ± 25* |
| CD4+CD45R+ | 177 ± 29 | 978 ± 144* | 264 ± 23 | 963 ± 151* | 450 ± 98 | 654 ± 58* | 344 ± 63 | 1,049 ± 130*# |
| CD8+CD45R− | 67 ± 15 | 284 ± 41* | 80 ± 43 | 201 ± 22* | 58 ± 7.0 | 96 ± 15 | 44 ± 12 | 113 ± 32* |
| CD8+CD45R+ | 238 ± 41 | 409 ± 73* | 204 ± 16 | 710 ± 142*# | 290 ± 51 | 305 ± 60 | 328 ± 46 | 505 ± 52*# |
The values (cells/µl) represent the means ± SE. a) Pathogen-positive control (n=8). b) Pathogen-positive treated (n=6). c) Pathogen-negative control (n=8). d) Pathogen-negative treated (n=6). * Compared with day 0 in the same group (P<0.01). # Compared with the values of the corresponding control groups (P<0.05).
Table 3B. Numbers of peripheral blood mononuclear cells (PBMCs) and CD45R−/CD45R+ T cell subpopulations in the healthy probiotic-treated and healthy control calves.
| HCa) |
HTb) |
|||
|---|---|---|---|---|
| Day 0 | Day 7 | Day 0 | Day 7 | |
| PBMCs | 4,500 ± 238 | 5,148 ± 476 | 3,808 ± 258 | 7,218 ± 365* |
| CD3+CD45R− | 1,621 ± 116 | 1,725 ± 198 | 1,498 ± 98 | 2,003 ± 491* |
| CD3+CD45R+ | 979 ± 71 | 1,114 ± 152 | 1,042 ± 45 | 1,815 ± 105*# |
| CD4+CD45R− | 128 ± 34 | 219 ± 51 | 108 ± 21 | 284 ± 22* |
| CD4+CD45R+ | 482 ± 81 | 439 ± 81 | 501 ± 71 | 1,307 ± 96*# |
| CD8+CD45R− | 52 ± 12 | 68 ± 11 | 50 ± 7.5 | 101 ± 14* |
| CD8+CD45R+ | 409 ± 50 | 502 ± 70 | 421 ± 22 | 693 ± 66* |
The values (cells/µl) represent the means ± SE. a) Healthy control calves without treatment (n=8). b) Healthy treated calves (received 3.0 g/100 kg of probiotic for 5 days; n=6). *Compared with day 0 in the same group (P<0.01). # Compared with the healthy controls on the same day (P<0.05).
Fig. 2.
Number of TLR2 (CD282) cells in the peripheral blood of the scouring PPT (n=8), PNT (n=6), HT (n=6) and HC (n=8) groups. Calves in the treated groups were given 3.0 g/100 kg BW of the probiotic once daily for 5 days, and calves without probiotic treatment served as corresponding controls. The values represent the mean ± SE. * Compared to day 0 in the same group. # Compared to the HC group (P<0.05). The first day of scouring occurrence and probiotic treatment was designated as day 0.
