Abstract
Kidney damage is a common sequela of several chronic pathologic conditions. Whether biomarkers of kidney damage are prognostic for more severe outcomes is unknown. We measured three urinary biomarkers (kidney injury molecule-1 [KIM-1], IL-18, and albumin) in 3010 individuals enrolled in the Health, Aging and Body Composition (Health ABC) study and used Cox proportional hazards models to investigate the associations of urinary KIM-1/creatinine (cr), IL-18/cr, and albumin/cr (ACR) with all-cause mortality and cardiovascular disease (CVD). Multivariable models adjusted for demographics, traditional CVD risk factors, and eGFR. Mean age of participants was 74 years, 49% of participants were men, and 41% of participants were black. During the median 12.4 years of follow-up, 1450 deaths and 797 CVD outcomes occurred. Compared with the lowest quartile, successive quartiles had the following adjusted hazard ratios (HRs; 95% confidence intervals [95% CIs]) for mortality: KIM-1/cr: (1.21; 1.03 to 1.41), (1.13; 0.96 to 1.34), and (1.28; 1.08 to 1.52); IL-18/cr: (1.02; 0.88 to 1.19), (1.16; 0.99 to 1.35), and (1.06; 0.90 to 1.25); ACR: (1.08; 0.91 to 1.27), (1.24; 1.06 to 1.46), and (1.63; 1.39 to 1.91). In similar analyses, only ACR quartiles associated with CVD: (1.19; 0.95 to 1.48), (1.35; 1.08 to 1.67), and (1.54; 1.24 to 1.91). Urinary KIM-1 had a modest association with all-cause mortality but did not associate with CVD, and urinary IL-18 did not associate with either outcome. In contrast, albuminuria strongly associated with all-cause mortality and CVD. Future studies should evaluate reasons for these differences in the prognostic importance of individual kidney injury markers.
Over the last decade, several novel urine biomarkers of acute tubular injury have been described. Before any increases in serum creatinine or decline in eGFR, these biomarkers have been shown to identify AKI,1 mediate kidney injury, and reflect tubular damage and apoptosis.2,3 They have also been shown to predict the severity of AKI and in-hospital mortality from multiple causes of AKI, including kidney transplantation, cardiac surgery, and septic shock.4–11
Although both urinary kidney injury molecule (KIM-1) and IL-18 were originally identified as markers of AKI, each marker has shown promise as an indicator of chronic tubular damage and progressive kidney disease. Urinary levels of KIM-1 are elevated in various forms of CKD and associated with the severity of pathology, including fibrosis and inflammation.12,13 Other studies have noted higher urinary IL-18 levels in patients with nephrotic syndrome and other forms of CKD compared with controls and the ability to predict severity of kidney disease.14,15
Recent work from our group has suggested that urinary KIM-1 and IL-18 may identify ambulatory persons who are at risk for kidney function decline as well as mortality. In the Multi-Ethnic Study of Atherosclerosis (MESA), elevated urinary KIM-1 levels were associated with kidney function decline using a case control design,16 whereas in the Women’s Interagency HIV Study (WIHS), elevated levels of urinary IL-18 and KIM-1 were each associated with both kidney function decline and all-cause mortality.17,18
However, to our knowledge, there are no large studies in the general population that have evaluated whether markers of tubular injury are associated with all-cause mortality or cardiovascular disease (CVD). On kidney biopsy, the severity of tubular atrophy and the severity of fibrosis are the most reliable pathologic features for prediction of progression to kidney failure in nearly all etiologies of kidney disease.19–21 Biomarkers of tubular injury, therefore, may also be associated with the consequences of CKD, such as CVD and mortality. There are also no studies that have compared associations of these newer tubular kidney injury markers (with the established urinary biomarker albuminuria) with all-cause mortality and CVD. In this analysis, we, therefore, evaluated the relationships of urinary KIM-1, IL-18, and albumin with all-cause and CVD in the Health, Aging and Body Composition (Health ABC) study, which had an elderly population at high risk for mortality and CVD.
Results
In total, 3010 participants had urine available for measurement of KIM-1, IL-18, and albumin to creatinine ratio (ACR). Median (interquartile range) levels of KIM-1, IL-18, and urine ACR (UACR) were 794 (406, 1442) pg/ml, 29 (13, 57) pg/ml, and 8.3 (4.6, 20.3) mg/g, respectively. Less than 1% of KIM-1, 5.25% of IL-18, 5.8% of albuminuria, and 5.9% of urine creatinine values were set to the lowest detection limit. Mean age of the population was 74 years, 49% of participants were men, 41% of participants were black, 24% of participants had diabetes, and 22% of participants had prevalent coronary heart disease (CHD); 17% of participants had eGFRcys<60 ml/min per 1.73 m2, 1.6% of participants had eGFRcys<30 ml/min per 1.73 m2, 0.3% of participants had eGFRcys<15 ml/min per 1.73 m2, and 19% of participants had UACR>30 mg/g (Table 1). Participants with higher urinary KIM-1/creatinine (cr) were more likely to be white, have diabetes or CHD, have higher levels of C-reactive protein (CRP) and UACR, and have lower levels of serum albumin and eGFRcys. Participants with higher IL-18/cr were more likely to be women, white, and smokers, have lower prevalence of CHD, and have higher levels of CRP, ACR, and eGFRcys (Supplemental Table 1). Participants with higher levels of UACR were more likely to be black, have diabetes, hypertension, and CHD, have higher CRP, and have lower eGFRcys (Supplemental Table 2). KIM-1 was significantly correlated with UACR (r=0.17, P<0.01), eGFRcys (r=−0.08, P<0.01), and IL-18 (r=0.19, P<0.01). IL-18 was also significantly correlated with eGFRcys (r=0.10, P<0.01) and ACR (r=0.17, P<0.01). The presence of eGFRcys<60 ml/min per 1.73 m2 was associated with higher KIM-1/cr and ACR (odds ratio [OR], 1.16; 95% confidence interval [95% CI], 1.06 to 1.28 and OR, 1.22; 95% CI, 1.16 to 1.29 per doubling) but not IL-18 (OR, 0.89; 95% CI, 0.81 to 0.97 per doubling).
