Abstract
Background
There is a reverse relationship between serum bilirubin level and incidence of stroke, heme oxygenase-1 (HO-1) can catalyze heme into bilirubin, it is unknown the association of HO-1 level with risk of stroke.
Methods
Sixty patients with stroke and fifty patients with transient ischemic attack (TIA) were recruited. Serum level of HO-1, total and direct bilirubin, alanine transaminase, live function, lipid profile and infection status of patients were measured.
Results
Significant differences were found between two groups in terms of serum levels of HO-1 (163.6±58.7 vs. 141.2±49.7, P=0.032), total bilirubin (10.1±4.6 vs. 15.8±2.7, P<0.001), direct bilirubin (3.2±2.1 vs. 5.9±1.2, P<0.001), fasting glucose (6.7±3.1 vs. 4.9±1.3, P<0.001), cholesterol (4.4±1.1 vs. 3.9±0.8, P=0.005) and diastolic blood pressure (DBP) (84.9±9.4 vs. 81.3±9.2, P=0.046). In multivariate analysis, serum direct bilirubin (OR, 2.83; P<0.001), total bilirubin (OR, 1.82, P=0.001), DBP (OR, 0.88, P=0.041), and fasting glucose (OR, 0.34, P<0.001) were independent predictors of stroke.
Conclusions
Serum HO-1 level is higher in patients with stroke than TIA, but the bilirubin level is lower in patients with stroke than TIA and is an independent predictor of stroke. Further studies are warranted to clarify the underlying link among HO-1, bilirubin and stroke.
Keywords: Heme oxygenase-1 (HO-1), stroke, bilirubin
Introduction
Heme oxygenases (HO) are microsomal enzymes that include heme oxygenase-1 (HO-1), HO-2 and HO-3. The activity of HO-1, also designated HSP32, is significantly induced by numerous stimuli, including heme, heavy metals, hormones, oxidative stress (1,2) and traumatic brain injury (3).
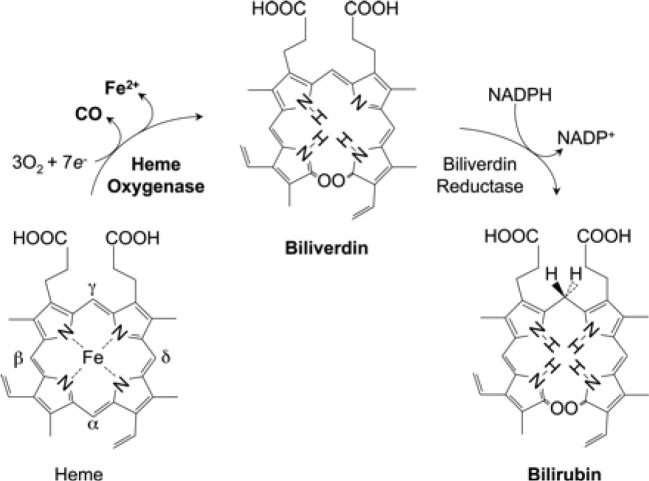
Tenhunen and coworkers (4) initially reported on the presence of HO in liver microsomes. HO catalyzes the first and rate-limiting step in the oxidative degradation of heme (Fe-protoporphyrin-IX) to carbon monoxide (CO), ferrous iron (Fe2+), and biliverdin-IX (Figure 1) (5-7). HO is not a heme protein in itself but uses heme as both its active center and substrate. CO activates cGMP to promote vasodilation, CO exerts a potent anti-inflammatory effect in various disease settings, and biliverdin is subsequently converted by biliberdin reductase to bilirubin (1,5,6), which can serve as an anti-oxidant, both of which may contribute to the reported protective role of HO-1 in cerebral ischemia and subarachnoid hemorrhage (5,8,9). In addition to its role in heme catabolism, HO-1 plays important roles in various pathophysiological states associated with cellular stress. Both HO-1 and bilirubin have the antiatherogenic properties (1,2,10).
Figure 1.
Heme is metabolized by heme oxygenase and biliverdin reductase with the production of CO, iron, biliverdin, and bilirubin. CO, carbon monoxide.