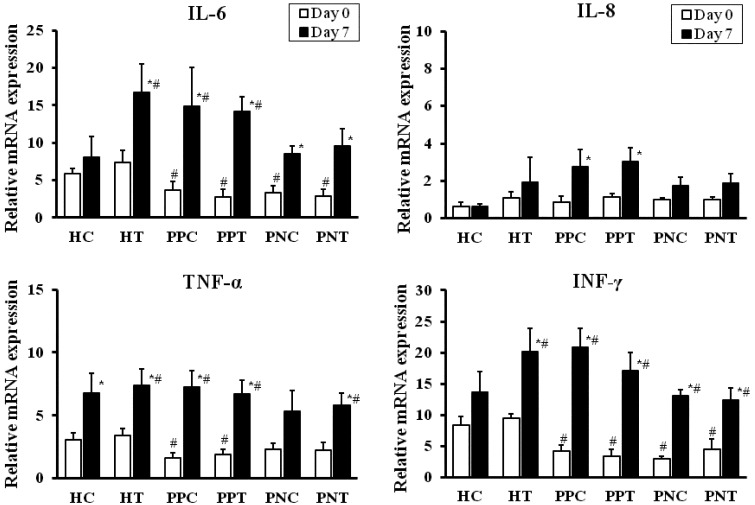
mRNA expression of cytokines: Compared with the value for the HC calves, the mRNA expression levels of interleukin (IL)-6 and interferon-gamma (IFN-γ) were lower (P<0.05) in the scouring PPT, PNT, PPC and PNC groups on day 0. The mRNA expression level of tumor necrosis factor-alpha (TNF-α) was lower (P<0.05) in the scouring PPT and PPC groups, but the values were greater in the scouring PNT, PNC, HT and HC groups on day 0 (Fig. 3). IL-6, IFN-γ and TNF-α mRNA expression was increased (P<0.05) in the PPT, PNT, PPC, PNC and HT groups on day 7. Furthermore, compared to day 0, the TNF-α mRNA expression level was higher (P<0.05) in the HC group on day 7. The mRNA expression level of IL-8 was similar between the scouring and healthy calves on day 0, but was elevated (P<0.05) in the scouring PPT and PPC groups on day 7. Compared to the HC group, the expression of IL-8 mRNA was slightly higher in the HT group on day 7, but the differences were not statistically significant.
Fig. 3.
Relative mRNA expression of IL-6, IL-8, TNF-α and IFN-γ in the peripheral blood of the scouring PPT (n=8), PPC (n=8), PNT (n=6), PNC (n=6), HT (n=6) and HC (n=8) groups. Calves in the treated groups were given 3.0 g/100 kg BW of the probiotic once daily for 5 days, and calves without probiotic treatment served as corresponding controls. The values represent the mean ± SE.* Compared to day 0 in the same group. # Compared to the HC group (P<0.05). The first day of scouring occurrence and probiotic treatment was designated as day 0.
DISCUSSION
We recently studied the effects of a probiotic consisting of L. plantarum, E. faecium and C. butyricum on the peripheral leukocyte subpopulations and cytokine mRNA expression levels in healthy weaned calves (in press). In the present study, peripheral leukocyte subpopulations and their cytokine mRNA expression levels were evaluated in the field in scouring and healthy pre-weaning calves to clarify the immune-stimulatory effects of the same probiotic agent.
Some reports have indicated that a probiotic including LAB reduced the rate of GIT disorders in calves [1, 26, 35, 36]. Increased intestinal LAB might stimulate the gut epithelial and associated lymphoid tissues and may activate local immune responses in the intestine [12]. It was also suggested that a decreased number of peripheral leukocytes, including CD8+ and WC1+ γδ T cells, might result in a high risk of calf scouring [27]. Similarly, a lower number of CD4+ and CD8+ cells and reduced cytokine mRNA expression levels were noted in neonates with diarrhea, compared with healthy children [9, 37]. In agreement with a previous report by Ohtsuka et al. [27], the numbers of CD4+, CD8+ and WC1+ γδ T cell subsets and of CD14+ and TLR2+ cells were significantly lower in scouring calves in the present study. Probiotics are known to affect the host immune system in different ways, including inducing greater antibody production and increasing epithelial barrier integrity, the upregulation of cell-mediated immunity, increased TLR signaling, enhanced dendritic cell (DC)-T cell interactions and heightened T cell associations [5, 18, 32]. The immune-stimulating properties of a Bacillus-based probiotic were suggested by the increase in peripheral blood CD8+ cells in scouring calves compared with controls [26]. Furthermore, the number of CD4+ cells increased in response to a probiotic consisting of C. butyricum in dairy cows [20]. In a study of calf PBMCs, the phagocytic activities of leukocytes were increased by a probiotic consisting of L. plantarum [18]. In addition, selective effects of a probiotic including Lactobacillus were reported for T cell activation, CD8+ cell activity and cytokine production [5]. Most peripheral T cell subpopulations have CD3 expressed on their surface, which might appear as a signal-transducing unit for the T cell receptor (TCR) [32]. Similar to our recent study using the same probiotic agent in healthy weaned calves, the proportion of CD3+CD45R+ T cells was marginally higher in the systemic circulation of pre-weaning scouring and HT calves. Similar to previous reports, a greater presence of CD45R+ T cells might facilitate