Abstract
Galectin-1, a β-galactoside–binding lectin, is involved in many physiologic and pathologic processes, including cell adhesion, differentiation, angiogenesis, and tumor progression. However, the role of galectin-1 in kidney cancer remains elusive. This study evaluated the role of galectin-1 in the progression and clinical prognosis of renal cell carcinoma. We found significant overexpression of galectin-1 in both kidney cancer cell lines and metastatic tissue specimens from patients with renal cell carcinoma. Knockdown of galectin-1 gene expression in renal cancer cell lines reduced cell invasion, clonogenic ability, and epithelial-mesenchymal transition in vitro; reduced tumor outgrowth in vivo; and inhibited the angiogenesis-inducing activity of these cells in vitro and in vivo. Galectin-1 knockdown decreased CXCR4 expression levels in kidney cancer cells, and restoration of CXCR4 expression in galectin-1–silenced cells rescued cell motility and clonogenic ability. Additional studies suggested that galectin-1 induced CXCR4 expression through activation of nuclear factor-κB (NF-κB). Analysis of patient specimens confirmed the clinical significance and positive correlation between galectin-1 and CXCR4 expression levels and revealed concomitant overexpression of galectin-1 and CXCR4 associated adversely with overall and disease-free survival. Our findings suggest that galectin-1 promotes tumor progression through upregulation of CXCR4 via NF-κB. The coordinated upregulation of galectin-1 and CXCR4 may be a novel prognostic factor for survival in patients with renal cell carcinoma and the galectin-1-CXCR4 axis may serve as a therapeutic target in this disease.
Keywords: renal carcinoma, survival, chemokine receptor
Renal cell carcinoma (RCC) accounts for approximately 4% of all adult malignancies.1 More than one third of patients will present with locally advanced or metastatic disease at the time of diagnosis.2 The 5-year survival rate of metastatic RCC is only 10% because of resistance to chemotherapy and radiation therapy.3 Although cytokine therapy with IFN-α and IL-2 is the gold standard treatment, its overall efficacy rate is limited by its significant toxicity.4 Recently, several molecular targeting drugs, including sunitinib and temsirolimus, have been approved for advanced RCC.4 However, treatment response is not long-standing and overall survival remains poor. Thus, the identification of novel molecular targets in RCC is urgently needed for the development of effective therapies.
Galectins are animal lectins defined by an affinity for β-galactosides and a highly conserved carbohydrate recognition domain of about 130 amino acids.5 Currently, 15 mammalian galectins have been classified into prototype, chimera-type, and tandem-repeat–type on the basis of their distinct biochemical structures.5 Galectin-1 (Gal-1), a homodimeric prototype of galectins, is implicated in many biologic processes, including cell differentiation, tissue development, pre-mRNA splicing, immunoregulation, and tumor progression.6 It acts through sugar-dependent interactions with β-galactoside–containing glycoconjugates extracellularly and via sugar-independent interactions with other proteins intracellularly. Increasing evidence reveals that Gal-1 is highly expressed in different tumor types, such as lung, ovarian, and prostate cancers,7–9 and is associated with poor prognosis and the metastatic phenotype. The upregulation of Gal-1 favors tumor growth and promotes tumor progression by modulating cell motility,10 inducing apoptosis of activated T cells,11 mediating cell adhesion,12 and participating in tumor angiogenesis.13 Furthermore, intracellular Gal-1 binds oncogenic H-Ras to enhance its anchorage to plasma membrane and activate the extracellular signal-regulated kinase (ERK) signaling pathway for neoplastic transformation.14 Recent studies report that Gal-1 knockdown can sensitize melanoma and lung cancer cells to chemotherapy.15,16
Previous studies have suggested that Gal-1 may serve as a potential marker for RCC.17,18 However, the detailed molecular mechanisms connecting Gal-1 to RCC remain largely unknown. The present study examined whether Gal-1 contributed to functional implications and clinical relevance in kidney cancer pathogenesis. Gal-1 was markedly upregulated in kidney cancer cell lines and tissue specimens. At the functional level, knockdown of Gal-1 significantly reduced invasiveness, clonogenicity, and epithelial-mesenchymal transition (EMT) of kidney cancer cells, as well as in tumor xenografts. Gal-1 silencing repressed angiogenesis-inducing activities of RCC cells both in vitro and in vivo. The results also revealed that Gal-1 might positively regulate CXCR4 expression via ERK/NF-κB activation to promote kidney cancer progression. In addition, concomitant overexpression of Gal-1 and CXCR4 could predict poorer survival rate in patients with RCC. We believe this is the first study to show that Gal-1 plays a critical role in renal oncogenesis via CXCR4 and that the Gal-1-CXCR4 axis highlights a novel mechanism that leads to an unfavorable clinical outcome in RCC.
Results
Gal-1 Was Highly Expressed in Human RCC Cell Lines and Tissues
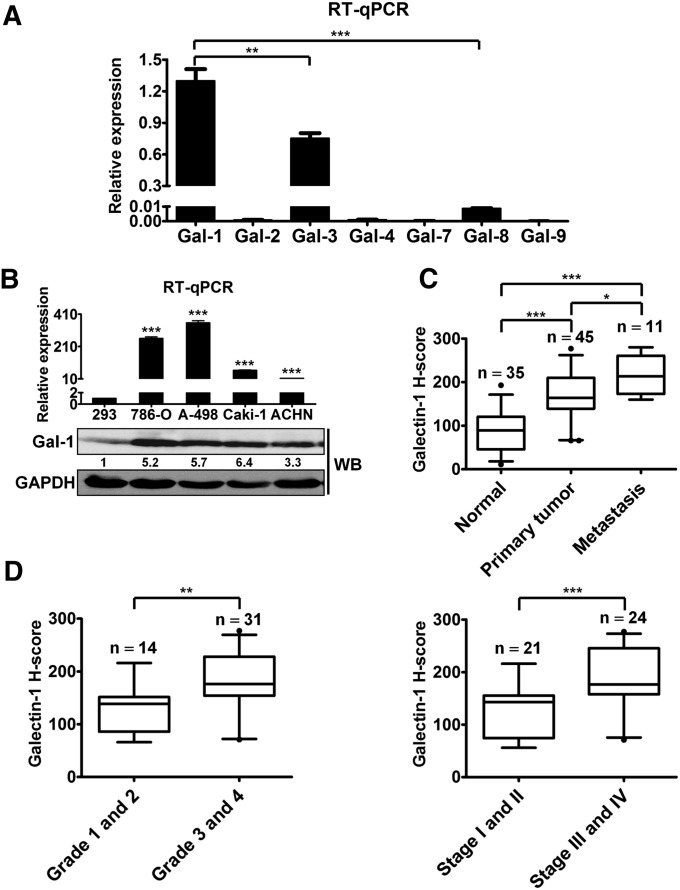
The RCC cell lines, 786-O and A-498, were used to examine the expression of the galectin family. As shown in Figure 1A, the highest expression of Gal-1 was found in A-498 and 786-O cells (data not shown). Moreover, quantitative RT-PCR (RT-qPCR) and Western blotting analysis indicated higher expressions of Gal-1 at both mRNA and protein levels in four RCC cell lines compared with levels in human embryonic kidney 293 cells (HEK293) (Figure 1B). To determine Gal-1 expression in human RCC tissues, IHC was conducted on a tissue microarray containing 35 normal kidney tissues, 45 RCC tissues, and 11 metastatic RCC tissues. Gal-1 expression was significantly higher in human RCC tissues with metastasis compared than in in primary tumor and normal kidney tissues (Figure 1C, Supplemental Figure 1A). In further investigation of the potential association of Gal-1 with RCC staging and grading, higher levels of Gal-1 were present in grade 3 and 4 subgroups and in stage III and IV subgroups (Figure 1D). Clinicopathologic analysis revealed that Gal-1 expression was significantly related to advanced tumor stage and grade, but not to age and sex (Supplemental Table 1). Thus, we suggest that Gal-1 was strongly associated with kidney cancer progression.
