Abstract
Little is known regarding the natural longitudinal changes in cardiac structure and function in CKD. We hypothesized that baseline CKD stage is associated with progressive worsening in cardiac structure and function. We conducted a prospective longitudinal study, recruiting 300 patients with stages 3–5 CKD from a major regional tertiary center and university teaching hospital in Hong Kong. Baseline CKD stages were studied in relation to natural longitudinal changes in echocardiographic and tissue Doppler imaging–derived parameters. Over 1 year, the prevalence of left ventricular (LV) hypertrophy increased from 40.3% to 48.9%, median left atrial volume index increased 4.8 (interquartile range [IQR], 2.1, 7.7) ml/m2 (P<0.001), peak systolic mitral annular velocity decreased 0.5 (IQR, −1.5, 0.5) cm/s (P<0.001), early diastolic mitral annular velocity decreased 0.5 (IQR, −1.5, 0.5) cm/s (P<0.001), and eGFR declined 2.0 (IQR, −5.0, 0.0) ml/min per 1.73 m2. CKD stages 4 and 5 were associated with more baseline abnormalities in cardiac structure and function and predicted greater longitudinal progression in LV mass index (odds ratio [OR], 3.02; 95% confidence interval [95% CI], 1.39 to 6.58), volume index (OR, 2.58; 95% CI, 1.18 to 5.62), and left atrial volume index (OR, 2.61; 95% CI, 1.20 to 5.69) and worse diastolic dysfunction grade (OR, 3.17; 95% CI, 1.16 to 8.69) compared with stage 3a in the fully adjusted analysis. In conclusion, more advanced CKD at baseline may be associated with larger longitudinal increases in LV mass and volume and greater deterioration in diastolic function.
Keywords: echocardiography, left ventricular hypertrophy, chronic kidney disease, heart disease
CKD is a global public health problem and a major risk factor for cardiovascular disease.1,2 Cardiovascular mortality is estimated to be at least 10- to 100-fold higher in patients with ESRD than in the age-, race-, and sex-matched general population.2 This higher mortality is attributed to an increased risk of developing accelerated atherosclerosis, vascular calcification, heart failure, and sudden cardiac death.3 Even earlier stages of CKD are associated with an increased cardiovascular risk.1,4,5 Of the various cardiovascular complications, left ventricular hypertrophy (LVH) is one of the most prevalent6,7 and is predictive of an increased mortality and cardiovascular death.8,9 More than 75% of patients with CKD already had established LVH at the time of dialysis initiation.10 The prevalence of LVH in patients with CKD who are not undergoing dialysis was lower, ranging from 32% to 75%.6,7,11,12 Notably, patients with an eGFR<30 ml/min per 1.73 m2 showed a >2-fold increased risk of having LVH compared with those with an eGFR≥60 ml/min per 1.73 m2,6 suggesting that worsening kidney function or uremia, per se, may be an important contributing factor for LVH in CKD. Indeed, several previous cross-sectional studies have consistently shown an important inverse relationship between eGFR and left ventricular (LV) mass index.6,7,11,12 We therefore hypothesized that patients with more advanced CKD are subjected to a more adverse cardiovascular risk profile, such as worse salt, volume, and BP control; more uremia, anemia, and inflammation; and more disturbed CKD-mineral bone disease. This risk profile predisposes to a more longitudinal progression and deterioration in cardiac structure and function compared with those who have less advanced CKD.
However, previous studies that examined cardiac structural and functional abnormalities in patients with CKD who are not undergoing dialysis were mostly cross-sectional. Recent longitudinal data from the Chronic Renal Insufficiency Cohort (CRIC) and the Initiating Dialysis Early and Late study included patients with very advanced CKD or those already receiving dialysis.13,14 In addition, the CRIC study did not standardize the time interval between baseline and follow-up echocardiography.14 Thus, the natural changes of cardiac structure and function in earlier CKD remain largely unknown. We conducted the CASCADE (A Study of Longitudinal ChAngeS of CArdiac Structure and Function in Chronic KiDney DisEase) study with an aim to examine the natural, longitudinal changes of cardiac structure and function over 1 year in stages 3–5 CKD. We hypothesized that baseline CKD stages would predict changes in cardiac structure and function over time.
Results
Participant Characteristics
Of the 300 recruited patients, 278 completed both baseline and 1-year assessment (Supplemental Figure 1). Characteristics of the study population are shown in Table 1. Underlying causes of CKD included chronic GN in 80 (28.8%) patients, diabetic nephropathy in 72 (25.9%), hypertensive nephropathy in 25 (9%), polycystic kidney disease in 25 (9%), obstructive uropathy in 7 (2.6%), tubulointerstitial nephritis in 1 (0.4%), and unknown in 68 (24.4%).
Table 1.
Characteristics of the study population (n=278)
| Characteristic | Value |
|---|---|
| Men, n (%) | 156 (56.1) |
| Age (yr) | 60±10 |
| Diabetes mellitus, n (%) | 115 (41.4) |
| Smoking status, n (%) | |
| Nonsmoker | 197 (70.9) |
| Ex-smoker | 52 (18.7) |
| Current smoker | 29 (10.4) |
| Body mass index (kg/m2) | 25.7±4.3 |
| Background atherosclerotic vascular complications, n (%) | 54 (19.5) |
| Background coronary artery disease, n (%) | 26 (9.4) |
| Background heart failure, n (%) | 8 (2.9) |
| Background ischemic or hemorrhagic cerebrovascular event, n (%) | 26 (9.4) |
| Systolic BP (mmHg) | 128±19 |
| Diastolic BP (mmHg) | 77±11 |
| Serum urea (mg/dl) | 252±134 |
| Serum creatinine (mg/dl) | 2.52±1.39 |
| eGFR (ml/min per 1.73 m2) | 33.6±17.8 |
| CKD stages, n (%) | |
| 3a | 61 (21.9) |
| 3b | 77 (27.7) |
| 4 | 92 (33.1) |
| 5 | 48 (17.3) |
| Hemoglobin (g/dl) | 12.0±2.1 |
| Serum albumin (g/dl) | 4.18±0.35 |
| Serum calcium (mg/dl) | 9.40±0.40 |
| Serum phosphate (mg/dl) | 3.78±0.77 |
| Fasting glucose (mg/dl) | 105±36 |
| Fasting total cholesterol (mg/dl) | 180±40 |
| Fasting triglyceride (mg/dl) | 135±88 |
| Total antihypertensive agents (n) | 2.58±1.25 |
| Use of calcium-channel blockers, n (%) | 183 (65.8) |
| Use of β-blockers, n (%) | 142 (51.1) |
| Use of renin-angiotensin-aldosterone system blockers, n (%) | 223 (80.3) |
| Use of statins, n (%) | 119 (42.8) |
| Use of aspirin, n (%) | 53 (19.1) |
| Use of erythropoietin-stimulating agent, n (%) | 10 (3.6) |
Continuous data are expressed as mean±SD and categorical data as number (percentage).
