Abstract
Noninvasive tests to differentiate the basis for acute dysfunction of the kidney allograft are preferable to invasive allograft biopsies. We measured absolute levels of 26 prespecified mRNAs in urine samples collected from kidney graft recipients at the time of for-cause biopsy for acute allograft dysfunction and investigated whether differential diagnosis of acute graft dysfunction is feasible using urinary cell mRNA profiles. We profiled 52 urine samples from 52 patients with biopsy specimens indicating acute rejection (26 acute T cell–mediated rejection and 26 acute antibody-mediated rejection) and 32 urine samples from 32 patients with acute tubular injury without acute rejection. A stepwise quadratic discriminant analysis of mRNA measures identified a linear combination of mRNAs for CD3ε, CD105, TLR4, CD14, complement factor B, and vimentin that distinguishes acute rejection from acute tubular injury; 10-fold cross-validation of the six-gene signature yielded an estimate of the area under the curve of 0.92 (95% confidence interval, 0.86 to 0.98). In a decision analysis, the six-gene signature yielded the highest net benefit across a range of reasonable threshold probabilities for biopsy. Next, among patients diagnosed with acute rejection, a similar statistical approach identified a linear combination of mRNAs for CD3ε, CD105, CD14, CD46, and 18S rRNA that distinguishes T cell–mediated rejection from antibody-mediated rejection, with a cross-validated estimate of the area under the curve of 0.81 (95% confidence interval, 0.68 to 0.93). Incorporation of these urinary cell mRNA signatures in clinical decisions may reduce the number of biopsies in patients with acute dysfunction of the kidney allograft.
Keywords: acute allograft rejection, mRNA, renal dysfunction
Acute graft dysfunction is a common complication after kidney transplantation. Serum creatinine, despite its inherent limitations, continues to remain the standard test to define allograft dysfunction. Physicians make decisions based on serum creatinine values and usually approach an increase in serum creatinine by considering prerenal, postrenal, and intrinsic renal causes of dysfunction during different time periods after transplantation. Among the intrinsic causes, acute T cell–mediated cellular rejection (ACR), acute antibody-mediated rejection (AMR), and acute tubular injury (ATI) because of ischemia-reperfusion injury or acute calcineurin inhibitor nephrotoxicity are the common causes of acute allograft dysfunction.1,2 Because acute rejection (AR) requires an increase in immunosuppressive therapy and ATI does not, it is imperative to differentiate AR from ATI. Although immunosuppressive drug levels and sensitization status may influence the perceived risk of AR, physicians do not accurately predict the histologic basis of acute graft dysfunction in most instances.3,4 Thus, there is continued reliance on allograft biopsies to confirm AR. Typically, acute allograft dysfunction, after excluding prerenal or postrenal causes with reasonable certainty, triggers the decision for an allograft biopsy.
Invasive kidney biopsies, despite becoming safer, still poses challenges, including bleeding and other complications, sampling errors, interobserver variability in interpretation, logistics, and costs.5,6 Therefore, there is a need to develop noninvasive tools for the differential diagnosis of acute dysfunction of the kidney allograft.
We have developed quantitative PCR (RT-PCR) assays to measure absolute levels of mRNA of immune products and showed that urinary cell mRNA profiles offer a noninvasive means of predicting ACR.7–10 The current study, while building on earlier studies of urinary cell mRNA profiling, advances the field in a number of important ways including (1) development of signatures discriminating AR from ATI and ACR from AMR, (2) introduction of a stepwise algorithm for the differential diagnosis of acute allograft dysfunction; and (3) application of decision analysis to calculate the net benefit across a range of reasonable threshold probabilities for kidney allograft biopsy.
Results
Patients and Samples
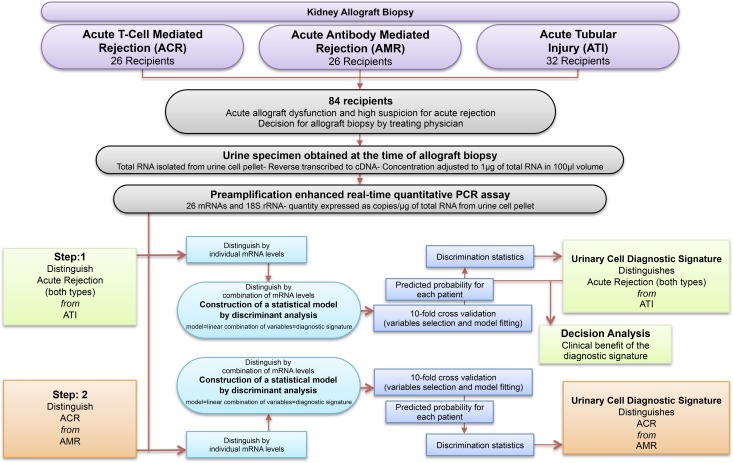
We measured absolute levels of mRNAs in 84 urine samples from 84 kidney transplant recipients who had undergone a clinically indicated (for-cause) kidney allograft biopsy at our institution to determine the cause of their acute allograft dysfunction (Figure 1). All 84 patients had either an elevation in the level of serum creatinine from baseline or persistently elevated serum creatinine levels that did not decrease as expected that prompted the treating physician to do a biopsy. Urine samples were collected at the time of a for-cause biopsy and before the initiation of any specific treatment. Among 84 biopsy-matched urine samples, 52 samples were from patients with biopsies showing AR (26 ACR and 26 AMR), and 32 samples were from patients with biopsies showing ATI without AR (Table 1). We selected biopsy-matched urine specimens at a ratio of 3:2 for AR:ATI and 1:1 for ACR:AMR. The 3:2 ratio is an approximation of the expected proportions of the biopsy diagnosis in consecutive biopsies performed for suspected AR. We used a 1:1 ratio of ACR:AMR to develop robust biomarkers for distinguishing these two major subtypes of AR. Urine from patients with less frequent findings, such as BK virus nephropathy or allergic interstitial nephritis, were not included. As illustrated in Figure 1, we used a two-step approach to develop the diagnostic signatures for the differential diagnosis of acute graft dysfunction. First, we sought to differentiate AR (both types; n=52) from ATI (n=32) with the use of urinary cell mRNA levels. Second, using the same assay results, we sought to differentiate ACR (n=26) from AMR (n=26).
