Abstract
Renal epithelial cells must maintain distinct protein compositions in their apical and basolateral membranes in order to perform their transport functions. The creation of these polarized protein distributions depends on sorting signals that designate the trafficking route and site of ultimate functional residence for each protein. Segregation of newly synthesized apical and basolateral proteins into distinct carrier vesicles can occur at the trans-Golgi network, recycling endosomes, or a growing assortment of stations along the cellular trafficking pathway. The nature of the specific sorting signal and the mechanism through which it is interpreted can influence the route a protein takes through the cell. Cell type–specific variations in the targeting motifs of a protein, as are evident for Na,K-ATPase, demonstrate a remarkable capacity to adapt sorting pathways to different developmental states or physiologic requirements. This review summarizes our current understanding of apical and basolateral trafficking routes in polarized epithelial cells.
Keywords: cell and transport physiology, epithelial, renal cell biology
Polarized epithelial cells separate an organism’s internal milieu from its external environment. To perform this barrier function, these cells possess an asymmetric design, with an apical membrane facing an “outside” lumen and a basolateral membrane facing neighboring cells and the basal lamina. These two distinct membrane domains are separated by intercellular junctional complexes, called tight junctions, which render the epithelial cell monolayer selectively permeable to solutes and fluid. A transporting epithelial tissue’s functional properties are determined in large measure by the inventories of ion channels, transporters, and pumps that are differentially localized to their apical and basolateral membranes and that account for the tissue’s capacity to mediate the net secretion or absorption of fluid and solutes. Differential sorting and directed targeting of membrane proteins to specific membrane domains in epithelial cells are thus necessary for the generation and maintenance of the biochemical polarity that is a prerequisite for their physiologic function.
Newly synthesized membrane proteins are packaged into transport vesicles at the trans-Golgi network (TGN) in preparation for their ultimate delivery to the plasma membrane. A protein’s route from the TGN to its designated plasma membrane localization can be direct, or it can involve stops at one or more endosomal compartments.1–3 In addition to the common endosomes that are present in all cell types, polarized epithelial cells also possess specialized endosomal compartments that participate in the trafficking of apical or basolateral proteins, specifically.4,5 The presence of these additional endosomal compartments is consistent with the more complicated sorting and trafficking processes that are required to generate and maintain polarity in these cells. In addition to being targeted directly to their sites of functional residence, proteins can also be mis-sorted or randomly delivered and then retained only in the correct compartment by interactions with cytoskeletal components or through other mechanisms that effectively immobilize the protein and prevent its departure. Additional trafficking processes provide acute regulation of cell surface expression once a protein is localized to the correct membrane domain. This is especially true of transport proteins in epithelial cells, whose activities are often tightly controlled in response to stress or environmental cues. The topic of transport protein regulation by trafficking has been the subject of many reviews.6,7 In the present review, we focus on the biosynthetic routes pursued by membrane proteins in polarized renal epithelial cells, the mechanisms that contribute to sorting, and the specializations that allow protein sorting pathways to be tailored to a cell’s physiologic requirements and developmental state.
Basolateral Sorting Signals
Basolateral sorting of both soluble secreted proteins and integral membrane proteins is directed primarily by signals embedded within the sorted protein’s primary structure (Table 1). The most common types of signals involved in the sorting of basolateral membrane proteins are tyrosine-based (NPxY or YxxØ) or dileucine (D/ExxxLL) motifs.8–12 These sequences are embedded within these proteins’ cytosol-facing domains and are similar in composition and geometry to the signals that drive endocytosis. In fact, basolateral signals can overlap with or serve as endocytosis signals,8–10,13–16 although this is not always the case.15,17,18 Additionally, basolateral sorting motifs involving a single leucine residue have been identified in several proteins, including stem cell factor,19 CD147,20 and amphiregulin (EExxxL).21 Protein components of the cellular trafficking machinery, including subunits of the clathrin adaptor complexes, mediate the interpretation of both tyrosine-based and dileucine targeting sequences.22–24 The adaptor protein (AP) complexes function as cargo receptors in the formation of clathrin-coated pits and vesicles.25–31 Tyrosine-based basolateral signals interact with µ1B, an epithelial-specific isoform of the µ1 subunit of the heterotetrameric AP complex.32–34 Less is known about the nature of signals that drive the targeting of proteins with multiple transmembrane domains, although some of these proteins are known to dimerize with single-pass membrane proteins that convey sorting information.35,36
Table 1.
Signals and mechanisms for sorting to the apical and basolateral membranes
| Sorting Signal | Protein | Presumed Sorting Mechanism | References |
|---|---|---|---|
| Basolateral | |||
| Tyrosine-based | Low-density lipoprotein receptor | Recycling, but not delivery, is μ1b-dependent | 10, 152 |
| Vesicular stomatitus virus G protein | μ1b-dependent | 153, 154 | |
| lgp120 | μ3a-dependent | 9, 155 | |
| Dileucine | Fc receptor FcRII-B2 | μ1b-independent | 11, 15, 156 |
| Mannose 6-phosphate receptor | μ1b-independent | 157, 158 | |
| E-cadherin | Rab11-mediated | 3, 12 | |
| Monoleucine | Stem cell factor | 19 | |
| CD147 | Clathrin-mediated; μ1b-dependent | 130 | |
| Amphiregulin | Recycling, but not delivery, is μ1b-dependent | 21 | |
| Apical | |||
| GPI-anchor | Decay accelerating factor | Lipid raft–associated | 40, 41 |
| Folate receptor | Lipid raft–associated | 43 | |
| N-Glycans | Clusterin (gp80) | Raft-independent | 46, 159 |
| gp114 | Galectin-3–mediated, raft-independent | 47, 61 | |
| Growth hormone | Independent of galectins 3 and 4 | 48 | |
| Erythropoietin | Cholesterol-dependent | 49, 160 | |
| Endolyn | Raft-independent | 50 | |
| O-Glycans | p75 neurotrophin receptor | Galectin-3–mediated, raft-independent | 53, 61 |
| Lactase phlorizin hydrolase | Galectin-3–mediated, raft-independent | 61 | |
| MUC1 | Raft-independent | 99, 161, 162 | |
| Podocalyxin | Transient lipid raft association | 52 | |
| Dipeptidyl peptidase IV | Lipid raft–associated | 55, 58 | |
| Sucrase isomaltase | Lipid raft–associated | 55, 56 | |
| Transmembrane domain | Neuraminidase | Lipid raft–associated | 67, 70 |
| Influenza hemagglutinin | Lipid raft–associated | 69, 163 | |
| Respiratory syncytial virus F protein | Lipid raft–associated | 71, 164 | |
| Sucrase isomaltase | Lipid raft–associated | 165 | |
| H,K-ATPase | Raft-independent | 72 |
Apical Sorting Signals
Apical signals are more diverse than their basolateral counterparts. Topologically, apical signals can reside in any domain of an apically sorted protein’s structure—extracellular, transmembrane, or cytosolic. Compositionally, apical signals can be based on amino acids, lipids, or carbohydrates. The apical membrane is divided into the ciliary region (which includes the periciliary base and the primary cilium itself) and the nonciliary membrane, and these domains are compositionally distinct from one another. This division adds further complexity to the process of apical sorting.37
The recognition that multiple glycosyl-phosphatidylinositol-anchored proteins (GPI-AP) are localized to the apical membranes of MDCK cells provided a first clue to the existence and nature of apical sorting signals.38 The sorting behaviors of chimeric proteins suggest that the GPI anchor is sufficient to specify sorting to the apical membrane.39,40 However, other reports indicate that the anchor is necessary to ensure apical sorting of GPI-APs, but is not in itself sufficient.41 The finding that GPI-APs oligomerize and partition into detergent-resistant membranes while trafficking to the apical membrane42,43 is consistent with the hypothesis that lipid rafts play critical roles in apical protein sorting.44,45 Affinity of GPI-APs for glycosphingolipid-enriched rafts is thought to cause their preferential incorporation into vesicles bound for the apical membrane.
Both N- and O-linked glycosylation have also been shown to serve as sorting signals for many apical proteins. The potential for N-glycans to act as apical sorting signals was first recognized for the glycoprotein gp80, which is missorted after treatment with N-glycosylation inhibitors.46 N-Glycans have since been shown to fulfill this function for many proteins in studies using glycosylation-deficient cell lines or mutagenesis.47–51 Insertion of a site for addition of N-glycans to the sequence of nonglycosylated rat growth hormone resulted in this protein being secreted apically rather than without polarity,48 whereas deletion of one N-glycan chain from erythropoietin impaired apical trafficking.49 Similarly, chimeric constructs containing the O-glycosylated region of the glycoprotein podocalyxin are endowed with a signal for apical targeting,52 and removal of O-glycans from neurotrophin receptors results in sorting to the basolateral rather than apical membrane.53 As for N-glycans, the importance of O-glycans for apical trafficking has been demonstrated using glycosylation inhibitors.54–58 However, studies on the roles of glycans as sorting signals have been complicated by nontargeting effects of their removal by biochemical or genetic means. For example, removal of a protein’s N-glycans can cause its retention in the endoplasmic reticulum (ER) due to improper folding.59 Thus, their exact role in apical sorting remains unclear.
