ABSTRACT
Histopathological and immunohistochemical examinations were made on a cutaneous tumor on the head of an 11-year-old female mixed-breed dog. The tumor was well demarcated and comprised multilobular structures of neoplastic epithelial cells with abundant plump peritumoral stromal cells. The neoplastic cells formed irregular cell cords or trabeculae and were arranged in characteristic palisades at the periphery. Immunohistochemically, neoplastic cells were positive for p63 and the several cytokeratins examined. In contrast, the plump peritumoral stromal cells were positive for vimentin and unevenly for nestin, a neuroepithelial stem cell protein. The stromal cells prominently proliferated in proximity to epithelial neoplastic cells, suggesting a close interaction between these two cell types.
Keywords: canine, nestin, stromal cell, trichoblastoma
The trichoblastoma is a benign cutaneous tumor that may derive from the hair germ of a developing follicle [3, 4, 9]. It is common in dogs and is histologically classified into several types, including ribbon, trabecular, granular cell and spindle cell [3, 9]. Most tumors contain a mixture of these patterns with a predominance of ribbon architecture [3, 5]. The neoplastic epithelial structures are embedded in a paucicellular fibrous stroma. The evidence of trichogenic differentiation is present in the form of abortive hair papillae formation (so called papillary mesenchymal bodies), consisting of the stroma forming into nests of neoplastic epithelial cells, reminiscent of the relationship between primitive hair germ and papilla. In humans, the trichoblastoma is a rare tumor, and five main patterns have been described as large nodular, small nodular, cribriform, racemiform and retiform [1].
Recently, we encountered an atypical case of canine trichoblastoma with prominent proliferation of plump peritumoral stromal cells with occasional expression of nestin. Nestin, a neuroepithelial stem cell protein, was initially identified as a marker for neuronal progenitor cells in the brain, but was subsequently found in mesenchymal stem cells from bone marrow, lung, muscle and pancreas [10]. In human cases of trichoblastoma, the nestin protein is usually found in the peritumoral stromal cells, but not in the epithelial component of these tumors [6, 8]. Brachelente et al. [2] reported nestin expression in the neoplastic epithelial cells of canine trichoblastoma. However, nestin expression in the peritumoral stromal cells was not mentioned in the study.
In this report, we describe the morphological and immunohistochemical findings of a case of trichoblastoma in a dog, which showed abundant plump stromal cell proliferation.
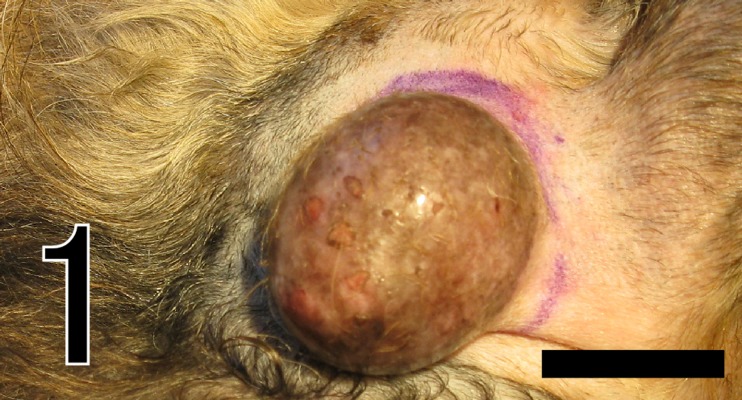
An 11-year-old mixed-breed female dog was brought to a local veterinary clinic, because of development of a cutaneous mass on the head (Fig. 1). The mass was round and non-ulcerated, sized 45 × 30 × 7 mm, and its cut surface was white and lobulated. Excision was performed, and the mass was submitted to our laboratory for histopathological examination. During the 7 months since surgical excision, no local recurrence or metastasis has been recognized.
Fig. 1.
