ABSTRACT
Regulatory T cells (Tregs) suppress the immune system and maintain the homeostasis of the immune system in healthy dogs. In septic patients, the percentage of circulating Tregs is increasing, which may cause the sepsis-induced immunosuppression. This study was performed to investigate the changes of the percentage of Tregs in total lymphocytes of the peripheral blood in the experimental canine endotoxemia model. The animals injected with a high dose lipopolysaccharide (LPS) induced severe leukopenia followed by leukocytosis, but the total lymphocytes number was relatively consistent. As a result of flow cytometric analysis, the percentage of Tregs in total lymphocytes of the peripheral blood was 8.45 ± 1.30% (day 0), and it temporarily decreased 2.54 ± 1.16% (day 1) and increased continuously until the end of the experiment (14.34 ± 4.10% on day 3 and 25.70 ± 7.39% on day 7), respectively. This study provides basic information in physiologic and immunologic changes in Tregs in dogs with sepsis model.
Keywords: canine endotoxemia model, immunosuppression, lipopolysaccharide (LPS), regulatory T cell, sepsis
Regulatory T cells (Tregs) play an important role in maintaining immunologic homeostasis and tolerance [16, 18]. Naturally occurring CD4 (+)/CD25 (+) regulatory T cells maintain the immunologic self-tolerance and inhibit the immune response by suppression of the expansion and the activation of self-reactive T cells, which is an important function of naturally occurring regulatory T cells [8, 15,16,17, 22].
Interestingly, in human patients with sepsis [9] and tumor [5] and murine study [13], Tregs seems to have harmful functions, which are directly related to the increased mortality by down regulating the immune system. In a report, sepsis surviving-mice that express a persistent expansion of Tregs were correlated to a long-term adaptive immune dysfunction and a susceptibility to secondary infection. That is, septic shock undermines the immune homeostasis by inducing an initial intense systemic inflammatory response that is rapidly followed by a negative feedback of anti-inflammatory process [6, 12]. Moreover, several studies reported that this inhibitory response may decrease a host resistance to the secondary nosocomial infections, thereby having a deleterious effect on patient’s outcome [7]. In veterinary medicine, however, studies about Tregs have been limited to cancers or feline immunodeficiency virus studies up to date [1, 3, 20, 21].
Thus, the goal of this study is to use flow cytometric analysis 1) to investigate the percentage of Tregs in total lymphocytes of the peripheral blood in normal 8 healthy beagles and 2) to investigate the changes of percentage of Tregs in canine endotoxemia as a septic model for 7 days compared with the control group using CD4, CD25 and FoxP3 specific monoclonal antibodies. To the author’s knowledge, this is the first Treg study related canine sepsis.
This experiment was approved from Chonbuk University Animal Care and Use Committee (CBU 2011-0026). Eight healthy male beagles (8–11 kg body weight, 1–2 years old) were divided as four dogs in control group injected with saline and the other 4 dogs in experimental group injected with high dose LPS (1 mg/kg) diluted in 5ml saline (LPS from Escherichia coli serotype O111:B4; Sigma, St. Louis, MO, U.S.A.) as we previously performed [19]. Blood samples were collected before LPS injection (day 0) and 1-, 3- and 7- day after via the jugular venipuncture. Three milliliters of blood were drawn into an EDTA tube for complete blood count (CBC) with an impedance cell counter (Vet ABC blood counter, ABX Diagnostics, Montpellier, France) and for flow cytometry analysis after peripheral blood mononuclear cells (PBMC) separation by Histopaque® 1077 (Sigma) [1]. Then, PBMCs were incubated with antibodies to the extracellular markers of anti-canine CD4 FITC (monoclonal, Serotec, Oxford, U.K.) and anti-canine CD25 PE (monoclonal, eBioscience, San Diego, CA, U.S.A.) in 1% BSA in PBS for 30 min at room temperature. Thereafter, samples were washed using a fixation/permeabilization buffer and incubated at 4°C for 12 hr. APC-conjugated anti-mouse/rat FoxP3 (clone FJK-16s, eBioscience) was used to stain intracellular markers of Tregs. APC-conjugated rat IgG2a (eBioscience) was used as the isotype control.