Mechanistically, lectin proteins are postulated to serve as the sorting receptors that transiently cluster N-glycosylated proteins into apically destined domains.60 Galectins 3, 4, and 9 are lectins that have been implicated in apical sorting in kidney and intestinal cells.61–64 The minimal mobility of glycosylated growth hormone in endosomes, as shown by fluorescence recovery after photobleaching, is consistent with the postulated role of lectin tethering in N-glycan–dependent sorting.65 Alternative theories suggest that N-glycans serve not as sorting signals but rather as structural supports that enhance protein folding or inhibit aggregation, thus allowing protein sequence-based signals to interact with protein receptors or lipid domains.66
Transmembrane domain sequences are responsible for the apical sorting of multiple viral single-pass membrane proteins, including hemagglutinin, neuraminidase, and the respiratory syncytial virus F protein.67–71 A transmembrane domain also appears to play a key role in apical sorting of the polytopic gastric H,K-ATPase protein.72 The α-subunits of the H,K-ATPase and Na,K-ATPase pumps share approximately 65% sequence identity,73 permitting the construction of structurally intact and functionally active chimeric pump proteins. A chimera in which the fourth transmembrane domain of the H,K-ATPase α-subunit was inserted into the complementary position of the basolateral Na,K-ATPase α-subunit was targeted to the apical membrane in transfected epithelial cells.72
As is the case for GPI-APs, all major classes of apical sorting signals have been implicated in inducing clustering of their associated proteins into detergent-resistant membrane domains. The role of clustering in driving biosynthetic apical sorting, however, is murky at best. Mutational analysis of the influenza virus hemagglutinin (HA) protein transmembrane domain showed that this sequence is required for the HA protein’s raft association, but not for its apical sorting.74 Furthermore, GPI-APs lose raft association when cells are subjected to mild levels of cholesterol depletion, but are still sorted to the apical membrane.75 Secreted forms of GPI-APs lacking the GPI linkage are targeted apically despite having lost both their raft association and the apical influence previously attributed to GPI.76 A 2005 review of epithelial trafficking suggested the novel unifying hypothesis that apical signals function as oligomerization-promoting factors.77 Support for this conjecture is supplied by data showing that a GPI-linked green fluorescent protein (GFP) construct was sorted to the apical membrane unless the GFP contained mutations known to abolish its tendency to oligomerize.43
The variable outcomes of seemingly straightforward experiments designed to test the activities of specific types of apical signals complicate the establishment of the role of any particular signal or class of signals in apical sorting. Some of this complexity may be attributable to the existence of redundant or multipart signals. One example of such a bipartite apical signal is found in podocalyxin. Individual removal of a heavily glycosylated region in the ectodomain or of a cytosolic region containing a C-terminal postsynaptic density 95/disc-large/zona occludens domain interaction motif produced only minimal effects on the protein’s steady state localization.52 In contrast, removing both of these apical signal domains resulted in podocalyxin protein that exhibits an entirely nonpolarized localization.52 The multiplicity of studies exploring the various classes of apical signals and the roles of lipid rafts clearly demonstrate that apical targeting is not the product of a simple binary “on-or-off switch” process. Further studies will be required to explore the individual functions of specific signal types, as well as the roles for oligomerization and for hierarchical and cooperative interplay in determining the mechanisms through which complex signals are interpreted.
Sorting Site for Biosynthetic Cargo
To understand the mechanisms mediating trafficking in polarized epithelial cells, it is necessary to elucidate the site at which basolateral proteins and apical proteins are separated from one another as they pursue their postsynthetic trafficking itineraries. Several studies address this issue using biochemical techniques or microscopy-based assays. By assessing the sialic acid content of the sugars attached to the basolaterally directed vesicular stomatitis virus glycoprotein (VSV-G), Fuller et al. demonstrated that this protein remains in physical contact with the apically directed influenza neuraminidase (whose enzymatic activity is capable of desialating VSV-G) through the late Golgi.78 Similarly, following infection of MDCK cells with two viruses that bud from opposite membranes—VSV-G from the basolateral membrane and HA from the apical membrane—Rindler and colleagues observed colocalization of VSV-G and HA within the same Golgi cisternae by colloidal gold immuno-electron microscopy.79 In contrast, work with GFP-tagged serglycin protein has suggested apical/basolateral separation as early as the cis Golgi or ER.80,81 Furthermore, studies suggest the existence of a novel class of detergent-resistant membrane domains that act as aggregators of apical proteins in the ER.82 Further research will be necessary to determine whether this type of early separation plays an obligate functional role in epithelial protein sorting.
One case of well established early segregation involves a specific class of apical proteins. GPI-APs have been shown in yeast to exit the ER in different vesicular carriers than other apical cargo.83 Most recently, the p24 family of transmembrane proteins was identified in yeast as the receptor/adaptor for GPI-APs, facilitating their incorporation into coat protein complex II–coated vesicles in the ER and regulating coat protein complex I–mediated retrograde transport of escaped, unremodeled GPI-APs.84 Because GPI-AP signals are chemically distinct from other sorting signals, which tend to be short amino acid sequences or post-translational modifications, it is perhaps not surprising that their sorting would be mechanistically and temporally distinct.
Despite these examples of early segregation, most sorting appears to occur at some point after proteins have passed through the late Golgi and reached the TGN. Evidence in favor of this argument comes from live imaging studies, which have revealed the separation of proteins into different vesicles upon or directly after their export from the TGN.85,86 In these experiments, progressive lateral separation of representative apical and basolateral proteins, concurrent with the exclusion of resident Golgi proteins from these segregated domains, culminated in the proteins exiting the Golgi in distinct carriers.
In addition to separation of apical and basolateral cargo, distinct sorting of some proteins bound for the same destination has also been observed within the TGN. Basolateral proteins are often classified according to the dependence of their sorting on the μ1B clathrin adaptor subunit. Two basolateral proteins—the μ1B-dependent VSV-G protein and μ1B-independent Na,K-ATPase—exit the TGN in separate carriers, demonstrating that the role for the TGN in epithelial sorting extends beyond the strict apical/basolateral dichotomy.87 μ1B is expressed in a subset of epithelial tissues and, as part of the AP1 clathrin coat adaptor complex, facilitates sorting of proteins that use tyrosine-based sorting signals (such as VSV-G) through the Rab8-positive common recycling endosome (CRE).88 As a complement to this pathway, the TGN-localized μ1A isoform was recently shown to facilitate TGN export of a GFP-tagged LDL-R construct, which contains a nonconventional tyrosine-based basolateral signal and does not typically traverse the endosomal system.89
A nonconventional route for apical transit that bypasses the Golgi apparatus has also been proposed for a subset of apical proteins.90,91 A GFP-tagged version of the serglycin protein was shown to traffic to the apical membrane in the presence of the Golgi transport–blocking drug brefeldin.92 Apical delivery was also observed when a KDEL ER retrieval sequence was appended to serglycin to prevent the protein’s progress along the classic secretory pathway from the ER to the Golgi.92 Recently, analysis of the glycosylation patterns of newly synthesized polycystin-2 suggested that the ciliary pool of this protein exits the Golgi at the cis compartment, rather than from the TGN.93 Additional studies focusing on newly synthesized cohorts of native proteins expressed at wild-type levels in untreated cells will be necessary to establish whether there is indeed a Golgi bypass route that functions as a bona fide apical transport pathway.
Sorting can also occur after proteins exit the TGN. Lipid raft–dependent and –independent classes of apical cargo segregate from one another by vesicle fission following TGN exit.85 Furthermore, as discussed below, a substantial number of post-Golgi protein sorting processes occur in the endosomal system. Finally, some proteins are sorted via a transcellular route. Although few proteins appear to pursue such pathways in renal epithelial cells, many apical proteins in hepatic cells are sent first to the basolateral membrane, followed by endocytosis and transport to the apical/canalicular membrane.94
Sorting through Endosomes
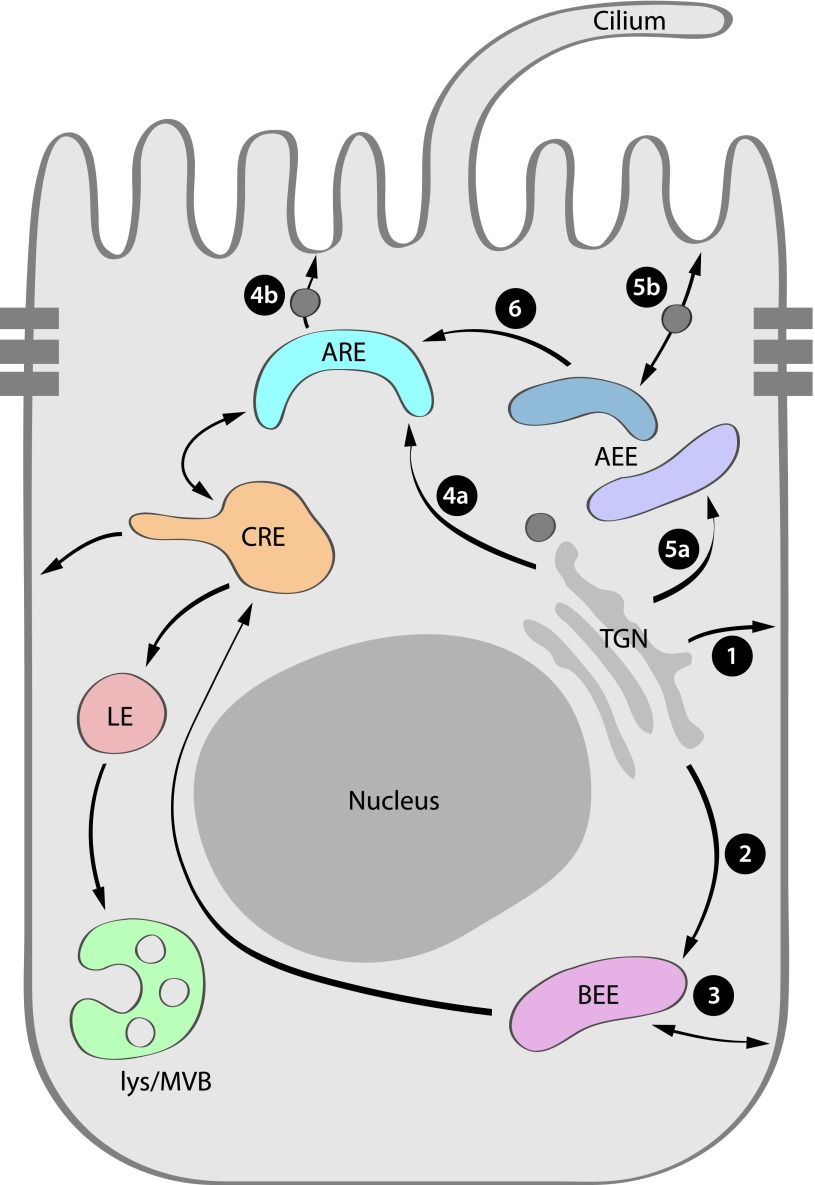
The endosomal system is an essential component of a cell’s trafficking network—both endocytic and exocytic—for membrane proteins, lipids, receptor-bound ligands, and various solutes (Figure 1). In polarized cells, proteins and lipids endocytosed from the apical and basolateral surface enter the apical (AEE) and basolateral early endosomes, respectively.95 From there, proteins can be sorted to the surface, targeted to the lysosomes for ultimate degradation, or transported to the CRE or apical recycling endosome (ARE). The ARE is a cup-shaped compartment in the subapical region of epithelial cells characterized by the presence of the small GTPase Rab11a and the motor protein Myosin Vb (MyoVb), and the absence of rapidly recycling transferrin receptor.96,97
Figure 1.