the regular activation of T cell subsets in probiotic-treated calves [26]. In contrast, bovine WC1+ γδ T cells are known to be immune suppressors and are involved in secreting IFN-γ [13, 28, 29]. WC1+ γδ T cells can respond to stimulation via the TCR and through CD3 stimulation, leading to the induction of IFN-γ expression in these cells [32]. Early production of IFN-γ by γδ T cells may have a role in linking innate and adaptive immune responses [29]. Furthermore, bovine γδ T lymphocyte subsets are activated indirectly through cytokines secreted by T cells, antigen-presenting cells and other accessory cells [13, 28, 31]. Our results show that the number of WC1+ γδ T cells increased in both the scouring and HT groups and was associated with increased IFN-γ expression. In addition, WC1+ γδ T cells were also greater in number in the HC group. This is in accordance with a previous report, which noted that γδ cell subsets were significantly larger in fetal and maturing calves than in mature animals [39].
Probiotics including Lactobacillus and C. butyricum induce the release of IL-6 and TNF-α, which are involved in nonspecific immune responses [11]. It was previously reported that pre-weaning calves given a probiotic had increased serum IgG and IFN-γ levels [35]. Furthermore, probiotics including E. faecium and C. butyricum or Lactobacillus induced the production of pro- and anti-inflammatory cytokines, including IL-6, IFN-γ and TNF-α, in human PBMCs [5, 14]. In the present study, increased IL-6, INF-γ and TNF-α mRNA expression was observed in the scouring treated, HT and HC groups. In contrast, IL-8 mRNA expression was unchanged, but it was elevated in the scouring groups. Greater expression of IL-8 is likely related to neutrophil function and/or inflammatory stimulation in bovines [2]. Therefore, IL-8 mRNA expression was changed only in scouring calves, but not in healthy calves. It was reported that TNF-α mRNA expression was related to age-related changes in intracellular cytokine production [9]. In the present study, a higher TNF-α mRNA expression level was also noted in the healthy control calves.
TLR expression is associated with bacterial immune stimulators, including LAB, in the GIT of calves [25]. In our preliminary study, it was revealed that calves given the probiotic had a stable ruminal pH compared to the controls. It was also reported that a significant increase in ruminal pH was correlated with increasing taxonomic units of Clostridia in the gastro-intestine of cattle [30]. Therefore, improved diversity and bacterial immigration might affect intestinal bacterial populations. However, the bacterial flora was not evaluated in the present study. In bovines, the expression of TLR2 was obvious on antigen-presenting cells with a high level expression on peripheral monocytes and gastrointestinal macrophages [19, 21, 25]. Moreover, bovine TLR2 cell surface molecules on monocyte-derived DCs and intestinal macrophages are most responsible for the recognition of Gram-positive bacteria [3, 6, 10]. In human PBMCs, monocytes presenting TLRs played an important role in Lactobacillus-induced immunological responses [5]. Mechanisms indicating a direct role for TLR2 include bacterial reorganization by epithelial macrophages in calves [25]. However, CD14+ cells are involved with the TLR4 and MD2 complex, which is essential for the response to most Gram-negative bacteria [19, 25]. Among probiotics, Lactobacillus strains have a rigid cell wall that is resistant to intracellular digestion and can stimulate intestinal macrophages presenting TLR2 to secrete a large quantity of cytokines [33]. In the present study, the numbers of CD14+ and TLR2+ cells were increased in the scouring and HT calves compared to the controls. A B-cell receptor complex including CD21 is present on mature B cells and ileal Peyer’s patch-derived B lymphocytes in young calves [16]. The stimulation of CD21+ cells is related to intestinal immune stimuli, and probiotics are known to trigger naive plasma cells to become IgA-producing CD21+ B cells [12, 24, 38]. In our study, the number of CD21+ cells was increased in the scouring and healthy probiotic-treated calves on day 7.