Echocardiographic and Other Clinical Characteristics of the Entire Cohort
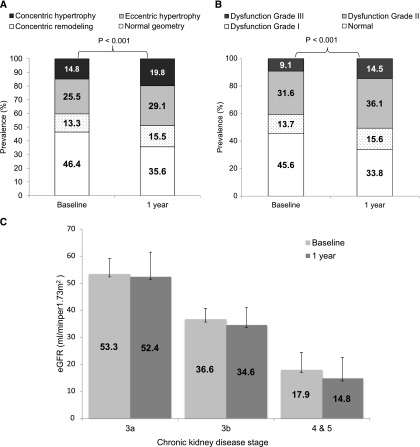
The entire cohort showed a significant increase in LV mass index (P<0.001) with an increased prevalence of LVH from 40.3% to 48.9% and worsening in geometric pattern over 1 year (Figure 1A). The entire cohort also showed a significant increase in LV volume index (P=0.004), left atrial volume index (LAVi) (P<0.001), and ratio of peak early transmitral flow velocity to early diastolic mitral annular velocity (E/Em) (P<0.001) with a significant decrease in ejection fraction (P=0.02), systolic mitral annular velocity (Sm) (P<0.001), early diastolic mitral annular velocity (Em) (P<0.001) (Table 2), and deterioration in diastolic dysfunction grades over 1 year (P<0.001) (Figure 1B). eGFR changed significantly by a median of −2.0 (interquartile range [IQR], 0 to −5.0) ml/min per 1.73 m2 (P<0.001) over 1 year in the entire cohort. Changes in systolic and diastolic BP were 3.75 (IQR, −9.00 to 18.00) mmHg and 0.50 (IQR, −7.50 to 8.10) mmHg, respectively, over 1 year. Other characteristics of the cohort at baseline and 1 year are compared in Supplemental Table 1.
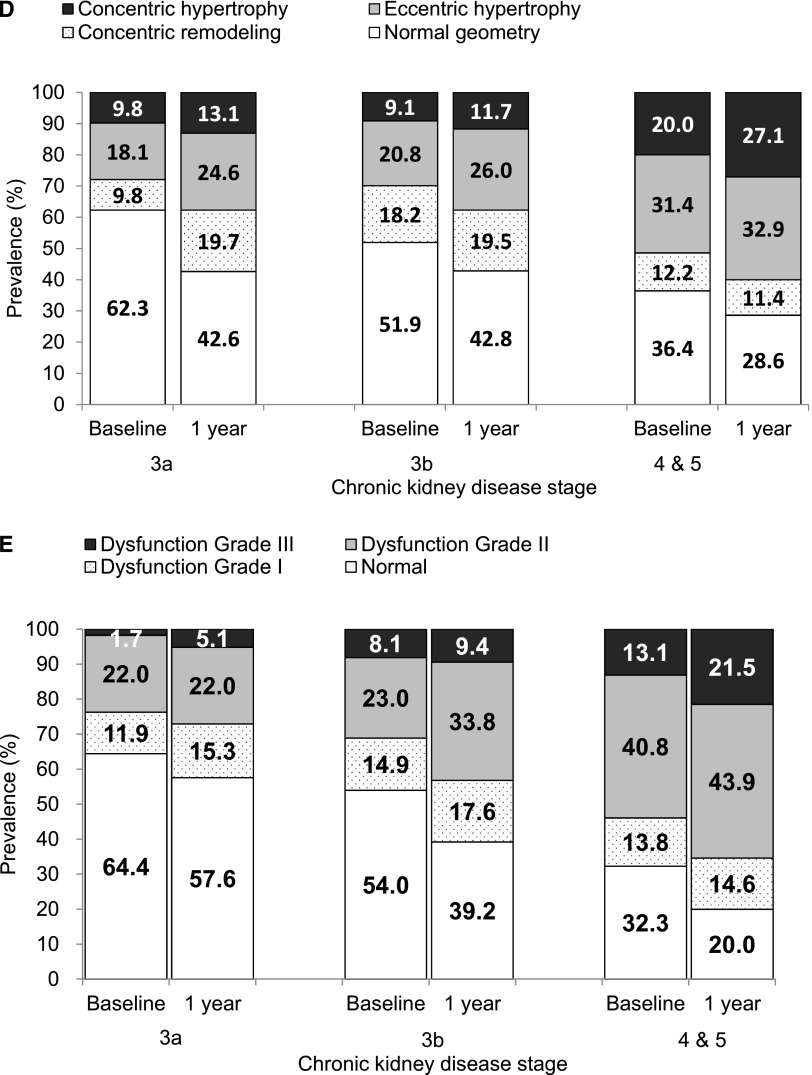
Figure 1.
(A) Increased prevalence of LV hypertrophy over 1 year. (B) Increased prevalence and severity of diastolic dysfunction over 1 year. (C) More advanced CKD showed more decline in eGFR over 1 year. (D) More advanced CKD showed greater increase in prevalence of LV hypertrophy and more deterioration in LV geometric pattern over 1 year. (E) More advanced CKD showed greater increase in prevalence and severity of diastolic dysfunction over 1 year. Diastolic function was graded according to the recommendation by the American Society of Echocardiography as normal, grades I (mild), II (moderate), and III (severe) diastolic dysfunction.22 Diastolic function was not graded in 10 patients with atrial fibrillation at baseline and in an additional 5 patients with new-onset atrial fibrillation at 1 year.
Table 2.