Apical and basolateral proteins pursue multiple biosynthetic routes to the plasma membrane following their exit from the TGN. This model depicts these routes as well as some endocytic pathways. Proteins targeted to the basolateral membrane can traffic directly from the TGN (1),87 or traffic through endosomes (2) before surface delivery (3).88,166 A similar direct route for apical proteins has not been observed. Endocytosed basolateral proteins traffic through the basolateral early endosome (BEE, 3) and CRE before recycling or degradation. Apically directed proteins traverse the apical early endosome (AEE, 4a and b) or the apical recycling endosome (ARE, 5a and b) before apical delivery. GPI-linked proteins, influenza HA protein, and other raft-dependent proteins traverse the AEE,98,99 while proteins such as endolyn and MUC1, whose trafficking depends on glycan residues, traffic through the ARE.99,167 Conversely, lactase phlorizin hydrolase and sucrase isomalatase traffic between the apical early and apical recycling endosomes (6).100 Proteins internalized from the apical membrane to the AEE can traffic to the ARE for recycling back to the surface, or to the CRE and late endosomes if they are targeted for degradation (unlabeled arrows). LE, late endosome; lys, lysosome; MVB, multivesicular body.
Multiple biosynthetic pathways passing through the endosomal system en route to the apical membrane have been observed. An elegant demonstration of trafficking of newly synthesized raft-dependent and raft-independent proteins through separate endosomal compartments was obtained by functionally inactivating the apical early and apical recycling endosomes.98,99 Overexpression of the dominant-negative acting MyoVb-tail domain selectively inhibited apical trafficking of the raft-independent apical protein endolyn, while horseradish peroxidase–mediated ablation of the AEE selectively inhibited apical trafficking of the raft-dependent influenza HA protein. Proteins with glycan-dependent signals are thought to traverse the ARE. Glycosylated growth hormone, which relies on N-glycans for its sorting, colocalizes with ARE markers en route to the apical surface, and its apical expression is decreased by overexpression of MyoVb-tail.65 In contrast, nonglycosylated GH did not localize to the ARE. In the case of the disaccharidases lactase phlorizin hydrolase and sucrase isomaltase—a raft-dependent protein and raft-independent protein, respectively—separation does not occur immediately after release from the TGN, but rather after sequential movement through Rab4-, Rab8-, and Rab11-positive endosomal compartments (or perhaps subcompartments).100
Mounting evidence demonstrates that, in addition to being separated into different endosomal compartments, cargoes are segregated into different subdomains within endosomes and that this lateral segregation facilitates cargo sorting (reviewed by Miaczynska and Zerial101). Using immuno-electron microscopy, Sonnichsen et al. demonstrated separation of resident Rab GTPases into distinct endosomal subdomains. Recycling endosomes exhibited Rab4- and Rab11-positive subdomains, and early endosomes possessed distinct Rab4 and Rab5 subdomains.102 Within early endosomes, recycling cargo segregates to tubular domains, while cargo headed for degradation remains in the spherical portion of the endosome.103 More recently, enrichment of recycling β2-adrenergic receptor was observed in tubular microdomains of endosomes that are stabilized by a local actin network.104 This localization depended on sorting information embedded within the β2-adrenergic receptor sequence, and it took place in different tubules and with different kinetics than did the bulk sorting of the rapidly recycling transferrin receptor. In polarized cells, apical and basolateral cargo segregated laterally in recycling endosomes in a phosphoinositide 3-kinase–dependent manner.105 This segregation was not observed when the same proteins were studied in nonpolarized cells. To fully establish the extent to which lateral segregation is operative and functionally important in sorting, it will be necessary to couple higher-resolution imaging of newly synthesized proteins with innovative assays capable of revealing and perturbing domain separations within endosomes, the Golgi network, and earlier trafficking compartments.
Cell-Specific Variations in Trafficking
Even when expressed in nonpolarized cells, apical and basolateral proteins can be separated into different cargo vesicles.86,106,107 This suggests that the machinery required to accomplish polarized sorting is expressed ubiquitously. However, polarized sorting can manifest highly cell type–specific patterns. The multiplicity of signal classes that are involved in apical and basolateral sorting, as well as the complex array of vesicular compartments that participate in the segregation of apical and basolateral proteins bearing these signals, allows for a plastic system in which distinct subsets of proteins can be redirected in response to environmental cues. Several proteins show cell-specific variations in trafficking.108–112 To demonstrate the remarkable capacity of the protein trafficking system to adapt to the physiologic requirements of particular cell states and tissues, we will focus on cell types in which the Na,K-ATPase complex exhibits an apical localization. As previously mentioned, the Na,K-ATPase complex localizes to the basolateral membrane of most polarized cells, where it plays an important role in the regulation of ion transport.
Cell Type–Specific Variations in Sorting
The Na,K-ATPase localizes to the apical membranes of cells of the retinal pigment epithelium (RPE)113–116 and the choroid plexus.117–120 Although these cells exhibit an apical distribution of this canonical basolateral protein, other proteins that serve as standard apical and basolateral markers retain their characteristic distributions.
Consistent with the vital role of the sodium pump and other transport proteins in determining the direction of solute flow, these distinct distributions of the Na,K-ATPase both reflect and determine the physiologic properties of their respective cell types. In renal tubular epithelial cells, basolateral sodium pump generates the transepithelial sodium gradients that drive most of the kidney’s absorptive fluxes of solutes and water.121 In the choroid plexus, the apical pool of Na,K-ATPase produces the secretory flux that is necessary for the generation of cerebral spinal fluid.122
One possible mechanism for differential localization may relate to ankyrin-fodrin complexes, which have been demonstrated to help link the Na,K-ATPase to the cytoskeleton and thus influence its localization. A role for basolateral ankyrin-fodrin in facilitating Na,K-ATPase basolateral localization has been suggested in MDCK and renal tubule cells.123,124 Conversely, ankyrin and fodrin were both found at the apical surface of choroid plexus and RPE cells, where ankyrin directly interacts with Na,K-ATPase.125,126 Fodrin and ankyrin also localize to the lateral membrane of choroid plexus cells, however,125 suggesting that the presence of these cytoskeletal linkers is not sufficient to specify Na,K-ATPase targeting. It is more likely that this cytoskeletal attachment plays a role in retention at the membrane, rather than directly determining the pump’s initial trafficking or ultimate distribution. It will be informative to more closely follow the biosynthetic route of native Na,K-ATPase protein in both renal and choroid plexus epithelial cells to parse out the alternate roles of selective targeting and selective retention in creating the distinct localizations of Na,K-ATPase these cell types exhibit. Recent advances in the generation of an immortalized choroid plexus cell line for use in in vitro experiments may prove extremely valuable in efforts to understand Na,K-ATPase trafficking in this tissue.127
In the case of RPE cells, the absence of epithelial-specific μ1B AP expression is associated with the aberrant apical localization of the coxsackie and adenovirus receptor protein.128 Interestingly, knockdown experiments in MDCK cells demonstrated that μ1B is required for correct recycling of coxsackie and adenovirus receptor to the basolateral membrane, but not for its initial biosynthetic delivery.128,129 Absence of μ1B cannot explain the apical localization of the Na,K-ATPase or the similarly “mis-sorted” CD147 in RPE cells, as the trafficking of these proteins is μ1B independent. While the underlying mechanism responsible for the apical accumulation of CD147 in RPE remains a mystery, apical CD147 appears to facilitate the apical accumulation of another protein, monocarboxylate transporter-1.130 In the case of the Na,K-ATPase, varying levels of the adherens junction protein E-cadherin were shown to affect the sodium pump’s apical versus basolateral localization in RPE cells. RPE cells expressing lower levels of E-cadherin exhibit more apical Na,K-ATPase than do cells possessing higher E-cadherin levels.131 The level of ankyrin and fodrin expression did not correlate with Na,K-ATPase localization in these cells.