Stroke is one of the leading cause of death and a major cause of disability in stroke patients. Transient ischemic attack (TIA) is a brief episode of neurologic dysfunction resulting from focal temporary cerebral ischemia not associated with cerebral infarction (11). It is reported that level of bilirubin is reversely associated with the ischemic cerebral infarction (2,12-15). However, little is known about the relationship of serum level of HO-1 with the risk of stroke or TIA. In this study, we compared the serum level of HO-1 in stroke patients with TIA patients in order to find the association of HO-1 with risk of stroke.
Methods and patients
Patients
The investigation was approved by the Clinical Research Ethics Committee of the hospital, and all patients gave written informed consent. Patients with first-ever or recurrent acute ischemic stroke or TIA consecutively admitted to hospital between May 2011 to June 2013 were studied.
Inclusion and exclusion conditions
The study inclusion criteria were well-documented clinical presentation and computed tomography or magnetic resonance imaging of brain, first or recurrent acute ischemic stroke or TIA occurring within seven days prior to admission; the capability and willingness to provide informed consent. The exclusion criteria for the study were hemorrhagic stroke, active liver disease, 2 times higher than normal value of alanine transaminase, bilirubin >34.2 umol/L, or albumin <3.5 mg/dL, hepatobiliary diseases, Gilbert syndrome (12), cancer, severe aphasia or physically unfit for physical examination, stroke or TIA over 7 days before admission, failure to give the informed consent.
Blood pressure was measured in a seated position with a mercury sphygmomanometer. Type 2 diabetes mellitus (DM2) was defined as fasting blood glucose over 7.8 mmol/L or self-reported DM2 or currently on medication for DM (16). Hypertension was defined as a systolic BP of at least 140 mmHg or a diastolic BP of at least 90 mmHg, or a self-reported diagnosis of hypertension and currently on medication to control BP (17). Infection was defined as white blood cell count >10×109/L and or neutrophile (%) >70%.
Laboratory test
Venous blood was taken within 24 hours after admission, and plasma was obtained by centrifugation at 1,000 g at 4 °C for 20 minutes. All aliquots were stored at –70 °C for study. Complete blood counts, fasting glucose, lipid profiles and liver function were measured by automated biochemical profiling. Serum HO-1 was measured with a commercial enzyme linked immunosorbent assay (ELISA) kit (Cusabio, catalog no CSB-E08266h, Barksdale, USA). Briefly, Add 100 µL prepared standards and samples in triplicate to wells of Anti-HO-1 immunoassay plate, incubate for 2 hours at 37 °C. Remove the liquid of each well, don’t wash. Add 100 µL of biotin-antibody HO-1 to each well. Incubate for 1 hour at 37 °C. Aspirate each well and wash, repeating the process two times for a total of three washes. Wash by filling each well with 200 µL of wash buffer and let it stand for 2 minutes. After the last wash, remove any remaining wash buffer. Add 100 µL of HRP-avidin to each well. Incubate for 1 hour at 37 °C. Add 90 µL of TMB substrate to each well. Incubate for 15 minutes at 37 °C in the dark. Add 50 µL of stop solution to each well, gently tap the plate to ensure thorough mixing. Determine the optical density of each well within 5 minutes with a microplate reader at 450 nm. Plot the HO-1 standard curve and calculate HO-1 sample concentrations.
Statistical analyses
Mean and SD was used to express the normal distributed data. Median (inter-quartile range, IQR) for non-normal distributed data. Categorical data were analyzed by the chi-squared test. Changed in levels of HO-1 and bilirubin levels were evaluated using 2-way repeated measures ANOVA. A multivariate logistic analysis was performed. A probability of less than 0.05 was considered as statistically significant. A software SPSS (13.0) was used for analysis.
Results
Patients’ characteristics
A total of 110 consecutive patients were enrolled in this study, 60 of 110 patients had stroke and 50 patients had TIA. Table 1 shows the baseline characteristics of all patients, all the data including HO-1, bilirubins and blood pressures are normally distributed. Table 2 demonstrates the results of univariate analysis of risk factors for stroke, serum level of HO-1, total and direct bilirubin, fasting glucose, cholesterol and diastolic blood pressure (DBP). A significant difference in both groups was found in terms of serum level of HO-1, total bilirubin, direct bilirubin, fasting glucose, and DBP (P<0.05, Table 2). In comparison with TIA, serum level of HO-1, fasting glucose, cholesterol, DBP were higher (P<0.001), whereas levels of total bilirubin and direct bilirubin were lower in patients with stroke (P<0.001). No significant difference in both groups was found in terms of age, sex, history of hypertension, diabetes mellitus, dyslipidemia and proportion of alcohol drinker and cigarette smoker, LDL, HDL, and systolic blood pressure (P>0.05).