Echocardiographic parameters and eGFR at baseline and 1 year and changes over 1 year
| Variable | Baseline | 1 Year | Changes over 1 Year | P Value |
|---|---|---|---|---|
| eGFR (ml/min per 1.73 m2) | 30.9±15.4 | 28.5±17.0 | −2.0 (−5.0, 0.0) | <0.001 |
| LV mass index (g/m2) | 105±32 | 112±34 | 6.4 (0.6, 13.1) | <0.001 |
| LV volume index (ml/m2) | 42.8±11.1 | 43.9±11.8 | 0.9 (−1.5, 3.6) | 0.004 |
| LV ejection fraction (%) | 70.0±6.8 | 69.0±6.9 | −1.0 (−6.0, 3.0) | 0.02 |
| Midwall FS (%) | 17.7±2.3 | 17.4±2.6 | −0.3 (−1.9, 0.9) | 0.004 |
| LAVi (ml/m2) | 30.6±12.6 | 35.3±13.7 | 4.8 (2.1, 7.7) | <0.001 |
| E (cm/s) | 81.2±21.5 | 82.7±21.9 | 1.0 (−9.0, 12.0) | 0.2 |
| A (cm/s) | 87.2±22.3 | 88.8±24.3 | 1.0 (−7.0, 12.0) | 0.1 |
| E/A | 0.97±0.3 | 1.00±0.5 | 0 (−0.1, 0.1) | 0.4 |
| Sm (cm/s) | 9.0±1.9 | 8.4±2.0 | −0.5 (−1.5, 0.5) | <0.001 |
| Em (cm/s) | 9.7±2.8 | 9.0±2.6 | −0.5 (−1.5, 0.5) | <0.001 |
| Am (cm/s) | 11.6±2.3 | 11.3±2.3 | −0.5 (−1.5, 1.0) | 0.06 |
| E/Em | 9.0±3.4 | 9.7±3.8 | 0.7 (−0.5, 2.0) | <0.001 |
Continuous data are expressed as mean±SD. Changes over 1 year expressed as median (IQR). FS, fractional shortening; A, peak late transmitral flow velocity; E, peak early transmitral flow velocity; E/A, ratio of early to late transmitral flow velocity.
CKD Stages in Relation to Echocardiographic Parameters at Baseline and 1 Year
We stratified patients into three CKD groups—stages 3a, 3b, and 4 and 5—by their baseline eGFR. Clinical and biochemical characteristics of the 3 groups are detailed in Supplemental Table 2. Table 3 compares eGFR and various echocardiographic parameters at baseline and 1 year, as well as longitudinal changes in various echocardiographic parameters over 1 year, among the three groups. Patients with stage 4 and 5 CKD at baseline showed significantly more decline in eGFR (P=0.08) (Figure 1C) and had greater increase in LV mass index (P=0.04), volume index (P=0.003), and LAVi (P=0.001) over 1 year compared with patients in stage 3a and 3b. The prevalence of LVH in stage 4 and 5 was 51.4% at baseline and increased to 60.0% at 1 year; this was the highest among the three groups (Figure 1D). The prevalence of diastolic dysfunction in stage 4 and 5 was 67.7% at baseline and 80.0% at 1 year; this was the highest among the three groups. The greatest increase was with grade II and III diastolic dysfunction, which was observed in 53.9% of patients with stage 4 and 5 CKD at baseline and increased to 65.4% at 1 year (Figure 1E).
Table 3.
Comparisons of eGFR and echocardiographic parameters at baseline and 1 year and changes over 1 year across the different CKD stages
| Variable | Stage 3a (n=61) | Stage 3b (n=77) | Stages 4 and 5 (n=140) | P Value |
|---|---|---|---|---|
| eGFR (ml/min per 1.73m2) | ||||
| Baseline | 53.3±5.8 | 36.6±4.1 | 17.9±6.5 | <0.001 |
| 1 yr | 52.4±9.0 | 34.6±6.4 | 14.8±7.7 | <0.001 |
| Change | −0.92 (−4.75, 3.35) | −1.85 (−4.49, 1.66) | −2.77 (−5.11, −0.86) | 0.01 |
| LV mass index (g/m2) | ||||
| Baseline | 95.5±22.7 | 95.6±25.4 | 114.8±36.5 | <0.001 |
| 1 yr | 100.2±21.4 | 100.5±25.5 | 122.6±38.5 | <0.001 |
| Change | 4.19 (0.66, 12.17) | 3.67 (−0.35, 9.58) | 7.15 (0.96, 12.81) | 0.04 |
| LV volume index (ml/m2) | ||||
| Baseline | 41.0±8.0 | 39.4±9.0 | 45.4±12.7 | <0.001 |
| 1 yr | 40.7±7.7 | 40.2±8.7 | 47.3±13.6 | <0.001 |
| Change | 0.06 (−2.33, 2.08) | 0.62 (−1.40, 3.22) | 1.63 (−0.70, 3.78) | 0.003 |
| LAVi (ml/m2) | ||||
| Baseline | 27.6±8.5 | 27.9±10.1 | 33.3±14.6 | 0.01 |
| 1 yr | 30.5±8.7 | 32.2±9.9 | 39.1±16.1 | <0.001 |
| Change | 3.41 (1.32, 4.91) | 4.58 (1.71, 6.78) | 5.43 (2.32, 8.40) | 0.001 |
| LV ejection fraction (%) | ||||
| Baseline | 69.7±6.5 | 71.4±5.8 | 69.3±7.4 | 0.06 |
| 1 yr | 69.6±5.0 | 69.4±5.4 | 68.5±8.2 | 0.2 |
| Change | 0.00 (−5.07, 4.00) | −1.85 (−6.46, 1.85) | −0.92 (−4.95, 3.82) | 0.3 |
| Midwall fraction shortening (%) | ||||
| Baseline | 18.0±2.4 | 17.9±1.9 | 17.6±2.5 | 0.2 |
| 1 yr | 17.9±2.0 | 17.7±2.3 | 16.9±2.9 | 0.01 |
| Change | −0.43 (−1.74, 1.71) | −0.07 (−1.67, 0.84) | −0.44 (−1.91, 0.69) | 0.4 |
| Sm (cm/s) | ||||
| Baseline | 9.2±1.7 | 9.0±1.8 | 8.8±2.0 | 0.9 |
| 1 yr | 8.3±1.8 | 8.4±2.0 | 8.4±2.2 | 0.7 |
| Change | −0.92 (−2.00, 0.46) | −0.80 (−1.38, 0.43) | −0.46 (−1.50, 0.50) | 0.2 |
| E (cm/s) | ||||
| Baseline | 81.7±19.1 | 77.5±19.4 | 83.2±23.3 | 0.2 |
| 1 yr | 82.2±19.6 | 80.9±18.5 | 83.8±24.5 | 0.6 |
| Change | 0.00 (−0.93, 8.65) | 2.18 (−6.73, 12.46) | 0.00 (−10.15, 11.21) | 0.3 |
| A (cm/s) | ||||
| Baseline | 77.9±18.5 | 84.3±19.4 | 93.1±23. 8 | 0.002 |
| 1 yr | 79.3±19.8 | 85.2±19.8 | 94.2±27.7 | 0.01 |
| Change | 2.57 (−8.00, 13.8) | −0.86 (−6.46, 5.60) | 2.00 (−6.87, 12.00) | 0.4 |
| E/A ratio | ||||
| Baseline | 1.1±0.3 | 1.0±0.3 | 0.9±0.3 | 0.06 |
| 1 yr | 1.1±0.5 | 1.0±0.2 | 1.0±0.7 | 0.6 |
| Change | −0.04 (−0.22, 0.13) | 0.02 (−0.09, 0.18) | −0.00 (−0.12, 0.08) | 0.2 |
| Em (cm/s) | ||||
| Baseline | 10.6±2.9 | 9.9±2.5 | 9.2±2.9 | 0.04 |
| 1 yr | 10.0±2.6 | 9.1±2.4 | 8.5±2.6 | 0.02 |
| Change | −0.50 (−1.39, 0.45) | −0.50 (−1.62, 0.46) | −0.50 (−1.50, 0.49) | 1.0 |
| Am (cm/s) | ||||
| Baseline | 11.0±1.6 | 11.4±2.0 | 11.9±2.6 | 0.04 |
| 1 yr | 10.7±1.9 | 11.0±2.5 | 11.7±2.6 | 0.02 |
| Change | −0.50 (−1.09, 0.50) | −0.45 (−1.50, 0.92) | −0.41 (−1.50, 1.38) | 0.9 |
| E/Em ratio | ||||
| Baseline | 8.1±2.5 | 8.2±2.6 | 9.8±4.0 | 0.001 |
| 1 yr | 8.6±2.7 | 9.4±3.5 | 10.6±4.8 | 0.02 |
| Change | 0.36 (−0.60, 1.57) | 0.91 (0.07, 2.06) | 0.61 (−0.50, 1.95) | 0.1 |
Comparisons of various echocardiographic parameters among the three groups at baseline and 1 year were done using general linear model, adjusting for age and sex. Changes over 1 year are expressed as median (IQR), and comparisons across three groups were done by Kruskal–Wallis test. Data at baseline and 1 year are expressed as mean±SD. A, peak late transmitral flow velocity; E, peak early transmitral flow velocity; E/A, ratio of early to late transmitral flow velocity.