Developmental Changes in Sorting
Studies performed on fetal kidneys revealed the presence of the Na,K-ATPase α1-subunit at the apical and lateral membranes (or distributed in a nonpolarized manner) of renal vesicles and newly formed collecting ducts in early stages of kidney organogenesis (Figure 2).132 This change in localization of active sodium pump is thought to play an important role in the formation of the lumen during tubulogenesis.133 Burrow and colleagues further showed that the timing of the postnatal switch to basolateral α1 distribution closely mirrored a switch in the expression of β-subunit isoforms. The sodium pump consists of two obligate subunits (α and β), as well as a regulatory component (γ). The catalytic α-subunit must assemble with the heavily glycosylated β-subunit in the ER in order to become catalytically active and to reach the cell surface.36,134–136 There are three α-subunit and two β-subunit isoforms; in mature tubules, the predominant heterodimer is α1β1. While β2 was expressed in the developing kidney, β1 protein was not observed, despite the presence of equivalent levels of β1 and β2 mRNA.133 β2 is also expressed in RPE,137 in the choroid plexus,138 and in tissue from patients with autosomal dominant polycystic kidney disease,139 where apical localization of Na,K-ATPase has been observed. These data, combined with the virtual lack of endogenous β2 expression in the adult kidney, suggest a role for β2 in guiding the apical accumulation of the Na,K-ATPase. The mechanism through which the β2-subunit might exert these effects remains to be determined.
Figure 2.
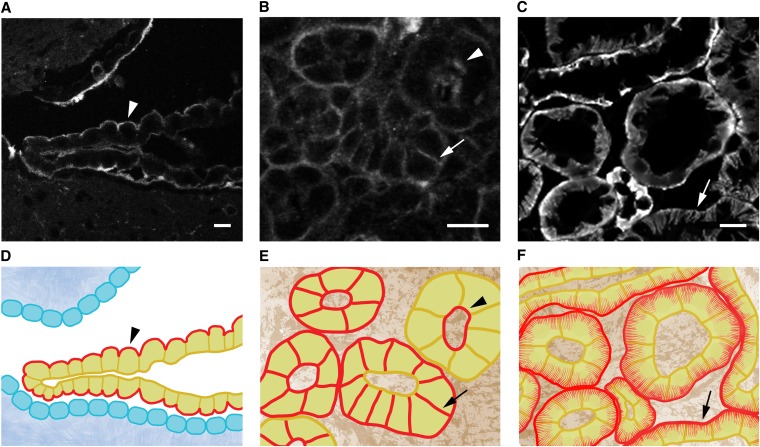
Na,K-ATPase displays distinct polarized localization in different tissues and developmental stages. (A–C) Immunolocalization of sodium pump α-subunit in murine choroid plexus (A), developing kidney (B), and adult kidney (C). Arrowheads indicate apical localization of α-subunit. Arrows indicate basolateral localization of α-subunit. (A) Sodium pump α-subunit localizes to the apical membrane of choroid plexus cells. (B) In developing kidneys harvested from embryonic day 17 mice, sodium pump α-subunit localizes to the apical membrane of the epithelial cells of the renal vesicles, which are the earliest structures in nephrogenesis. In newly developing collecting ducts, sodium pump α-subunit localizes to both apical and basolateral membranes or—in more mature collecting ducts—to basolateral membranes only. (C) Sodium pump α-subunit localizes to the basolateral infoldings of adult kidney tubules. (D–F) Schematic views of sodium pump localization in choroid plexus (D), the developing kidney (E), and the adult kidney (F). Sodium pump α-subunit is depicted in red. Bar=10 μm.
Epithelial cells can manifest tissue type–specific or developmental stage–dependent idiosyncrasies in their sorting behaviors. The mechanisms responsible for these behaviors remain largely uncharacterized. Clearly, however, while much of the polarized sorting machinery is present in all cell types, the regulated expression of distinct effector proteins can play a role in fine-tuning protein trafficking to a cell’s specific physiology and developmental state.
Impaired Protein Trafficking and Disease
Perturbations in the complicated network of subcellular trafficking pathways are implicated in many diseases.140 Pathogenic defects in trafficking include inverted polarity, disrupted recycling and intracellular retention, as highlighted in the following examples of renal pathology. In Dent disease, mutations in the chloride channel ClC-5 result in redistribution of H+-ATPase to the basolateral membrane rather than the apical membrane of proximal tubule cells.141,142 The ClC-5 mutation also impairs the function of the endocytic system, contributing to proteinuria. Bartter syndrome can be caused by mutations that perturb the trafficking or the distribution of the ClC-Kb channel or the renal outer medullary K+ channel.143 Mutations resulting in decreased internalization and downregulation of the epithelial sodium channel cause Liddle syndrome.144 The resulting constitutive activity of the epithelial sodium channel at the apical membrane leads to excessive sodium retention and pseudohyperaldosteronism.145 In the case of nephrogenic diabetes insipidus, mutations in the gene encoding the collecting duct water channel aquaporin-2 can produce a functional but misrouted channel that fails to reach the plasma membrane.146,147 Without aquaporin-2 at the apical membrane, the water permeability of collecting tubule epithelial cells is decreased and excess water is lost in urine. Disruption of apico-basal polarity has been associated with polycystic kidney disease.148 Mislocalization of Na,K-ATPase, the epidermal growth factor receptor, polycystin-1 and other proteins has been observed, although these trafficking defects may be consequences rather than causes of polycystic kidney disease pathogenesis.149–151
Conclusion
Polarized sorting of membrane proteins in epithelial cells is vital for the generation and maintenance of the cell polarity, which is in turn necessary for numerous aspects of epithelial cell function. Recent data contribute to our understanding of the vast complexity of sorting signals, regulators, and compartments that together control sorting to the apical and basolateral membranes. The ER, Golgi, and endosomal structures have all been observed to function as sorting sites along the biosynthetic pathway, with increasing evidence for cargo being separated into distinct subdomains within these compartments. Future studies will continue to uncover and further define apical and basolateral trafficking pathways and will reveal how they are altered to fit the physiology of different tissues. Given the importance of apico-basal polarity for normal kidney function, this research holds important implications for our understanding of the pathogenesis and therapy of renal disease.
Acknowledgments
The authors would like to thank all of the members of the Caplan Laboratory for helpful discussions. This work is supported by National Science Foundation Graduate Research Fellowship Grant No. DGE-1122492 (E.H.S.) and National Institutes of Health grants DK17433 and DK072612 (M.J.C.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Futter CE, Connolly CN, Cutler DF, Hopkins CR: Newly synthesized transferrin receptors can be detected in the endosome before they appear on the cell surface. J Biol Chem 270: 10999–11003, 1995 [DOI] [PubMed] [Google Scholar]
- 2.Laird V, Spiess M: A novel assay to demonstrate an intersection of the exocytic and endocytic pathways at early endosomes. Exp Cell Res 260: 340–345, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Lock JG, Stow JL: Rab11 in recycling endosomes regulates the sorting and basolateral transport of E-cadherin. Mol Biol Cell 16: 1744–1755, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sheff DR, Daro EA, Hull M, Mellman I: The receptor recycling pathway contains two distinct populations of early endosomes with different sorting functions. J Cell Biol 145: 123–139, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown PS, Wang E, Aroeti B, Chapin SJ, Mostov KE, Dunn KW: Definition of distinct compartments in polarized Madin-Darby canine kidney (MDCK) cells for membrane-volume sorting, polarized sorting and apical recycling. Traffic 1: 124–140, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Welling PA, Weisz OA: Sorting it out in endosomes: An emerging concept in renal epithelial cell transport regulation. Physiology (Bethesda) 25: 280–292, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muth TR, Caplan MJ: Transport protein trafficking in polarized cells. Annu Rev Cell Dev Biol 19: 333–366, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Le Bivic A, Sambuy Y, Patzak A, Patil N, Chao M, Rodriguez-Boulan E: An internal deletion in the cytoplasmic tail reverses the apical localization of human NGF receptor in transfected MDCK cells. J Cell Biol 115: 607–618, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hunziker W, Harter C, Matter K, Mellman I: Basolateral sorting in MDCK cells requires a distinct cytoplasmic domain determinant. Cell 66: 907–920, 1991 [DOI] [PubMed] [Google Scholar]
- 10.Matter K, Hunziker W, Mellman I: Basolateral sorting of LDL receptor in MDCK cells: The cytoplasmic domain contains two tyrosine-dependent targeting determinants. Cell 71: 741–753, 1992 [DOI] [PubMed] [Google Scholar]