Figure 1.
Flowchart showing the two-step approach for the discovery and validation of urinary cell diagnostic signatures for the differential diagnosis of acute kidney graft dysfunction. We measured urinary cell transcript levels from 84 kidney transplant recipients with acute allograft dysfunction with the use of preamplification-enhanced real-time quantitative PCR assays using a customized amplicon to construct the standard curve and quantified mRNA abundance as copies per microgram of total RNA obtained from urinary cells. We used individual transcripts as variables to construct statistical models using discriminant analysis. In each model, the linear combination of variables yielded a discriminant score that constituted the diagnostic signature. We used a two-step approach to develop the diagnostic signatures. In the first step, we sought to differentiate AR (both types; n=52) from ATI (n=32). In the second step, with the use of the same PCR assay results, we sought to differentiate ACR (n=26) from AMR (n=26). We used 10-fold cross-validation to validate both the models.
Table 1.
Characteristics of kidney allograft recipients
| Variables | ACR | AMR | ATI | Pa Value |
|---|---|---|---|---|
| Kidney allograft recipients, N | 26 | 26 | 32 | — |
| Urine specimens, N | 26 | 26 | 32 | — |
| At the time of transplant | ||||
| Age (yr), mean (SD) | 49 (14) | 47 (13) | 51 (15) | 0.50 |
| Women, N (%) | 8 (31) | 11 (42) | 13 (41) | 0.60 |
| Racial categories (black/other categories), N (%) | 11/15 (42/58) | 8/18 (31/69) | 9/23 (28/72) | 0.50 |
| Cause of end stage kidney disease, N (%) | ||||
| Diabetes mellitus | 7 (27) | 4 (15) | 6 (19) | 0.90 |
| Hypertension | 6 (23) | 7 (27) | 8 (24) | |
| GN | 3 (12) | 5 (20) | 6 (19) | |
| Others | 6 (23) | 4 (15) | 6 (19) | |
| Unknown | 4 (15) | 6 (23) | 6 (19) | |
| Donor information | ||||
| Age (yr), mean (SD) | 43 (21) | 45 (13) | 53 (11) | 0.10 |
| Women, N (%) | 15 (68) | 10 (45) | 12 (44) | 0.20 |
| Racial categories (black/other categories), N (%) | 4/22 (15/85) | 8/18 (31/69) | 5/27 (16/84) | 0.50 |
| Deceased donor organ, N (%) | 10 (38) | 14 (54) | 19 (59) | 0.30 |
| Human leukocyte antigen mismatches, mean (SD) | 4 (1.2) | 5 (1.6) | 4 (1.8) | 0.10 |
| Cold ischemia time (deceased donors; h), median (IQR) | 21 (12–29) | 24 (12–26) | 28 (18–33) | 0.50 |
| Induction therapy, N (%) | ||||
| Lymphocyte-depleting Thymoglobulin | 16 (62) | 16 (62) | 27b (84) | 0.02c |
| Lymphocyte-nondepleting IL-2 receptor antibody | 4 (15) | 8 (30) | 5 (16) | 0.01d |
| None | 6 (23) | 2 (8) | 0 (0) | |
| After transplant and before the index allograft biopsy | ||||
| Delayed graft function, N (%) | 8 (31) | 5 (19) | 19 (59) | 0.01 |
| Maintenance immunosuppression, N (%) | ||||
| Tacrolimus | 26 (100) | 26 (100) | 32 (100) | — |
| Mycophenolate | 26 (100) | 26 (100) | 32 (100) | — |
| Corticosteroids | 16 (62) | 17 (65) | 7 (22) | 0.001 |
| AR, N (%) | 2 (8) | 2 (8) | 0 (0) | — |
| Bacterial urinary tract infections,e N (%) | 9 (35) | 8 (31) | 5 (16) | 0.20 |
| BK virus nephropathy, N (%) | 0 (0) | 1 (4) | 0 (0) | — |
| Cytomegalovirus disease, N (%) | 0 (0) | 0 (0) | 0 (0) | — |
| At the time of the index allograft biopsyf | ||||
| Time from transplantation to biopsy (mo), median (IQR) | 4.4 (0.6–10.5) | 16.2 (0.5–34.4) | 1.2 (0.6–2.8) | 0.03g |
| Biopsy within 1 mo of transplantation, N (%) | 10 (39) | 9 (35) | 12 (38) | <0.001 |
| Biopsy between 1 and 12 mo of transplantation, N (%) | 10 (39) | 3 (12) | 18 (56) | |
| Biopsy beyond 12 mo of transplantation, N (%) | 6 (23) | 14 (54) | 2 (6) | |
| Indication for biopsy (creatinine increase/delayed graft function), N (%) | 25/1 (96/4) | 23/3 (88/12) | 24/8 (75/25) | 0.10 |
| Serum creatinine (mg/dl), median (IQR) | 3.20 (1.90–4.33) | 2.62 (2.01–4.29) | 3.10 (2.46–5.12) | 0.30 |