Table 4 describes the multiple logistic regression analysis of “progressors” in various echocardiographic parameters. Compared with stage 3a (reference group), patients with stages 4 and 5 CKD were independently predictive of being “progressors” in LV mass index (P=0.01), LV volume index (P=0.02), LAVi (P=0.02), and diastolic dysfunction grades (P=0.03) in the fully adjusted models controlling for other known factors associated with LV abnormalities, including age, sex, diabetes, background coronary artery disease, study baseline systolic BP, hemoglobin, serum albumin, LDL cholesterol, baseline use of renin-angiotensin-aldosterone system blockers, LV mass index, and change in systolic BP over 1 year. Supplemental Table 3 presents the multiple logistic regression analysis of progressors in various echocardiographic parameters in relation to eGFR as a continuous variable. Results were essentially very similar to those reported in Table 4 with eGFR stratified into three CKD groups.
Table 4.
Multiple logistic regression analysis of progressors in various cardiac structural and functional parameters in relation to baseline CKD stages
| Variable | Odds Ratio (95% CI), P Value | ||
|---|---|---|---|
| Stage 3a (n=61) | Stage 3b (n=77) | Stages 4 and 5 (n=140) | |
| Progressor of LVMi | |||
| Adjusted for age and sex | Reference | 0.93 (0.46 to 1.87), P=0.8 | 2.50 (1.32 to 4.74), P=0.01 |
| Plus baseline LVMi | Reference | 0.91 (0.45 to 1.85), P=0.8 | 2.71 (1.41 to 5.23), P=0.003 |
| Adjusted model | Reference | 0.94 (0.45 to 1.94), P=0.9 | 3.24 (1.50 to 6.98), P=0.003 |
| Fully adjusted model | Reference | 0.83 (0.39 to 1.75), P=0.6 | 2.78 (1.25 to 6.19), P=0.01 |
| Progressor of LVVi | |||
| Adjusted for age and sex | Reference | 1.66 (0.83 to 3.34), P=0.2 | 2.67 (1.40 to 5.06), P=0.003 |
| Plus baseline LVMi and LVVi | Reference | 1.62 (0.80 to 3.28), P=0.2 | 2.77 (1.43 to 5.38), P=0.003 |
| Adjusted model+baseline LVVi | Reference | 1.59 (0.76 to 3.33), P=0.2 | 2.62 (1.21 to 5.65), P=0.02 |
| Fully adjusted model+baseline LVVi | Reference | 1.63 (0.77 to 3.47), P=0.2 | 2.70 (1.22 to 5.99), P=0.02 |
| Progressor of LAVi | |||
| Adjusted for age and sex | Reference | 2.23 (1.10 to 4.54), P=0.03 | 3.28 (1.71 to 6.34), P<0.001 |
| Plus baseline LVMi and LAVi | Reference | 2.23 (1.09 to 4.55), P=0.03 | 3.21 (1.64 to 6.28), P=0.001 |
| Adjusted model+baseline LAVi | Reference | 1.99 (0.95 to 4.17), P=0.07 | 2.61 (1.22 to 5.63), P=0.01 |
| Fully adjusted model+baseline LAVi | Reference | 2.12 (0.99 to 4.56), P=0.05 | 2.50 (1.13 to 5.54), P=0.02 |
| Progressor of Sm | |||
| Adjusted for age and sex | Reference | 0.88 (0.44 to 1.77), P=0.7 | 0.62 (0.33 to 1.16), P=0.1 |
| Plus baseline LVMi+Sm | Reference | 0.90 (0.44 to 1.83), P=0.8 | 0.58 (0.30 to 1.12), P=0.1 |
| Adjusted model+baseline Sm | Reference | 0.87 (0.41 to 1.82), P=0.7 | 0.59 (0.27 to 1.26), P=0.2 |
| Fully adjusted model+baseline Sm | Reference | 0.81 (0.38 to 1.73), P=0.6 | 0.57 (0.26 to 1.26), P=0.2 |
| Progressor of ejection fraction | |||
| Adjusted for age and sex | Reference | 1.47 (0.74 to 2.91), P=0.3 | 1.28 (0.69 to 2.39), P=0.4 |
| Plus baseline LVMi+ejection fraction | Reference | 1.24 (0.56 to 2.76), P=0.6 | 1.59 (0.75 to 3.37), P=0.2 |
| Adjusted model+baseline ejection fraction | Reference | 1.20 (0.53 to 2.73), P=0.7 | 1.42 (0.60 to 3.32), P=0.4 |
| Fully adjusted model+baseline ejection fraction | Reference | 1.12 (0.48 to 2.59), P=0.8 | 1.38 (0.57 to 3.38), P=0.5 |
| Progressor of mwFS | |||
| Adjusted for age and sex | Reference | 0.69 (0.35 to 1.37), P=0.3 | 0.85 (0.46 to 1.58), P=0.6 |
| Plus baseline LVMi+mwFS | Reference | 0.73 (0.35 to 1.54), P=0.4 | 0.76 (0.38 to 1.52), P=0.4 |
| Adjusted model+baseline mwFS | Reference | 0.81 (0.38 to 1.77), P=0.6 | 0.98 (0.44 to 2.17), P=1.0 |
| Fully adjusted model+baseline mwFS | Reference | 0.79 (0.36 to 1.74), P=0.6 | 0.90 (0.40 to 2.07), P=0.8 |
| Progressor in diastolic dysfunction gradea | |||
| Adjusted for age and sex | Reference | 2.37(0.98 to 5.75), P=0.06 | 2.71(1.19 to 6.18), P=0.02 |
| Plus baseline LVMi+diastolic function grade | Reference | 2.60 (1.03 to 6.53), P=0.04 | 3.14 (1.31 to 7.53), P=0.01 |
| Adjusted model+baseline diastolic function grade | Reference | 2.48 (0.96 to 6.39),. P=0.06 | 3.34 (1.26 to 8.91), P=0.02 |
| Fully adjusted model+baseline diastolic function grade | Reference | 2.35 (0.87 to 6.37), P=0.09 | 2.84 (1.02 to 7.89), P=0.05 |
“Progressor” was defined as those in the upper 50th percentile for changes in LVMi, LVVi, or LAVi over 1 year and those with changes over 1 year in the lower 50th percentile for Sm and ejection fraction. Adjusted model: adjusted for known factors associated with LV abnormalities, including age, sex, diabetes, background coronary artery disease, baseline systolic BP, hemoglobin, serum albumin, LDL cholesterol, baseline LVMi, and change in systolic BP over 1 year. Fully adjusted model: adjusted for all factors above plus medications, including use of renin-angiotensin-aldosterone system blockers, β-blockers, calcium-channel blockers, and diuretics. 95% CI, 95% confidence interval; LVMi, LV volume index; LVV, LV volume index; mwFS, midwall fractional shortening.
“Progressor” in diastolic dysfunction grade was defined as deterioration in diastolic function over 1 year by 1 or more grades according to the diastolic dysfunction grading by the American Society of Echocardiography using a combination of echocardiographic parameters, including LAVi, average of septal and lateral Em, and E/Em ratio. Patients who already had the most severe form of diastolic dysfunction (grade III) at baseline were not considered in the diastolic dysfunction progression analysis.
Discussion