Table 1.
Characteristics of participants by quartiles of KIM-1 standardized to urine creatinine
| Characteristics | KIM-1 Standardized to Urine Creatinine (ng/mg) | P Value for Linear Trend | All | |||
|---|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | |||
| Range | 29–497 | 498–814 | 815–1239 | 1240–71,535 | ||
| N | 751 | 756 | 750 | 753 | 3010 | |
| Age (yr) | 73.4 (2.8) | 73.4 (2.9) | 73.8 (2.9) | 73.8 (2.9) | <0.001 | 73.6 (2.9) |
| Men | 418 (56) | 357 (47) | 331 (44) | 361 (48) | 0.001 | 1467 (49) |
| Race/Ethnicity | ||||||
| White | 286 (38) | 356 (47) | 520 (69) | 602 (80) | <0.001 | 1764 (59) |
| Black | 465 (62) | 400 (53) | 230 (31) | 151 (20) | 1246 (41) | |
| Site | ||||||
| Memphis | 359 (48) | 353 (47) | 383 (51) | 409 (54) | 0.003 | 1504 (50) |
| Pittsburgh | 392 (52) | 403 (53) | 367 (49) | 344 (46) | 1506 (50) | |
| Education | ||||||
| Less than high school | 251 (34) | 209 (28) | 156 (21) | 140 (19) | <0.001 | 756 (25) |
| High school graduate | 214 (29) | 244 (32) | 261 (35) | 260 (35) | 979 (33) | |
| Postsecondary school | 284 (38) | 300 (40) | 332 (44) | 351 (47) | 1267 (42) | |
| Diabetes | 151 (22) | 181 (25) | 154 (21) | 212 (29) | 0.01 | 701 (24) |
| Hypertension | 517 (69) | 482 (64) | 477 (64) | 516 (69) | 0.89 | 1992 (66) |
| SBP (mmHg) | 136 (21) | 135 (21) | 136 (20) | 136 (22) | 0.66 | 136 (21) |
| Smoking | ||||||
| Never | 341 (46) | 339 (45) | 339 (45) | 291 (39) | 0.06 | 1310 (44) |
| Former | 72 (10) | 68 (9) | 75 (10) | 95 (13) | 310 (10) | |
| Current | 337 (45) | 348 (46) | 336 (45) | 364 (49) | 1385 (46) | |
| Prevalent heart failure | 22 (3) | 22 (3) | 21 (3) | 28 (4) | 0.41 | 93 (3) |
| Prevalent CHD | 143 (19) | 162 (22) | 147 (20) | 191 (26) | 0.01 | 643 (22) |
| CRP | 1.63 [0.97, 2.97] | 1.69 [1.01, 3.17] | 1.62 [0.99, 2.92] | 1.79 [1.01, 3.48] | 0.03 | 1.68 [0.99, 3.13] |
| Albumin (g/dl) | 4.02 (0.32) | 3.98 (0.31) | 3.96 (0.30) | 3.96 (0.33) | <0.001 | 3.98 (0.31) |
| LDL (mg/dl) | 122 (33) | 123 (35) | 122 (34) | 119 (37) | 0.03 | 122 (35) |
| HDL (mg/dl) | 54 (17) | 55 (18) | 54 (17) | 53 (17) | 0.04 | 54 (17) |
| eGFRcys (ml/min per 1.73 m2) | 80 (20) | 80 (20) | 80 (21) | 75 (23) | <0.001 | 79 (21) |
| eGFRcys<60 | 108 (14) | 104 (14) | 112 (15) | 185 (25) | <0.001 | 509 (17) |
| UACR (mg/g) | 6.88 [3.78, 16.92] | 7.45 [4.37, 16.61] | 8.30 [4.74, 18.03] | 11.38 [5.78, 33.41] | <0.001 | 8.26 [4.55, 20.31] |
| UACR>30 | 117 (16) | 118 (16) | 121 (16) | 202 (27) | <0.001 | 558 (19) |
Data are presented as mean (SD), n (%), or median [interquartile range].
All-Cause Mortality and Urine Biomarkers
There were 1450 deaths and median follow-up of 12.4 years.
KIM-1/cr
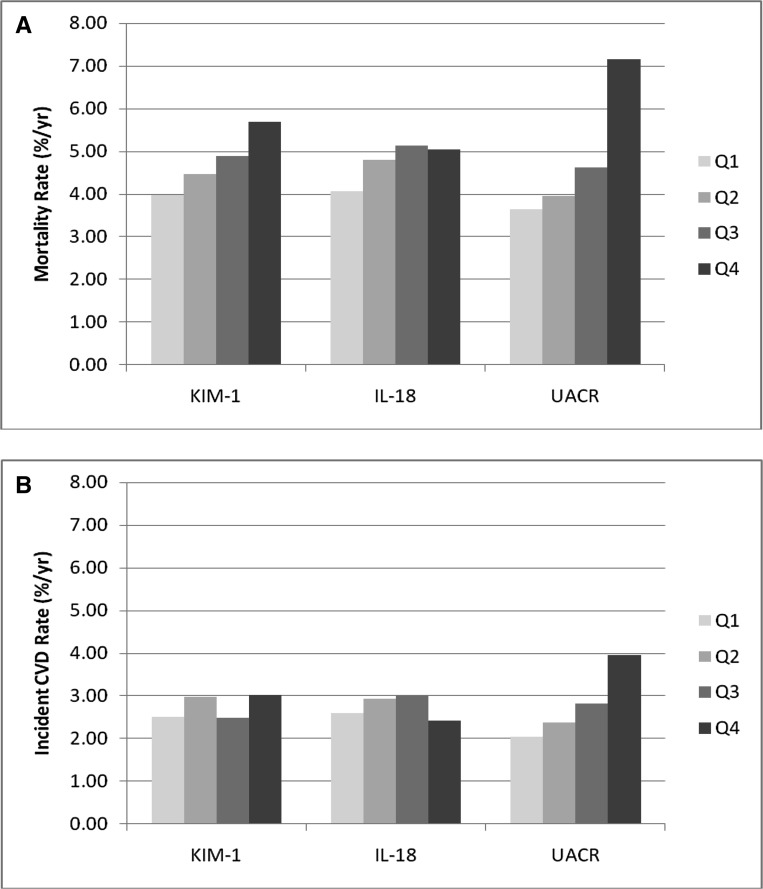
The mortality rate was highest in the highest quartile of KIM-1/cr relative to the other three quartiles (Figure 1A). In continuous models, KIM-1/cr was associated with an increased risk of mortality in demographic- and risk factor-adjusted models, but this finding was attenuated after adjustment for eGFRcys and UACR. In the quartile analysis, the top quartile of KIM-1/cr was associated with approximately a 50% higher mortality risk compared with the lowest quartile after adjustment for risk factors and remained independently associated with a 28% higher risk of mortality after adjustment for UACR and eGFRcys (Table 2).