- 11.Hunziker W, Fumey C: A di-leucine motif mediates endocytosis and basolateral sorting of macrophage IgG Fc receptors in MDCK cells. EMBO J 13: 2963–2969, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miranda KC, Khromykh T, Christy P, Le TL, Gottardi CJ, Yap AS, Stow JL, Teasdale RD: A dileucine motif targets E-cadherin to the basolateral cell surface in Madin-Darby canine kidney and LLC-PK1 epithelial cells. J Biol Chem 276: 22565–22572, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Brewer CB, Roth MG: A single amino acid change in the cytoplasmic domain alters the polarized delivery of influenza virus hemagglutinin. J Cell Biol 114: 413–421, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prill V, Lehmann L, von Figura K, Peters C: The cytoplasmic tail of lysosomal acid phosphatase contains overlapping but distinct signals for basolateral sorting and rapid internalization in polarized MDCK cells. EMBO J 12: 2181–2193, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matter K, Yamamoto EM, Mellman I: Structural requirements and sequence motifs for polarized sorting and endocytosis of LDL and Fc receptors in MDCK cells. J Cell Biol 126: 991–1004, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simonsen A, Bremnes B, Nordeng TW, Bakke O: The leucine-based motif DDQxxLI is recognized both for internalization and basolateral sorting of invariant chain in MDCK cells. Eur J Cell Biol 76: 25–32, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Haass C, Koo EH, Capell A, Teplow DB, Selkoe DJ: Polarized sorting of beta-amyloid precursor protein and its proteolytic products in MDCK cells is regulated by two independent signals. J Cell Biol 128: 537–547, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas DC, Roth MG: The basolateral targeting signal in the cytoplasmic domain of glycoprotein G from vesicular stomatitis virus resembles a variety of intracellular targeting motifs related by primary sequence but having diverse targeting activities. J Biol Chem 269: 15732–15739, 1994 [PubMed] [Google Scholar]
- 19.Wehrle-Haller B, Imhof BA: Stem cell factor presentation to c-Kit. Identification of a basolateral targeting domain. J Biol Chem 276: 12667–12674, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Deora AA, Gravotta D, Kreitzer G, Hu J, Bok D, Rodriguez-Boulan E: The basolateral targeting signal of CD147 (EMMPRIN) consists of a single leucine and is not recognized by retinal pigment epithelium. Mol Biol Cell 15: 4148–4165, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gephart JD, Singh B, Higginbotham JN, Franklin JL, González A, Fölsch H, Coffey RJ: Identification of a novel mono-leucine basolateral sorting motif within the cytoplasmic domain of amphiregulin. Traffic 12: 1793–1804, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohno H, Stewart J, Fournier MC, Bosshart H, Rhee I, Miyatake S, Saito T, Gallusser A, Kirchhausen T, Bonifacino JS: Interaction of tyrosine-based sorting signals with clathrin-associated proteins. Science 269: 1872–1875, 1995 [DOI] [PubMed] [Google Scholar]
- 23.Boll W, Gallusser A, Kirchhausen T: Role of the regulatory domain of the EGF-receptor cytoplasmic tail in selective binding of the clathrin-associated complex AP-2. Curr Biol 5: 1168–1178, 1995 [DOI] [PubMed] [Google Scholar]
- 24.Heilker R, Manning-Krieg U, Zuber JF, Spiess M: In vitro binding of clathrin adaptors to sorting signals correlates with endocytosis and basolateral sorting. EMBO J 15: 2893–2899, 1996 [PMC free article] [PubMed] [Google Scholar]
- 25.Pearse BM: Receptors compete for adaptors found in plasma membrane coated pits. EMBO J 7: 3331–3336, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glickman JN, Conibear E, Pearse BM: Specificity of binding of clathrin adaptors to signals on the mannose-6-phosphate/insulin-like growth factor II receptor. EMBO J 8: 1041–1047, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sosa MA, Schmidt B, von Figura K, Hille-Rehfeld A: In vitro binding of plasma membrane-coated vesicle adaptors to the cytoplasmic domain of lysosomal acid phosphatase. J Biol Chem 268: 12537–12543, 1993 [PubMed] [Google Scholar]
- 28.Sorkin A, Carpenter G: Interaction of activated EGF receptors with coated pit adaptins. Science 261: 612–615, 1993 [DOI] [PubMed] [Google Scholar]
- 29.Sorkin A, McKinsey T, Shih W, Kirchhausen T, Carpenter G: Stoichiometric interaction of the epidermal growth factor receptor with the clathrin-associated protein complex AP-2. J Biol Chem 270: 619–625, 1995 [DOI] [PubMed] [Google Scholar]
- 30.Kirchhausen T, Bonifacino JS, Riezman H: Linking cargo to vesicle formation: receptor tail interactions with coat proteins. Curr Opin Cell Biol 9: 488–495, 1997 [DOI] [PubMed] [Google Scholar]
- 31.Langer JD, Stoops EH, Béthune J, Wieland FT: Conformational changes of coat proteins during vesicle formation. FEBS Lett 581: 2083–2088, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Ohno H, Tomemori T, Nakatsu F, Okazaki Y, Aguilar RC, Foelsch H, Mellman I, Saito T, Shirasawa T, Bonifacino JS: Mu1B, a novel adaptor medium chain expressed in polarized epithelial cells. FEBS Lett 449: 215–220, 1999 [DOI] [PubMed] [Google Scholar]
- 33.Fölsch H, Ohno H, Bonifacino JS, Mellman I: A novel clathrin adaptor complex mediates basolateral targeting in polarized epithelial cells. Cell 99: 189–198, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Sugimoto H, Sugahara M, Fölsch H, Koide Y, Nakatsu F, Tanaka N, Nishimura T, Furukawa M, Mullins C, Nakamura N, Mellman I, Ohno H: Differential recognition of tyrosine-based basolateral signals by AP-1B subunit mu1B in polarized epithelial cells. Mol Biol Cell 13: 2374–2382, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Geering K, Theulaz I, Verrey F, Häuptle MT, Rossier BC: A role for the beta-subunit in the expression of functional Na+-K+-ATPase in Xenopus oocytes. Am J Physiol 257: C851–C858, 1989 [DOI] [PubMed] [Google Scholar]
- 36.Gottardi CJ, Caplan MJ: Molecular requirements for the cell-surface expression of multisubunit ion-transporting ATPases. Identification of protein domains that participate in Na,K-ATPase and H,K-ATPase subunit assembly. J Biol Chem 268: 14342–14347, 1993 [PubMed] [Google Scholar]
- 37.Garcia-Gonzalo FR, Reiter JF: Scoring a backstage pass: Mechanisms of ciliogenesis and ciliary access. J Cell Biol 197: 697–709, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lisanti MP, Sargiacomo M, Graeve L, Saltiel AR, Rodriguez-Boulan E: Polarized apical distribution of glycosyl-phosphatidylinositol-anchored proteins in a renal epithelial cell line. Proc Natl Acad Sci U S A 85: 9557–9561, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown DA, Crise B, Rose JK: Mechanism of membrane anchoring affects polarized expression of two proteins in MDCK cells. Science 245: 1499–1501, 1989 [DOI] [PubMed] [Google Scholar]
- 40.Lisanti MP, Caras IW, Davitz MA, Rodriguez-Boulan E: A glycophospholipid membrane anchor acts as an apical targeting signal in polarized epithelial cells. J Cell Biol 109: 2145–2156, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paladino S, Sarnataro D, Zurzolo C: Detergent-resistant membrane microdomains and apical sorting of GPI-anchored proteins in polarized epithelial cells. Int J Med Microbiol 291: 439–445, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Brown DA, Rose JK: Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell 68: 533–544, 1992 [DOI] [PubMed] [Google Scholar]
- 43.Paladino S, Sarnataro D, Pillich R, Tivodar S, Nitsch L, Zurzolo C: Protein oligomerization modulates raft partitioning and apical sorting of GPI-anchored proteins. J Cell Biol 167: 699–709, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simons K, van Meer G: Lipid sorting in epithelial cells. Biochemistry 27: 6197–6202, 1988 [DOI] [PubMed] [Google Scholar]
- 45.Simons K, Wandinger-Ness A: Polarized sorting in epithelia. Cell 62: 207–210, 1990 [DOI] [PubMed] [Google Scholar]
- 46.Urban J, Parczyk K, Leutz A, Kayne M, Kondor-Koch C: Constitutive apical secretion of an 80-kD sulfated glycoprotein complex in the polarized epithelial Madin-Darby canine kidney cell line. J Cell Biol 105: 2735–2743, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Le Bivic A, Garcia M, Rodriguez-Boulan E: Ricin-resistant Madin-Darby canine kidney cells missort a major endogenous apical sialoglycoprotein. J Biol Chem 268: 6909–6916, 1993 [PubMed] [Google Scholar]
- 48.Scheiffele P, Peränen J, Simons K: N-glycans as apical sorting signals in epithelial cells. Nature 378: 96–98, 1995 [DOI] [PubMed] [Google Scholar]
- 49.Kitagawa Y, Sano Y, Ueda M, Higashio K, Narita H, Okano M, Matsumoto S, Sasaki R: N-glycosylation of erythropoietin is critical for apical secretion by Madin-Darby canine kidney cells. Exp Cell Res 213: 449–457, 1994 [DOI] [PubMed] [Google Scholar]
- 50.Ihrke G, Bruns JR, Luzio JP, Weisz OA: Competing sorting signals guide endolyn along a novel route to lysosomes in MDCK cells. EMBO J 20: 6256–6264, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martínez-Maza R, Poyatos I, López-Corcuera B, N úñez E, Giménez C, Zafra F, Aragón C: The role of N-glycosylation in transport to the plasma membrane and sorting of the neuronal glycine transporter GLYT2. J Biol Chem 276: 2168–2173, 2001 [DOI] [PubMed] [Google Scholar]
- 52.Yu C-Y, Chen J-Y, Lin Y-Y, Shen K-F, Lin W-L, Chien C-L, ter Beest MB, Jou TS: A bipartite signal regulates the faithful delivery of apical domain marker podocalyxin/Gp135. Mol Biol Cell 18: 1710–1722, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yeaman C, Le Gall AH, Baldwin AN, Monlauzeur L, Le Bivic A, Rodriguez-Boulan E: The O-glycosylated stalk domain is required for apical sorting of neurotrophin receptors in polarized MDCK cells. J Cell Biol 139: 929–940, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gouyer V, Leteurtre E, Delmotte P, Steelant WF, Krzewinski-Recchi MA, Zanetta JP, Lesuffleur T, Trugnan G, Delannoy P, Huet G: Differential effect of GalNAcalpha-O-bn on intracellular trafficking in enterocytic HT-29 and Caco-2 cells: correlation with the glycosyltransferase expression pattern. J Cell Sci 114: 1455–1471, 2001 [DOI] [PubMed] [Google Scholar]