An interaction between probiotics and the host immune system leads to the interface of intestinal immune cells, such as CD14+ and TLR+ cell macrophages or DCs and to the release of cytokine stimulators by T and B cells [6, 8, 14]. The activation and balanced response of T cells is an important component of the recovery from GIT disease in cattle [25, 38]. Probiotics contribute to homeostasis of the intestinal bacterial flora and result in scouring recovery in young calves [26, 35, 36].
In the present study, a probiotic was administed to pre-weaning scouring calves for 5 days, and stool samples were examined to discover the underlying cause of scouring. The scouring PPT and PPC or PNT and PNC groups revealed a greater number of peripheral leukocytes and higher cytokine mRNA expression levels on day 7, although the immune marker values were slightly higher in the PPT and PPC groups compared with PNT and PNC groups. Higher immune marker values on day 7 in the both pathogen-positive and pathogen-negative scouring groups (PPT and PNT) might be induced by probiotic administration and scouring causers. Furthermore, the increased numbers of circulating immune cells in HT group indicated the immune-stimulatory effects of the probiotic, as suggested by previous studies [7, 20]. On the other hand, the scouring calves given no probiotic (PPC and PNC groups) exhibited greater expression of immune markers on day 7, which might be associated with the usual reaction of immune system through the GIT disorders [37]. The recovery of scouring in the calves given probiotic was in agreement with that in previous reports [1, 12, 19, 26]. From these findings, probiotic administration to the scouring calves might be effective in both pathogen-positive and pathogen-negative cases. In this study, serum IgG was not analyzed. However, it was previously suggested that the serum IgG concentration was increased by probiotic administration in pre-weaning calves [36].
In conclusion, the administration of a bacteria-based probiotic resulted in increased numbers of CD3+ T cells; CD4+, CD8+ and WC1+ γδ T cells; and CD14+, CD21+ and TLR2+ cells in scouring PPT, PNT and HT calves, compared with healthy controls. Moreover, higher IL-6, INF-γ and TNF-α mRNA expression levels were noted in peripheral leukocytes, and the probiotic-treated calves recovered from scouring. These findings demonstrate that the administration of a probiotic elicits immunological changes in the systemic circulation of scouring and healthy calves. Considering the effects of probiotics on immune markers in healthy calves, the repeated treatment of healthy pre-weaning calves with a 5-day probiotic regimen could elevate the levels of reduced immune markers, thereby reducing the incidence of scouring in young calves.
Acknowledgments
We acknowledge the technical support of Dr. Chika Seki at Miyarisan Pharmaceutical Co., Ltd. (Tokyo, Japan).