Figure 1.
Mortality and CVD by urinary injury markers. (A) Mortality rates by quartiles of KIM-1, IL-18, and ACR. (B) CVD by quartiles of KIM-1, IL-18, and ACR. CVD was defined by a CHD event and/or stroke. Q, quartile.
Table 2.
Association of KIM-1, IL-18, and UACR with mortality
| Exposure Variable | Unadjusted | Demographic-Adjusteda | Risk Factor–Adjustedb | Adjusted for Baseline Kidney Functionc | Adjusted for Time-Dependent Kidney Functiond | Adjusted for Rapid Decline in Kidney Functione |
|---|---|---|---|---|---|---|
| KIM-1 standardized to UCr (ng/mg) | ||||||
| Continuous (per doubling) | 1.09 (1.04, 1.14) | 1.15 (1.10, 1.19) | 1.10 (1.05, 1.15) | 1.02 (0.97, 1.07) | 1.06 (1.00, 1.11) | 1.02 (0.97, 1.08) |
| P value | <0.001 | <0.001 | <0.001 | 0.36 | 0.04 | 0.48 |
| Quartiles | ||||||
| <497 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 497–814 | 1.15 (0.98, 1.34) | 1.27 (1.08, 1.48) | 1.21 (1.04, 1.42) | 1.21 (1.03, 1.41) | 1.24 (1.06, 1.45) | 1.09 (0.92, 1.30) |
| 815–1239 | 1.06 (0.90, 1.24) | 1.29 (1.09, 1.52) | 1.17 (0.99, 1.38) | 1.13 (0.96, 1.34) | 1.16 (0.98, 1.36) | 1.03 (0.86, 1.23) |
| ≥1240 | 1.39 (1.20, 1.62) | 1.77 (1.51, 2.09) | 1.46 (1.23, 1.72) | 1.28 (1.08, 1.52) | 1.33 (1.12, 1.57) | 1.35 (1.14, 1.60) |
| P valuef | <0.001 | <0.001 | <0.001 | 0.02 | 0.007 | 0.10 |
| IL-18 standardized to UCr (pg/mg) | ||||||
| Continuous (per doubling) | 0.99 (0.95, 1.03) | 1.04 (1.00, 1.09) | 1.03 (0.99, 1.08) | 0.99 (0.95, 1.03) | 0.98 (0.93, 1.03) | 1.02 (0.97, 1.07) |
| P value | 0.99 | 0.04 | 0.16 | 0.54 | 0.88 | 0.37 |
| Quartiles | ||||||
| <19 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 19–31 | 0.98 (0.84, 1.14) | 1.04 (0.90, 1.21) | 1.05 (0.90, 1.22) | 1.02 (0.88, 1.19) | 1.05 (0.90, 1.22) | 1.06 (0.89, 1.26) |
| 32–55 | 1.05 (0.91, 1.22) | 1.25 (1.07, 1.45) | 1.21 (1.04, 1.41) | 1.16 (0.99, 1.35) | 1.16 (0.99, 1.35) | 1.20 (1.00, 1.26) |
| ≥56 | 0.95 (0.82, 1.11) | 1.21 (1.03, 1.42) | 1.15 (0.98, 1.35) | 1.06 (0.90, 1.25) | 1.06 (0.90, 1.25) | 1.02 (0.84, 1.23) |
| P valuef | 0.60 | 0.01 | 0.07 | 0.26 | 0.31 | 0.16 |
| ACR (mg/g) | ||||||
| Continuous (per doubling) | 1.14 (1.12, 1.16) | 1.13 (1.11, 1.16) | 1.12 (1.09, 1.14) | 1.11 (1.08, 1.13) | 1.09 (1.06, 1.12) | 1.09 (1.06, 1.13) |
| P value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| >30 versus <30 mg/g | 1.95 (1.73, 2.21) | 1.84 (1.62, 2.08) | 1.67 (1.47, 1.90) | 1.57 (1.38, 1.79) | 1.43 (1.25, 1.62) | 1.56 (1.34, 1.80) |
| P value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Quartiles | ||||||
| <4.6 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 4.6–8.3 | 1.06 (0.90, 1.25) | 1.07 (0.91, 1.26) | 1.06 (0.90, 1.26) | 1.08 (0.91, 1.27) | 1.04 (0.88, 1.23) | 1.02 (0.85, 1.22) |
| 8.4–20.3 | 1.26 (1.07, 1.47) | 1.26 (1.08, 1.48) | 1.23 (1.05, 1.45) | 1.24 (1.06, 1.46) | 1.15 (0.98, 1.35) | 1.11 (0.92, 1.33) |
| >20.3 | 2.06 (1.77, 2.40) | 1.95 (1.67, 2.26) | 1.71 (1.46, 2.00) | 1.63 (1.39, 1.91) | 1.46 (1.25, 1.71) | 1.52 (1.27, 1.81) |
| P valuef | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
UCr, urine creatinine.