- 55.Naim HY, Joberty G, Alfalah M, Jacob R: Temporal association of the N- and O-linked glycosylation events and their implication in the polarized sorting of intestinal brush border sucrase-isomaltase, aminopeptidase N, and dipeptidyl peptidase IV. J Biol Chem 274: 17961–17967, 1999 [DOI] [PubMed] [Google Scholar]
- 56.Alfalah M, Jacob R, Preuss U, Zimmer KP, Naim H, Naim HY: O-linked glycans mediate apical sorting of human intestinal sucrase-isomaltase through association with lipid rafts. Curr Biol 9: 593–596, 1999 [DOI] [PubMed] [Google Scholar]
- 57.Spodsberg N, Alfalah M, Naim HY: Characteristics and structural requirements of apical sorting of the rat growth hormone through the O-glycosylated stalk region of intestinal sucrase-isomaltase. J Biol Chem 276: 46597–46604, 2001 [DOI] [PubMed] [Google Scholar]
- 58.Slimane TA, Lenoir C, Sapin C, Maurice M, Trugnan G: Apical secretion and sialylation of soluble dipeptidyl peptidase IV are two related events. Exp Cell Res 258: 184–194, 2000 [DOI] [PubMed] [Google Scholar]
- 59.Vagin O, Kraut JA, Sachs G: Role of N-glycosylation in trafficking of apical membrane proteins in epithelia. Am J Physiol Renal Physiol 296: F459–F469, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Delacour D, Koch A, Jacob R: The role of galectins in protein trafficking. Traffic 10: 1405–1413, 2009 [DOI] [PubMed] [Google Scholar]
- 61.Delacour D, Cramm-Behrens CI, Drobecq H, Le Bivic A, Naim HY, Jacob R: Requirement for galectin-3 in apical protein sorting. Curr Biol 16: 408–414, 2006 [DOI] [PubMed] [Google Scholar]
- 62.Delacour D, Gouyer V, Zanetta J-P, Drobecq H, Leteurtre E, Grard G, Moreau-Hannedouche O, Maes E, Pons A, André S, Le Bivic A, Gabius HJ, Manninen A, Simons K, Huet G: Galectin-4 and sulfatides in apical membrane trafficking in enterocyte-like cells. J Cell Biol 169: 491–501, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mishra R, Grzybek M, Niki T, Hirashima M, Simons K: Galectin-9 trafficking regulates apical-basal polarity in Madin-Darby canine kidney epithelial cells. Proc Natl Acad Sci U S A 107: 17633–17638, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mo D, Costa SA, Ihrke G, Youker RT, Pastor-Soler N, Hughey RP, Weisz OA: Sialylation of N-linked glycans mediates apical delivery of endolyn in MDCK cells via a galectin-9-dependent mechanism. Mol Biol Cell 23: 3636–3646, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mattila PE, Youker RT, Mo D, Bruns JR, Cresawn KO, Hughey RP, Ihrke G, Weisz OA: Multiple biosynthetic trafficking routes for apically secreted proteins in MDCK cells. Traffic 13: 433–442, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rodriguez-Boulan E, Gonzalez A: Glycans in post-Golgi apical targeting: sorting signals or structural props? Trends Cell Biol 9: 291–294, 1999 [DOI] [PubMed] [Google Scholar]
- 67.Kundu A, Avalos RT, Sanderson CM, Nayak DP: Transmembrane domain of influenza virus neuraminidase, a type II protein, possesses an apical sorting signal in polarized MDCK cells. J Virol 70: 6508–6515, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang XF, Compans RW, Chen S, Lamb RA, Arvan P: Polarized apical targeting directed by the signal/anchor region of simian virus 5 hemagglutinin-neuraminidase. J Biol Chem 272: 27598–27604, 1997 [DOI] [PubMed] [Google Scholar]
- 69.Lin S, Naim HY, Rodriguez AC, Roth MG: Mutations in the middle of the transmembrane domain reverse the polarity of transport of the influenza virus hemagglutinin in MDCK epithelial cells. J Cell Biol 142: 51–57, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Barman S, Nayak DP: Analysis of the transmembrane domain of influenza virus neuraminidase, a type II transmembrane glycoprotein, for apical sorting and raft association. J Virol 74: 6538–6545, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brock SC, Heck JM, McGraw PA, Crowe JE, Jr: The transmembrane domain of the respiratory syncytial virus F protein is an orientation-independent apical plasma membrane sorting sequence. J Virol 79: 12528–12535, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dunbar LA, Aronson P, Caplan MJ: A transmembrane segment determines the steady-state localization of an ion-transporting adenosine triphosphatase. J Cell Biol 148: 769–778, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shull GE, Lingrel JB: Molecular cloning of the rat stomach (H+ + K+)-ATPase. J Biol Chem 261: 16788–16791, 1986 [PubMed] [Google Scholar]
- 74.Tall RD, Alonso MA, Roth MG: Features of influenza HA required for apical sorting differ from those required for association with DRMs or MAL. Traffic 4: 838–849, 2003 [DOI] [PubMed] [Google Scholar]
- 75.Lipardi C, Nitsch L, Zurzolo C: Detergent-insoluble GPI-anchored proteins are apically sorted in fischer rat thyroid cells, but interference with cholesterol or sphingolipids differentially affects detergent insolubility and apical sorting. Mol Biol Cell 11: 531–542, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pang S, Urquhart P, Hooper NM: N-glycans, not the GPI anchor, mediate the apical targeting of a naturally glycosylated, GPI-anchored protein in polarised epithelial cells. J Cell Sci 117: 5079–5086, 2004 [DOI] [PubMed] [Google Scholar]
- 77.Rodriguez-Boulan E, Kreitzer G, Müsch A: Organization of vesicular trafficking in epithelia. Nat Rev Mol Cell Biol 6: 233–247, 2005 [DOI] [PubMed] [Google Scholar]
- 78.Fuller SD, Bravo R, Simons K: An enzymatic assay reveals that proteins destined for the apical or basolateral domains of an epithelial cell line share the same late Golgi compartments. EMBO J 4: 297–307, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rindler MJ, Ivanov IE, Plesken H, Rodriguez-Boulan E, Sabatini DD: Viral glycoproteins destined for apical or basolateral plasma membrane domains traverse the same Golgi apparatus during their intracellular transport in doubly infected Madin-Darby canine kidney cells. J Cell Biol 98: 1304–1319, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tveit H, Dick G, Skibeli V, Prydz K: A proteoglycan undergoes different modifications en route to the apical and basolateral surfaces of Madin-Darby canine kidney cells. J Biol Chem 280: 29596–29603, 2005 [DOI] [PubMed] [Google Scholar]
- 81.Vuong TT, Prydz K, Tveit H: Differences in the apical and basolateral pathways for glycosaminoglycan biosynthesis in Madin-Darby canine kidney cells. Glycobiology 16: 326–332, 2006 [DOI] [PubMed] [Google Scholar]
- 82.Alfalah M, Wetzel G, Fischer I, Busche R, Sterchi EE, Zimmer K-P, Sallmann H-P, Naim HY: A novel type of detergent-resistant membranes may contribute to an early protein sorting event in epithelial cells. J Biol Chem 280: 42636–42643, 2005 [DOI] [PubMed] [Google Scholar]
- 83.Muñiz M, Morsomme P, Riezman H: Protein sorting upon exit from the endoplasmic reticulum. Cell 104: 313–320, 2001 [DOI] [PubMed] [Google Scholar]
- 84.Castillon GA, Aguilera-Romero A, Manzano-Lopez J, Epstein S, Kajiwara K, Funato K, Watanabe R, Riezman H, Muñiz M: The yeast p24 complex regulates GPI-anchored protein transport and quality control by monitoring anchor remodeling. Mol Biol Cell 22: 2924–2936, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jacob R, Naim HY: Apical membrane proteins are transported in distinct vesicular carriers. Curr Biol 11: 1444–1450, 2001 [DOI] [PubMed] [Google Scholar]
- 86.Keller P, Toomre D, Díaz E, White J, Simons K: Multicolour imaging of post-Golgi sorting and trafficking in live cells. Nat Cell Biol 3: 140–149, 2001 [DOI] [PubMed] [Google Scholar]
- 87.Farr GA, Hull M, Mellman I, Caplan MJ: Membrane proteins follow multiple pathways to the basolateral cell surface in polarized epithelial cells. J Cell Biol 186: 269–282, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ang AL, Taguchi T, Francis S, Fölsch H, Murrells LJ, Pypaert M, Warren G, Mellman I: Recycling endosomes can serve as intermediates during transport from the Golgi to the plasma membrane of MDCK cells. J Cell Biol 167: 531–543, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gravotta D, Carvajal-Gonzalez JM, Mattera R, Deborde S, Banfelder JR, Bonifacino JS, Rodriguez-Boulan E: The clathrin adaptor AP-1A mediates basolateral polarity. Dev Cell 22: 811–823, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Prydz K, Tveit H, Vedeler A, Saraste J: Arrivals and departures at the plasma membrane: direct and indirect transport routes. Cell Tissue Res 352: 5–20, 2013 [DOI] [PubMed] [Google Scholar]
- 91.Marie M, Sannerud R, Avsnes Dale H, Saraste J: Take the ‘A’ train: on fast tracks to the cell surface. Cell Mol Life Sci 65: 2859–2874, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tveit H, Akslen LKA, Fagereng GL, Tranulis MA, Prydz K: A secretory Golgi bypass route to the apical surface domain of epithelial MDCK cells. Traffic 10: 1685–1695, 2009 [DOI] [PubMed] [Google Scholar]