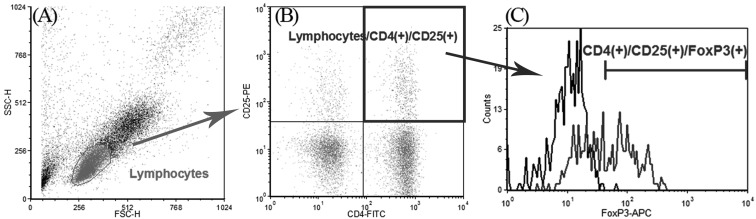
Lymphocytes were discriminated by forward- and light-scatter (Fig. 1A), and Tregs were confirmed by using anti-CD4, -CD25 (Fig. 1B) and -FoxP3 antibodies (Fig. 1D) compared with an irrelevant isotype-matched antibody (mouse IgG, control for FoxP3 staining). CD4 (+)/CD25 (+)/FoxP3 (+) cells were calculated among gated lymphocytes compared with CD4 (+)/CD25 (+)/isotype control (+) cells. Absolute numbers of lymphocytes were calculated by multiplying percentage of Tregs by absolute lymphocyte counts in CBC. Flow cytometry analysis was performed by a FACS Calibur flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, CA, U.S.A.). Data were acquired from a minimum of 10,000 events. Data analyses were performed using FCS Express (FCS Express Version 4, De Novo Software). For statistics, data were analyzed by Student’s t-test with Levene’s test for equality of varience (parametric) or Mann-Whitney U test (non-parametric) after Shapiro-Wilk normalization test in SPSS software (SPSS Inc., Chicago, IL, U.S.A.). Data are represented as mean ± S.D. A probability (P) value of <0.05 was considered to be statistically significant.
Fig. 1.
Identification of canine Tregs using CD4, CD25 and FoxP3 antibody. Lymphocytes were discriminated in terms of forward- and light-scatter (A) and CD4, CD25 double positive cells were recognized after gating lymphocytes (B) and then Foxp3 positive cells were calculated from the gated cells in B, compared with an irrelevant isotype-matched antibody (mouse IgG, control for FoxP3 staining, black line) (C). FoxP3 positive area was calculated including less than 1% of isotype-matched antibody stained cells.
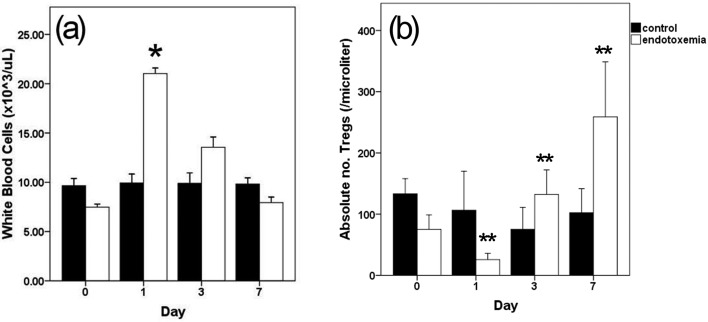
After high concentrations of LPS were injected into the blood vessels, dogs in the experimental group showed fever, vomiting and diarrhea within an hour, which are the common symptoms of canine endotoxemia [19, 23]. Before the LPS injection, the mean total white blood cell (WBC) counts of 8 male beagles were 8.56 ± 1.97 × 103/µl. In the experimental group, however, leukocytosis was observed one day after the LPS injection (WBC counts, 21.03 × 103/µl, P<0.05 vs. control) (Fig. 2A). Total lymphocytes numbers on days 0, 1, 3 and 7 were not significantly time dependently changed (0.95 ± 0.03, 0.85 ± 0.02, 0.88 ± 0.03 and 0.93 ± 0.02 × 103/µl, respectively).
Fig. 2.
Changes in total leukocyte counts (a) and absolute number of CD4 (+)/CD25 (+)/FoxP3 (+) T cells in total lymphocytes by flow cytometry analysis (b) during 7 days of LPS injection. Leukocytosis was presented at 1 day after LPS injection. The absolute number of CD4 (+)/CD25 (+)/FoxP3 (+) T cells was decreased in 1 day after LPS injection, but continuously increased until day 7. Data represent mean ± SD *P<0.05, **P<0.01 versus control group at each time.
In terms of Tregs population in this model, the percentage of Tregs was 8.45 ± 1.30% of total lymphocytes in peripheral blood before LPS injection (1.24 ± 0.43% of blood, absolute count of 75.2 ± 23.7/µl). This is similar Treg population result compared to a previous study (1.00% of peripheral blood from under 2 years of dogs) [14]. After LPS injection, the percentage of CD4 (+)/CD25 (+)/FoxP3 (+) T cells in total lymphocytes decreased on the first day after the LPS injection (LPS, 2.54 ± 1.16%; control, 8.54 ± 2.60; P<0.05 vs. control). And, after that, it increased gradually on day 3 and day 7 after the LPS injection, respectively, compared with control group (14.34 ± 4.10%, P<0.001 vs. control: 7.27 ± 0.95) and 7 (25.70 ± 7.39%, P<0.001 vs. control: 7.40 ± 0.92) (Fig. 2B). The absolute numbers of Tregs in peripheral blood were 25.8 ± 10.2/µl (day 1, P<0.001 vs. control: 106.4 ± 63.7), 132.2 ± 40.4/µl (day 3, P<0.01 vs. control: 75.4 ± 35.7) and 258.9 ± 89.9/µl (day 7, P<0.001 vs. control: 102.3 ± 39.3).