| Urine protein-to-creatinine ratio, median (IQR) | 0.56 (0.3–2.4) | 1.03 (0.4–2.9) | 0.31 (0.2–0.9) | 0.10 |
| Serum tacrolimus trough (ng/ml), median (IQR) | 5.2 (4.6–8.7) | 6.5 (4.4–8.9) | 9.3 (7.8–10.4) | <0.001h |
| Biopsy information | ||||
| AMR (I/II/III)i | — | 1/25/0 | — | — |
| ACR (IA/IB/IIA/IIB/III)i | 5/15/4/2/0 | — | — | — |
| ATI | — | 32 | ||
| Focal necrosis/isometric vacuolization, N (%) | 9/20 (28/63) | |||
| Positive staining for complement split product C4d, N (%) | 0 (0) | 26 (100) | 0 (0) | — |
| Concomitant interstitial fibrosis/tubular atrophy (moderate to severe), N (%) | 3 (12) | 7 (27) | 1 (3) | 0.10 |
| Antibodies to one or more donor-specific human leukocyte antigens | ||||
| Data available, N (%) | 13 (50) | 22 (85) | 25 (78) | |
| MFI of the highest rank donor-specific bead<1000 | 3 (23) | 0 (0) | 17 (68) | <0.001 |
| MFI=1000–3000 | 6 (46) | 2 (9) | 4 (16) | |
| MFI=3000–10,000 | 3 (23) | 10 (46) | 3 (12) | |
| MFI>10,000 | 1 (8) | 10 (46) | 1 (4) | |
| Urine specimens | ||||
| Collected on the day of biopsy, N (%) | 18 (69) | 18 (69) | 21 (66) | |
| Collected 1 d before/1 d after biopsy, N (%) | 3/5 (12/19) | 4/4 (15/15) | 2/9 (6/28) | 0.70 |
| Urine volume (ml), median (IQR) | 45 (28–70) | 43 (28–55) | 35 (25–45) | 0.10 |
| Urinary cell total RNA quantity (μg), median (IQR) | 2.1 (1.2–3.4) | 1.0 (0.6–2.5) | 1.2 (0.5–2.3) | 0.10 |
| Urinary cell total RNA purity (OD260/OD280 ratio),j median (IQR) | 1.97 (1.93–2.02) | 1.96 (1.91–1.99) | 1.93 (1.84–1.99) | 0.10 |
IQR, interquartile range; MFI, mean fluorescent intensity.
P value derived by chi-squared test for categorical variables or Kruskal–Wallis test for continuous variables.
Includes one patient with alemtuzumab (Campath-1H) induction.
P value based on chi-squared test of independence for three rows (lymphocyte-depleting induction immunosuppression, lymphocyte-nondepleting induction, and no induction) and three columns (ACR, AMR, and ATI).
P value based on chi-squared test of independence for two rows (induction immunosuppression and no induction) and three columns (ACR, AMR, and ATI).
Defined as the presence of ≥105 colony forming units per milliliter of urine.
Three patients (two AMR patients and one ATI patient) had BK virus replication (≥1 copy of BK virus VP1 mRNA per picogram total RNA from urinary cells) in the urine collected at the time of biopsy. None of the three patients had BK virus nephropathy (negative for SV40 staining in the biopsy tissue).
P<0.05 by Dunn’s test for AMR versus ATI.
P<0.05 by Dunn’s test for ACR versus ATI and AMR versus ATI.
Based on the Banff 2007 update of the Banff 1997 diagnostic categories for renal allograft biopsies.
Ratio of OD (absorbance of ultraviolet light) at 260 and 280 nm. Pure RNA has a ratio of approximately 2.
Urine volume and quantity and purity of total RNA isolated from the urinary cells did not vary across the three diagnostic categories (Table 1). The quantity and purity of total RNA and the absolute levels of housekeeping/reference gene 18S ribosomal RNA (rRNA) were not related to the time from transplantation to biopsy/urine collection (Supplemental Figure 1).
mRNA Levels in Urinary Cells
We designed gene-specific oligonucleotide primers and TaqMan probes (Supplemental Table 1) and measured absolute levels of 26 prespecified mRNAs and 18S rRNA in urinary cells using preamplification-enhanced real-time quantitative PCR assays.10 We designed the 26-member mRNA panel to be mechanistically informative and include mRNAs encoding proteins implicated in innate as well as adaptive immunity.
Table 2 shows the median (interquartile range) absolute copy number per microgram of total RNA of all 26 mRNAs measured and the levels of 18S rRNA in the urinary cells from all 84 patients. Box plots of the levels are illustrated in Supplemental Figure 2.
Table 2.