Figure 1.
Gal-1 was highly expressed in RCC cell lines and tissue specimens. (A) The mRNA levels of galectin members in A-498 cells were detected by RT-qPCR. The delta Ct method (2−ΔCt; ΔCt=galectins−GAPDH) was used to calculate the relative expressions of galectin members. Data are mean±SD. (B) Analysis of Gal-1 mRNA and protein levels in HEK293 cells and cultured human RCC cell lines was performed by RT-qPCR (upper panel) and Western blotting (WB) (lower panel). Data are mean±SD. (C) Immunohistochemical staining showed distinct expressions of Gal-1 in RCC tissues with metastasis (n=11) compared with normal kidney tissues (n=35) and primary tumor tissues (n=45). (D) Gal-1 expression in RCC significantly correlated with advanced tumor grade (left panel) and staging (right panel). The box represents median and interquartile ranges, and error bars present 1.5-fold×the interquartile range below the 25th and above the 75th percentile. Results are representative of three independent experiments. GAPDH, glyceraldehyde-3-phosphate dehydrogenase. *P<0.05; **P<0.01, ***P<0.001.
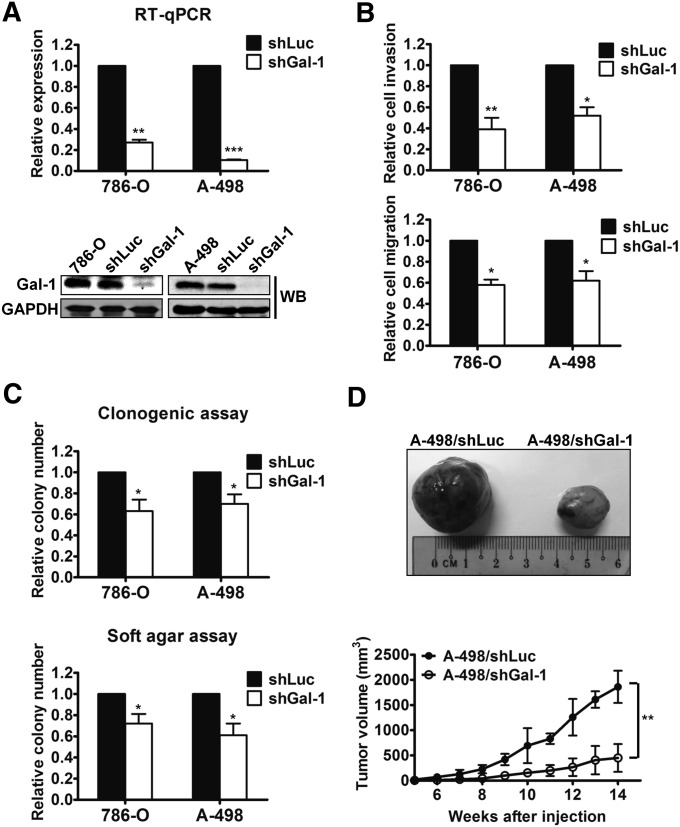
Knockdown of Gal-1 in RCC Cells Reduced Cell Invasion, Clonogenic Ability, and EMT In Vitro and Tumor Growth In Vivo
Because up to 75% of patients with sporadic clear cell RCC have aberrant von Hippel-Lindau (VHL) tumor suppressor gene,3 two VHL-mutant kidney cancer cell lines (786-O and A-498) were used in the following experiments. To investigate the role of Gal-1 in RCC progression, the vesicular stomatitis virus-G pseudotyped lentivirus–short hairpin RNA (shRNA) system was used to silence Gal-1 in both cell lines. Decreased Gal-1 expression was observed in cells infected with lentivirus carrying Gal-1 shRNA (shGal-1) compared with cells infected with the shLuc virus (Figure 2A). There was no difference in cell proliferation in vitro between the cells trandsuced by lentivirus expressing shRNA directed against luciferase (shLuc) and by shGal-1 (Supplemental Figure 1B). To determine the functional effects of Gal-1 on cell invasion and migration of kidney cancer cells, transwells with or without Matrigel coating were used. Both migratory and invasive abilities were obviously attenuated in shGal-1–infected 786-O and A-498 cells (Figure 2B). Analyzing the colony-forming ability of kidney cancer cells expressing shLuc or shGal-1 in anchorage-dependent (clonogenic assay) and anchorage-independent (soft agar assay) conditions, Gal-1 silencing significantly decreased the clonogenicity of RCC cells in both conditions (Figure 2C). Similar results were also found in another Gal-1–knockdown clone of A-498 cells (shGal-1#426; data not shown). In addition, we analyzed several EMT-associated markers, including E-cadherin and its repressors (Slug, Snail, and ZEB-1) in Gal-1–knockdown A-498 cells. Under suppression of Gal-1, expression of E-cadherin was increased, while expression of vimentin and transcriptional repressors of E-cadherin were reduced in RCC cells (Supplemental Figure 1, C and D). To examine the in vivo tumorigenicity mediated by Gal-1, shLuc- or shGal-1–transduced A-498 cells were inoculated subcutaneously into nude mice. Notably, Gal-1 knockdown in A-498 cells exhibited remarkable deceleration in tumor growth (Figure 2D). Consistent with in vitro data, reversion of EMT was also observed in the tumors of A-498/shGal-1 mice (Supplemental Figure 1, E and F). These data revealed that Gal-1 might promote RCC tumorigenicity by enhancing cell motility, clonogenicity, and EMT in vitro and tumor growth in vivo.
Figure 2.
Inhibition of Gal-1 in RCC cells decreased the migration, invasion, clonogenic abilities in vitro, and tumor growth in vivo. (A) Knockdown of Gal-1 in 786-O and A-498 cells was suppressed by lentiviral-mediated shRNA. shLuc served as a negative control. Both mRNA and protein levels of Gal-1 were detected using RT-qPCR and Western blotting (WB), respectively. (B) Invasiveness (upper panel) and migration (lower panel) of shGal-1–infected 786-O and A-498 cells were examined. Relative invasion or migration ability was normalized to that of cells transduced with shLuc. (C) Clonogenic assay (upper panel) and soft agar assay (lower panel) were used to evaluate the effect of Gal-1 knockdown on colony-forming ability. (D) shLuc- or shGal-1–infected A-498 cells (5×106 cells/100 μl) were injected subcutaneously into 6- to 8-week-old nude mice in each experiment group (n=4). Tumor volumes were presented as means±SD. Results are representative of three independent experiments. Data are mean±SD. GAPDH, glyceraldehyde-3-phosphate dehydrogenase. *P<0.05; **P<0.01; ***P<0.001.
Gal-1 Silencing Reduced Angiogenesis-Inducing Activities of RCC Cells In Vitro and In Vivo
To investigate the effects of Gal-1 silencing on angiogenesis in vitro, we collected conditioned media from A-498 cells infected with shLuc or shGal-1 and then applied for human umbilical vein endothelial cell (HUVEC) culture to analyze endothelial cell proliferation, migration, and tube formation. Proliferation rate, migrating capability, and tube formation decreased significantly when HUVECs were cultured by concentrated conditioned media from A-498/shGal-1 cells (Supplemental Figure 2, A–C). Matrigel plug assay was further performed to examine the effects of Gal-1 knockdown on angiogenesis-inducing activities of RCC cells in vivo. As shown in Supplemental Figure 2D, Matrigel plugs containing supernatants of A-498/shLuc appeared reddish in color, whereas plugs containing supernatants of A-498/shGal-1 were transparent. In addition, VEGF expression was significantly decreased in tumor mass from mice injected with shGal-1–infected A-498 cells (Supplemental Figure 1, E and F). These data suggest that Gal-1 in RCC cells may promote tumor angiogenesis both in vitro and in vivo.