The cutaneous neoplasm on the head. Bar=5 mm
The mass was fixed in 10% neutral-buffered formalin, embedded in paraffin, sectioned and stained with hematoxylin and eosin, periodic acid-Schiff (PAS) and Masson trichrome stain. Immunohistochemistry (IHC) was performed by the immunoenzyme polymer method using the primary antibodies shown in Table 1 [11]. Peroxidase-conjugated anti-mouse (Histofine Simple Stain MAX-PO (M); Nichirei Corp., Tokyo, Japan) or anti-rabbit (Histofine Simple Stain MAX-PO (R); Nichirei) immunoglobulin (Ig) G was used as secondary antibodies. After immunoreaction, the sections were colorized with diaminobenzidine (DAB) and followed by counterstaining by Mayer’s hematoxylin with/without PAS reaction. Immunohistochemical staining was performed on this case as well as five cases of common type trichoblastoma (ribbon type) and five normal canine skin tissues for comparison. Double-labeled immunohistochemistry was also performed using anti-cytokeratin (CK) AE1/AE3 and anti-vimentin antibodies. This assay used FITC-conjugated goat anti-mouse IgG (EY Laboratories, San Maeto, CA, U.S.A.) and rhodamine-conjugated goat anti-rabbit IgG (Chemicon, Temecula, CA, U.S.A.) to label anti-CK AE1/AE3 and anti-vimentin, respectively. Fluorescence was analyzed using a FSX100 fluorescence microscope (Olympus, Tokyo, Japan).
Table 1. Primary antibodies and immunostaining protocol in the current study.
| Antibodya) | Clone | Dilution | Source | Positive reactions in canine tissuesb) | Antigen retrievald) |
|---|---|---|---|---|---|
| Pan-CK | AE1/AE3 | 1:50 | Dako Denmark A/S., Glostrup, Denmark |
Epidermis, ORS, AG, SG, Hair medullac) | MW, 95°C, 10 min |
| Pan-CK | KL1 | 1:100 | Immunotech., Marselle, France |
Epidermis (except for the basal layer) ORS (innner cells), Hair medulla |
MW, 90°C, 10 min |
| Pan-CK | CAM5.2 | prediluted | Becton Dicknson., Heidelberg, Germany |
IRS, AG (luminal cells) | Proteinase K, 37°C, 10 min |
| CK14 | LL002 | 1:100 | Serotec., Weisbeden, Germany |
Epidermis (basal layer), ORS, AG (myoepithelial cells) SG, Hair medulla |
MW, 90°C, 10 min |
| CK18 | Ks 18.4 | prediluted | Progen Biotechnik., heidelberg, Germany |
AG (luminal cells) | Proteinase K, 37°C, 11 min |
| E-cadherin | 4A2C7 | 1:100 | Invitrogen., Camarillo, CA, USA |
Epithelial cells | AC, 121°C, 20 min |
| Vimentin | SP20 | 1:100 | Nichirei Corp., Tokyo, Japan |
Mesenchimal cells | MW, 95°C, 10 min |
| SMA | 1A4 | 1:100 | Dako Denmark A/S., Glostrup, Denmark |
Smooth muscle cells | No treatment |
| p63 | 4A4 | 1:150 | Thermo Fisher Scientific., Waltham, MA |
Epidermis (basal layer), ORS, AG (myoepithelial cells) SG (reserve cells), Hair matrix cells |
AC, 121°C, 20 min |
| Adipophilin | AP125 | 1:50 | Dako Denmark A/S., Glostrup, Denmark |
SG (sebocytes) | MW, 90°C, 10 min |
| MelanA | A103 | 1:50 | Dako Denmark A/S., Glostrup, Denmark |
Melanocytes | MW, 95°C, 10 min |
| S-100a | Polyclonal | 1:100 | Dako Denmark A/S., Glostrup, Denmark |
Neuron cells | MW, 90°C, 10 min |
| Nestin | Polyclonal | 1:200 | Millipore., Billerica, USA |
Middle portion of hair follicle | Tripsin, 37°C, 30 min |
| Ki67 | MIB-1 | 1:100 | Dako Denmark A/S., Glostrup, Denmark |
Epidermis (basal layer) | AC, 121°C, 20 min |
a) CK=cytokeratin; SMA=α-smooth muscle actin, b) IRS=inner root sheath; ORS=outer root sheath; AG=apocrin sweat gland; SG=sebaceous gland, c) The positivity against CK AE1/AE3 is variable between indidual. Most consistent are shown in this table. d) Trypsin=0.1% trypsin (Dako A/S); MW=microwave, citrate buffer (PH 6.0). Proteinase K=0.4 mg/ml proteinase K (Wako Pure Chemical Industries Ltd. Osaka; Japan), AC=autocleve, citrate buffer (PH 6.0); Pepsin=0.4% pepsin (Sigma-Aldrich Co., St. Louis, MO, U.S.A.).