Adjusted for age, sex, race, site, and education.
Additionally adjusted for diabetes, hypertension, SBP, smoking, prevalent heart failure, prevalent CHD, albumin, and CRP.
Risk factor-adjusted for eGFRcys and UACR at baseline.
Risk factor-adjusted for baseline UACR and eGFRcys (time-dependent).
Risk factor-adjusted for baseline UACR and rapid kidney function decline (∆eGFRcys≥3 ml/min per 1.73 m2 per year).
P value for trend.
IL-18/cr
Quartiles of IL-18/cr had a weak association with mortality (Figure 1A). In continuous models, IL-18/cr was modestly associated with mortality in demographic models, but this finding was attenuated by adjustment for kidney-related risk factors. In quartile analyses, the top two quartiles were also modestly associated with higher mortality, but these findings were attenuated and no longer significant after adjustment for risk factors eGFRcys and UACR (Table 2).
UACR
Higher quartiles of UACR had increased mortality rates compared with the lowest quartile (Figure 1A). In continuous models, higher UACR was associated with all-cause mortality in unadjusted and fully adjusted models (Table 2). In quartile analyses, the top two quartiles were independently associated with a 24% and 63% increased risk of mortality compared with the lowest quartile, respectively.
CVD and Urine Biomarkers
There were 797 CVD outcomes with median follow-up of 11.8 years. Associations of KIM-1/cr and IL-18/cr were weak for the CVD outcome (Figure 1B). In adjusted models, we found no association between either KIM-1/cr or IL-18/cr with CVD. In contrast, UACR was a risk factor for CVD in all unadjusted and adjusted models (Table 3).
Table 3.
Association of KIM-1, IL-18, and UACR with CVD
| Exposure Variable | Unadjusted | Demographic-Adjusteda | Risk Factor-Adjustedb | Adjusted for Kidney Functionc |
|---|---|---|---|---|
| KIM-1 standardized to UCr (ng/mg) | ||||
| Continuous (per doubling) | 1.05 (0.99, 1.12) | 1.09 (1.03, 1.16) | 1.03 (0.96, 1.10) | 0.96 (0.90, 1.03) |
| P value | 0.12 | 0.08 | 0.44 | 0.21 |
| Quartiles | ||||
| <497 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 497–814 | 1.20 (0.98, 1.47) | 1.28 (1.04, 1.56) | 1.20 (0.98, 1.47) | 1.19 (0.97, 1.46) |
| 815–1239 | 1.01 (0.82, 1.25) | 1.14 (0.92, 1.42) | 1.02 (0.82, 1.27) | 0.99 (0.79, 1.23) |
| ≥1240 | 1.21 (0.99, 1.49) | 1.38 (1.11, 1.72) | 1.10 (0.88, 1.37) | 0.97 (0.77, 1.22) |
| P valued | 0.11 | 0.02 | 0.29 | 0.17 |
| IL-18 standardized to UCr (pg/mg) | ||||
| Continuous (per doubling) | 0.98 (0.93, 1.04) | 1.02 (0.97, 1.08) | 1.00 (0.95, 1.06) | 0.97 (0.92, 1.02) |
| P value | 0.50 | 0.46 | 0.91 | 0.25 |
| Quartiles | ||||
| <19 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 19–31 | 1.13 (0.92, 1.38) | 1.17 (0.96, 1.44) | 1.18 (0.96, 1.44) | 1.15 (0.94, 1.41) |
| 32–55 | 1.17 (0.95, 1.43) | 1.30 (1.06, 1.60) | 1.25 (1.01, 1.53) | 1.20 (0.98, 1.48) |
| ≥56 | 0.94 (0.76, 1.16) | 1.11 (0.89, 1.39) | 1.05 (0.84, 1.31) | 0.98 (0.78, 1.22) |
| P valued | 0.14 | 0.09 | 0.13 | 0.12 |
| ACR (mg/g) | ||||
| Continuous (per doubling) | 1.13 (1.10, 1.16) | 1.12 (1.09, 1.16) | 1.09 (1.06, 1.13) | 1.08 (1.05, 1.12) |
| P value | <0.001 | <0.001 | <0.001 | <0.001 |
| >30 versus <30 mg/g | 1.80 (1.52, 2.13) | 1.73 (1.46, 2.05) | 1.48 (1.24, 1.76) | 1.39 (1.16, 1.66) |
| P value | <0.001 | <0.001 | <0.001 | <0.001 |
| Quartiles | ||||
| <4.6 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 4.6–8.3 | 1.19 (0.96, 1.48) | 1.20 (0.96, 1.49) | 1.18 (0.95, 1.47) | 1.19 (0.95, 1.48) |
| 8.5–20.3 | 1.40 (1.13, 1.74) | 1.41 (1.14, 1.75) | 1.34 (1.08, 1.66) | 1.35 (1.08, 1.67) |
| >20.3 | 2.01 (1.63, 2.47) | 1.93 (1.57, 2.38) | 1.61 (1.30, 2.00) | 1.54 (1.24, 1.91) |
| P valued | <0.001 | <0.001 | <0.001 | 0.001 |
Adjusted for age, sex, race, site, and education.
Additionally adjusted for diabetes, hypertension, SBP, smoking, prevalent heart failure, prevalent CHD, albumin, and CRP.
Additionally adjusted for eGFRcys and UACR.
P value for trend.
Interactions
We found no interaction of KIM-1/cr or IL-18/cr with eGFR, UACR, sex, or race for the outcomes of either mortality or CVD (P>0.05 for all).
Sensitivity Analyses
KIM-1/cr on a linear scale (but not in quartiles) showed a relatively small but significant association with progression of kidney disease in fully adjusted analyses using linear mixed models (Supplemental Table 3). There was no association, however, of KIM-1/cr with rapid progression (Supplemental Table 4). IL-18/cr was not associated with progression using either outcome, whereas ACR was associated with progression of CKD using either method (Supplemental Tables 3 and 4).