To the best of our knowledge, this prospective study is the first to examine the natural longitudinal changes of cardiac structure and function in stages 3–5 CKD over 1 year. LV mass index and volume index increased significantly and both systolic and diastolic function worsened (as denoted by a significant reduction in ejection fraction and Sm and by decreasing Em, increasing E/Em ratio, increasing LAVi, and worsening diastolic function grades, respectively) in a cohort of patients with stages 3–5 CKD who showed a median eGFR change of −2 (IQR, 0 to −5) ml/min per 1.73 m2 over a 1-year period.
The other key novel finding is that more severe CKD stages at baseline predicted greater longitudinal increase in LV mass index and volume index over 1 year. This relationship remained significant independent of age, sex, baseline cardiac abnormalities, and other important confounding covariates. Our results contrast the CRIC longitudinal study showing no significant change in LV mass, but worsening of ejection fraction between advanced CKD and ESRD, with the mean interval between the two echocardiograms being 2 years.14 The exact explanation for these differences is not clear, but they may be partly due to differences in study design, sample size, and population. Comparatively, our study has a larger sample size than the CRIC longitudinal echocardiography study14 and may thus be more powered to detect a significant difference in LV mass index and other echocardiographic parameters over 1 year. Our study also differs from the CRIC longitudinal echocardiography study that examined patients with stage 5 CKD who progressed to ESRD. Our study has several other notable and important strengths in being prospectively designed, having a standardized time frame between baseline and 1-year echocardiograms in all patients, including a contemporary cohort of patients with stages 3–5 CKD, and having very low dropout and a missing echocardiography rate (approximately 7%, including death as a reason for dropout). In addition, our study represents the first and the largest longitudinal study to date that incorporated tissue Doppler imaging in the serial assessment of systolic and diastolic function in patients with CKD who are not undergoing dialysis. Tissue Doppler imaging represents a more sensitive and accurate method for functional assessment than conventional flow Doppler imaging.15 Furthermore, all echocardiograms were analyzed by a single experienced cardiologist who demonstrated good intraobserver reproducibility. Thus, our findings should be generalizable to other CKD populations.
Our findings of more LVH and dilatation with more advanced CKD stages at baseline were consistently demonstrated after 1 year, clearly confirming that cardiac structural abnormalities and their progression were closely related to the severity of kidney dysfunction, with more deterioration seen in stages 4 and 5 than in stages 3a and 3b. Furthermore, a greater increase in the prevalence of concentric hypertrophy was observed over 1 year with CKD stages 4 and 5 (7.1%) than with stages 3a (3.3%) and 3b (2.6% increase). These novel findings further extend the study by Go and coworkers and explain why more advanced CKD was associated with greater risk of mortality and cardiovascular events.1 Our observations are well in keeping with several previous cross-sectional analyses, including the CRIC study, showing an important inverse relation between eGFR and the degree of LVH.6,7,11,12,16,17 The prevalence of LVH in our cohort at baseline (40.3%) appeared similar to that noted in some earlier reports,7,11,12 although it was lower compared with the CRIC cross-sectional study.6 Previous studies demonstrated the importance of LVH in predicting adverse clinical outcomes and cardiovascular events in CKD.18,19 Studies in dialysis patients also showed that further increase in LV mass was associated with more adverse clinical outcomes compared with patients with LV mass regression.20,21 Notably, accompanying these structural changes, we observed a significant worsening in diastolic function parameters (namely, decreased Em, increased E/Em ratio, increased LAVi) but not systolic function (as denoted by ejection fraction and Sm) across stages 3a, 3b, and 4 and 5 CKD at both baseline and 1 year. This observation differed from that of the CRIC study, which showed a very similar prevalence of diastolic dysfunction, defined using flow Doppler imaging across the different stages of nondialysis CKD and that did not increase further with progression to ESRD.6 It is well recognized that diastolic function is best defined using a combination of echocardiographic parameters, namely Em, E/Em ratio, and LAVi, rather than relying on a single parameter.22 According to use of combination of these echocardiographic parameters to grade diastolic function,22 patients with stages 4 and 5 CKD showed the highest prevalence of diastolic dysfunction at baseline (67.7%) and the prevalence increased to 80% after 1 year. More than half of the patients with stages 4 and 5 CKD had grades II and III diastolic dysfunction at baseline, and that increased further to 65.4% at 1 year. Furthermore, patients with stages 4 and 5 CKD at baseline showed more longitudinal deterioration in diastolic function grades over 1 year compared with patients who had stages 3a and 3b CKD. Considering only patients with normal diastolic function and those with grades I and II diastolic dysfunction, stages 4 and 5 CKD were independently associated with more deterioration in diastolic dysfunction grades in the fully adjusted multiple logistic regression analysis compared with stages 3a and 3b. These findings for the first time demonstrate the importance of baseline CKD stages in predicting longitudinal progression of diastolic dysfunction.