- 93.Hoffmeister H, Babinger K, Gürster S, Cedzich A, Meese C, Schadendorf K, Osten L, de Vries U, Rascle A, Witzgall R: Polycystin-2 takes different routes to the somatic and ciliary plasma membrane. J Cell Biol 192: 631–645, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bartles JR, Feracci HM, Stieger B, Hubbard AL: Biogenesis of the rat hepatocyte plasma membrane in vivo: comparison of the pathways taken by apical and basolateral proteins using subcellular fractionation. J Cell Biol 105: 1241–1251, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sheff DR, Kroschewski R, Mellman I: Actin dependence of polarized receptor recycling in Madin-Darby canine kidney cell endosomes. Mol Biol Cell 13: 262–275, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lapierre LA, Kumar R, Hales CM, Navarre J, Bhartur SG, Burnette JO, Provance DW, Jr, Mercer JA, Bähler M, Goldenring JR: Myosin vb is associated with plasma membrane recycling systems. Mol Biol Cell 12: 1843–1857, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lapierre LA, Dorn MC, Zimmerman CF, Navarre J, Burnette JO, Goldenring JR: Rab11b resides in a vesicular compartment distinct from Rab11a in parietal cells and other epithelial cells. Exp Cell Res 290: 322–331, 2003 [DOI] [PubMed] [Google Scholar]
- 98.Cresawn KO, Potter BA, Oztan A, Guerriero CJ, Ihrke G, Goldenring JR, Apodaca G, Weisz OA: Differential involvement of endocytic compartments in the biosynthetic traffic of apical proteins. EMBO J 26: 3737–3748, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mattila PE, Kinlough CL, Bruns JR, Weisz OA, Hughey RP: MUC1 traverses apical recycling endosomes along the biosynthetic pathway in polarized MDCK cells. Biol Chem 390: 551–556, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cramm-Behrens CI, Dienst M, Jacob R: Apical cargo traverses endosomal compartments on the passage to the cell surface. Traffic 9: 2206–2220, 2008 [DOI] [PubMed] [Google Scholar]
- 101.Miaczynska M, Zerial M: Mosaic organization of the endocytic pathway. Exp Cell Res 272: 8–14, 2002 [DOI] [PubMed] [Google Scholar]
- 102.Sönnichsen B, De Renzis S, Nielsen E, Rietdorf J, Zerial M: Distinct membrane domains on endosomes in the recycling pathway visualized by multicolor imaging of Rab4, Rab5, and Rab11. J Cell Biol 149: 901–914, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mayor S, Presley JF, Maxfield FR: Sorting of membrane components from endosomes and subsequent recycling to the cell surface occurs by a bulk flow process. J Cell Biol 121: 1257–1269, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Puthenveedu MA, Lauffer B, Temkin P, Vistein R, Carlton P, Thorn K, Taunton J, Weiner OD, Parton RG, von Zastrow M: Sequence-dependent sorting of recycling proteins by actin-stabilized endosomal microdomains. Cell 143: 761–773, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Thompson A, Nessler R, Wisco D, Anderson E, Winckler B, Sheff D: Recycling endosomes of polarized epithelial cells actively sort apical and basolateral cargos into separate subdomains. Mol Biol Cell 18: 2687–2697, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Müsch A, Xu H, Shields D, Rodriguez-Boulan E: Transport of vesicular stomatitis virus G protein to the cell surface is signal mediated in polarized and nonpolarized cells. J Cell Biol 133: 543–558, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yoshimori T, Keller P, Roth MG, Simons K: Different biosynthetic transport routes to the plasma membrane in BHK and CHO cells. J Cell Biol 133: 247–256, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gundersen D, Powell SK, Rodriguez-Boulan E: Apical polarization of N-CAM in retinal pigment epithelium is dependent on contact with the neural retina. J Cell Biol 121: 335–343, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Marmorstein AD, Bonilha VL, Chiflet S, Neill JM, Rodriguez-Boulan E: The polarity of the plasma membrane protein RET-PE2 in retinal pigment epithelium is developmentally regulated. J Cell Sci 109: 3025–3034, 1996 [DOI] [PubMed] [Google Scholar]
- 110.Philp NJ, Wang D, Yoon H, Hjelmeland LM: Polarized expression of monocarboxylate transporters in human retinal pigment epithelium and ARPE-19 cells. Invest Ophthalmol Vis Sci 44: 1716–1721, 2003 [DOI] [PubMed] [Google Scholar]
- 111.Fanelli A, Grollman EF, Wang D, Philp NJ: MCT1 and its accessory protein CD147 are differentially regulated by TSH in rat thyroid cells. Am J Physiol Endocrinol Metab 285: E1223–E1229, 2003 [DOI] [PubMed] [Google Scholar]
- 112.Gill RK, Saksena S, Alrefai WA, Sarwar Z, Goldstein JL, Carroll RE, Ramaswamy K, Dudeja PK: Expression and membrane localization of MCT isoforms along the length of the human intestine. Am J Physiol Cell Physiol 289: C846–C852, 2005 [DOI] [PubMed] [Google Scholar]
- 113.Joseph DP, Miller SS: Apical and basal membrane ion transport mechanisms in bovine retinal pigment epithelium. J Physiol 435: 439–463, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Miller SS, Edelman JL: Active ion transport pathways in the bovine retinal pigment epithelium. J Physiol 424: 283–300, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Miller SS, Steinberg RH, Oakley B, 2nd: The electrogenic sodium pump of the frog retinal pigment epithelium. J Membr Biol 44: 259–279, 1978 [DOI] [PubMed] [Google Scholar]
- 116.Quinn RH, Miller SS: Ion transport mechanisms in native human retinal pigment epithelium. Invest Ophthalmol Vis Sci 33: 3513–3527, 1992 [PubMed] [Google Scholar]
- 117.Quinton PM, Wright EM, Tormey JM: Localization of sodium pumps in the choroid plexus epithelium. J Cell Biol 58: 724–730, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Masuzawa T, Ohta T, Kawamura M, Nakahara N, Sato F: Immunohistochemical localization of Na+, K+-ATPase in the choroid plexus. Brain Res 302: 357–362, 1984 [DOI] [PubMed] [Google Scholar]
- 119.Praetorius J: Water and solute secretion by the choroid plexus. Pflugers Arch 454: 1–18, 2007 [DOI] [PubMed] [Google Scholar]
- 120.Siegel GJ, Holm C, Schreiber JH, Desmond T, Ernst SA: Purification of mouse brain (Na+ + K+)-ATPase catalytic unit, characterization of antiserum, and immunocytochemical localization in cerebellum, choroid plexus, and kidney. J Histochem Cytochem 32: 1309–1318, 1984 [DOI] [PubMed] [Google Scholar]
- 121.Muth TR, Dunbar LA, Cortois-Coutry N, Roush DL, Caplan MJ: Sorting and trafficking of ion transport proteins in polarized epithelial cells. Curr Opin Nephrol Hypertens 6: 455–459, 1997 [DOI] [PubMed] [Google Scholar]
- 122.Zeuthen T, Wright EM: An electrogenic NA+/K+ pump in the choroid plexus. Biochim Biophys Acta 511: 517–522, 1978 [DOI] [PubMed] [Google Scholar]
- 123.Nelson WJ, Hammerton RW: A membrane-cytoskeletal complex containing Na+,K+-ATPase, ankyrin, and fodrin in Madin-Darby canine kidney (MDCK) cells: Implications for the biogenesis of epithelial cell polarity. J Cell Biol 108: 893–902, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Morrow JS, Cianci CD, Ardito T, Mann AS, Kashgarian M: Ankyrin links fodrin to the alpha subunit of Na,K-ATPase in Madin-Darby canine kidney cells and in intact renal tubule cells. J Cell Biol 108: 455–465, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Marrs JA, Napolitano EW, Murphy-Erdosh C, Mays RW, Reichardt LF, Nelson WJ: Distinguishing roles of the membrane-cytoskeleton and cadherin mediated cell-cell adhesion in generating different Na+,K(+)-ATPase distributions in polarized epithelia. J Cell Biol 123: 149–164, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gundersen D, Orlowski J, Rodriguez-Boulan E: Apical polarity of Na,K-ATPase in retinal pigment epithelium is linked to a reversal of the ankyrin-fodrin submembrane cytoskeleton. J Cell Biol 112: 863–872, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Monnot AD, Zheng W: Culture of choroid plexus epithelial cells and in vitro model of blood-CSF barrier. Methods Mol Biol 945: 13–29, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Diaz F, Gravotta D, Deora A, Schreiner R, Schoggins J, Falck-Pedersen E, Rodriguez-Boulan E: Clathrin adaptor AP1B controls adenovirus infectivity of epithelial cells. Proc Natl Acad Sci U S A 106: 11143–11148, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Carvajal-Gonzalez JM, Gravotta D, Mattera R, Diaz F, Perez Bay A, Roman AC, Schreiner RP, Thuenauer R, Bonifacino JS, Rodriguez-Boulan E: Basolateral sorting of the coxsackie and adenovirus receptor through interaction of a canonical YXXPhi motif with the clathrin adaptors AP-1A and AP-1B. Proc Natl Acad Sci U S A 109: 3820–3825, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Deora AA, Philp N, Hu J, Bok D, Rodriguez-Boulan E: Mechanisms regulating tissue-specific polarity of monocarboxylate transporters and their chaperone CD147 in kidney and retinal epithelia. Proc Natl Acad Sci U S A 102: 16245–16250, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Burke JM, Cao F, Irving PE: High levels of E-/P-cadherin: Correlation with decreased apical polarity of Na/K ATPase in bovine RPE cells in situ. Invest Ophthalmol Vis Sci 41: 1945–1952, 2000 [PubMed] [Google Scholar]