We noted some degree of attenuation in the relationship of KIM-1 with mortality when we adjusted for either baseline kidney function (ACR and eGFRcys) or time-dependent changes in eGFRcys (Table 2). In additional analyses that adjusted for serum bicarbonate, parathyroid hormone, phosphorus, and hemoglobin, there was no significant difference in the results (Supplemental Table 5). Similarly, in analyses that adjusted for hemoglobin A1C and time-dependent changes in systolic BP (SBP), there was no significant difference in the results (Supplemental Table 6).
Discussion
To our knowledge, this study is one of the largest studies to date and one of the first in the general population that has evaluated the relationship of tubular injury markers with risk for mortality and CVD. Our results showed that urinary KIM-1/cr was moderately associated with mortality in demographic- and risk factor-adjusted models; however, there was attenuation after adjustment for eGFR and ACR, resulting in a modest independent association of urinary KIM-1/cr with mortality. We noted no independent association between urinary KIM-1/cr and CVD or between urinary IL-18/cr and either all-cause mortality or CVD. In contrast to the tubular injury markers, we noted a significant and consistent relationship of UACR with both mortality and CVD.
Few studies have evaluated the association between urinary markers of tubular injury and longitudinal outcomes in stable ambulatory patients. In patients with CKD, urinary neutrophil gelatinase-associated lipocalin (NGAL) has been associated with more rapid progression of kidney disease in an observational study of 96 individuals.22 Similarly, NGAL was associated with development of incident CKD in the Atherosclerosis Risk in Communities study.23 In MESA, both urinary IL-18 and KIM-1 were associated with development of incident CKD and/or rapid progression of kidney disease using a case control design,16 whereas among HIV-infected women in WIHS, elevated levels of urinary IL-18 and KIM-1 were each associated with kidney function decline.18 In one of the only outpatient studies to evaluate mortality, urinary IL-18 and KIM-1 were also associated with mortality in WIHS.17
We hypothesized that urinary markers of tubular injury might be associated with mortality for the following reasons. First, CKD is a well accepted risk factor for mortality as well as CVD,24 and therefore, subclinical kidney injury may also be a risk factor for these outcomes. Second, prior literature has suggested that markers of tubular injury reflect pathologic damage,12,13,15 which may result in tubular dysfunction. In turn, tubular dysfunction may be associated with adverse outcomes through pathways, including mineral metabolism, erythropoeisis, acid–base regulation, and urinary concentration ability. Thus, the specific tubular pathway may lead to mortality risk. Third, the markers of tubular injury may reflect the overall severity of kidney disease or the global burden of CKD risk factors, such as hypertension or diabetes, and thus, they may be a risk factor for all-cause mortality or CVD. Fourth, these biomarkers may reflect global deterioration or injury processes throughout the body. Increased kidney injury or fibrosis may parallel similar processes in lungs, heart, and other organs.
Our results show that urinary KIM-1 is independently related to mortality, suggesting that tubular injury biomarkers may associate with clinically important long-term outcomes in ambulatory persons. We noted some attenuation in the relationship after adjustment for baseline ACR and eGFRcys as well as time-dependent eGFRcys. This attenuation suggests that a component, but not all, of the relationship between KIM-1 and mortality may be through its association with baseline kidney disease and progression of kidney disease. We noted no attenuation in the relationship of KIM-1/cr with mortality after adjusting for several markers of tubulointerstitial function and/or severity of kidney disease risk factors. We acknowledge, however, the possibility of residual confounding, particularly given that tubulointerstitial function and severity or risk factors are difficult to ascertain. The association of KIM-1 with mortality was, however, modest. The lack of a stronger independent association between urinary KIM-1 and mortality and the absence of significant associations between urinary KIM-1 and incident CVD and between urinary IL-18 with either mortality or incident CVD may be because of one of several reasons. First, urinary IL-18 and KIM-1 concentrations may not be accurate proxies for either severity of kidney disease or tubular dysfunction, partly because they are inducible biomarkers released in response to stress. Indeed, it is possible that other urine injury markers, such as NGAL,25 may be better proxies for tubulointerstitial disease. NGAL has not, however, been assayed in the Health ABC study. Second, these markers may also not be stable enough or have adequate precision in ambulatory elders to capture a process that accelerates mortality risk. Third, the general population may not be the ideal population to evaluate these relationships, because most individuals do not have substantial kidney damage. However, we noted no difference in the associations by level of eGFR or UACR.
In contrast to our results with the tubular injury markers, we noted a strong relationship between higher UACR and both mortality and CVD. These findings are consistent with prior studies,26 including those studies in the elderly,27 which have suggested that ACR is a risk factor for these outcomes. Although the exact mechanism relating albuminuria to these outcomes is unknown, it has been hypothesized that albuminuria may be a manifestation of generalized endothelial dysfunction, the severity of CVD risk factors, such as hypertension or diabetes, or the severity of underlying kidney disease.24 In addition, albuminuria is a well recognized risk factor for progression of kidney disease through several mechanisms, including inhibition of regeneration of podocytes in the glomerular tuft.28 Progressive kidney disease, in turn, may be associated with CVD and mortality. Albuminuria is also associated with progressive proximal tubular dysfunction and injury,29–31 and, therefore, it may be a better marker of total kidney damage compared with markers that only incorporate tubular injury.
Our study has several strengths and limitations. The strengths include a large study in an older population with detailed ascertainment of risk factors, covariates, and outcomes and the ability to adjust for well accepted measures of kidney function, including eGFR and ACR. Our measurements of urinary tubular injury have external validity, because they were associated with expected participant characteristics, such as measures of kidney function (UACR and eGFR) and presence of diabetes. We used a laboratory that has extensive experience with these measures. The limitations include the observational nature of the study, which results in our inability to ascribe cause–effect relationships and the possibility of residual confounding. In addition, we do not have detailed measures of either tubular function or kidney pathology. Finally, our urine biomarkers may have degraded during long-term storage, which could lead to measurement error that would bias to weaker results.