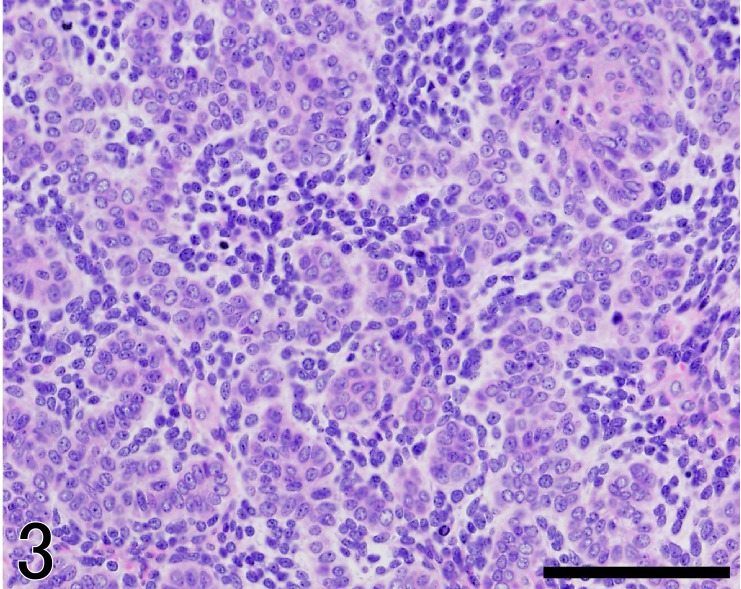
The mass was well demarcated and comprised many lobules (Fig. 2). Each lobule was composed of epithelial neoplastic cells together with abundant plump stromal cells. The neoplastic cells formed irregular cell cords or trabeculae and were arranged in the characteristic palisades at the periphery. These neoplastic cells were mainly composed of trichoblastic cells with small ovoid nuclei and scant cytoplasm (Fig. 3). The second minor population of tumor cells consisted of cells with small monomorphic nuclei and PAS-positive granules in the clear cytoplasm, resembling outer root sheath cells. Mitotic activity of neoplastic cells was moderate, at 4 mitoses per 10 high-power fields.
Fig. 2.