Levels of mRNA in urinary cells
| Type of mRNA | ACR (copies/μg total RNA, median [IQR]; n=26) | AMR (copies/μg total RNA, median [IQR]; n=26) | ATI (copies/μg total RNA, median [IQR]; n=32) | P Value (Kruskal–Wallis Test) | P Value (Dunn’s Test) | ||
|---|---|---|---|---|---|---|---|
| ACR Versus AMR | ACR Versus ATI | AMR Versus ATI | |||||
| CD3ε | 27,350 (15,525–96,400) | 2690 (875–8540) | 382 (108–1415) | <0.001 | <0.05 | <0.05 | <0.05 |
| Granzyme B | 17,400 (4105–33,950) | 2240 (708–5610) | 444 (190–2105) | <0.001 | <0.05 | <0.05 | >0.05 |
| Perforin | 12,950 (4745–48,125) | 1920 (407–6443) | 21 (84–1313) | <0.001 | <0.05 | <0.05 | <0.05 |
| FoxP3 | 541 (371–1080) | 119 (13–288) | 12.5 (12.5–12.5) | <0.001 | <0.05 | <0.05 | <0.05 |
| OX40 | 4885 (1007–11,450) | 422 (127–2033) | 235 (12.5–385) | <0.001 | <0.05 | <0.05 | >0.05 |
| CD105 | 32,550 (13,750–55,750) | 6890 (1785–16,450) | 2950 (663–8398) | <0.001 | <0.05 | <0.05 | >0.05 |
| CD146 | 983 (630–2923) | 573 (126–1323) | 316 (56–750) | <0.01 | >0.05 | <0.05 | >0.05 |
| von Willebrand factor | 1245 (523–2680) | 718 (121–1960) | 316 (88–1096) | <0.01 | >0.05 | <0.05 | >0.05 |
| IgJ | 1210 (571–4525) | 523 (149–3660) | 72 (12.5–192) | <0.001 | >0.05 | <0.05 | <0.05 |
| PSMB10 | 214,500 (66,525–484,500) | 28,900 (9345–58,975) | 28,550 (6253–44,150) | <0.001 | <0.05 | <0.05 | >0.05 |
| TRIB1 | 227,000 (145,250–452,750) | 62,450 (18,900–176,500) | 86,200 (18,750–242,500) | <0.01 | <0.05 | <0.05 | >0.05 |
| TLR4 | 25,200 (5363–67,525) | 6800 (975–19,725) | 4140 (444–13,775) | <0.01 | <0.05 | <0.05 | >0.05 |
| CD14 | 290,500 (117,025–699,250) | 21,500 (9548–88,500) | 55,950 (3083–182,250) | <0.001 | <0.05 | <0.05 | >0.05 |
| Complement factor 3 | 16,700 (6028–43,550) | 6445 (1233–13,950) | 2120 (745–8318) | <0.001 | <0.05 | <0.05 | >0.05 |
| Complement factor 5 | 806 (374–1733) | 260 (46–535) | 97 (12.5–390) | <0.001 | <0.05 | <0.05 | >0.05 |
| Properdin | 68,500 (19,600–208,500) | 8960 (3165–47,425) | 21,450 (1858–59,400) | <0.01 | <0.05 | <0.05 | >0.05 |
| Complement factor B | 31,900 (14,200–58,925) | 27,150 (14,150–51,475) | 15,250 (6085–26,575) | 0.01 | >0.05 | <0.05 | >0.05 |
| CD55 | 571,000 (321,500–1,403,000) | 175,500 (63,075–530,000) | 173,500 (44,475–891,750) | <0.01 | <0.05 | <0.05 | >0.05 |
| CD46 | 749,500 (328,000–1,438,000) | 173,500 (83,100–411,750) | 272,000 (60,075–526,500) | <0.001 | <0.05 | <0.05 | >0.05 |
| Vimentin | 1,430,000 (556,000–3,810,000) | 390,000 (91,100–800,500) | 295,500 (53,350–844,250) | <0.001 | <0.05 | <0.05 | >0.05 |
| NKCC2 | 3130 (967–7133) | 1031 (214–3480) | 1110 (191–2863) | <0.01 | <0.05 | <0.05 | >0.05 |
| E-cadherin | 19,200 (7248–41,700) | 7160 (3443–15,675) | 5310 (1246–14,675) | <0.01 | <0.05 | <0.05 | >0.05 |
| IL-6 | 1307 (576–10,433) | 328 (47–2598) | 151 (12.5–1935) | <0.01 | <0.05 | <0.05 | >0.05 |
| CXCL13 | 7920 (3428–50,600) | 437 (12.5–3865) | 78 (12.5–459) | <0.001 | <0.05 | <0.05 | >0.05 |
| CD20 | 3360 (644–6805) | 449 (133–2088) | 89 (12.5–403) | <0.001 | <0.05 | <0.05 | <0.05 |
| TGFβ1 | 117,000 (44,350–207,250) | 27,950 (5410–68,750) | 18,050 (2978–46,675) | <0.001 | <0.05 | <0.05 | >0.05 |
| 18S rRNA | 1.12×1010 (6.68×109–3.62×1010) | 4.15×109 (1.97×109–9.45×109) | 4.28×109 (1.40×109–1.19×1010) | <0.01 | <0.05 | <0.05 | >0.05 |
The 26 mRNAs and 18S rRNA were quantified using gene-specific primers and probes by real-time PCR assay. Median absolute copy numbers per microgram of total RNA (IQR; interquartile range) of each mRNA measure and 18S rRNA are shown. P values were calculated using Kruskal–Wallis test of no differences among the ACR, AMR, and ATI biopsy groups. After Kruskal–Wallis test, pairwise comparisons among the three groups were done using the Dunn’s test. P values<0.05 were considered statistically significant. The rationale for the mRNAs selected for inclusion in the mRNA panel is provided in the Supplemental Appendix.
The levels of all 26 mRNAs and the levels of 18S rRNA were significantly different (P<0.05) in urinary cells from the patients with biopsies showing ACR, AMR, or ATI by Kruskal–Wallis test. Pairwise comparisons using Dunn’s test showed that urinary cell levels of mRNA for CD3ε, perforin, FoxP3, and CD20 were significantly different between ACR and AMR, between ACR and ATI, and between AMR and ATI. Pairwise comparisons also showed that the levels of 18 rRNA were significantly different between ACR and AMR and between ACR and ATI but not between AMR and ATI (Table 2).