In conclusion, urinary KIM-1 had a modest independent association with all-cause mortality, whereas urinary IL-18 was not associated with all-cause mortality or CVD. In contrast, albuminuria was strongly and independently associated with all-cause mortality and CVD. Future studies should evaluate reasons for differences in the prognostic importance of tubular versus glomerular injury markers, whether tubular injury markers are associated with mortality or CVD in the setting of more advanced CKD, and whether tubular injury markers are associated with progression of CKD.
Concise Methods
Design and Participants
The Health ABC cohort comes from a National Institute on Aging-sponsored study that enrolled 3075 well functioning men and women ages 70–79 years from two clinical sites in Memphis, Tennessee and Pittsburgh, Pennsylvania. Participant eligibility required self-reported ability to walk 0.25 miles, climb 10 steps, and perform basic activities of daily living without difficulty, the absence of life-threatening illness, and plans to remain in the geographic area for at least 3 years. Participants underwent a half-day evaluation that included medical history, physical activity assessment, physical examination, and radiographic tests. The study was approved by the institutional review boards at the University of Tennessee Health Science Center and the University of Pittsburgh. In addition, the present study was approved by University of California at San Francisco, San Francisco Veterans Affairs Medical Center, and Tufts Medical Center committees on human research.
Exposure Variables
The primary exposure variables were urinary KIM-1, IL-18, and albumin, which were measured concurrently from previously frozen baseline urine samples stored at −80°C. The kidney injury biomarkers were measured at the Cincinnati Children’s Hospital Medical Center Biomarker Laboratory. All urine specimens were in continuous storage without prior freeze–thaw. Laboratory personnel were blinded to clinical information about the participants. The urine KIM-1 ELISA was constructed using commercially available reagents (Duoset DY1750; R&D Systems, Inc., Minneapolis, MN).32 Urine IL-18 was measured using a commercially available ELISA kit (Medical & Biologic Laboratories Co., Nagoya, Japan).33 The microalbumin assay is based on a particle-enhanced turbidimetric inhibition immunoassay adapted to a clinical chemistry system, which allows direct quantitation of albumin in urine samples (Siemens, Newark, DE). Urine creatinine was measured by a modified Jaffe method on a clinical chemistry analyzer (Siemens).34 To account for urine volume, the biomarker levels were standardized to urine creatinine.1 Intra- and interassay coefficients of variation for the urine measures were IL-18, 7.2%/7.5%; KIM-1, 5.6%/4.9%; albumin, 2.3%/6%; and creatinine, 0.6%/1.1%. Minimum detectable values for each biomarker were KIM-1, 59 ng/ml; IL-18, 12.5 pg/ml; albumin, 1.3 mg/dl; and urine creatinine, 0.05 mg/dl. Values that were below detectable were set to the lowest detectable value.
Outcome Variables
Mortality
Follow-up for this analysis was until 2011 and occurred every 6 months by either telephone or annual visits to clinical centers. Deaths were ascertained by review of local obituaries, reports to the clinical centers by family members, or means of the semiannual contacts. Immediate and underlying causes of death were determined by a central adjudication committee on the basis of review of the death certificate, all recent hospital records, and interview with the next of kin. Date of death was taken from the death certificate.35
CVD
CVD was defined by either first CHD event and/or stroke after enrollment. Follow-up was until 2011. CHD was defined by coronary death or any overnight hospitalization in an acute care hospital for acute myocardial infarction. Incident stroke was defined as fatal and nonfatal stroke events. Participants were questioned about any hospitalizations for CHD or stroke every 6 months. When an event was reported, hospital records were collected and verified by a Health ABC Disease Adjudicator at each site.
Covariates
Covariates included sociodemographic factors (age, sex, race, clinical site, and education level); lifestyle factors (current smoking [defined by current versus former or never]); comorbid conditions (diabetes [defined by the use of hypoglycemic agents (self-report), fasting plasma glucose>126 mg/dl, or a 2-hour oral glucose tolerance test>200 mg/dl]); hypertension (defined by either self-report plus use of antihypertensive medications or measured SBP>140 mmHg or diastolic BP>90 mmHg); heart failure and CHD (defined as myocardial infarction, angina, or coronary artery bypass); physical exam findings (including SBP); and blood tests (including serum albumin and CRP). Cystatin C was measured by a particle-enhanced immunonephelometric assay (N Latex Cystatin C) using a BNII Nephelometer (Dade Behring, Inc., Deerfield, IL). GFR was estimated using the 2012 Chronic Kidney Disease Epidemiology equation for cystatin C, which includes age and sex.36
Statistical Analyses
Participants were divided into quartiles of KIM-1/cr (quartiles of IL-18 and UACR are provided in Supplemental Tables 1 and 2), and the distributions of covariates across groups were evaluated. Baseline characteristics of participants were compared using t or chi-squared tests as appropriate. Spearman correlations between KIM-1/cr, IL-18/cr, eGFRcys, and UACR were calculated. We evaluated the association of KIM-1/cr, IL-18/cr, and UACR with the presence of CKD defined by eGFRcys<60 ml/min per 1.73 m2 using multivariable logistic regression analyses adjusted for demographics and CVD risk factors. CVD event and death rates were calculated per 100 person-years and reported as percent per person-year. Cox proportional hazards models were used to investigate the associations of KIM-1/cr, IL-18/cr, and UACR with both all-cause mortality and CVD events, while censoring for non-CVD mortality. Models were nested and adjusted in stages for (1) age, sex, race, site, and education status; (2) traditional CVD risk factors (diabetes, hypertension, SBP, smoking, prevalent CHD, prevalent heart failure, serum albumin, and CRP); and (3) eGFRcys and UACR in the tubular injury models and eGFRcys in albuminuria models. Given the skewed distribution of the predictors, models were initially evaluated with the exposure variable transformed to the log base 2, presented per doubling, and subsequently, presented in quartiles. UACR was also evaluated using the clinical cutpoint of 30 mg/g. The proportional hazards assumption was satisfied for all models, with P values for Schoenfeld residuals>0.20. We evaluated interactions of the tubular injury markers with eGFR> or ≤60 ml/min per 1.73 m2, ACR>30 mg/g or ≤30 mg/g, sex, and race for the outcomes of mortality and CVD. We conducted analyses using S-Plus (version 8.0; Tibco, Seattle, WA) and SPSS statistical software (version 16.0.2; SPSS, Inc., Chicago, IL).