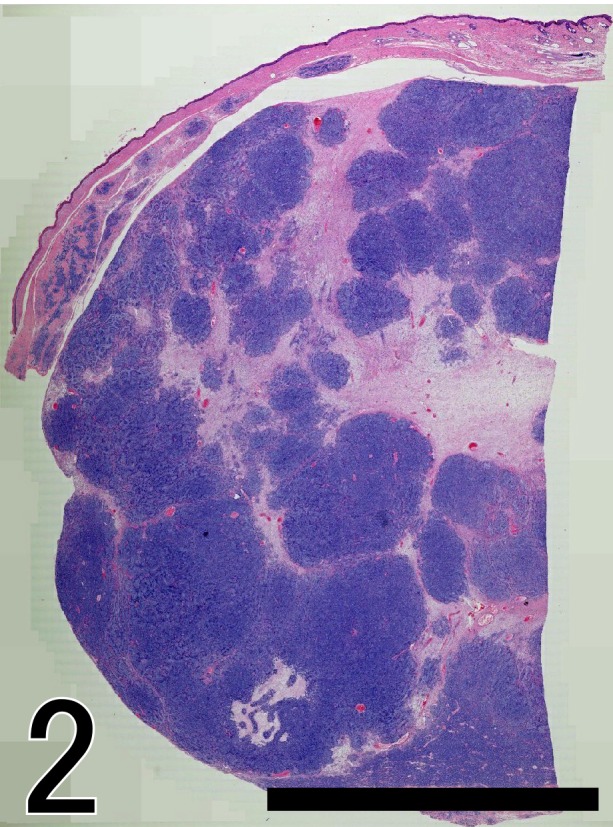
The cutaneous neoplasm is well-demarcated and densely cellular. The mass is comprised of variably sized lobules. Hematoxylin and Eosin (HE) stain. Bar=1,000 µm.
Fig. 3.
The tumor composed of neoplastic basaloid cells together with a lot of plump stromal cells morphologically resembling hair papilla cells. HE. Bar=100 µm.
On the other hand, characteristic stromal cells prominently proliferated around these neoplastic cells accompanied by scant collagen fibers (Fig. 3). These cells were characterized by plump, oval or elongated nuclei and eosinophilic scant cytoplasm. Cytological atypia of these cells was mild and showed moderate mitotic activity, at 3 mitoses per 10 high-power fields. These plump stromal cells were located in the entire peritumoral stroma. Interlobar stroma was composed of plump stromal cells, elongated spindle stromal cells and scant collagen fibers.
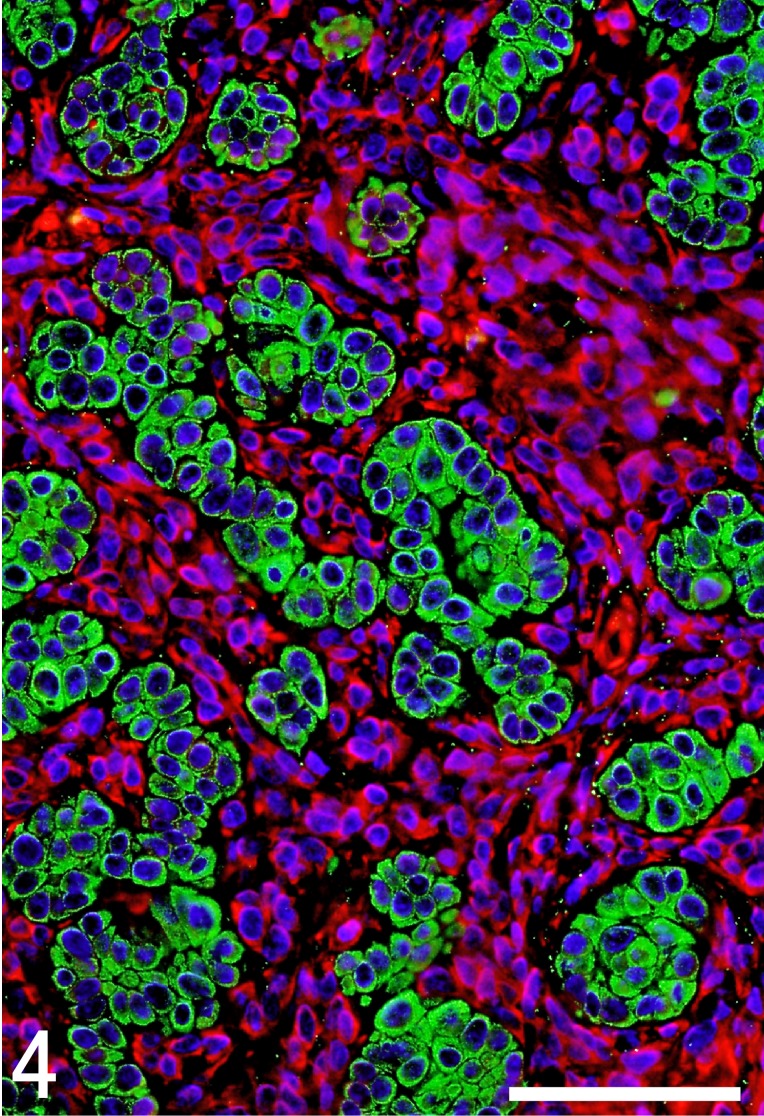
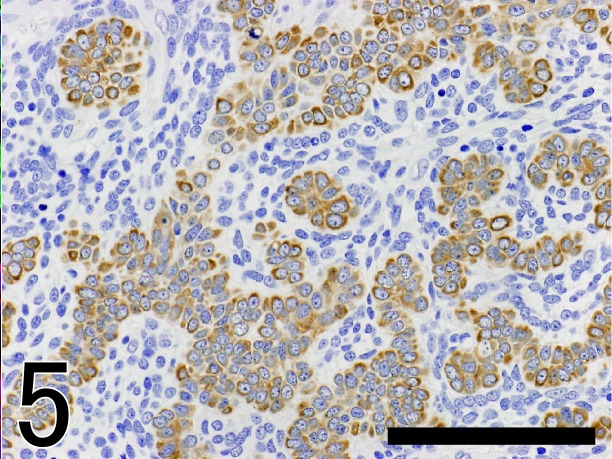
The results of immunohistochemical examinations of the present case and other five trichoblastomas are summarized in Table 2. In the present case, neoplastic epithelial cells were consistently positive for CK AE1/AE3 (Fig. 4), CK 14 (Fig. 5) and p63, but negative for CK CAM5.2, CK 18, vimentin, SMA, adipophilin, melan A, S-100a and nestin. However, only the central part of the neoplastic epithelial cells resembling outer root sheath cells was positive for CK KL-1. Plump stromal cells were negative for all cytokeratins examined, E-cadherin, SMA, p63, adipophilin, melan A and S-100a, but were consistently