- 132.Minuth WW, Gross P, Gilbert P, Kashgarian M: Expression of the α-subunit of Na/K-ATPase in renal collecting duct epithelium during development. Kidney Int 31: 1104–1112, 1987 [DOI] [PubMed] [Google Scholar]
- 133.Burrow CR, Devuyst O, Li X, Gatti L, Wilson PD: Expression of the beta2-subunit and apical localization of Na+-K+-ATPase in metanephric kidney. Am J Physiol 277: F391–F403, 1999 [DOI] [PubMed] [Google Scholar]
- 134.Caplan MJ, Forbush B, 3rd, Palade GE, Jamieson JD: Biosynthesis of the Na,K-ATPase in Madin-Darby canine kidney cells. Activation and cell surface delivery. J Biol Chem 265: 3528–3534, 1990 [PubMed] [Google Scholar]
- 135.Jaunin P, Jaisser F, Beggah AT, Takeyasu K, Mangeat P, Rossier BC, Horisberger JD, Geering K: Role of the transmembrane and extracytoplasmic domain of beta subunits in subunit assembly, intracellular transport, and functional expression of Na,K-pumps. J Cell Biol 123: 1751–1759, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Béguin P, Hasler U, Staub O, Geering K: Endoplasmic reticulum quality control of oligomeric membrane proteins: Topogenic determinants involved in the degradation of the unassembled Na,K-ATPase alpha subunit and in its stabilization by beta subunit assembly. Mol Biol Cell 11: 1657–1672, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ruiz A, Bhat SP, Bok D: Expression and synthesis of the Na,K-ATPase beta 2 subunit in human retinal pigment epithelium. Gene 176: 237–242, 1996 [DOI] [PubMed] [Google Scholar]
- 138.Watts AG, Sanchez-Watts G, Emanuel JR, Levenson R: Cell-specific expression of mRNAs encoding Na+,K(+)-ATPase alpha- and beta-subunit isoforms within the rat central nervous system. Proc Natl Acad Sci U S A 88: 7425–7429, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wilson PD, Devuyst O, Li X, Gatti L, Falkenstein D, Robinson S, Fambrough D, Burrow CR: Apical plasma membrane mispolarization of NaK-ATPase in polycystic kidney disease epithelia is associated with aberrant expression of the beta2 isoform. Am J Pathol 156: 253–268, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.De Matteis MA, Luini A: Mendelian disorders of membrane trafficking. N Engl J Med 365: 927–938, 2011 [DOI] [PubMed] [Google Scholar]
- 141.Günther W, Lüchow A, Cluzeaud F, Vandewalle A, Jentsch TJ: ClC-5, the chloride channel mutated in Dent’s disease, colocalizes with the proton pump in endocytotically active kidney cells. Proc Natl Acad Sci U S A 95: 8075–8080, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Moulin P, Igarashi T, Van der Smissen P, Cosyns J-P, Verroust P, Thakker RV, Scheinman SJ, Courtoy PJ, Devuyst O: Altered polarity and expression of H+-ATPase without ultrastructural changes in kidneys of Dent’s disease patients. Kidney Int 63: 1285–1295, 2003 [DOI] [PubMed] [Google Scholar]
- 143.Naesens M, Steels P, Verberckmoes R, Vanrenterghem Y, Kuypers D: Bartter’s and Gitelman’s syndromes: from gene to clinic. Nephron Physiol 96: 65–78, 2004 [DOI] [PubMed] [Google Scholar]
- 144.Hansson JH, Nelson-Williams C, Suzuki H, Schild L, Shimkets R, Lu Y, Canessa C, Iwasaki T, Rossier B, Lifton RP: Hypertension caused by a truncated epithelial sodium channel gamma subunit: genetic heterogeneity of Liddle syndrome. Nat Genet 11: 76–82, 1995 [DOI] [PubMed] [Google Scholar]
- 145.Rossier BC, Pradervand S, Schild L, Hummler E: Epithelial sodium channel and the control of sodium balance: Interaction between genetic and environmental factors. Annu Rev Physiol 64: 877–897, 2002 [DOI] [PubMed] [Google Scholar]
- 146.Deen PM, Croes H, van Aubel RA, Ginsel LA, van Os CH: Water channels encoded by mutant aquaporin-2 genes in nephrogenic diabetes insipidus are impaired in their cellular routing. J Clin Invest 95: 2291–2296, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mulders SM, Knoers NV, Van Lieburg AF, Monnens LA, Leumann E, Wühl E, Schober E, Rijss JP, Van Os CH, Deen PM: New mutations in the AQP2 gene in nephrogenic diabetes insipidus resulting in functional but misrouted water channels. J Am Soc Nephrol 8: 242–248, 1997 [DOI] [PubMed] [Google Scholar]
- 148.Wilson PD: Apico-basal polarity in polycystic kidney disease epithelia. Biochim Biophys Acta 1812: 1239–1248, 2011 [DOI] [PubMed] [Google Scholar]
- 149.Wilson PD, Sherwood AC, Palla K, Du J, Watson R, Norman JT: Reversed polarity of Na(+) -K(+) -ATPase: mislocation to apical plasma membranes in polycystic kidney disease epithelia. Am J Physiol 260: F420–F430, 1991 [DOI] [PubMed] [Google Scholar]
- 150.Du J, Wilson PD: Abnormal polarization of EGF receptors and autocrine stimulation of cyst epithelial growth in human ADPKD. Am J Physiol 269: C487–C495, 1995 [DOI] [PubMed] [Google Scholar]
- 151.Lebeau C, Hanaoka K, Moore-Hoon ML, Guggino WB, Beauwens R, Devuyst O: Basolateral chloride transporters in autosomal dominant polycystic kidney disease. Pflugers Arch 444: 722–731, 2002 [DOI] [PubMed] [Google Scholar]
- 152.Gan Y, McGraw TE, Rodriguez-Boulan E: The epithelial-specific adaptor AP1B mediates post-endocytic recycling to the basolateral membrane. Nat Cell Biol 4: 605–609, 2002 [DOI] [PubMed] [Google Scholar]
- 153.Thomas DC, Brewer CB, Roth MG: Vesicular stomatitis virus glycoprotein contains a dominant cytoplasmic basolateral sorting signal critically dependent upon a tyrosine. J Biol Chem 268: 3313–3320, 1993 [PubMed] [Google Scholar]
- 154.Fölsch H, Pypaert M, Maday S, Pelletier L, Mellman I: The AP-1A and AP-1B clathrin adaptor complexes define biochemically and functionally distinct membrane domains. J Cell Biol 163: 351–362, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Stephens DJ, Banting G: Specificity of interaction between adaptor-complex medium chains and the tyrosine-based sorting motifs of TGN38 and lgp120. Biochem J 335: 567–572, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Roush DL, Gottardi CJ, Naim HY, Roth MG, Caplan MJ: Tyrosine-based membrane protein sorting signals are differentially interpreted by polarized Madin-Darby canine kidney and LLC-PK1 epithelial cells. J Biol Chem 273: 26862–26869, 1998 [DOI] [PubMed] [Google Scholar]
- 157.Johnson KF, Kornfeld S: A His-Leu-Leu sequence near the carboxyl terminus of the cytoplasmic domain of the cation-dependent mannose 6-phosphate receptor is necessary for the lysosomal enzyme sorting function. J Biol Chem 267: 17110–17115, 1992 [PubMed] [Google Scholar]
- 158.Distel B, Bauer U, Le Borgne R, Hoflack B: Basolateral sorting of the cation-dependent mannose 6-phosphate receptor in Madin-Darby canine kidney cells. Identification of a basolateral determinant unrelated to clathrin-coated pit localization signals. J Biol Chem 273: 186–193, 1998 [DOI] [PubMed] [Google Scholar]
- 159.Graichen R, Lösch A, Appel D, Koch-Brandt C: Glycolipid-independent sorting of a secretory glycoprotein to the apical surface of polarized epithelial cells. J Biol Chem 271: 15854–15857, 1996 [DOI] [PubMed] [Google Scholar]
- 160.Maruyama M, Kishimoto M, Ishida K, Watanabe Y, Nishikawa M, Masuda S, Sasaki R, Takakura Y: Cholesterol is required for the polarized secretion of erythropoietin in Madin-Darby canine kidney cells. Arch Biochem Biophys 438: 174–181, 2005 [DOI] [PubMed] [Google Scholar]
- 161.Huet G, Hennebicq-Reig S, de Bolos C, Ulloa F, Lesuffleur T, Barbat A, Carrière V, Kim I, Real FX, Delannoy P, Zweibaum A: GalNAc-alpha-O-benzyl inhibits NeuAcalpha2-3 glycosylation and blocks the intracellular transport of apical glycoproteins and mucus in differentiated HT-29 cells. J Cell Biol 141: 1311–1322, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kinlough CL, McMahan RJ, Poland PA, Bruns JB, Harkleroad KL, Stremple RJ, Kashlan OB, Weixel KM, Weisz OA, Hughey RP: Recycling of MUC1 is dependent on its palmitoylation. J Biol Chem 281: 12112–12122, 2006 [DOI] [PubMed] [Google Scholar]
- 163.Scheiffele P, Roth MG, Simons K: Interaction of influenza virus haemagglutinin with sphingolipid-cholesterol membrane domains via its transmembrane domain. EMBO J 16: 5501–5508, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Brown G, Jeffree CE, McDonald T, Rixon HWM, Aitken JD, Sugrue RJ: Analysis of the interaction between respiratory syncytial virus and lipid-rafts in Hep2 cells during infection. Virology 327: 175–185, 2004 [DOI] [PubMed] [Google Scholar]
- 165.Jacob R, Alfalah M, Grünberg J, Obendorf M, Naim HY: Structural determinants required for apical sorting of an intestinal brush-border membrane protein. J Biol Chem 275: 6566–6572, 2000 [DOI] [PubMed] [Google Scholar]
- 166.Cancino J, Torrealba C, Soza A, Yuseff MI, Gravotta D, Henklein P, Rodriguez-Boulan E, González A: Antibody to AP1B adaptor blocks biosynthetic and recycling routes of basolateral proteins at recycling endosomes. Mol Biol Cell 18: 4872–4884, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Potter BA, Ihrke G, Bruns JR, Weixel KM, Weisz OA: Specific N-glycans direct apical delivery of transmembrane, but not soluble or glycosylphosphatidylinositol-anchored forms of endolyn in Madin-Darby canine kidney cells. Mol Biol Cell 15: 1407–1416, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]