Development of a Six-Gene Urinary Cell Diagnostic Signature to Differentiate AR from ATI
Receiver-operating characteristic (ROC) curve analyses of the individual urinary cell mRNA measures to differentiate AR (both types) from ATI are shown in Supplemental Table 2. We used stepwise quadratic discriminant analysis to develop a linear combination of variables that best predicted the diagnostic groups.11 Because several patients in the ATI group had FoxP3 mRNA levels below the detection limit of the assay, we did not include it as an independent variable but used all other measures of 25 mRNAs and 18S rRNA as independent variables in the analysis. A six-gene model of natural logarithm (ln)-transformed mRNA values of CD3ε, CD105, TLR4, CD14, Complement factor B, and Vimentin emerged as the parsimonious model yielding the diagnostic signature that distinguished AR from ATI: (0.52×lnCD3ε)+(1.02×lnCD105)+(0.81×lnTLR4)+(−1.16×lnCD14)+(0.28×lnComplement Factor B)+(−0.79×lnVimentin), where the unit of measurement in the PCR assay is copies per microgram of total RNA. This diagnostic signature better differentiated AR from ATI than any single mRNA measure (e.g., versus CD3ε [area under the ROC (AUC)=0.88], likelihood ratio test, P<0.001). The diagnostic signature also outperformed other variables: time from transplantation to biopsy (AUC=0.65), serum creatinine (AUC=0.59), and tacrolimus trough levels (AUC=0.77).
Internal Validation of the Six-Gene Urinary Cell Diagnostic Signature
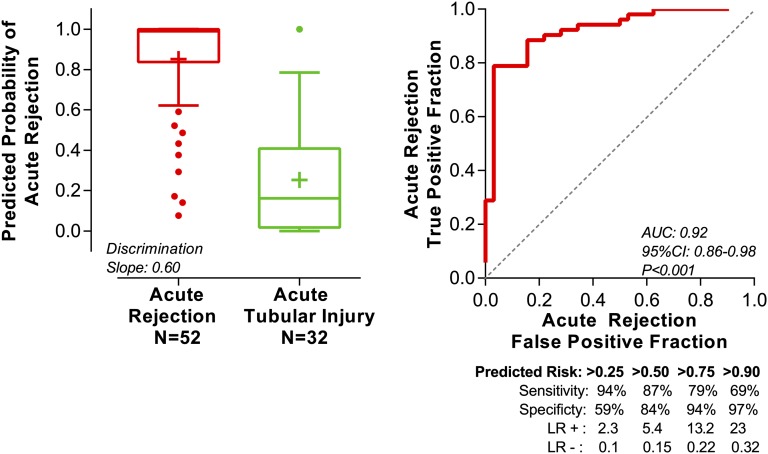
We did 10-fold cross-validation to internally validate the six-gene diagnostic signature (Figure 2). We used the predicted probability for each patient from the cross-validation to construct an ROC curve. Our six-gene model yielded a cross-validated estimate of the AUC of 0.92 (95% confidence interval, 0.86 to 0.98; P<0.001). This estimate is the estimate of the expected value of the AUC in an independent sample not used for deriving the diagnostic signature.
Figure 2.
Predicted probability of AR from the 10-fold cross-validation of the six-gene urinary cell diagnostic signature. We measured absolute levels of 26 mRNAs and 18S rRNA in the urinary cells from 84 kidney graft recipients. We used quadratic discriminant function analysis to derive linear combination of mRNAs to better differentiate 52 AR biopsies (ACR and AMR; n=52 patients) from 32 ATI biopsies (n=32 patients) than any single mRNA measure. A linear combination of six mRNAs (CD3ε, CD105, TLR4, CD14, Complement Factor B, and Vimentin) emerged as the parsimonious model and yielded a discriminant score that constituted the diagnostic signature. We did 10-fold cross-validation to internally validate the six-gene diagnostic signature. The entire study cohort of 84 patients was randomly divided into 10 equal groups. Within each of 10 groups, the proportion of samples (AR versus ATI) was similar to the undivided cohort. At the first run, group 1 (10% of samples) was excluded, and a signature was derived from the remaining nine groups (90% of samples), including both variables selection and model fitting. Next, this newly derived signature was applied to samples of group 1 to predict their diagnostic outcome. In the second run, group 2 was excluded, and a signature was derived from the remaining nine groups (90% of samples), including both variables selection and model fitting. This newly derived signature was applied to samples of group 2 (10% of samples) to predict their diagnostic outcome. This iteration was done for all 10 groups. Thus, all observations were used for both deriving and validating a model, and each observation was used for validation exactly one time. Accordingly, the predicted probability for an individual patient was derived from a model that did not include any data from that patient. We used the predicted probability for each patient from the cross-validation to construct an ROC curve. The left panel shows the box plot of predicted probability of AR from the cross-validation. The horizontal line within each box represents the median, and the plus symbol represents the mean. The bottom and top of each box represent 1.5 times the interquartile range. The values beyond 1.5 times the interquartile range are shown as dots. The discrimination slope is the difference between the means of the predicted probabilities of the two groups. The right panel shows the ROC curve of the predicted probability for each patient from the cross-validation to diagnose AR. The sensitivity (true positive fraction), specificity (false positive fraction), likelihood ratio of a positive test (LR+; sensitivity/1−specificity), and likelihood ratio of a negative test (LR−; 1−sensitivity/specificity) for various cutpoints of predicted risks are shown beneath the x axis. The AUC is the estimate of the expected value in an independent sample not used for deriving the diagnostic signature.