REFERENCES
- 1.Abe F., Ishibashi N., Shimamura S.1995. Effect of administration of bifidobacteria and lactic acid bacteria to newborn calves and piglets. J. Dairy Sci. 78: 2838–2846. doi: 10.3168/jds.S0022-0302(95)76914-4 [DOI] [PubMed] [Google Scholar]
- 2.Beecher C., Daly M., Berry D. P., Klostermann K., Flynn J., Meaney W., Hill C., McCarthy T. V., Ross R. P., Giblin L.2009. Administration of a live culture of Lactococcus lactis DPC 3147 into the bovine mammary gland stimulates the local host immune response, particularly IL-1 beta and IL-8 gene expression. J. Dairy Res. 76: 340–348. doi: 10.1017/S0022029909004154 [DOI] [PubMed] [Google Scholar]
- 3.Boneca I. G., Dussurgetc O., Cabanesc D., Nahori M. A., Sousac S., Lecuit M., Psylinakis E., Bouriotis V., Hugot J. P., Giovannini M., Coyle A., Bertin J., Namane A., Rousselle J. C., Cayet N., Pre’vost M. C., Balloy V., Chignard M., Philpott D. J., Cossart P., Girardin S. E.2007. A critical role for peptidoglycan N-deacetylation in Listeria evasion from the host innate immune system. Proc. Natl. Acad. Sci. U.S.A. 104: 997–1002. doi: 10.1073/pnas.0609672104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borchers A. T., Selmi C., Meyers F. J., Keen C. L., Gershwin M. E.2009. Probiotics and immunity. J. Gastroenterol. 44: 26–46. doi: 10.1007/s00535-008-2296-0 [DOI] [PubMed] [Google Scholar]
- 5.Dong H., Rowland I., Tuohy K. M., Thomas L. V., Yaqoob P.2010. Selective effects of Lactobacillus casei Shirota on T cell activation, natural killer cell activity and cytokine production. Clin. Exp. Immunol. 161: 378–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drakes M., Blanchard T., Czinn S.2004. Bacterial probiotic modulation of dendritic cells. Infect. Immun. 72: 3299–3309. doi: 10.1128/IAI.72.6.3299-3309.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Emmanuel D. G., Jafari A., Beauchemin K. A., Leedle J. A., Ametaj B. N.2007. Feeding live cultures of Enterococcus faecium and Saccharomyces cerevisiae induces an inflammatory response in feedlot steers. J. Anim. Sci. 85: 233–239. doi: 10.2527/jas.2006-216 [DOI] [PubMed] [Google Scholar]
- 8.Foligne B., Zoumpopoulou1 G., Dewulf J., BenYounes A., Chareyre F., Sirard J. C., Pot B., Grangette C.2007. A key role of dendritic cells in probiotic functionality. PLoS ONE 2: e313. doi: 10.1371/journal.pone.0000313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gasparoni A., Ciardelli L., Avanzini A., Castellazzi A. M., Carini R., Rondini G., Chirico G.2003. Age-related changes in intracellular TH1/TH2 cytokine production, immunoproliferative T lymphocyte response and natural killer cell activity in newborns, children and adults. Biol. Neonate 84: 297–303. doi: 10.1159/000073638 [DOI] [PubMed] [Google Scholar]
- 10.Gao Q., Lili Q., Tianxing W., Wang J.2012a Clostridium butyricum activates TLR2-mediated MyD88-independent signaling pathway in HT-29 cells. Mol. Cell. Biochem. 361: 31–37. doi: 10.1007/s11010-011-1084-y [DOI] [PubMed] [Google Scholar]
- 11.Gao Q., Lili Q., Tianxing W., Wang J.2012b An important role of interleukin-10 in counteracting excessive immune response in HT-29 cells exposed to Clostridium butyricum. BMC Microbiol. 12: 100. doi: 10.1186/1471-2180-12-100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heczko P. B., Strus M., Kochan P.2006. Critical evaluation of probiotic activity of lactic acid bacteria and their effects. J. Physiol. Pharmacol. 57: 5–12 [PubMed] [Google Scholar]