Table 1. Basic characteristics of patients with stroke or TIA.
| Variable | Stroke | TIA | P value |
|---|---|---|---|
| Age (years) | 68.3±11.9 | 70.9±12.7 | 0.275 |
| Male, n (%) | 41 (68.3) | 36 (72.0) | 0.676 |
| Hypertension, n (%) | 38 (63.3) | 35 (70.0) | 0.461 |
| DM, n (%) | 25 (41.7) | 24 (48.0) | 0.506 |
| Dyslipidemia, n (%) | 17 (28.3) | 10 (20.0) | 0.312 |
| Current smoker, n (%) | 20 (33.3) | 16 (32.0) | 0.88 |
| Alcohol drinker, n (%) | 17 (28.3) | 14 (28.0) | 0.97 |
| Infection, n (%) | 37 (61.7) | 31 (62.1) | 0.971 |
| HO-1 (μmol/L) | 163.6±58.7 | 141.2±49.7 | 0.032 |
| Total bilirubin (μmol/L) | 10.1±4.6 | 15.8±2.7 | <0.001 |
| direct bilirubin (μmol/L) | 3.2±2.1 | 5.9±1.2 | <0.001 |
| ALT (IU/L) | 15.6±5.2 | 16.3±3.6 | 0.452 |
| Fasting glucose (mmol/L) | 6.7±3.1 | 4.9±1.3 | <0.001 |
| Cholesterol (mmol/L) | 4.4±1.1 | 3.9±0.8 | 0.005 |
| LDL (mmol/L) | 2.7±0.8 | 2.4±0.8 | 0.052 |
| HDL (mmol/L) | 1.2±0.2 | 1.1±0.2 | 0.903 |
| SBP (mmHg) | 129.9±16.4 | 128.1±9.9 | 0.475 |
| DBP (mmHg) | 84.9±9.4 | 81.3±9.2 | 0.046 |
| WBC (×109) | 6.6±2.3 | 5.6±1.5 | 0.01 |
| Neutrophile % | 61.4±9.9 | 60.4±9.1 | 0.587 |
Data are presented as n (%) or means ± SD; TIA, transient ischemic attack; DM, diabetes mellitus; HO-1, heme oxygenase-1; ALT, alanine transaminase; LDL, low density lipoprotein; HDL, high density lipoprotein; SBP, systolic blood pressure; DBP, diastolic blood pressure; WBC, white blood cell count.
Table 2. Univariate analysis of risk factors for stroke.
| Variable | 95% CI | P value |
|---|---|---|
| Heme oxygenase-1 | (1.96,42.95) | 0.032 |
| Total bilirubin | (–7.09,–4.29) | 0.001 |
| Direct bilirubin | (–3.33,–2.07) | 0.001 |
| Fasting glucose | (0.905,2.66) | <0.001 |
| Cholesterol | (0.154,0.856) | 0.005 |
| DBP | (0.072,7.11) | 0.046 |
CI, confidence interval; DBP, diastolic blood pressure.
In order to study the association of serum HO-1, bilirubin levels with stroke, multivariate logistic regression analysis was performed. In regression model, following adjustment for age, gender, history of hypertension, DM, dyslipidemia, HDL, LDL, serum levels of direct bilirubin, total bilirubin, fasting glucose, white blood cell count, and DBP were significant independent predictor of stroke (P<0.05; Table 3).
Table 3. Multivariate regression analysis for stroke.
| Variable | Odds ratio | 95% CI | P value |
|---|---|---|---|
| Direct bilirubin | 2.83 | 1.595-5.017 | <0.001 |
| Total bilirubin | 1.82 | 1.289-2.555 | 0.001 |
| White blood cell count | 0.35 | 0.152-0.811 | 0.014 |
| Glucose | 0.34 | 0.190-0.612 | <0.001 |
| DBP | 0.88 | 0.001-0.995 | 0.041 |
CI, confidence interval; DBP, diastolic blood pressure.