Sensitivity Analyses
We performed several sensitivity analyses to explore potential mechanisms relating kidney injury markers to outcomes. First, given that the association of urine injury markers with outcomes may be through their effects on progression of kidney disease, we evaluated the association of the injury markers with progression of kidney disease using logistic regression and linear mixed models and whether the relationships were attenuated after adjustment for time-dependent changes in eGFRcys. Cystatin C was measured at baseline, year 3, and year 10 in the Health ABC study, and the cystatin C values were calibrated to each other. Change in kidney function was defined as eGFRcys change in milliliters per minute per 1.73 m2 per year, and rapid kidney function decline was defined as eGFRcys loss of >3 ml/min per 1.73 m2 per year; prior studies have shown that this rate is associated with increased CVD morbidity and mortality, independent of baseline eGFR.37,38 Second, given that urinary injury markers may reflect tubulointerstitial dysfunction, which in turn, is associated with CVD or mortality, we evaluated whether adjustment for baseline serum bicarbonate, parathyroid hormone, serum phosphorus, and serum hemoglobin attenuated the results. Third, because urine injury markers may reflect the severity of diabetes or hypertension, we evaluated whether adjustment for baseline hemoglobin A1C or time-dependent SBP, respectively, attenuated the results.
Disclosures
C.R.P. is a coinventor on a patent for the use of IL-18 as a marker of AKI (no financial value).
Supplementary Material
Acknowledgments
This research was supported by National Institute on Aging Grants R01-AG027002 and R01-AG028050; National Institute on Aging Contracts N01-AG-6-2101, N01-AG-6-2103, and N01-AG-6-2106; and National Institute of Nursing Research Grant R01-NR012459. This study was also supported, in part, by the Intramural Research Program of the National Institutes of Health, National Institute on Aging.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013070713/-/DCSupplemental.
References
- 1.McMahon GM, Waikar SS: Biomarkers in nephrology: Core Curriculum 2013. Am J Kidney Dis 62: 165–178, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Melnikov VY, Ecder T, Fantuzzi G, Siegmund B, Lucia MS, Dinarello CA, Schrier RW, Edelstein CL: Impaired IL-18 processing protects caspase-1-deficient mice from ischemic acute renal failure. J Clin Invest 107: 1145–1152, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Melnikov VY, Faubel S, Siegmund B, Lucia MS, Ljubanovic D, Edelstein CL: Neutrophil-independent mechanisms of caspase-1-and IL-18-mediated ischemic acute tubular necrosis in mice. J Clin Invest 110: 1083–1091, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parikh CR, Jani A, Melnikov VY, Faubel S, Edelstein CL: Urinary interleukin-18 is a marker of human acute tubular necrosis. Am J Kidney Dis 43: 405–414, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Parikh CR, Jani A, Mishra J, Ma Q, Kelly C, Barasch J, Edelstein CL, Devarajan P: Urine NGAL and IL-18 are predictive biomarkers for delayed graft function following kidney transplantation. Am J Transplant 6: 1639–1645, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Parikh CR, Mishra J, Thiessen-Philbrook H, Dursun B, Ma Q, Kelly C, Dent C, Devarajan P, Edelstein CL: Urinary IL-18 is an early predictive biomarker of acute kidney injury after cardiac surgery. Kidney Int 70: 199–203, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Parikh CR, Coca SG, Thiessen-Philbrook H, Shlipak MG, Koyner JL, Wang Z, Edelstein CL, Devarajan P, Patel UD, Zappitelli M, Krawczeski CD, Passik CS, Swaminathan M, Garg AX, TRIBE-AKI Consortium : Postoperative biomarkers predict acute kidney injury and poor outcomes after adult cardiac surgery. J Am Soc Nephrol 22: 1748–1757, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV: Kidney Injury Molecule-1 (KIM-1): A novel biomarker for human renal proximal tubule injury. Kidney Int 62: 237–244, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Han WK, Waikar SS, Johnson A, Betensky RA, Dent CL, Devarajan P, Bonventre JV: Urinary biomarkers in the early diagnosis of acute kidney injury. Kidney Int 73: 863–869, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J, Mori K, Barasch J, Devarajan P: Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 365: 1231–1238, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Nickolas TL, O’Rourke MJ, Yang J, Sise ME, Canetta PA, Barasch N, Buchen C, Khan F, Mori K, Giglio J, Devarajan P, Barasch J: Sensitivity and specificity of a single emergency department measurement of urinary neutrophil gelatinase-associated lipocalin for diagnosing acute kidney injury. Ann Intern Med 148: 810–819, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nepal M, Bock GH, Sehic AM, Schultz MF, Zhang PL: Kidney injury molecule-1 expression identifies proximal tubular injury in urate nephropathy. Ann Clin Lab Sci 38: 210–214, 2008 [PubMed] [Google Scholar]