Clinical Benefit of the Six-Gene Urinary Cell Diagnostic Signature
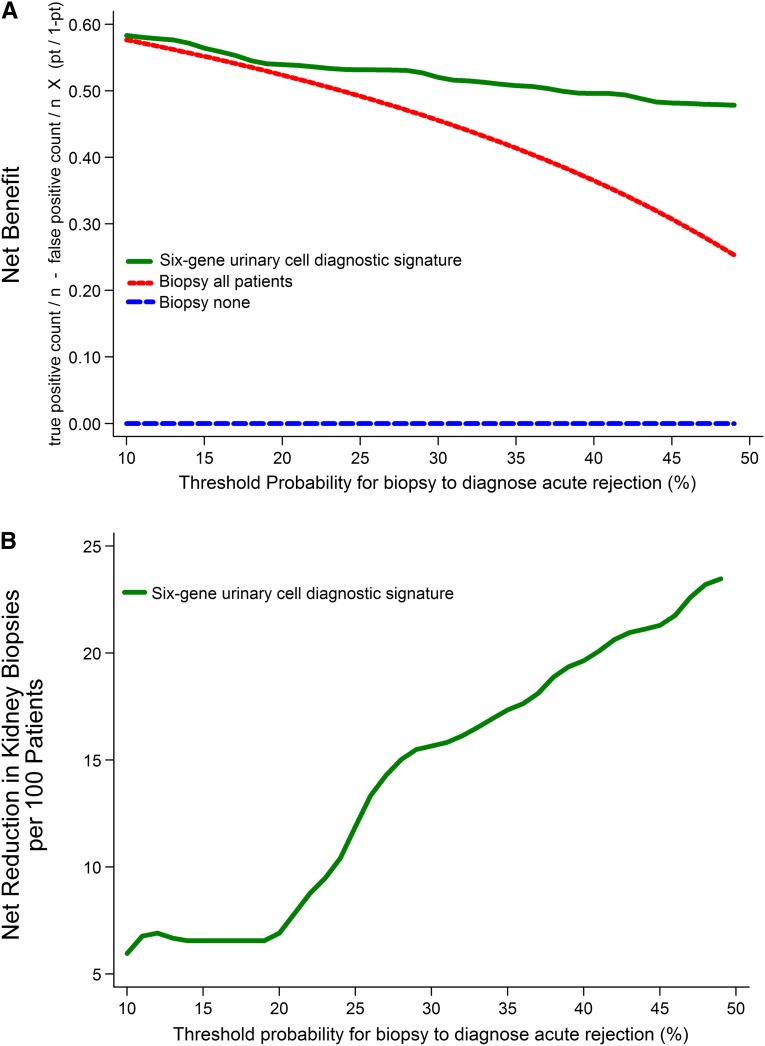
We used decision curve analysis (Figure 3) to assess whether the signature was clinically beneficial.12 This analysis depicts the net benefit of the signature at various threshold probabilities (pt), the minimum expected probability of AR at which the physician in consultation with the patient opts for a biopsy to diagnose AR (e.g., if a physician will do a biopsy when the probability of AR in a patient is 20% but will not do a biopsy if the probability is 19%, then the pt is 20%; this pt may vary among physicians). Our analysis showed that, across a range of reasonable threshold probabilities from 10% to 50%, the highest net benefit was for the diagnostic signature. The net reduction in avoidable biopsies per 100 patients when using the diagnostic signature is shown in Figure 3, lower panel.
Figure 3.
Decision curve analysis to assess the clinical benefit of the six-gene urinary cell diagnostic signature to differentiate AR from ATI. We used the predicted probability for each patient from the 10-fold cross-validation in decision curve analysis to quantify the clinical benefit of the diagnostic signature in terms of the number of unnecessary biopsies that can be avoided in the diagnosis of AR. In the upper panel, the y axis represents the net benefit ((true positive count/n)−(false positive count/n)×[pt/(1−pt)]), where true positive count is the number of patients with AR, false positive count is the number of patients with ATI, n is the total number of patients, and pt is the threshold probability. Here, pt/(1−pt) is the ratio of the harms of false positive to false negative results. Of 84 patients that we studied, 52 (62%) patients had AR. This proportion of AR is a reasonable approximation of the expected incidence of AR in consecutive for-cause (diagnostic) biopsies done to identify the cause of acute graft dysfunction. The green line is the net benefit of the urinary cell diagnostic signature. This strategy is compared with the biopsy all patients strategy (red line), which is essentially the current approach. The blue line, which represents no net benefit, is the biopsy none strategy. The decision curve plot depicts that, among patients who present with acute graft dysfunction, within a reasonable physician/patient threshold probability for doing a biopsy to diagnose AR, the use of urinary cell diagnostic signature is beneficial compared with the current biopsy all patients strategy. In the lower panel, for each threshold probability on the x axis, the corresponding value on the y axis represents the net reduction in avoidable biopsies per 100 patients when using the diagnostic signature.
Development and Validation of a Five-Gene Urinary Cell Diagnostic Signature to Differentiate ACR from AMR
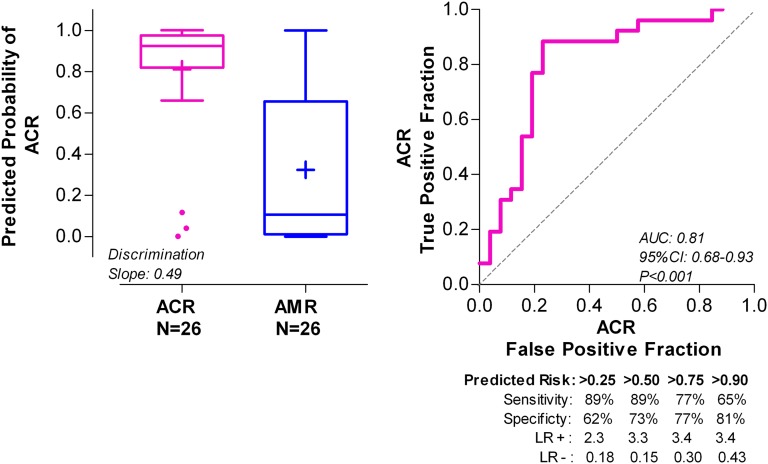
After distinguishing AR from ATI noninvasively using the six-gene diagnostic signature, we next determined if the two types of ARs, ACR and AMR, could be differentiated without the need for an invasive biopsy (Figure 1). The diagnostic value of individual mRNAs to differentiate ACR from AMR, ascertained using the ROC curve analysis, is shown in Supplemental Table 3. A five-gene model of ln-transformed mRNA values of CD3ε, CD105, CD14, CD46, and18S rRNA emerged as the parsimonious model, yielding the following diagnostic signature: (0.67×lnCD3ε)+(−1.18×lnCD105)+(1.30×lnCD14)+(−0.83×lnCD46)+(0.45×ln18S). This diagnostic signature better differentiated ACR from AMR than any other single mRNA measure (e.g., versus CD3ε [AUC=0.87], likelihood ratio test, P<0.001). Ten-fold cross-validation of this five-gene model yielded an estimate of the AUC of 0.81 (95% confidence interval, 0.68 to 0.93; P<0.001) (Figure 4).