Discussion
The major finding of our study showed that serum level of HO-1 was higher in patients with stroke than TIA, and serum level of both total and direct bilirubin was lower in patients with stroke than TIA. The underlying pathophysiological role has not been understood and requires further investigation. Regarding the level of human serum HO-1 with cardiovascular disorder, Idriss et al. (18) reported that HO-1 is raised in stable coronary artery disease and acute coronary artery syndromes. To the best of our knowledge, no data are available for the distribution of human HO-1 in literature, and this is the first paper to report the association of HO-1 level with the risk of stroke.
Tenhunen and coworkers (4) were the first to report on the presence of HO in liver microsomes capable of degrading heme to bilirubin, and this activity was subsequently dissociated from cytochrome P-450 (5-7,9). Three isoenzymes of HO-1 have been found, HO-2 and HO-3 are constitutively expressed isoforms, HO-1 is induced in most cells, oxidative conditions, heme, cytokines and NO donors can induce HO-1 gene expression (5). HO-1 catalyzes the rate-limiting step in the heme catabolism, forming CO, iron, and biliverdin that is subsequently reduced to bilirubin (1,2). In our study, we found that the HO-1 level was higher in stroke group than TIA group, but the total and direct bilirubin level were lower in stroke group than TIA group. High level of HO-1 can be explained that stroke patients have been exposed to a high degree of oxidative stress such as atherosclerosis, infection, hypertension, which leads to an upregulation of HO-1.
Morsi et al. (19) reported that HO-1 expression and its activity in human endothelia cells are present only in advanced atherosclerosis and the degree of its expression increases with severity of atherosclerosis. Expressed HO-1 in atherosclerotic lesions can ameliorate oxidative stress and inhibit inflammatory processes in the vessel wall (1). The leukocyte mRNA expression of HO-1 is reported to be associated with the severity of coronary heart disease (20). HO-1 level may reflect the general status of patients’ oxidative stress. Serum bilirubin might have some protective function against risk of stroke in men (21).
Numerous studies showed that there is a reverse relationship of bilirubin level with incidence of strokes (2,6-9), which is true in our study, the bilirubin level in stroke patients was lower than TIA patients. However, high serum level of HO-1 in stroke patients in our group were expected to catalyze heme and produce high level of bilirubin theoretically, but surprisingly, the results of serum level of bilirubin was lower, the controversial relationships of HO-1 and bilirubin with the risk of stroke have not been completely understood, and require further study. At least, the activity of HO-1 may not be linearly related to the formation of bilirubin; another reason may be the upregulated HO-1 enzyme may not be fully activated.
Stroke can be caused by ruptured unstable carotid plaques (11), or thrombosis formation in cerebral arteries. It is reported that approximately 70% of the patients with stroke are cerebral infarction, 15% were hemorrhagic stroke and 15% were TIA (22). Patients with TIA may fully progress into stroke (22). Oxidative stress such as infection induces more circulating neutrophils and macrophages in blood, produces more inducible cytokines and activates HO-1 activity, moreover, immune responses to infection contribute significantly to the formation of atherogenesis. Blood circulation in small atherosclerotic arteries becomes slower especially on condition of low systemic blood pressure and or dehydration, and thereby causes the ischemic stroke.
We have to mention that a limitation was existed in our study. A small number of patients included in current study, many confounder factors of patients, and lack of variables of stroke prognosis may bias the results, and account for the discordance of HO-1 and bilirubin level with risk of stroke. A NIH Stroke Scale should be included in future study.