On the other hand, there was no significant difference in the longitudinal changes of systolic function among the three groups, suggesting that worsening diastolic dysfunction accounts for the primary functional abnormality associated with increasing LVH and dilatation in progressive CKD. Consistently, more severe CKD at baseline was also significantly predictive of greater longitudinal increase in left atrial volume independent of age, sex, and other confounding covariates. Left atrial enlargement is regarded as a morphophysiologic expression of diastolic dysfunction and provides important structural and diastolic functional assessment complementary to LV mass and systolic function.23,24 Left atrial volume also displays additional prognostic value beyond that provided by other echocardiographic parameters in dialysis patients.25–27 Thus, in keeping with earlier cross-sectional studies that showed greater impairment of diastolic function than systolic function in patients with CKD who were not undergoing dialysis,28,29 our longitudinal study demonstrated that more advanced CKD stages at baseline were associated with more deterioration in diastolic function over time but not systolic function. Our observations provide important novel insights into the nature of structural and functional changes of the myocardium with CKD progression. We hypothesized that increasing salt and fluid retention with worsening BP control are probably important contributing factors to more LV structural changes, remodeling, and greater diastolic dysfunction in advanced CKD. As shown by our data, the greater use of calcium-channel blockers and diuretics, as well as more eccentric and concentric hypertrophy with worsening CKD, favored this hypothesis. Uremia, anemia, hypoalbuminemia, and disordered mineral metabolism also probably contribute to more adverse LV structural and diastolic functional parameters with worsening CKD. Further study will be needed to elucidate the relative contributions of these different factors.
Our study has some limitations that are worth noting. Even though our study is so far the largest to examine longitudinal changes of cardiac structure and function in CKD, our sample size may still be regarded as slightly underpowered for attempting to evaluate between-group differences in the changes in individual systolic and diastolic functional parameters over 1 year. In addition, we did not have urine protein or albumin excretion data in this cohort.
In conclusion, this prospective longitudinal study demonstrated a significant deterioration in cardiac structure and systolic and diastolic function over 1 year in patients with stages 3–5 CKD. More advanced CKD stages at baseline may be associated with more longitudinal progression in LV mass and volume and diastolic dysfunction but not systolic dysfunction. These findings form an important rationale for early institution of therapeutic strategies in CKD to prevent progressive cardiac hypertrophy, dilatation, and diastolic dysfunction. Our observations provide important novel insights to planning future clinical trials that aim to retard cardiac structural and functional alterations and improve cardiovascular outcomes in CKD.
Concise Methods
Study Design
This is a prospective study with 1-year longitudinal follow-up. The institutional review board and ethics committee of the Hong Kong West Cluster approved the study protocol. All patients provided written informed consent before study entry. Altogether, 300 eligible patients with stages 3–5 CKD were recruited consecutively from the Renal Outpatient Clinic of a major regional tertiary care and university teaching hospital in Hong Kong between May and December 2011. Included were patients with stages 3–5 CKD defined according to the Kidney Disease Improving Global Outcomes guideline, which classified eGFR of 45–59, 30–44, 15–29, and <15 ml/min per 1.73 m2 as stages 3a, 3b, 4, and 5, respectively.30 We used the abbreviated Modification of Diet in Renal Disease equation to estimate GFR.31 We excluded patients with hypertrophic cardiomyopathy, underlying malignancy, chronic liver disease, systemic lupus erythematosus, chronic rheumatic heart disease, congenital heart disease, other significant valvular heart disease, and refusal to give consent. The enrollment of study patients is shown in Supplemental Figure 1.
Data Collection
We collected data on smoking status, diabetes mellitus, background kidney disease, coronary artery disease, heart failure, ischemic or hemorrhagic cerebrovascular events, atherosclerotic vascular complications, and medications on study entry. With a mercury sphygmomanometer, systolic and diastolic BP were measured twice: when patients presented to the center for study echocardiography at baseline and then at 1 year. Measurements were made on either arm after the patient had rested for 15 minutes, and readings were averaged to give the final systolic and diastolic BP.
Echocardiography and Tissue Doppler Imaging
Transthoracic echocardiography was performed using a Vivid-I ultrasonographic system (GE Healthcare) with an M3S multifrequency transducer according to the guidelines of the American Society of Echocardiography at baseline and 1 year.22,32 All examinations were performed, and images were analyzed offline by a single experienced cardiologist at both time points, blinded to all clinical details of patients, using the EchoPAC software (GE-vingmed). All images were analyzed after both baseline and first year echocardiogram images were acquired but were not read in tandem. The cardiologist was blinded to the first versus second echocardiogram reading of all patients. Three consecutive cardiac cycles were performed and average values of the three were recorded for all echocardiographic parameters.