- 13.Hoek A., Rutten V. P., Kool J., Arkesteijn G. J., Bouwstra R. J., Van Rhijn I., Koets A. P.2009. Subpopulations of bovine WC1(+) gammadelta T cells rather than CD4(+)CD25(high) Foxp3(+) T cells act as immune regulatory cells ex vivo. Vet. Res. 40: 06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hua M. C., Lin T. Y., Lai M. W., Kong M. S., Chang H. J., Chen C. C.2010. Probiotic Bio-Three induces Th1 and anti-inflammatory effects in PBMC and dendritic cells. World J. Gastroenterol. 16: 3529–3540. doi: 10.3748/wjg.v16.i28.3529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishihara N., Chu D. C., Akachi S., Juneja L. R.2001. Improvement of intestinal microflora balance and prevention of digestive and respiratory organ diseases in calves by green tea extracts. Livest. Prod. Sci. 68: 217–229. doi: 10.1016/S0301-6226(00)00233-5 [DOI] [Google Scholar]
- 16.Neill J. D., Ridpath J. F., Liebler-Tinorio E.2006. Global gene expression profiling of bovine immature B cells using serial analysis of gene expression. Anim. Biotechnol. 17: 21–31. doi: 10.1080/10495390500460957 [DOI] [PubMed] [Google Scholar]
- 17.Kalliomäki M. A., Walker W. A.2005. Physiologic and pathologic interactions of bacteria with gastrointestinal epithelium. Gastroenterol. Clin. North Am. 34: 383–399. doi: 10.1016/j.gtc.2005.05.007 [DOI] [PubMed] [Google Scholar]
- 18.Kawakami S., Tomoya Y., Naota N.2010. Leukocyte phagocytic activity with or without probiotics in Holstein calves. Res. J. Biol. Sci. 5: 13–16. doi: 10.3923/rjbsci.2010.13.16 [DOI] [Google Scholar]
- 19.Kawakami S., Tomoya Y., Naota N., Cia Y.2010. Feeding of lactic acid bacteria and yeast on growth and diarrhea of Holstein calves. Journal of Animal and Veterinary Advances 9: 1112–1114. doi: 10.3923/javaa.2010.1112.1114 [DOI] [Google Scholar]
- 20.Kohiruimaki M., Ohtuka H., Tanami E., Kitagawa M., Masui M., Ando T., Kawamura S.2008. Effects of active egg white product/ Clostridium butyricum Miyairi 588 additive on peripheral leukocyte populations in periparturient dairy cows. J. Vet. Med. Sci. 70: 321–323. doi: 10.1292/jvms.70.321 [DOI] [PubMed] [Google Scholar]
- 21.Kwong L. S., Parsons R., Patterson R., Coffey T. J., Thonur L., Changd J. S., Russell G., Haig D., Werling D., Hope J. C.2011. Characterisation of antibodies to bovine toll-like receptor (TLR)-2 and cross-reactivity with ovine TLR2. Vet. Immunol. Immunopathol. 139: 313–318. doi: 10.1016/j.vetimm.2010.10.014 [DOI] [PubMed] [Google Scholar]
- 22.Lassen B., Viltrop A., Raaperi K., Jarvis T.2009. Eimeria and Cryptosporidium in Estonian dairy farms in regard to age, species, and diarrhea. Vet. Parasitol. 166: 212–219. doi: 10.1016/j.vetpar.2009.08.022 [DOI] [PubMed] [Google Scholar]
- 23.Livak K. J., Schmitge T. D.2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCt method. Methods 25: 402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 24.Macpherson A. J., Uhr T.2004. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science 303: 1662–1665. doi: 10.1126/science.1091334 [DOI] [PubMed] [Google Scholar]
- 25.Malmuthuge N., Li M., Fries P., Griebel P. J., Guan L. L.2012. Regional and age dependent changes in gene expression of toll-like receptors and key antimicrobial defence molecules throughout the gastrointestinal tract of dairy calves. Vet. Immunol. Immunopathol. 146: 18–26. doi: 10.1016/j.vetimm.2012.01.010 [DOI] [PubMed] [Google Scholar]
- 26.Novak K. N., Davis E., Wehnes C. A., Shields D. R., Coalsonb J. A., Smitha A. H., Rehberger T. G.2012. Effect of supplementation with an electrolyte containing a Bacillus-based direct-fed microbial on immune development in dairy calves. Res. Vet. Sci. 92: 427–434. doi: 10.1016/j.rvsc.2011.04.008 [DOI] [PubMed] [Google Scholar]