In this study, we found transient decrease and persistent increment of Tregs in dogs after LPS stimulation. The idea of the whole study was to observe how much the percentage or numbers of Tregs would change in a canine endotoxemia model. Since CARS (compensatory anti-inflammatory response syndrome) followed by profound pro-inflammatory state in septic crisis results in immunosuppressive state that cannot protect host from the primary infections of secondary nosocomial infections [2, 13], determining the host immune response whether it is up-regulated or down-regulated is very useful to predict the outcome of the sepsis. Specifically, Tregs, a subset of T lymphocytes, play an important role in maintaining the immune system and are essential for the active suppression [1, 4, 13]. Transient diminished Tregs after one day of canine endotoxemia may be because of hyperinflammatory response in early stage of the sepsis. However, on days 3 and 7, there were significant continuous increase of percentages of Tregs. This can be explained the compensatory anti-inflammatory response in the later stage of sepsis [2]. Understanding kinetics of immune functional changes is an important clue to understand septic scenario and to predict the outcome. This kinetics of immune response is accordance with previous reports which revealed that the immune failure could occur within the first week of the sepsis in a rodent model [11] and human septic patients [10]. This result can be used as a preliminary study to observe the kinetics of the canine immune status in sepsis. Further studies to investigate increased Tregs function should be performed in the future.
Acknowledgments
ACKNOWLEDGMENT. This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (KRF-2011-0011105).
REFERENCES
- 1.Biller B. J., Elmslie R. E., Burnett R. C., Avery A. C., Dow S. W.2007. Use of FoxP3 expression to identify regulatory T cells in healthy dogs and dogs with cancer. Vet. Immunol. Immunopathol. 116: 69–78. doi: 10.1016/j.vetimm.2006.12.002 [DOI] [PubMed] [Google Scholar]
- 2.Bone R. C.1996. Sir Isaac Newton, sepsis, SIRS, and CARS. Crit. Care Med. 24: 1125–1128. doi: 10.1097/00003246-199607000-00010 [DOI] [PubMed] [Google Scholar]
- 3.Burton J. H., Mitchell L., Thamm D. H., Dow S. W., Biller B. J.2011. Low-dose cyclophosphamide selectively decreases regulatory T cells and inhibits angiogenesis in dogs with soft tissue sarcoma. J. Vet. Intern. Med. 25: 920–926. doi: 10.1111/j.1939-1676.2011.0753.x [DOI] [PubMed] [Google Scholar]
- 4.Fontenot J. D., Gavin M. A., Rudensky A. Y.2003. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 4: 330–336. doi: 10.1038/ni904 [DOI] [PubMed] [Google Scholar]
- 5.Horiuchi Y., Tominaga M., Ichikawa M., Yamashita M., Jikumaru Y., Nariai Y., Nakajima Y., Kuwabara M., Yukawa M.2009. Increase of regulatory T cells in the peripheral blood of dogs with metastatic tumors. Microbiol. Immunol. 53: 468–474. doi: 10.1111/j.1348-0421.2009.00144.x [DOI] [PubMed] [Google Scholar]
- 6.Hotchkiss R. S., Karl I. E.2003. The pathophysiology and treatment of sepsis. N. Engl. J. Med. 348: 138–150. doi: 10.1056/NEJMra021333 [DOI] [PubMed] [Google Scholar]
- 7.Kessel A., Bamberger E., Masalha M., Toubi E.2009. The role of T regulatory cells in human sepsis. J. Autoimmun. 32: 211–215. doi: 10.1016/j.jaut.2009.02.014 [DOI] [PubMed] [Google Scholar]
- 8.Maloy K. J., Powrie F.2001. Regulatory T cells in the control of immune pathology. Nat. Immunol. 2: 816–822. doi: 10.1038/ni0901-816 [DOI] [PubMed] [Google Scholar]
- 9.Monneret G., Debard A. L., Venet F., Bohe J., Hequet O., Bienvenu J., Lepape A.2003. Marked elevation of human circulating CD4+CD25+ regulatory T cells in sepsis-induced immunoparalysis. Crit. Care Med. 31: 2068–2071. doi: 10.1097/01.CCM.0000069345.78884.0F [DOI] [PubMed] [Google Scholar]