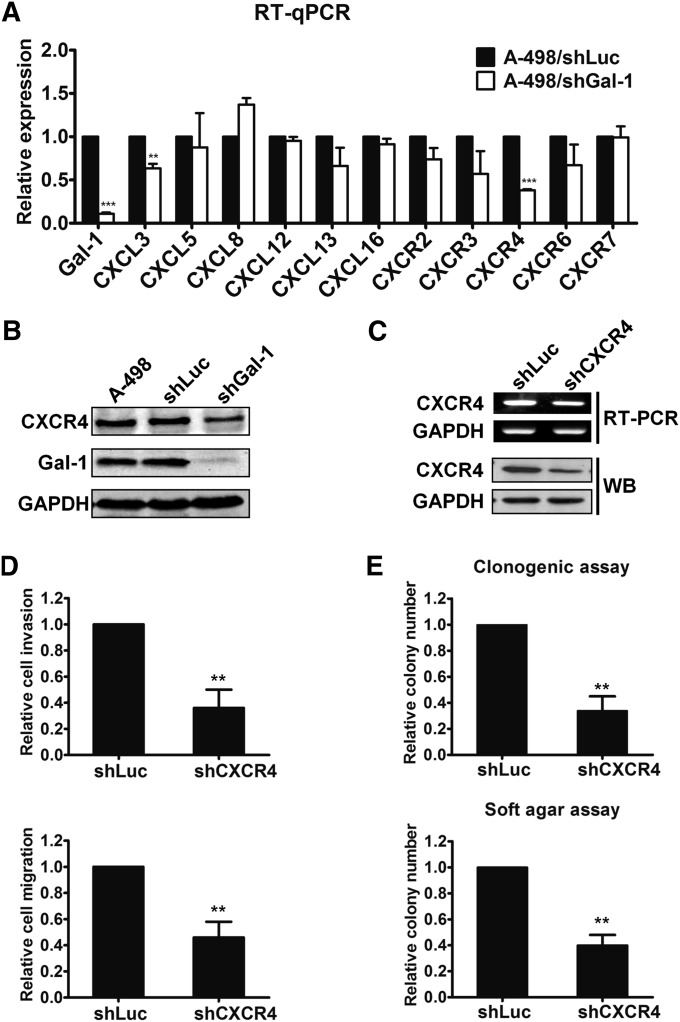
Knockdown of Gal-1 Downregulated CXCR4 Expression in A-498 Cells
To investigate the molecular mechanisms of Gal-1 on tumor progression, RT-qPCR was used to screen chemokines and chemokine receptors that were associated with tumorigenesis. Results showed that silencing of Gal-1 in A-498 cells significantly repressed CXCR4 expression at the mRNA level (Figure 3A, Supplemental Figure 3A). Western blotting was then used to confirm the reduction of CXCR4 protein caused by Gal-1 knockdown (Figure 3B). To further explore the role of CXCR4 in RCC, CXCR4 expression was repressed by lentivirus-delivered shRNA in A-498 cells. The CXCR4 mRNA and protein levels were significantly lower in shCXCR4-infected A-498 cells than in the control cells (Figure 3C). CXCR4 knockdown resulted in significant inhibition of cell invasion and migration and clonogenic ability in vitro (Figure 3, D and E). Thus, Gal-1 might positively regulate CXCR4 expression to promote tumor progression in RCC.
Figure 3.
Knockdown of Gal-1 downregulated CXCR4. (A) The effects of Gal-1 silencing on cancer-associated genes expression were detected by RT-qPCR. (B) Validation of CXCR4 protein expression after Gal-1 knockdown was demonstrated by Western blotting (WB). (C) CXCR4 was silenced by the lentiviral shCXCR4 infection in A-498 cells. Both mRNA and protein levels of CXCR4 were detected by RT-PCR and Western blotting, respectively. (D) The effects of CXCR4 knockdown on cell invasion (upper panel) and migration (lower panel) were examined in A-498 cells. Relative invasion or migration ability was normalized to that of cells transduced with shLuc. (E) The colony-forming ability of CXCR4-infected A-498 cells was evaluated by clonogenic (upper panel) and soft agar assays (lower panel). Results are representative of three independent experiments. Data are mean±SD. GAPDH, glyceraldehyde-3-phosphate dehydrogenase. **P<0.01; ***P<0.001.
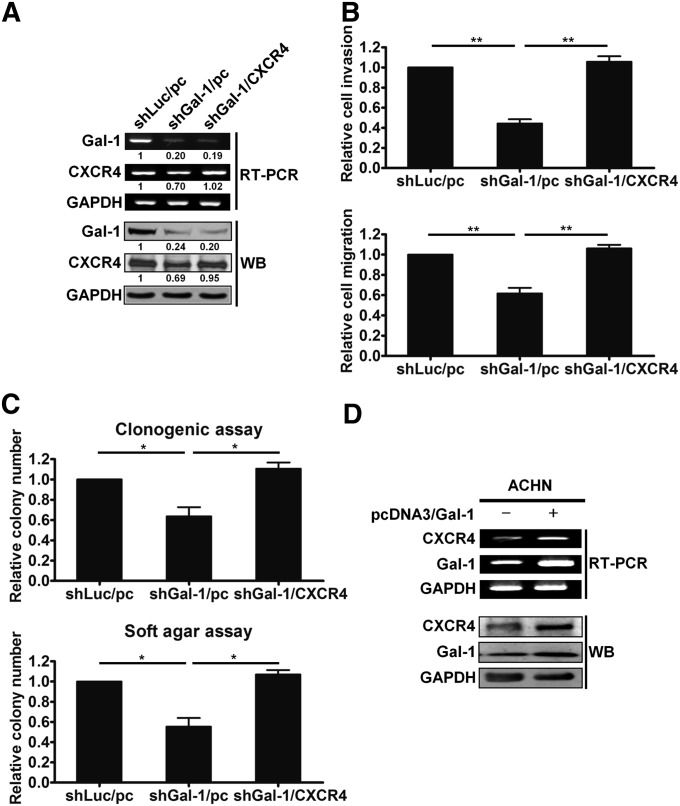
Gal-1 Promoted Cell Motility and Clonogenicity of RCC Cells by Upregulating CXCR4 Expression
To further confirm whether Gal-1 enhanced RCC aggressiveness by activating CXCR4, pcDNA3-CXCR4 or pcDNA3 empty vector was transiently transfected into shGal-1–transduced A-498 cells (shGal-1/CXCR4 or shGal-1/pc). The expression of CXCR4 was restored in shGal-1/CXCR4 cells by reexpressing CXCR4 (Figure 4A). However, both mRNA and protein levels of Gal-1 were not affected, implying that CXCR4 was downstream of Gal-1. The restoration of CXCR4 in shGal-1–infected A-498 cells also rescued the decreased cell invasion and migration caused by Gal-1 knockdown (Figure 4B). The colony-forming abilities were also recovered in shGal-1/CXCR4 cells (Figure 4C).
Figure 4.
Gal-1 facilitated cell motility and clonogenic survival via CXCR4 activation. (A) pcDNA3 empty vector or CXCR4 overexpression plasmid was transfected into A-498/shGal-1 cells. RT-PCR and Western blotting (WB) were used to detect mRNA and protein levels of transfectants, respectively. (B) A-498/shGal-1 cells ectopically expressing CXCR4 were tested for invasive (upper panel) and migratory (lower panel) abilities. (C) Clonogenic assay (upper panel) and soft agar assay (lower panel) were evaluated in A-498/shGal-1 cells reexpressing CXCR4. (D) The effects of Gal-1 overexpression on CXCR4 expression in ACHN cells were examined by RT-PCR and Western blotting. Results are representative of three independent experiments. Data are mean±SD. GAPDH, glyceraldehyde-3-phosphate dehydrogenase. *P<0.05; **P<0.01.