LV ejection fraction was calculated by the modified biplane Simpson method.33 LV mass was obtained by a formula recommended by the American Society of Echocardiography32 and indexed by body surface area. LV hypertrophy was defined as LV mass indexed by body surface area>115 g/m2 in men and >95 g/m2 in women. Relative wall thickness was calculated as two times the posterior wall thickness in end-diastole divided by LV internal dimensions in end-diastole. LV geometric patterns were defined as normal geometry, concentric remodeling, eccentric hypertrophy, and concentric hypertrophy on the basis of relative wall thickness and LV mass index.32 Left atrial volume was assessed by tracing the left atrial endocardial borders and height of left atrium in apical two-chamber and four-chamber views at end-systole and was indexed by body surface area.32 Mitral inflow velocities were obtained using pulsed-wave Doppler imaging as described previously for peak E-wave and A-wave velocity.34 Tissue Doppler imaging was performed with sample volumes placed at septal and lateral mitral annulus during systole and diastole to estimate Sm, Em, and late diastolic mitral annular velocity (Am) as previously described from apical four-chamber view.35,36 The final Sm, Em, and Am represented the mean values at septal and lateral mitral annulus. The ratio of E/Em was used as an estimate of LV filling pressure.37 Diastolic dysfunction was defined as an LAVi≥34 ml/m2 and an Em<9 cm/s, with severity graded as I (mild), II (moderate), and III (severe) if E/Em ratio was ≤8, 9–2, and ≥13, respectively, according to the American Society of Echocardiography.22 Ten patients with atrial fibrillation at baseline and an additional 5 patients with new-onset atrial fibrillation at 1 year were excluded from grading of diastolic function. Echocardiographic images of 10 patients were analyzed on two separate occasions 10 days apart by the same observer to test for reproducibility of various measurements. The intraobserver tests showed excellent reproducibility, with intraclass correlation coefficients (95% confidence intervals ) of 0.99 (0.98 to 0.99) for LV mass, 0.98 (0.98 to 0.99) for left atrial volume, 0.99 (0.98 to 0.99) for LV volume, 0.99 (0.95 to 1.00) for ejection fraction, 0.98 (0.97 to 0.99) for peak E wave, 0.96 (0.93 to 0.99) for peak A wave, 0.97 (0.94 to 0.97) for Sm, 0.96 (0.84 to 0.99) for Em, and 0.98 (0.92 to 1.00) for Am.
Biochemical Analysis
EDTA and heparinized blood samples were collected at baseline and 1 year at the time of echocardiography for measurement of serum urea, creatinine, calcium, phosphorus and albumin, urate, fasting glucose, total cholesterol, LDL cholesterol, triglyceride, and blood hemoglobin in a standard hospital biochemistry laboratory.
Statistical Analyses
Continuous data are expressed as mean±SD or median (IQR), depending on the distribution of data, and categorical data are expressed as number (percentage). Comparisons in the various parameters between baseline and 1 year were performed by the paired t test for continuous data or McNemar test for categorical data. General linear model was used to compare differences in various parameters at baseline and 1 year among the three CKD groups, adjusting for age and sex. Changes in the various parameters over 1 year are expressed as median (IQR) because of non-normal distribution, and comparisons among the three groups were done using the Kruskal–Wallis test.
Changes in LV mass index, volume index, LAVi, ejection fraction, midwall fractional shortening, and Sm over 1 year were stratified by the median. “Progressors” in LV mass index, volume index, and LAVi were defined as those with changes in the corresponding echocardiographic parameters over 1 year in the upper 50th percentile. Progressors in ejection fraction, midwall fractional shortening, and Sm were defined as those with changes in ejection fraction, midwall fractional shortening, and Sm over 1 year in the lower 50th percentile. Progressors in diastolic dysfunction grade referred to those with diastolic function that deteriorated by ≥1 grade, defined using a combination of LAVi, Em, and E/Em according to the American Society of Echocardiography.18 Patients with improved or the same diastolic dysfunction grade after 1 year were classified as nonprogressors in diastolic dysfunction. Multiple logistic regression analysis was performed to determine the importance of baseline CKD stages in predicting progressors in various echocardiographic parameters, adjusting for age, sex, baseline echocardiographic parameters, and other confounding covariates in a stepwise fashion. Statistical analysis was performed using SPSS software, version 20.0 (SPSS, Inc., Chicago, IL). A two-sided P value <0.05 was considered to indicate statistical significance.
Disclosures
A.Y.-M.W. received speaker honoraria from Sanofi, Fresinius Kabi, and Roche Diagnostics; received grants from Baxter, Sanofi, and AbbVie; and has served on the advisory board of Sanofi.
Supplementary Material
Acknowledgments
We acknowledge Sharon Wong, Sharon Cheung, Eunice Shiu, and Anne Geursen-Banzhaf for assisting in the study coordination and clinical data collection. We are indebted to all patients who participated in this study.
This study was supported by an independent research grant from Sanofi Renal, Cambridge, Massachusetts.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013080899/-/DCSupplemental.
References
- 1.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW, American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention : Kidney disease as a risk factor for development of cardiovascular disease — A statement from the American Heart Association councils on kidney in cardiovascular disease, high blood pressure research, clinical cardiology, and epidemiology and prevention. Circulation 108: 2154–2169, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Herzog CA, Asinger RW, Berger AK, Charytan DM, Díez J, Hart RG, Eckardt KU, Kasiske BL, McCullough PA, Passman RS, DeLoach SS, Pun PH, Ritz E: Cardiovascular disease in chronic kidney disease. A clinical update from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 80: 572–586, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT, Gansevoort RT, Chronic Kidney Disease Prognosis Consortium : Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet 375: 2073–2081, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alonso A, Lopez FL, Matsushita K, Loehr LR, Agarwal SK, Chen LY, Soliman EZ, Astor BC, Coresh J: Chronic kidney disease is associated with the incidence of atrial fibrillation: The Atherosclerosis Risk in Communities (ARIC) study. Circulation 123: 2946–2953, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park M, Hsu CY, Li Y, Mishra RK, Keane M, Rosas SE, Dries D, Xie D, Chen J, He J, Anderson A, Go AS, Shlipak MG, Chronic Renal Insufficiency Cohort (CRIC) Study Group : Associations between kidney function and subclinical cardiac abnormalities in CKD. J Am Soc Nephrol 23: 1725–1734, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levin A, Singer J, Thompson CR, Ross H, Lewis M: Prevalent left ventricular hypertrophy in the predialysis population: Identifying opportunities for intervention. Am J Kidney Dis 27: 347–354, 1996 [DOI] [PubMed] [Google Scholar]
- 8.Foley RN, Parfrey PS, Harnett JD, Kent GM, Murray DC, Barré PE: The prognostic importance of left ventricular geometry in uremic cardiomyopathy. J Am Soc Nephrol 5: 2024–2031, 1995 [DOI] [PubMed] [Google Scholar]