Figure 4.
Predicted probability of ACR from the 10-fold cross-validation of the five-gene urinary cell diagnostic signature. After the differentiation of AR from ATI in the first step (Figure 1), in the second step and among patients diagnosed with AR biopsies, we derived (using the same assay results) another urinary cell diagnostic signature to better differentiate ACR biopsies (n=26 patients) from AMR biopsies (n=26 patients) than any single mRNA measure. By quadratic discriminant function analysis, a linear combination of four mRNAs (CD3ε, CD105, CD14, and CD46) and 18S rRNA emerged as the parsimonious model and yielded a discriminant score that constituted the diagnostic signature. We did 10-fold cross-validation to internally validate the five-gene diagnostic signature. The left panel shows the box plot of predicted probability of ACR biopsies from the cross-validation. The right panel shows the ROC curve of the five-gene urinary cell diagnostic signature to diagnose ACR. The AUC is the estimate of the expected value in an independent sample not used for deriving the diagnostic signature.
Other Attributes of the Urinary Cell Diagnostic Signatures
We examined whether the signature is diagnostic in patients induced with different types of induction therapy. The six-gene signature distinguishes AR from ATI in patients induced with lymphocyte-depleting antibodies (P<0.001) and patients induced with anti–IL-2 receptor antibodies or no induction (P<0.01) (Table 3). Our analysis also showed that the five-gene signature distinguishes ACR from AMR in patients induced with lymphocyte-depleting antibodies (P<0.001) and patients induced with anti–IL-2 receptor antibodies or no induction (P<0.001) (Table 3).
Table 3.
Urinary cell diagnostic signature score: subgroup analysis
| Variables | Six-Gene Signature Score (AR versus ATI) Median Valuea | Five-Gene Signature Score (ACR versus AMR) Median Valuea | ||||||
|---|---|---|---|---|---|---|---|---|
| N | AR (n=52) | ATI (n=32) | P Value | N | ACR (n=26) | AMR (n=26) | P Value | |
| Biopsy within 1 mo of transplantation | 31 | 1.15 | −1.47 | <0.001 | 19 | 10.18 | 7.87 | <0.001 |
| Biopsy beyond 1 mo of transplantation | 53 | 0.77 | −1.41 | <0.001 | 33 | 10.21 | 8.10 | <0.001 |
| P value | 0.7 | 0.6 | 0.7 | 0.3 | ||||
| Men | 52 | 0.98 | −1.45 | <0.001 | 33 | 10.29 | 7.95 | <0.001 |
| Women | 32 | 0.77 | −1.46 | <0.001 | 19 | 10.11 | 7.88 | <0.01 |
| P value | 0.7 | 0.7 | 0.7 | 0.6 | ||||
| Black | 28 | 1.08 | −1.36 | <0.001 | 19 | 10.34 | 8.05 | <0.001 |
| Others | 56 | 0.77 | −1.46 | <0.001 | 33 | 10.08 | 7.90 | <0.001 |
| P value | 0.7 | 0.8 | 0.5 | 0.6 | ||||
| Black donor | 17 | 1.61 | −1.87 | <0.01 | 12 | 10.29 | 8.38 | <0.01 |
| Other donor | 67 | 0.77 | −1.45 | <0.001 | 40 | 10.18 | 7.87 | <0.001 |
| P value | 0.6 | 0.3 | 0.9 | 0.2 | ||||
| Living donor | 41 | 1.11 | −1.45 | <0.001 | 27 | 10.33 | 8.70 | <0.001 |
| Deceased donor | 43 | 0.47 | −1.46 | <0.001 | 25 | 10.11 | 7.87 | <0.001 |
| P value | 0.1 | 0.7 | 0.5 | 0.04 | ||||
| History of delayed graft function | 26 | 1.30 | −1.34 | <0.001 | 13 | 10.17 | 7.95 | <0.01 |
| No delayed graft function | 58 | 0.77 | −1.46 | <0.001 | 39 | 10.22 | 7.92 | <0.001 |
| P value | 0.9 | 0.6 | 0.9 | 0.7 | ||||