In addition, transfection of Gal-1 cDNA plasmid into low-expression Gal-1 cells, ACHN and HEK293, led to increased expression of both Gal-1 and CXCR4 at mRNA and protein levels (Figure 4D, Supplemental Figure 3B). Gal-1–overexpressing ACHN cells exhibited increased cell invasion, migration, and clonogenic cell survival (Supplemental Figure 3, C and D). Further, ACHN/pc or ACHN/Gal-1 cells were intravenously injected into the tail vein of athymic nude mice to examine the effects of Gal-1 on the metastatic activity in vivo. As shown in Supplemental Figure 3E, Gal-1 overexpression increased the number of pulmonary metastatic foci. These results suggest that Gal-1–enhanced tumorigenicity might be mediated by CXCR4 activation.
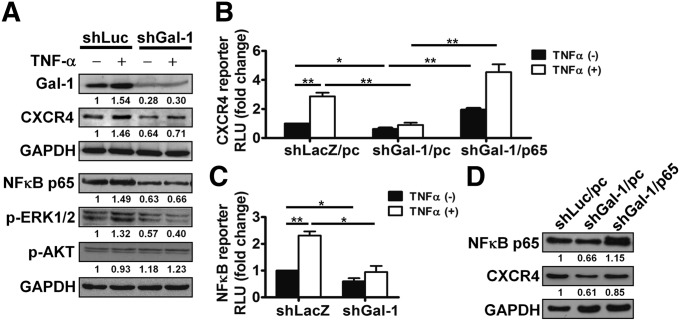
Induction of CXCR4 by TNF-α Was Modulated by Gal-1 and ERK/NF-κB Activation
Several cytokines and growth factors, such as VEGF,19 TGF-β1,20 and TNF-α,21 could increase CXCR4 expression. In patients with RCC, plasma TNF-α is significantly higher and associated with tumor size, grade, and stage.22 Therefore, we sought to determine whether Gal-1 was involved in the transcriptional upregulation of CXCR4 induced by TNF-α. As shown in Figure 5, A and B, TNF-α increased protein levels of Gal-1 and CXCR4 and induced CXCR4 promoter activity in shLuc- or shLacZ-infected A-498 control cells. In addition, Gal-1 silencing significantly blocked the induction of Gal-1 and CXCR4 at protein levels (Figure 5A) and CXCR4 promoter activity (Figure 5B) by TNF-α treatment.
Figure 5.
TNF-α–induced CXCR4 expression was mediated by Gal-1 and ERK/NF-κB activation. (A) A-498 cells transduced with shLuc or shGal-1 virus were treated with or without TNF-α (50 ng/ml) for 30 minutes and then analyzed by Western blotting. (B) A-498 cells expressing shLacZ or shGal-1 (1×105/ml) were cotransfected with pcDNA3 empty vector (pc) or pFLAG-p65 (p65), pGL2-CXCR4-luc, and pRL-SV40 Renilla plasmid. After 24 hours of transfection, the cells were treated with or without TNF-α (25 ng/ml) for 2 days and then analyzed by luciferase assay to evaluate CXCR4 promoter activity. (C) shLacZ- or shGal-1–infected A-498 cells (1×105/ml) were transfected with pGL3-NF-κB-luc and pRL-SV40 Renilla plasmid. After 24 hours of transfection, the cells were treated with or without TNF-α (25 ng/ml) for 2 days. Quantification of firefly and Renilla activities was measured. (D) A-498 cells expressing shLuc or shGal-1 were transfected with pcDNA3 empty vector (pc) or pFLAG-p65 (p65). NF-κB p65 and CXCR4 protein levels were determined by Western blotting. RLU, relative luciferase unit. Results are representative of three independent experiments. Data are mean±SD. GAPDH, glyceraldehyde-3-phosphate dehydrogenase. *P<0.05; **P<0.01.
To further clarify the molecular mechanisms of Gal-1–mediated CXCR4 expression, shLuc- and shGal-1–infected A-498 cells treated with or without recombinant human TNF-α for 30 minutes were analyzed by Western blotting. TNF-α significantly enhanced both NF-κB p65 and phospho-ERK1/2 signaling in shLuc-infected A-498 cells. However, the increased levels from TNF-α treatment were much lower in Gal-1–knockdown cells (Figure 5A). The phosphorylation of AKT was not activated by TNF-α (Figure 5A).
A previous study indicated that NF-κB promotes breast cancer cell motility by upregulating CXCR4 expression.23 Thus, luciferase reporter assay was used to determine the involvement of NF-κB in Gal-1–induced CXCR4 expression. NF-κB luciferase activity was slightly reduced in shGal-1–transduced cells without TNF-α treatment (Figure 5C). Upon stimulation of TNF-α, repression of Gal-1 significantly reduced TNF-α–induced NF-κB luciferase activity. Moreover, decreased CXCR4 promoter activity and protein expression in Gal-1–knockdown cells were reversed by transiently transfecting with pFLAG-p65 overexpression plasmid (Figure 5, B and D). These data revealed that Gal-1 might modulate CXCR4 expression via ERK signaling and NF-κB activation.
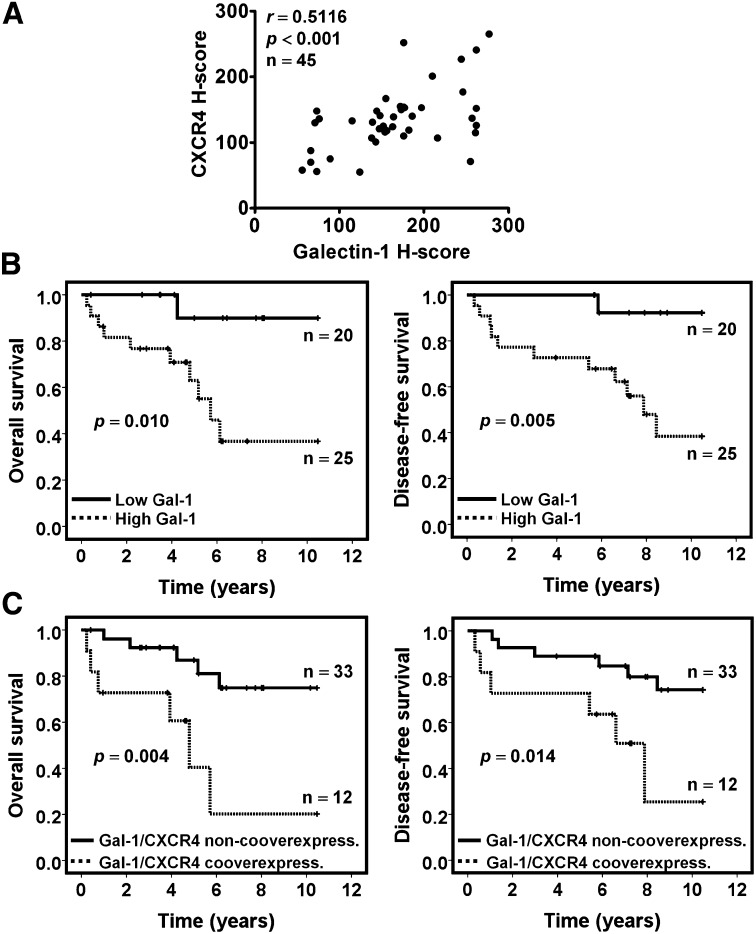
Clinical Correlation of Gal-1 with CXCR4 and Patient Outcome
Given that Gal-1 might regulate CXCR4 expression in RCC, immunohistochemical analysis was done to evaluate the relationship between Gal-1 expression and CXCR4 expression in kidney cancer tissues (n=45). Gal-1 expression was significantly consistent with CXCR4 expression in kidney cancer tissues (Figure 6A). There were also higher levels of CXCR4 expression in RCC tissues that correlated with advanced tumor stage and grade (Supplemental Figure 4A). Kaplan–Meier analysis and log-rank test were conducted to determine overall survival (OS) and disease-free survival (DFS). Patients with RCC expressing higher Gal-1 expression had significantly reduced OS (Figure 6B, left panel) and DFS (Figure 6B, right panel) rates than those with low Gal-1 expression. In addition, higher CXCR4 expression was associated with shorter OS and DFS (Supplemental Figure 4B).