- 10.Monneret G., Lepape A., Voirin N., Bohe J., Venet F., Debard A. L., Thizy H., Bienvenu J., Gueyffier F., Vanhems P.2006. Persisting low monocyte human leukocyte antigen-DR expression predicts mortality in septic shock. Intensive Care Med. 32: 1175–1183. doi: 10.1007/s00134-006-0204-8 [DOI] [PubMed] [Google Scholar]
- 11.Muenzer J. T., Davis C. G., Chang K., Schmidt R. E., Dunne W. M., Coopersmith C. M., Hotchkiss R. S.2010. Characterization and Modulation of the Immunosuppressive Phase of Sepsis. Infect. Immun. 78: 1582–1592. doi: 10.1128/IAI.01213-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Munford R. S., Pugin J.2001. Normal responses to injury prevent systemic inflammation and can be immunosuppressive. Am. J. Respir. Crit. Care Med. 163: 316–321. doi: 10.1164/ajrccm.163.2.2007102 [DOI] [PubMed] [Google Scholar]
- 13.Nascimento D. C., Alves-Filho J. C., Sonego F., Fukada S. Y., Pereira M. S., Benjamim C., Zamboni D. S., Silva J. S., Cunha F. Q.2010. Role of regulatory T cells in long-term immune dysfunction associated with severe sepsis. Crit. Care Med. 38: 1718–1725. doi: 10.1097/CCM.0b013e3181e78ad0 [DOI] [PubMed] [Google Scholar]
- 14.Rissetto K. C., Rindt H., Selting K. A., Villamil J. A., Henry C. J., Reinero C. R.2010. Cloning and expression of canine CD25 for validation of an anti-human CD25 antibody to compare T regulatory lymphocytes in healthy dogs and dogs with osteosarcoma. Vet. Immunol. Immunopathol. 135: 137–145. doi: 10.1016/j.vetimm.2010.02.002 [DOI] [PubMed] [Google Scholar]
- 15.Sakaguchi S., Sakaguchi N., Asano M., Itoh M., Toda M.1995. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 155: 1151–1164 [PubMed] [Google Scholar]
- 16.Sakaguchi S., Ono M., Setoguchi R., Yagi H., Hori S., Fehervari Z., Shimizu J., Takahashi T., Nomura T.2006. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol. Rev. 212: 8–27. doi: 10.1111/j.0105-2896.2006.00427.x [DOI] [PubMed] [Google Scholar]
- 17.Shevach E. M.2002. CD4+ CD25+ suppressor T cells: more questions than answers. Nat. Rev. Immunol. 2: 389–400 [DOI] [PubMed] [Google Scholar]
- 18.Shevach E. M., DiPaolo R. A., Andersson J., Zhao D. M., Stephens G. L., Thornton A. M.2006. The lifestyle of naturally occurring CD4+ CD25+ Foxp3+ regulatory T cells. Immunol. Rev. 212: 60–73. doi: 10.1111/j.0105-2896.2006.00415.x [DOI] [PubMed] [Google Scholar]
- 19.Song R., Kim J., Yu D., Park C., Park J.2012. Kinetics of IL-6 and TNF-α changes in a canine model of sepsis induced by endotoxin. Vet. Immunol. Immunopathol. 146: 143–149. doi: 10.1016/j.vetimm.2012.02.008 [DOI] [PubMed] [Google Scholar]
- 20.Tominaga M., Horiuchi Y., Ichikawa M., Yamashita M., Okano K., Jikumaru Y., Nariai Y., Kadosawa T.2010. Flow cytometric analysis of peripheral blood and tumor-infiltrating regulatory T cells in dogs with oral malignant melanoma. J. Vet. Diagn. Invest. 22: 438–441. doi: 10.1177/104063871002200317 [DOI] [PubMed] [Google Scholar]
- 21.Vahlenkamp T. W., Tompkins M. B., Tompkins W. A.2004. Feline immunodeficiency virus infection phenotypically and functionally activates immunosuppressive CD4+CD25+ T regulatory cells. J. Immunol. 172: 4752–4761 [DOI] [PubMed] [Google Scholar]
- 22.Yagi H., Nomura T., Nakamura K., Yamazaki S., Kitawaki T., Hori S., Maeda M., Onodera M., Uchiyama T., Fujii S., Sakaguchi S.2004. Crucial role of FOXP3 in the development and function of human CD25+CD4+ regulatory T cells. Int. Immunol. 16: 1643–1656. doi: 10.1093/intimm/dxh165 [DOI] [PubMed] [Google Scholar]
- 23.Yu D.H., Kim B., Park J.2012. Pathophysiologic and Immunologic Changes in a Canine Endotoxemia over a Period of 24 Hours. J. Vet. Med. Sci. 74: 537–544. doi: 10.1292/jvms.11-0321 [DOI] [PubMed] [Google Scholar]