Conclusions
In summary, our study demonstrates that serum HO-1 level is higher in stroke patients than TIA patients, infection rate is associated with stroke patients. However, bilirubin level is lower in stroke patients. HO-1 may be a general marker to reflect the oxidative stress of patients. Underlying mechanism of HO-1 versus bilirubin with risk of stroke deserves further investigation. A larger sample size of patients from multiple medical centers with long-term follow up will detect significant findings in the risk of stroke.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Platt JL, Nath KA. Heme oxygenase: protective gene or Trojan horse. Nat Med 1998;4:1364-5 [DOI] [PubMed] [Google Scholar]
- 2.Novotný L, Vítek L.Inverse relationship between serum bilirubin and atherosclerosis in men: a meta-analysis of published studies. Exp Biol Med (Maywood) 2003;228:568-71 [DOI] [PubMed] [Google Scholar]
- 3.Okubo S, Xi G, Keep RF, et al. Cerebral hemorrhage, brain edema, and heme oxygenase-1 expression after experimental traumatic brain injury. Acta Neurochir Suppl 2013;118:83-7 [DOI] [PubMed] [Google Scholar]
- 4.Tenhunen R, Marver HS, Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci U S A 1968;61:748-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ren S, Liu H, Licad E, et al. Expression of rat liver tryptophan 2,3-dioxygenase in Escherichia coli: structural and functional characterization of the purified enzyme. Arch Biochem Biophys 1996;333:96-102 [DOI] [PubMed] [Google Scholar]
- 6.Ren S, Correia MA. Heme: a regulator of rat hepatic tryptophan 2,3-dioxygenase? Arch Biochem Biophys 2000;377:195-203 [DOI] [PubMed] [Google Scholar]
- 7.Stocker R, Perrella MA. Heme oxygenase-1: a novel drug target for atherosclerotic diseases? Circulation 2006;114:2178-89 [DOI] [PubMed] [Google Scholar]
- 8.Sharp FR, Zhan X, Liu DZ. Heat shock proteins in the brain: role of Hsp70, Hsp 27, and HO-1 (Hsp32) and their therapeutic potential. Transl Stroke Res 2013;4:685-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abraham NG, Kappas A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol Rev 2008;60:79-127 [DOI] [PubMed] [Google Scholar]
- 10.Juan SH, Cheng TH, Lin HC, et al. Mechanism of concentration-dependent induction of heme oxygenase-1 by resveratrol in human aortic smooth muscle cells. Biochem Pharmacol 2005;69:41-8 [DOI] [PubMed] [Google Scholar]
- 11.Ren S, Fan X, Peng L, et al. Expression of NF-κB, CD68 and CD105 in carotid atherosclerotic plaque. J Thorac Dis 2013;5:771-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perlstein TS, Pande RL, Creager MA, et al. Serum total bilirubin level, prevalent stroke, and stroke outcomes: NHANES 1999-2004. Am J Med 2008;121:781-788.e1. [DOI] [PMC free article] [PubMed]
- 13.Li RY, Cao ZG, Zhang JR, et al. Decreased serum bilirubin is associated with silent cerebral infarction. Arterioscler Thromb Vasc Biol 2014;34:946-51 [DOI] [PubMed] [Google Scholar]
- 14.Luo Y, Li J, Zhang J, et al. Elevated bilirubin after acute ischemic stroke linked to the stroke severity. Int J Dev Neurosci 2013;31:634-8 [DOI] [PubMed] [Google Scholar]
- 15.Xu T, Zhang J, Xu T, et al. Association of serum bilirubin with stroke severity and clinical outcomes. Can J Neurol Sci 2013;40:80-4 [DOI] [PubMed] [Google Scholar]
- 16.Copeland KC, Silverstein J, Moore KR, et al. Management of newly diagnosed type 2 Diabetes Mellitus (T2DM) in children and adolescents. Pediatrics 2013;131:364-82 [DOI] [PubMed] [Google Scholar]
- 17.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014;311:507-20 [DOI] [PubMed] [Google Scholar]
- 18.Idriss NK, Lip GY, Balakrishnan B, et al. Plasma haemoxygenase-1 in coronary artery disease. A comparison with angiogenin, matrix metalloproteinase-9, tissue inhibitor of metalloproteinase-1 and vascular endothelial growth factor. Thromb Haemost 2010;104:1029-37 [DOI] [PubMed] [Google Scholar]
- 19.Morsi WG, Shaker OG, Ismail EF, et al. HO-1 and VGEF gene expression in human arteries with advanced atherosclerosis. Clin Biochem 2006;39:1057-62 [DOI] [PubMed] [Google Scholar]
- 20.Vítek L.Does hyperbilirubinemia protect from coronary heart disease? Am J Cardiol 2001;88:1218. [DOI] [PubMed] [Google Scholar]
- 21.Kimm H, Yun JE, Jo J, et al. Low serum bilirubin level as an independent predictor of stroke incidence: a prospective study in Korean men and women. Stroke 2009;40:3422-7 [DOI] [PubMed] [Google Scholar]
- 22.Mohr JP, Albers GW, Amarenco P, et al. American Heart Association Prevention Conference. IV. Prevention and Rehabilitation of Stroke. Etiology of stroke. Stroke 1997;28:1501-6 [DOI] [PubMed] [Google Scholar]