Figure 6.
Expression of Gal-1 was associated with CXCR4 expression and poor prognosis in RCC. (A) Results of the association between Gal-1 and CXCR4 were determined by immunohistochemistry in patients with RCC (n=45). (B) The overall survival (left panel) and disease-free survival (right panel) were calculated using Kaplan–Meier analysis according to low and high Gal-1. High Gal-1 protein expression was determined above the median H-score values. (C) Kaplan–Meier plots for overall survival (left panel) and disease-free survival (right panel) were stratified by concomitant overexpression of Gal-1 and CXCR4 (n=12) compared with the nonconcomitant overexpression of the two proteins (n=33). The P values were calculated using log-rank test.
Furthermore, we analyzed the prognostic potential of Gal-1 expression in patients with RCC. Multivariate analysis revealed that Gal-1 overexpression was an independent prognostic factor for OS and DFS (Supplemental Tables 2 and 3) in patients with RCC, with calculated ORs of 9.07 (95% confidence interval, 1.12 to 73.37) and 10.41 (95% confidence interval, 1.30 to 83.21), respectively. In an examination of the prognostic indicator of the Gal-1–CXCR4 axis in patients with RCC, results showed that patients with tumors concomitantly overexpressing Gal-1 and CXCR4 had the worst OS and DFS (Figure 6C). These data provide information that Gal-1 expression in RCC represented a novel prognostic factor for kidney cancer patients and that Gal-1 and CXCR4 co-overexpression might be a predictor of poor outcome in patients with RCC.
Discussion
The tumor microenvironment consists of tumor cells and stromal cells that can produce several inflammatory mediators, such as cytokines, chemokines, and galectins, to facilitate tumor development and metastatic progression.24 Gal-1 has been recently found as an important contributor to angiogenesis, immunosuppression, and metastasis within the tumor microenvironment.5 Intriguingly, Gal-1 either promotes or inhibits cell growth according to its localization, cell type, and cell activation status.6 Extracellular Gal-1 has inhibitory effects on the proliferation of neuroblastoma and lymphoma cells.25,26 In contrast, suppression of Gal-1 in glioma cells arrests cell growth, implying that endogenous Gal-1 has a growth-promoting role.27 Furthermore, the silencing of Gal-1 significantly reduces metastatic potential in murine breast and colon cancer cells.28 These observations imply that endogenous Gal-1 may play an important role in RCC progression. Although it has been reported that metastatic RCC tissues with poor prognosis show higher Gal-1 expression,18 the molecular mechanisms of Gal-1 in progression of RCC still remain unclear.
Within the tumor microenvironment, a complex network of chemokines and their receptors affects the initiation and progression of tumors.29 Recent findings indicate that Gal-1 in cancer-associated fibroblasts can promote the metastasis of oral squamous cell carcinoma by modulating monocyte chemotactic protein-1 (MCP-1/CCL2).30 The present study demonstrates for the first time that Gal-1 contributes to kidney cancer progression via activation of CXCR4, which is the most common chemokine receptor overexpressed in human cancers. Interaction of CXCR4 with its sole ligand CXCL12 (also called stromal-derived factor-1α) is reportedly involved in the regulation of chemotaxis, invasion, and organ-specific metastases.31–33 We found that levels of CXCL12 mRNA and protein in RCC cell lines and tumor tissues were very low (data not shown). The results were consistent with those of other studies indicating that both RCC cell lines and human RCC specimens exhibit elevated expressions of CXCR4, but not CXCL12, compared with normal kidney specimens.34,35 The strong expression of CXCR4 in RCC is associated with metastasis and is predictive of worse prognosis.36,37 The results here further show that patients with metastatic RCC have higher CXCR4 and Gal-1 coexpression than those with primary tumors (data not shown).
The expression of CXCR4 is stimulated by several proinflammatory cytokines and growth factors. TNF-α regulates CXCR4 expression via NF-κB signaling in ovarian cancer cells,21 while epidermal growth factor can enhance CXCR4 expression via PI3-K/PTEN/AKT/mTOR signaling pathway.38 In the present study, TNF-α induced NF-κB translocation and further activate CXCR4 mRNA expression in a Gal-1–dependent manner. Furthermore, Gal-1 has been identified as a potent interacting partner of oncogenic H-Ras to promote malignant transformation.14 Meanwhile, TNF-α–induced Ras activation has been shown to be important in the hepatic proliferation of liver injury.39 Hence, we propose that extracellular stimuli (e.g., TNF-α) may elicit Gal-1 expression and Ras activation, thereby enhancing H-Ras-Gal-1 interaction and subsequently activating the ERK/NF-κB/CXCR4 axis (Supplemental Figure 4C).
The VHL protein is involved in the regulation of the transcription factor called hypoxia-inducible factor (HIF) for ubiquitin-dependent degradation in the presence of oxygen.40 Because clear cell RCC cells lack functional VHL protein, HIF-α subunits accumulate and heterodimerize with HIF-β. This heterodimer translocates into the nucleus, binds to hypoxia response elements, and transcriptionally activates the target genes involved in angiogenesis, proliferation, and glucose metabolism. Recent findings suggest that HIF-1 directly regulates Gal-1 expression as well as CXCR4.37,41
Two VHL-mutant cell lines (786-O and A-498) exhibit higher expression of both Gal-1 and CXCR4 than VHL-wild type cell lines (Caki-1 and ACHN) in the present study (Figure 1B, Supplemental Figure 3F). Interestingly, either in Gal-1–knockdown A-498 cells or in Gal-1–overexpressing ACHN cells, Gal-1 can regulate CXCR4 expression to promote kidney cancer progression (Figures 3B and 4D). In addition, Gal-1 regulates the expression of hypoxia-related genes that are implicated in angiogenesis in glioblastoma.42 Thus, the involvement of Gal-1 in HIF-induced CXCR4 expression is worthy of further investigations.
Recent studies indicate that the CXCL12-CXCR4 axis maintains a cancer stem cell population and confers drug resistance to pancreatic cancer cells and non–small cell lung cancer cells, suggesting that these chemoresistant CXCR4+ stem-like subpopulations have high tumorigenic and metastatic properties.43,44 Gal-1 also mediates chemoresistance and radioresistance in melanoma and cervical cancer cells, respectively.15,45 Because Gal-1 is an important upstream regulator of ERK, NF-κB, and CXCR4, targeting Gal-1 may be a more effective strategy for combination cancer therapy.
In conclusion, Gal-1 promotes kidney cancer progression by activating ERK1/2, NF-κB, and CXCR4. The coordinated expression of Gal-1 and CXCR4 may be predictive of worse prognosis in patients with RCC. Therefore, Gal-1 could be a novel therapeutic target and a valuable prognostic marker for RCC.