positive for vimentin (Fig. 4). In addition, these peritumoral stromal cells unevenly expressed nestin (Fig. 6). Both neoplastic epithelial cells and plump stromal cells expressed Ki67 at a high rate (Fig. 7). In normal canine skin tissues, nestin expression was not detected. Peritumoral stromal cells in other five cases of trichoblastoma consisted of two types of stromal cells, plump cells and thin fibroblasts. The proportion of the two cell types varied from lesion to lesion, although the later was constantly predominant. These stromal cells showed either no expression or very rare expression of nestin and low positive rate for Ki67.
Table 2. Reslts of immunostaining of the neoplastic epithelial cells and stromal cellsa).
| Cell type | AE1/AE3 | KL1 | CAM5.2 | CK14 | CK18 | E-cadherin | Vimentin | SMA | p63 | Adipophilin | MelanA | S-100a | Nestin | Ki67b) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neoplasticepithelial cells | ||||||||||||||
| Present case | + | + (>5%)c) | – | + | – | + | – | – | + | – | – | – | – | 6.4% |
| Common type trichoblastoma (n=5) | + | – | – | + | – | + | – | – | + | – | – | – | – | 6.2 ± 4.7%d) |
| Stromal cells | ||||||||||||||
| Present case | – | – | – | – | – | – | + | – | – | – | – | – | + (>10%) | 9.4% |
| Common type trichoblastoma (n=5) | – | – | – | – | – | – | + | – | – | – | – | – | –/±e) | 1.0 ± 0.6%d) |
a) –=negative; +=positive, b) positive rate for Ki67, c) Only central part of epithelial cells resembling outer root sheath cells is positive for KL-1, d) mean ± SD, e) A few number of mesenchymal cells are positive for nestin (2 of 5).