- 9.Stack AG, Saran R: Clinical correlates and mortality impact of left ventricular hypertrophy among new ESRD patients in the United States. Am J Kidney Dis 40: 1202–1210, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Foley RN, Parfrey PS, Harnett JD, Kent GM, Martin CJ, Murray DC, Barre PE: Clinical and echocardiographic disease in patients starting end-stage renal disease therapy. Kidney Int 47: 186–192, 1995 [DOI] [PubMed] [Google Scholar]
- 11.Paoletti E, Bellino D, Cassottana P, Rolla D, Cannella G: Left ventricular hypertrophy in nondiabetic predialysis CKD. Am J Kidney Dis 46: 320–327, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Nardi E, Palermo A, Mulè G, Cusimano P, Cottone S, Cerasola G: Left ventricular hypertrophy and geometry in hypertensive patients with chronic kidney disease. J Hypertens 27: 633–641, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Whalley GA, Marwick TH, Doughty RN, Cooper BA, Johnson DW, Pilmore A, Harris DC, Pollock CA, Collins JF, Investigators IES, IDEAL Echo Substudy Investigators : Effect of early initiation of dialysis on cardiac structure and function: Results from the echo substudy of the IDEAL trial. Am J Kidney Dis 61: 262–270, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Bansal N, Keane M, Delafontaine P, Dries D, Foster E, Gadegbeku CA, Go AS, Hamm LL, Kusek JW, Ojo AO, Rahman M, Tao K, Wright JT, Xie D, Hsu CY, Investigators CS, CRIC Study Investigators : A longitudinal study of left ventricular function and structure from CKD to ESRD: The CRIC study. Clin J Am Soc Nephrol 8: 355–362, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu CM, Sanderson JE, Marwick TH, Oh JK: Tissue Doppler imaging: A new prognosticator for cardiovascular diseases. J Am Coll Cardiol 49: 1903–1914, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Cioffi G, Tarantini L, Frizzi R, Stefenelli C, Russo TE, Selmi A, Toller C, Furlanello F, de Simone G: Chronic kidney disease elicits excessive increase in left ventricular mass growth in patients at increased risk for cardiovascular events. J Hypertens 29: 565–573, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Eckardt KU, Scherhag A, Macdougall IC, Tsakiris D, Clyne N, Locatelli F, Zaug MF, Burger HU, Drueke TB: Left ventricular geometry predicts cardiovascular outcomes associated with anemia correction in CKD. J Am Soc Nephrol 20: 2651–2660, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nardi E, Palermo A, Mulè G: Inappropriately high left ventricular mass: Marker of very high cardiovascular risk in patients with chronic kidney disease? Hypertens Res 35: 800–801, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Chang JM, Chen SC, Huang JC, Su HM, Chen HC: Anemia and left ventricular hypertrophy with renal function decline and cardiovascular events in chronic kidney disease [published online ahead of print February 19, 2013]. Am J Med Sci 10.1097/MAJ.0b013e31827981be [DOI] [PubMed] [Google Scholar]
- 20.Zoccali C, Benedetto FA, Mallamaci F, Tripepi G, Giacone G, Stancanelli B, Cataliotti A, Malatino LS: Left ventricular mass monitoring in the follow-up of dialysis patients: Prognostic value of left ventricular hypertrophy progression. Kidney Int 65: 1492–1498, 2004 [DOI] [PubMed] [Google Scholar]
- 21.London GM, Pannier B, Guerin AP, Blacher J, Marchais SJ, Darne B, Metivier F, Adda H, Safar ME: Alterations of left ventricular hypertrophy in and survival of patients receiving hemodialysis: Follow-up of an interventional study. J Am Soc Nephrol 12: 2759–2767, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A: Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr 22: 107–133, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Tsang TS, Barnes ME, Gersh BJ, Bailey KR, Seward JB: Left atrial volume as a morphophysiologic expression of left ventricular diastolic dysfunction and relation to cardiovascular risk burden. Am J Cardiol 90: 1284–1289, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Pritchett AM, Mahoney DW, Jacobsen SJ, Rodeheffer RJ, Karon BL, Redfield MM: Diastolic dysfunction and left atrial volume: A population-based study. J Am Coll Cardiol 45: 87–92, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Patel RK, Jardine AG, Mark PB, Cunningham AF, Steedman T, Powell JR, McQuarrie EP, Stevens KK, Dargie HJ, Jardine AG: Association of left atrial volume with mortality among ESRD patients with left ventricular hypertrophy referred for kidney transplantation. Am J Kidney Dis 55: 1088–1096, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim SJ, Han SH, Park JT, Kim JK, Oh HJ, Yoo DE, Yoo TH, Kang SW, Choi KH: Left atrial volume is an independent predictor of mortality in CAPD patients. Nephrol Dial Transplant 26: 3732–3739, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Tripepi G, Benedetto FA, Mallamaci F, Tripepi R, Malatino L, Zoccali C: Left atrial volume in end-stage renal disease: A prospective cohort study. J Hypertens 24: 1173–1180, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Fathi R, Isbel N, Haluska B, Case C, Johnson DW, Marwick TH: Correlates of subclinical left ventricular dysfunction in ESRD. Am J Kidney Dis 41: 1016–1025, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Hayashi SY, Rohani M, Lindholm B, Brodin LA, Lind B, Barany P, Alvestrand A, Seeberger A: Left ventricular function in patients with chronic kidney disease evaluated by colour tissue Doppler velocity imaging. Nephrol Dial Transplant 21: 125–132, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Kidney Disease Improving Global Outcomes (KDIGO) Chronic Kidney Disease Work Group : KDIGO Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kid Int Suppl 3:150, 2013 [Google Scholar]
- 31.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D, Modification of Diet in Renal Disease Study Group : A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ, Grp CQW, Chamber Quantification Writing Group. American Society of Echocardiography’s Guidelines and Standards Committee. European Association of Echocardiography : Recommendations for chamber quantification: A report from the American Society of Echocardiography's guidelines and standards committee and the chamber quantification writing group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 18: 1440–1463, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Otterstad JE, Froeland G, St John Sutton M, Holme I: Accuracy and reproducibility of biplane two-dimensional echocardiographic measurements of left ventricular dimensions and function. Eur Heart J 18: 507–513, 1997 [DOI] [PubMed] [Google Scholar]
- 34.Oki T, Tabata T, Yamada H, Wakatsuki T, Shinohara H, Nishikado A, Iuchi A, Fukuda N, Ito S: Clinical application of pulsed Doppler tissue imaging for assessing abnormal left ventricular relaxation. Am J Cardiol 79: 921–928, 1997 [DOI] [PubMed] [Google Scholar]
- 35.Nagueh SF, Middleton KJ, Kopelen HA, Zoghbi WA, Quiñones MA: Doppler tissue imaging: A noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol 30: 1527–1533, 1997 [DOI] [PubMed] [Google Scholar]
- 36.Sohn DW, Chai IH, Lee DJ, Kim HC, Kim HS, Oh BH, Lee MM, Park YB, Choi YS, Seo JD, Lee YW: Assessment of mitral annulus velocity by Doppler tissue imaging in the evaluation of left ventricular diastolic function. J Am Coll Cardiol 30: 474–480, 1997 [DOI] [PubMed] [Google Scholar]
- 37.Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, Tajik AJ: Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: A comparative simultaneous Doppler-catheterization study. Circulation 102: 1788–1794, 2000 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.