Concise Methods
Cell Lines and Mice
Human RCC cell lines 786-O (American Type Culture Collection [ATCC] no. CRL-1932) and A-498 (ATCC no. HTB-44) were obtained from Bioresource Collection and Research Center (Hsinchu, Taiwan). Human RCC cell lines Caki-1 (ATCC no. HTB-46) and ACHN (ATCC no. CRL-1611) were purchased from ATCC. The HEK293 and HEK293T cell lines were given by Dr. Jason C. Huang (National Yang-Ming University). HUVECs were obtained from Lifeline Cell Technology (Walkersville, MD) and cultured in VascuLife Basal Medium (Lifeline Cell Technology) containing 2% FBS, 10 mM l-glutamine, 0.2% EnGS, 5 ng/ml rhEGF, 1 μg/ml hydrocortisone hemisuccinate, 0.75 units/ml heparin sulfate, and 50 μg/ml ascorbic acid (Lifeline Cell Technology). Male nude mice (BALB/c AnN.Cg-Foxn1nu/CrlNarl) were purchased from the National Laboratory Animal Center (Taipei, Taiwan) and maintained under specific pathogen-free (SPF) conditions in the Animal Center of National Yang-Ming University according to the regulations of the Animal Care Committee of National Yang-Ming University.
Tissue Microarrays and Immunohistochemistry
All specimens and patient information were obtained after informed consent according to the institutional review board guidelines of the Tri-Service General Hospital, National Defense Medical Center (TSGH-IRB-098–05–221 and TSGH-IRB-2–101–05–001). Constructed tissue microarray sections containing 100 individual 2-mm diameter samples per array were subjected to immunohistochemical analysis. The detailed clinicopathologic features of 45 patients with kidney cancer are shown in Supplemental Table 4. After deparaffinization, the tissue sections were subjected to antigen retrieval in 10 mM citrate buffer (pH 6.0) by microwave treatment for 15 minutes. The samples were subsequently immersed in 3% H2O2 for 5 minutes to block endogenous peroxidase and then incubated with Gal-1 or CXCR4 primary antibodies diluted in blocking buffer at 4°C overnight. The slides were processed using LSAB+System-HRP kit (DAKO) according to the manufacturer’s protocol, and counterstained using hematoxylin. A digital pathology system for scoring stained cells was processed by Aperio ImageScope (Aperio Technologies, Inc.) for analysis. Tumor regions were hand-annotated on TMA slide images using Aperio’s annotation software (ImageScope v10; Aperio Technologies, Inc.). Annotated data were analyzed by the positive pixel count algorithm (ImageScope). The immunostaining results were scored by the product of the percentage of immunopositive cells (0–100) multiplied by staining intensity score (0, 1, 2, and 3) to yield scores of 0–300.46
RT-PCR and RT-qPCR
Total cellular RNA was extracted by TRIzol reagent (Invitrogen), and 5 μg of extracted RNA was used to synthesize first-strand cDNA using SuperScript III First-Strand Synthesis System (Invitrogen) according to the manufacturer’s protocol for RT-PCR. Quantitative RT-PCR was performed by Fast SYBR Green Master Mix (Applied Biosystems) according to the manufacturer’s instructions, using an ABI7700 System (Applied Biosystems). Values were normalized against the glyceraldehyde-3-phosphate dehydrogenase mRNA level. Each experiment was repeated at least three times to confirm reproducibility, with the reaction in triplicate wells for each sample. The specific primers used in RT-PCR or RT-qPCR are described in Supplemental Table 5.
Western Blotting
Total cellular proteins were extracted in the lysis buffer. Equal amounts (40 μg) were separated on 13% SDS-PAGE, electroblotted onto nitrocellulose membrane (Millipore), probed with the indicated antibodies and horseradish peroxidase–conjugated secondary antibodies, and analyzed by enhanced chemiluminescence. The antibodies used in this study are described in Supplemental Table 6.
Establishment of Gal-1 or CXCR4 Knockdown Cells
For Gal-1 or CXCR4 knockdown, pLKO.1 plasmid containing shRNA-targeting human Gal-1 (shGal-1) or CXCR4 (shCXCR4), purchased from the National RNAi Core Facility (Taiwan), was introduced into HEK293T cells with lentiviral packaging vectors pMD.G and pCMV-ΔR8.91. The viral supernatants were harvested and used to transduce RCC cells. Then, 5×104/ml 786-O and A-498 cells were infected with the collected viruses for 24 hours in the presence of 8 μg/ml protamine sulfate, followed by puromycin selection (4 μg/ml) for 9 days. The specific target sequences by shGal-1 or shCXCR4 are listed in Supplemental Table 7. A control vector expressing shRNA against luciferase or LacZ (pLKO.1-shLuc or -shLacZ) was used as a negative control for knockdown validation.
In Vitro Cell Proliferation
Briefly, 1×103 786-O or A-498 cells transduced with shLuc or shGal-1 were cultured in 96-well microtiter plates in a total volume of 100 μl/well. On days 0, 2, and 4, cell proliferation was measured by CellTiter 96 aqueous nonradioactive cell proliferation assay based on the manufacturer’s protocol (Promega).
Cell Migration and Invasion Assays
Tumor cell migration and invasion assays were assessed by Transwell assay (Costar, 8-μm pore; Corning). For the invasion assay, each insert was coated with Matrigels (BD Biosciences). A total of 2×104 cells was resuspended in 100 μl 0.5% FBS-cultured medium and plated in the upper chamber. The lower chamber was filled with 800 μl complete medium (cultured medium supplemented with 10% FBS). After incubation at 37°C for 22 hours, the invaded or migrated cells on the lower surfaces of inserts were fixed with methanol for 10 minutes. The Matrigels and nonpenetrating cells were mechanically wiped using cotton swabs. Subsequently, the chambers were stained with Liu stain (Muto) and counted under light microscopy (×100 magnifications).
Anchorage-Independent Growth Assay
Cells, 2×103 786-O or 1×103 A-498, were suspended in 0.33% Bacto-agar (Sigma-Aldrich) and then layered over 0.5% Bacto agar in six-well plates. On day 30, the colonies were counted after fixing with methanol and staining with Giemsa.
Clonogenic Assay
shLuc- or shGal-1–infected cells (1×102) were plated in six-well plates and incubated for 10 days. The cells were then fixed with methanol and stained with 0.5% crystal violet. Plates with colonies were rinsed with tap water and left to dry at room temperature. The colonies were counted using a microscope.
In Vivo Tumor Growth
To establish a xenograft tumor model, shLuc- or shGal-1–infected A-498 cells (5×106/100 μl cells) were implanted subcutaneously into the abdominal flanks of 6- to 8-week-old male athymic nude mice in groups of four. The tumor size was measured with a caliper and calculated as length×width×height (in mm3) every week.
HUVEC Proliferation Assay
HUVECs were plated at 5×103 cells/well in 96-well plates and incubated overnight. Cells were serum starved for 12 hours and then treated with 200-fold–concentrated supernatants from shLuc- or shGal-1–infected A-498 cells for 48 hours. The rate of cell proliferation was determined using cell proliferation ELISA (Roche) via bromodeoxyuridine incorporation into new synthesized DNA according to the manufacturer’s protocol.
HUVEC Migration Assay
HUVECs migration assay was performed on the IBIDI insert (Ibidi). A total of 2×104/70 μl HUVECs were seeded into an IBIDI insert. After 12 hours, the insert was gently removed, creating a gap of approximately 500 μm, and the cells were treated with 200-fold–concentrated conditioned media obtained from A-498/shLuc and A-498/shGal-1 cells. Images were captured at 0, 6, and 12 hours of incubation, and cell migration was quantified using ImageJ software.
Tube Formation Assay
Ten μl Matrigels (BD Biosciences) was pipetted into the lower chambers of an IBIDI μ-Slide Angiogenesis (Ibidi) and hardened for 30 minutes at 37°C. The cell suspension (50 μl; 2×105 cells/ml) was grown in the presence of 200-fold–concentrated conditioned media from A-498/shLuc and A-498/shGal-1 cells in μ-slide precoated with Matrigel. After 18 hours of seeding, pictures were taken and the images were analyzed using ImageJ software. Network formation was quantified by measuring the total tube length.