Fig. 4.
Double-labeled immunofluorescence microscopy of the tumor tissue. Green color indicates positive staining for CK clone AE1/AE3. Red color shows positive staining for vimentin. Nuclei are colored blue with 4,6-diamino-2-phenylindole. Neoplastic epithelial cells are positive for CK AE1/AE3, but not for vimentin. In contrast, stromal plump stromal cells are positive for vimentin, but not for CK AE1/AE3. Bar=50 µm.
Fig. 5.
Immunostaining of CK14 in the tumor tissue. Neoplastic epithelial cells are positive for CK14. Bar=100 µm.
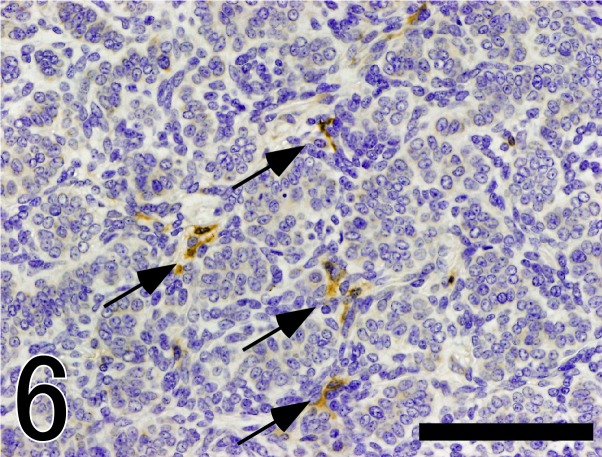
Fig. 6.
Immunostaining of nestin in the tumor tissue. A part of peritumoral plump stromal cells express nestin (arrow). Bar=50 µm.
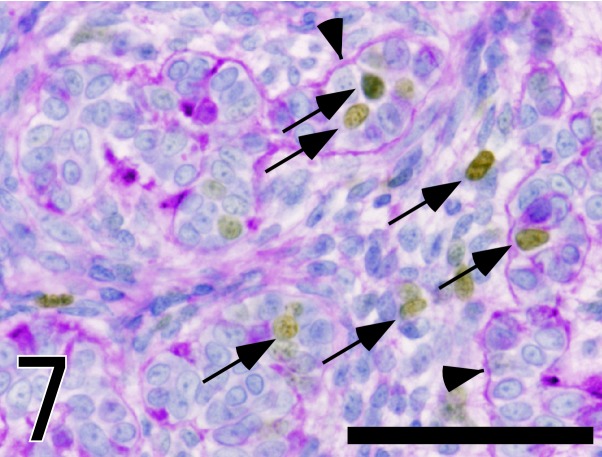
Fig. 7.
Immunostaining of Ki67 with Mayer’s hematoxylin stain and PAS reaction in the tumor tissue. Brown color shows Ki67 positive nuclei (arrows). PAS-positive basement membrane (arrowheads) encircled epithelial components. Bar=50 µm.
On the basis of histopathological findings, the tumor was diagnosed as a trichoblastoma. However, the present case was not typical, because of accompanying prominent proliferation of plump peritumoral stromal cells.
The development of adnexa is the result of intimate interaction between trichoblastic cells of the epidermis and mesenchymal cells. The precursor of the hair follicle is a local thickening of the embryonic epidermis, also known as a placode, in the development of dermal papilla cells. Soon after, a dense collection of plump mesenchymal cells of the future follicular papilla forms beneath the placode. The basal cells become trichoblastic cells of the hair follicle that will give rise to the specialized components of the follicle, the hair shaft, the internal root sheath and the external root sheath [1]. Benign adnexal neoplasms are thought to recapitulate the embryonic development of adnexal structures [10]. The peritumoral stroma in our case was composed of abundant plump cells and scant collagen fibers, while, in contrast, stromal cells were mainly thin spindle cells with thick collagen fiber in the other trichoblastomas. Moreover, some of the peritumoral stromal cells expressed the neuroepithelial stem cell protein, nestin. Therefore, plump appearances of the peritumoral stromal cells in canine trichoblastoma might be reminiscent of embryonic mesenchymal cells or follicle dermal papilla cells.