- 27.Ohtsuka H., Ono M., Saruyama Y., Mukai M., Kohiruimaki M., Kawamura S.2011. Comparison of the peripheral blood leukocyte population between Japanese Black and Holstein calves. Anim. Sci. J. 82: 93–98. doi: 10.1111/j.1740-0929.2010.00833.x [DOI] [PubMed] [Google Scholar]
- 28.Park Y. H., Yoo H. S., Yoon J. W., Yang S. J., An J. S., Davis W. C.2000. Phenotypic and functional analysis of bovine gammadelta lymphocytes. J. Vet. Sci. 1: 39–48 [PubMed] [Google Scholar]
- 29.Price S. J., Sopp P., Howard C. J., Hope J. C.2007. Workshop cluster 1+ gammadelta T-cell receptor+ T cells from calves express high levels of interferon-gamma in response to stimulation with interleukin-12 and −18. Immunology 120: 57–65. doi: 10.1111/j.1365-2567.2006.02477.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li R. W., Wu S., Ransom L., Baldwin R. L., Li W., Li C.2012. Perturbation dynamics of the rumen microbiota in response to exogenous butyrate. PLoS ONE 7: e29392. doi: 10.1371/journal.pone.0029392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rogers A. N., Vanburen D. G., Hedblom E. E., Tilahun M. E., Telfer J. C., Baldwin C. L.2005. Gammadelta T cell function varies with the expressed WC1 co-receptor. J. Immunol. 174: 3386–3393 [DOI] [PubMed] [Google Scholar]
- 32.Sathiyaseelan T., Rogers A., Baldwin C. L.2002. Response of bovine gammadelta T cells to activation through CD3. Vet. Immunol. Immunopathol. 90: 155–168. doi: 10.1016/S0165-2427(02)00244-1 [DOI] [PubMed] [Google Scholar]
- 33.Shida K., Kiyoshima-Shibata J., Kaji R., Nagaoka M., Nanno M.2009. Peptidoglycan from lactobacilli inhibits interleukin-12 production by macrophages induced by Lactobacillus casei through toll-like receptor 2-dependent and independent mechanisms. Immunology 128: e858–869. doi: 10.1111/j.1365-2567.2009.03095.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shida K., Nanno M., Nagata S.2011. Flexible cytokine production by macrophages and T cells in response to probiotic bacteria: a possible mechanism by which probiotics exert multifunctional immune regulatory activities. Gut Microbes 2: 109–114. doi: 10.4161/gmic.2.2.15661 [DOI] [PubMed] [Google Scholar]
- 35.Signorini M. L., Soto L. P., Zbrun M. V., Sequeira G. J., Rosmini M. R., Frizzo L. S.2012. Impact of probiotic administration on the health and fecal microbiota of young calves: A meta-analysis of randomized controlled trials of lactic acid bacteria. Res. Vet. Sci. 93: 250–258. doi: 10.1016/j.rvsc.2011.05.001 [DOI] [PubMed] [Google Scholar]
- 36.Sun P., Wang J. Q., Zhang H. T.2010. Effects of bacillus subtilis natto on performance and immune function of pre-weaning calves. J. Dairy Sci. 93: 5851–5855. doi: 10.3168/jds.2010-3263 [DOI] [PubMed] [Google Scholar]
- 37.Wang Y., Dennehy P. H., Keyserling H. L., Tang K., Gentsch J. R., Glass R. I., Jiang B.2007. Rotavirus infection alters peripheral T-cell homeostasis in children with acute diarrhea. J. Virol. 81: 3904–3912. doi: 10.1128/JVI.01887-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weiss D. J., Evanson O. A., Souza C. D.2006. Mucosal immune response in cattle with subclinical johne’s disease. Vet. Pathol. 43: 127–135. doi: 10.1354/vp.43-2-127 [DOI] [PubMed] [Google Scholar]
- 39.Wilson R. A., Zolnai A., Rudas P., Frenyo L. V.1996. T-cell subsets in blood and lymphoid tissues obtained from fetal calves, maturing calves, and adult bovine. Vet. Immunol. Immunopathol. 53: 49–60. doi: 10.1016/0165-2427(95)05543-6 [DOI] [PubMed] [Google Scholar]