In Vivo Matrigel Plug Assay
Eight-week-old male C57BL/6 mice were subcutaneously injected with 300 μl of Matrigel containing supernatants from A-498/shLuc or A-498/shGal-1 cells. Matrigel plugs were surgically removed on day 7 and photographed.
Luciferase Assay
shLuc- or shGal-1–infected cells (1×105/ml) were transiently cotransfected with pGL3-NF-κB-luc or pGL2-CXCR4-luc (given by Dr. Mien-Chie Hung from MD Anderson Cancer Center, Houston, TX) and pRL-SV40 (Promega) using the jetPEI transfection reagent (Polyplus). Firefly and Renilla luciferase activities were measured 48 hours after transfection using the Dual-Luciferase Reporter Assay System (Promega) according to the manufacturer’s recommendation.
Experimental Metastasis In Vivo
ACHN/pc or ACHN/Gal-1 cells (1×106) were suspended in 100 μl of PBS and injected intravenously via lateral tail vein of 8-week-old athymic nude mice. After 30 days, lungs of mice were removed and fixed in 10% formalin.
Statistical Analyses
Data were expressed as the mean±SD. All statistical analyses were conducted using one-way ANOVA and t test by the SPSS statistical software program (version 17.0; SPSS, Inc.). A two-tailed chi-squared test was used to test the significance of the association between Gal-1 expression and clinicopathologic characteristics. Survival durations were analyzed using the Kaplan–Meier method and compared by the log-rank test in the patient groups. A Cox proportional hazard model was used for univariate and multivariate analyses. Gal-1 and CXCR4 dual staining were evaluated for any association using Spearman correlation method. Statistical significance was set at P<0.05.
Disclosure
None.
Supplementary Material
Acknowledgments
We thank Drs. Mien-Chie Hung for kindly providing the pGL2-CXCR4-luc plasmid, Jason C. Huang for kindly providing the HEK293T and HEK293 cell lines, and L.P. Ting for the pFLAG-p65 plasmid.
This study was supported by the National Science Council (101-2321-B-010-005, 102-2321-B-010-004), Ministry of Education (Aim for the Top University Plan), Tri-Service General Hospital and National Defense Medical Center (TSGHC101060), Taoyuan Armed Forces General Hospital (AFTYGH-10028, AFTYGH-10131), Cheng-Hsin General Hospital (102F218C23), and Taipei City Hospital, Taiwan.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013070773/-/DCSupplemental.
References
- 1.Siegel R, Naishadham D, Jemal A: Cancer statistics, 2012. CA Cancer J Clin 62: 10–29, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Custódio S, Joaquim A, Peixoto V, Macedo JE, Faria AL, Macias E, Rego S, Araújo A: Metastatic renal cell carcinoma: The importance of immunohistochemistry in differential diagnosis. Case Rep Oncol 5: 30–34, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eisengart LJ, MacVicar GR, Yang XJ: Predictors of response to targeted therapy in renal cell carcinoma. Arch Pathol Lab Med 136: 490–495, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Escudier B: Emerging immunotherapies for renal cell carcinoma. Ann Oncol 23[Suppl 8]: viii35–40, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Yang RY, Rabinovich GA, Liu FT: Galectins: Structure, function and therapeutic potential. Expert Rev Mol Med 10: e17, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Camby I, Le Mercier M, Lefranc F, Kiss R: Galectin-1: A small protein with major functions. Glycobiology 16: 137R–157R, 2006 [DOI] [PubMed] [Google Scholar]
- 7.van den Brûle FA, Waltregny D, Castronovo V: Increased expression of galectin-1 in carcinoma-associated stroma predicts poor outcome in prostate carcinoma patients. J Pathol 193: 80–87, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Szoke T, Kayser K, Baumhakel JD, Trojan I, Furak J, Tiszlavicz L, Horvath A, Szluha K, Gabius HJ, Andre S: Prognostic significance of endogenous adhesion/growth-regulatory lectins in lung cancer. Oncology 69: 167–174, 2005 [DOI] [PubMed] [Google Scholar]
- 9.van den Brûle F, Califice S, Garnier F, Fernandez PL, Berchuck A, Castronovo V: Galectin-1 accumulation in the ovary carcinoma peritumoral stroma is induced by ovary carcinoma cells and affects both cancer cell proliferation and adhesion to laminin-1 and fibronectin. Lab Invest 83: 377–386, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Camby I, Belot N, Lefranc F, Sadeghi N, de Launoit Y, Kaltner H, Musette S, Darro F, Danguy A, Salmon I, Gabius HJ, Kiss R: Galectin-1 modulates human glioblastoma cell migration into the brain through modifications to the actin cytoskeleton and levels of expression of small GTPases. J Neuropathol Exp Neurol 61: 585–596, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Kovács-Sólyom F, Blaskó A, Fajka-Boja R, Katona RL, Végh L, Novák J, Szebeni GJ, Krenács L, Uher F, Tubak V, Kiss R, Monostori E: Mechanism of tumor cell-induced T-cell apoptosis mediated by galectin-1. Immunol Lett 127: 108–118, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Horiguchi N, Arimoto K, Mizutani A, Endo-Ichikawa Y, Nakada H, Taketani S: Galectin-1 induces cell adhesion to the extracellular matrix and apoptosis of non-adherent human colon cancer Colo201 cells. J Biochem 134: 869–874, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Thijssen VL, Postel R, Brandwijk RJ, Dings RP, Nesmelova I, Satijn S, Verhofstad N, Nakabeppu Y, Baum LG, Bakkers J, Mayo KH, Poirier F, Griffioen AW: Galectin-1 is essential in tumor angiogenesis and is a target for antiangiogenesis therapy. Proc Natl Acad Sci U S A 103: 15975–15980, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paz A, Haklai R, Elad-Sfadia G, Ballan E, Kloog Y: Galectin-1 binds oncogenic H-Ras to mediate Ras membrane anchorage and cell transformation. Oncogene 20: 7486–7493, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Mathieu V, Le Mercier M, De Neve N, Sauvage S, Gras T, Roland I, Lefranc F, Kiss R: Galectin-1 knockdown increases sensitivity to temozolomide in a B16F10 mouse metastatic melanoma model. J Invest Dermatol 127: 2399–2410, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Chung LY, Tang SJ, Sun GH, Chou TY, Yeh TS, Yu SL, Sun KH: Galectin-1 promotes lung cancer progression and chemoresistance by upregulating p38 MAPK, ERK, and cyclooxygenase-2. Clin Cancer Res 18: 4037–4047, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Dihazi H, Müller C, Asif AR, Flad T, Elmaouhoub A, Müller GA: Whole cell profiling and identification of galectin-1 as a potential marker of renal cell carcinoma. Proteomics Clin Appl 1: 200–214, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Masui O, White NM, DeSouza LV, Krakovska O, Matta A, Metias S, Khalil B, Romaschin AD, Honey RJ, Stewart R, Pace K, Bjarnason GA, Siu KW, Yousef GM: Quantitative proteomic analysis in metastatic renal cell carcinoma reveals a unique set of proteins with potential prognostic significance. Mol Cell Proteomics 12: 132–144, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salcedo R, Wasserman K, Young HA, Grimm MC, Howard OM, Anver MR, Kleinman HK, Murphy WJ, Oppenheim JJ: Vascular endothelial growth factor and basic fibroblast growth factor induce expression of CXCR4 on human endothelial cells: In vivo neovascularization induced by stromal-derived factor-1alpha. Am J Pathol 154: 1125–1135, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen S, Tuttle DL, Oshier JT, Knot HJ, Streit WJ, Goodenow MM, Harrison JK: Transforming growth factor-beta1 increases CXCR4 expression, stromal-derived factor-1alpha-stimulated signalling and human immunodeficiency virus-1 entry in human monocyte-derived macrophages. Immunology 114: 565–574, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kulbe H, Hagemann T, Szlosarek PW, Balkwill FR, Wilson JL: The inflammatory cytokine tumor necrosis factor-alpha regulates chemokine receptor expression on ovarian cancer cells. Cancer Res 65: 10355–10362, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Yoshida N, Ikemoto S, Narita K, Sugimura K, Wada S, Yasumoto R, Kishimoto T, Nakatani T: Interleukin-6, tumour necrosis factor alpha and interleukin-1beta in patients with renal cell carcinoma. Br J Cancer 86: 1396–1400, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Helbig G, Christopherson KW, 2nd, Bhat-Nakshatri P, Kumar S, Kishimoto H, Miller KD, Broxmeyer HE, Nakshatri H: NF-kappaB promotes breast cancer cell migration and metastasis by inducing the expression of the chemokine receptor CXCR4. J Biol Chem 278: 21631–21638, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Mantovani A, Allavena P, Sica A, Balkwill F: Cancer-related inflammation. Nature 454: 436–444, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Kopitz J, von Reitzenstein C, André S, Kaltner H, Uhl J, Ehemann V, Cantz M, Gabius HJ: Negative regulation of neuroblastoma cell growth by carbohydrate-dependent surface binding of galectin-1 and functional divergence from galectin-3. J Biol Chem 276: 35917–35923, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Suzuki O, Nozawa Y, Abe M: The regulatory roles of cell surface sialylation and N-glycans in human B cell lymphoma cell adhesion to galectin-1. Int J Oncol 28: 155–160, 2006 [PubMed] [Google Scholar]
- 27.Yamaoka K, Mishima K, Nagashima Y, Asai A, Sanai Y, Kirino T: Expression of galectin-1 mRNA correlates with the malignant potential of human gliomas and expression of antisense galectin-1 inhibits the growth of 9 glioma cells. J Neurosci Res 59: 722–730, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Ito K, Ralph SJ: Inhibiting galectin-1 reduces murine lung metastasis with increased CD4(+) and CD8 (+) T cells and reduced cancer cell adherence. Clin Exp Metastasis 29: 561–572, 2012 [DOI] [PubMed] [Google Scholar]
- 29.Ali S, Lazennec G: Chemokines: Novel targets for breast cancer metastasis. Cancer Metastasis Rev 26: 401–420, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu MH, Hong HC, Hong TM, Chiang WF, Jin YT, Chen YL: Targeting galectin-1 in carcinoma-associated fibroblasts inhibits oral squamous cell carcinoma metastasis by downregulating MCP-1/CCL2 expression. Clin Cancer Res 17: 1306–1316, 2011 [DOI] [PubMed] [Google Scholar]
- 31.Müller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, Verástegui E, Zlotnik A: Involvement of chemokine receptors in breast cancer metastasis. Nature 410: 50–56, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Murakami T, Maki W, Cardones AR, Fang H, Tun Kyi A, Nestle FO, Hwang ST: Expression of CXC chemokine receptor-4 enhances the pulmonary metastatic potential of murine B16 melanoma cells. Cancer Res 62: 7328–7334, 2002 [PubMed] [Google Scholar]
- 33.Phillips RJ, Burdick MD, Lutz M, Belperio JA, Keane MP, Strieter RM: The stromal derived factor-1/CXCL12-CXC chemokine receptor 4 biological axis in non-small cell lung cancer metastases. Am J Respir Crit Care Med 167: 1676–1686, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Schrader AJ, Lechner O, Templin M, Dittmar KE, Machtens S, Mengel M, Probst-Kepper M, Franzke A, Wollensak T, Gatzlaff P, Atzpodien J, Buer J, Lauber J: CXCR4/CXCL12 expression and signalling in kidney cancer. Br J Cancer 86: 1250–1256, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang L, Chen W, Gao L, Yang Q, Liu B, Wu Z, Wang Y, Sun Y: High expression of CXCR4, CXCR7 and SDF-1 predicts poor survival in renal cell carcinoma. World J Surg Oncol 10: 212, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang L, Wang L, Yang B, Yang Q, Qiao S, Wang Y, Sun Y: Strong expression of chemokine receptor CXCR4 by renal cell carcinoma cells correlates with metastasis. Clin Exp Metastasis 26: 1049–1054, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Staller P, Sulitkova J, Lisztwan J, Moch H, Oakeley EJ, Krek W: Chemokine receptor CXCR4 downregulated by von Hippel-Lindau tumour suppressor pVHL. Nature 425: 307–311, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Phillips RJ, Mestas J, Gharaee-Kermani M, Burdick MD, Sica A, Belperio JA, Keane MP, Strieter RM: Epidermal growth factor and hypoxia-induced expression of CXC chemokine receptor 4 on non-small cell lung cancer cells is regulated by the phosphatidylinositol 3-kinase/PTEN/AKT/mammalian target of rapamycin signaling pathway and activation of hypoxia inducible factor-1alpha. J Biol Chem 280: 22473–22481, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Isayama F, Froh M, Yin M, Conzelmann LO, Milton RJ, McKim SE, Wheeler MD: TNF alpha-induced Ras activation due to ethanol promotes hepatocyte proliferation independently of liver injury in the mouse. Hepatology 39: 721–731, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Baldewijns MM, van Vlodrop IJ, Vermeulen PB, Soetekouw PM, van Engeland M, de Bruïne AP: VHL and HIF signalling in renal cell carcinogenesis. J Pathol 221: 125–138, 2010 [DOI] [PubMed] [Google Scholar]
- 41.Zhao XY, Chen TT, Xia L, Guo M, Xu Y, Yue F, Jiang Y, Chen GQ, Zhao KW: Hypoxia inducible factor-1 mediates expression of galectin-1: The potential role in migration/invasion of colorectal cancer cells. Carcinogenesis 31: 1367–1375, 2010 [DOI] [PubMed] [Google Scholar]
- 42.Le Mercier M, Mathieu V, Haibe-Kains B, Bontempi G, Mijatovic T, Decaestecker C, Kiss R, Lefranc F: Knocking down galectin 1 in human hs683 glioblastoma cells impairs both angiogenesis and endoplasmic reticulum stress responses. J Neuropathol Exp Neurol 67: 456–469, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Singh S, Srivastava SK, Bhardwaj A, Owen LB, Singh AP: CXCL12-CXCR4 signalling axis confers gemcitabine resistance to pancreatic cancer cells: A novel target for therapy. Br J Cancer 103: 1671–1679, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jung MJ, Rho JK, Kim YM, Jung JE, Jin YB, Ko YG, Lee JS, Lee SJ, Lee JC, Park MJ: Upregulation of CXCR4 is functionally crucial for maintenance of stemness in drug-resistant non-small cell lung cancer cells. Oncogene 32: 209–221, 2013 [DOI] [PubMed] [Google Scholar]
- 45.Huang EY, Chen YF, Chen YM, Lin IH, Wang CC, Su WH, Chuang PC, Yang KD: A novel radioresistant mechanism of galectin-1 mediated by H-Ras-dependent pathways in cervical cancer cells. Cell Death Dis 3: e251, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pierceall WE, Wolfe M, Suschak J, Chang H, Chen Y, Sprott KM, Kutok JL, Quan S, Weaver DT, Ward BE: Strategies for H-score normalization of preanalytical technical variables with potential utility to immunohistochemical-based biomarker quantitation in therapeutic response diagnostics. Anal Cell Pathol (Amst) 34: 159–168, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.