We suspect that neoplastic epithelial cells might relate to proliferation of the stromal cells. The clonal analysis might provide valuable information regarding the nature of stromal cells as confirmed in human mammary phyllodes tumor [7].
This paper is the first report of canine trichoblastoma with abundant plump stromal cells showing expression of nestin.
Acknowledgments
This work was supported in part by the Grant-in-Aid for Challenging Exploratory Research (25660237) from the Japan Society for the Promotion of Science (JSPS).
REFERENCES
- 1.Ackerman A. B., Reddy V. B., Soyer H. P.2001. Trichoblastoma. pp. 425–622. In: Neoplasms with Follicular Differentiation. Lea & Febiger, Philadelphia. [Google Scholar]
- 2.Brachelente C., Porcellato I., Sforna M., Lepri E., Mechelli L., Bongiovanni L.2013. The contribution of stem cells to epidermal and hair follicle tumours in the dog. Vet. Dermatol. 24: 188–194. doi: 10.1111/j.1365-3164.2012.01101.x [DOI] [PubMed] [Google Scholar]
- 3.Goldschmidt M. H., Dunstan R. W., Stannard A. A., von Tscharner C., Walder E. J., Yager J. A.1998. Histological classification of epithelial and melanocytic tumors of the skin of domestic animals, 2nd ser., vol. 3. Armed Forces Institute of Pathology. Washington, D.C. [Google Scholar]
- 4.Gross T. L., Ihrke P. J., Walder E. J., Affolter V. K.2005. Skin Diseases of the Dog and Cat. 2nd ed. Blackwell Publishing, Oxford. [Google Scholar]
- 5.Meuten D. J.2008. Tumors in Domestic Animals, 4th ed. Wiley-Blackwell, Ames. [Google Scholar]
- 6.Misago N., Mori T., Narisawa Y.2010. Nestin expression in stromal cells of trichoblastoma and basal cell carcinoma. J. Eur. Acad. Dermatol. Venereol. 24: 1354–1358. doi: 10.1111/j.1468-3083.2010.03641.x [DOI] [PubMed] [Google Scholar]
- 7.Noguchi S., Motomura K., Inaji H., Imaoka S., Koyama H.1993. Clonal analysis of fibroadenoma and phyllodes tumor of the breast. Cancer Res. 53: 4071–4074 [PubMed] [Google Scholar]
- 8.Sellheyer K., Krahl D.2011. Does the peritumoral stroma of basal cell carcinoma recapitulate the follicular connective tissue sheath? J. Cutan. Pathol. 38: 551–559. doi: 10.1111/j.1600-0560.2011.01686.x [DOI] [PubMed] [Google Scholar]
- 9.Sharif M., Reinacher M.2006. Clear Cell Trichoblastomas in Two Dogs. J. Vet. Med. A. Physiol. Pathol. Clin. Med. 53: 352–354. doi: 10.1111/j.1439-0442.2006.00834.x [DOI] [PubMed] [Google Scholar]
- 10.Tiede S., Kloepper J. E., Ernst N., Poeggeler B., Kruse C., Paus R.2009. Nestin in human skin: exclusive expression in intramesenchymal skin compartments and regulation by leptin. J. Invest. Dermatol. 129: 2711–2720. doi: 10.1038/jid.2009.148 [DOI] [PubMed] [Google Scholar]
- 11.Yasuno K., Nishiyama S., Suetsugu F., Ogihara K., Madarame H., Shirota K.2009. Cutaneous clear cell adnexal carcinoma in a dog: special reference to cytokeratin expression. J. Vet. Med. Sci. 71: 1513–1517. doi: 10.1292/jvms.001513 [DOI] [PubMed] [Google Scholar]