- 13.van Timmeren MM, van den Heuvel MC, Bailly V, Bakker SJ, van Goor H, Stegeman CA: Tubular kidney injury molecule-1 (KIM-1) in human renal disease. J Pathol 212: 209–217, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Kiliś-Pstrusińska K, Medyńska A, Zwolińska D, Wawro A: Interleukin-18 in urine and serum of children with idiopathic nephrotic syndrome. Kidney Blood Press Res 31: 122–126, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Matsumoto K, Kanmatsuse K: Elevated interleukin-18 levels in the urine of nephrotic patients. Nephron 88: 334–339, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Peralta CA, Katz R, Bonventre JV, Sabbisetti V, Siscovick D, Sarnak M, Shlipak MG: Associations of urinary levels of kidney injury molecule 1 (KIM-1) and neutrophil gelatinase-associated lipocalin (NGAL) with kidney function decline in the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Kidney Dis 60: 904–911, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peralta CA, Scherzer R, Grunfeld C, Abraham A, Tien PC, Devarajan P, Bennett M, Butch AW, Anastos K, Cohen MH, Nowicki M, Sharma A, Young MA, Sarnak M, Parikh CR, Shlipak MG: Urinary biomarkers of kidney injury are associated with all-cause mortality in the Women's Interagency HIV Study [published online ahead of print December 3, 2013]. HIV Med 10.1111/hiv.12113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shlipak MG, Scherzer R, Abraham A, Tien PC, Grunfeld C, Peralta CA, Devarajan P, Bennett M, Butch AW, Anastos K, Cohen MH, Nowicki M, Sharma A, Young MA, Sarnak MJ, Parikh CR: Urinary markers of kidney injury and kidney function decline in HIV-infected women. J Acquir Immune Defic Syndr 61: 565–573, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Howie AJ, Ferreira MA, Adu D: Prognostic value of simple measurement of chronic damage in renal biopsy specimens. Nephrol Dial Transplant 16: 1163–1169, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Nath KA: Tubulointerstitial changes as a major determinant in the progression of renal damage. Am J Kidney Dis 20: 1–17, 1992 [DOI] [PubMed] [Google Scholar]
- 21.Takebayashi S, Kiyoshi Y, Hisano S, Uesugi N, Sasatomi Y, Meng J, Sakata N: Benign nephrosclerosis: Incidence, morphology and prognosis. Clin Nephrol 55: 349–356, 2001 [PubMed] [Google Scholar]
- 22.Bolignano D, Lacquaniti A, Coppolino G, Donato V, Campo S, Fazio MR, Nicocia G, Buemi M: Neutrophil gelatinase-associated lipocalin (NGAL) and progression of chronic kidney disease. Clin J Am Soc Nephrol 4: 337–344, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhavsar NA, Köttgen A, Coresh J, Astor BC: Neutrophil gelatinase-associated lipocalin (NGAL) and kidney injury molecule 1 (KIM-1) as predictors of incident CKD stage 3: The Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis 60: 233–240, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW, American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention : Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 108: 2154–2169, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Liu KD, Yang W, Anderson AH, Feldman HI, Demirjian S, Hamano T, He J, Lash J, Lustigova E, Rosas SE, Simonson MS, Tao K, Hsu CY, Chronic Renal Insufficiency Cohort (CRIC) study investigators : Urine neutrophil gelatinase-associated lipocalin levels do not improve risk prediction of progressive chronic kidney disease. Kidney Int 83: 909–914, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT, Chronic Kidney Disease Prognosis Consortium : Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet 375: 2073–2081, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rifkin DE, Katz R, Chonchol M, Fried LF, Cao J, de Boer IH, Siscovick DS, Shlipak MG, Sarnak MJ: Albuminuria, impaired kidney function and cardiovascular outcomes or mortality in the elderly. Nephrol Dial Transplant 25: 1560–1567, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peired A, Angelotti ML, Ronconi E, la Marca G, Mazzinghi B, Sisti A, Lombardi D, Giocaliere E, Della Bona M, Villanelli F, Parente E, Ballerini L, Sagrinati C, Wanner N, Huber TB, Liapis H, Lazzeri E, Lasagni L, Romagnani P: Proteinuria impairs podocyte regeneration by sequestering retinoic acid. J Am Soc Nephrol 24: 1756–1768, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Donadelli R, Abbate M, Zanchi C, Corna D, Tomasoni S, Benigni A, Remuzzi G, Zoja C: Protein traffic activates NF-kB gene signaling and promotes MCP-1-dependent interstitial inflammation. Am J Kidney Dis 36: 1226–1241, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Donadelli R, Zanchi C, Morigi M, Buelli S, Batani C, Tomasoni S, Corna D, Rottoli D, Benigni A, Abbate M, Remuzzi G, Zoja C: Protein overload induces fractalkine upregulation in proximal tubular cells through nuclear factor kappaB- and p38 mitogen-activated protein kinase-dependent pathways. J Am Soc Nephrol 14: 2436–2446, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Morigi M, Macconi D, Zoja C, Donadelli R, Buelli S, Zanchi C, Ghilardi M, Remuzzi G: Protein overload-induced NF-kappaB activation in proximal tubular cells requires H(2)O(2) through a PKC-dependent pathway. J Am Soc Nephrol 13: 1179–1189, 2002 [PubMed] [Google Scholar]
- 32.Chaturvedi S, Farmer T, Kapke GF: Assay validation for KIM-1: Human urinary renal dysfunction biomarker. Int J Biol Sci 5: 128–134, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krawczeski CD, Goldstein SL, Woo JG, Wang Y, Piyaphanee N, Ma Q, Bennett M, Devarajan P: Temporal relationship and predictive value of urinary acute kidney injury biomarkers after pediatric cardiopulmonary bypass. J Am Coll Cardiol 58: 2301–2309, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Larsen K: Creatinine assay by a reaction-kinetic principle. Clin Chim Acta 41: 209–217, 1972 [DOI] [PubMed] [Google Scholar]
- 35.Cesari M, Penninx BW, Newman AB, Kritchevsky SB, Nicklas BJ, Sutton-Tyrrell K, Rubin SM, Ding J, Simonsick EM, Harris TB, Pahor M: Inflammatory markers and onset of cardiovascular events: Results from the Health ABC study. Circulation 108: 2317–2322, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS, CKD-EPI Investigators : Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 367: 20–29, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rifkin DE, Shlipak MG, Katz R, Fried LF, Siscovick DS, Chonchol M, Newman AB, Sarnak MJ: Rapid kidney function decline and mortality risk in older adults. Arch Intern Med 168: 2212–2218, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shlipak MG, Katz R, Kestenbaum B, Siscovick D, Fried L, Newman A, Rifkin D, Sarnak MJ: Rapid decline of kidney function increases cardiovascular risk in the elderly. J Am Soc Nephrol 20: 2625–2630, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.