| Induction immunosuppression | 76 | 1.01 | −1.46 | <0.001 | 44 | 10.11 | 7.98 | <0.001 |
| No induction immunosuppression | 8 | 0.77 | —b | — | 8 | 11.06 | 7.40c | — |
| P value | 0.9 | — | 0.2 | — | ||||
| Lymphocyte-depleting induction immunosuppression (Thymoglobulin)d | 59 | 1.11 | −1.50 | <0.001 | 32 | 10.18 | 8.31 | <0.001 |
| Lymphocyte-nondepleting induction (IL-2 receptor antibody) or no induction | 25 | 0.62 | −1.11 | <0.01 | 20 | 10.20 | 7.90 | <0.001 |
| P value | 0.5 | 0.1 | 0.7 | 0.7 | ||||
| Maintenance immunosuppression with corticosteroids | 40 | 0.77 | −1.45 | <0.001 | 33 | 10.61 | 7.87 | <0.001 |
| Maintenance immunosuppression without corticosteroids | 44 | 1.30 | −1.46 | <0.001 | 19 | 10.09 | 8.65 | <0.001 |
| P value | 0.4 | 0.8 | 0.4 | 0.1 | ||||
| History of bacterial urinary tract infection | 22 | 0.77 | −1.27 | <0.01 | 17 | 10.0 | 8.0 | <0.001 |
| No bacterial urinary tract infection | 62 | 1.01 | −1.46 | <0.001 | 35 | 10.3 | 7.9 | <0.001 |
| P value | 0.8 | 0.5 | 0.4 | 0.6 | ||||
| ACR, Banff 4 IA/IB | 20 | 1.36 | — | — | 20 | 10.09 | — | — |
| ACR, Banff 4 IIA/IIB | 6 | 0.49 | — | — | 6 | 11.14 | — | — |
| P value | 0.3 | — | — | 0.1 | ||||
| Concomitant interstitial fibrosis/tubular atrophy, nil to mild | 73 | 0.77 | −1.46 | <0.001 | 42 | 10.25 | 7.87 | <0.001 |
| Concomitant interstitial fibrosis/tubular atrophy, moderate to severe | 11 | 1.36 | −0.55c | — | 10 | 9.66 | 8.65 | 0.02 |
| P value | 0.9 | — | 0.8 | 0.3 | ||||
Median values of the two diagnostic signature scores are shown for different subgroups of patient variables. P values are derived using the Mann–Whitney test.
There were no patients in the ATI and no induction immunosuppression group.
n≤2. Hence, no statistical comparisons were done.
Includes one patient with alemtuzumab (Campath) induction.
Tacrolimus and mycophenolate were used as maintenance immunosuppressive therapy with or without additional corticosteroids (Table 1). The signature discriminated AR from ATI in patients managed with or without corticosteroid maintenance therapy (P<0.001 for both groups). The signature also distinguished ACR from AMR in patients managed with (P<0.001) or without (P<0.001) corticosteroid maintenance therapy (Table 3).
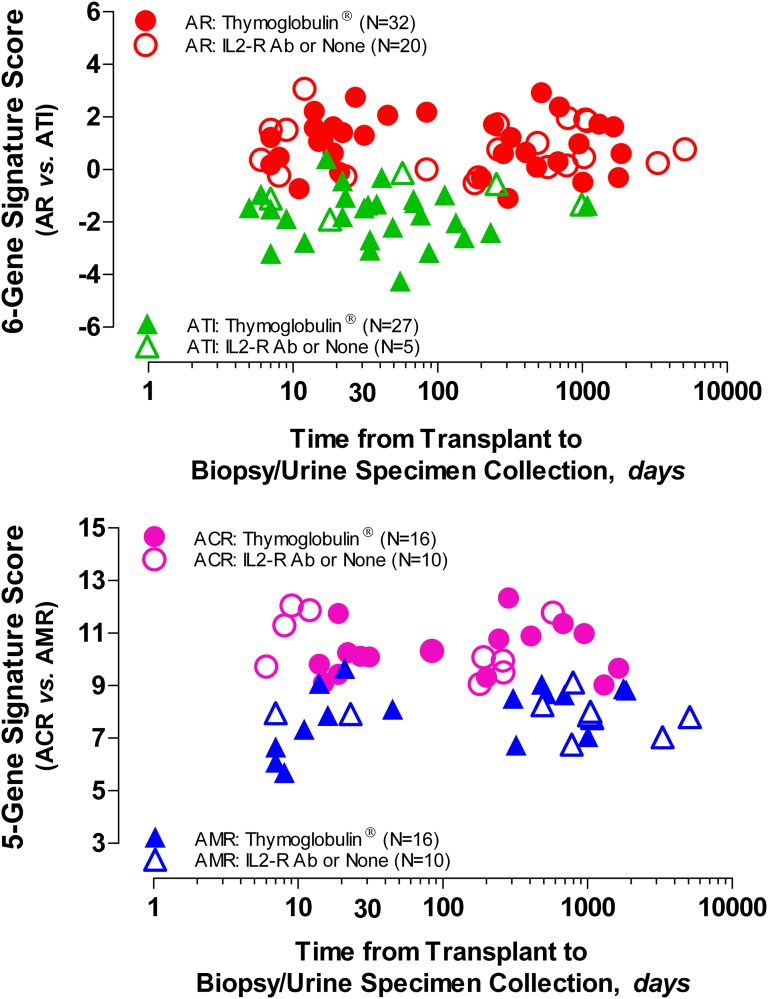
We examined whether the two diagnostic signatures are associated with time from transplantation to biopsy/urine collection (Supplemental Table 4). Our evaluation showed that there was no significant relationship between the signatures and the time from transplantation to biopsy in patients induced with depleting or nondepleting antibodies (Figure 5) (Spearman’s correlation, P>0.05).
Figure 5.
Relationship between the urinary cell diagnostic signature score and the time from transplantation to biopsy/urine sample collection. The diagnostic signature score is represented on the y axis (upper panel, six-gene signature; lower panel, five-gene signature), and time from transplantation to biopsy/urine sample collection, in logarithmic scale, is represented on the x axis. Induction immunosuppression therapy with lymphocyte-depleting Thymoglobulin (including one patient with alemtuzumab) is shown as closed symbols, whereas induction with lymphocyte-nondepleting IL-2 receptor antibody or no induction therapy is shown as open symbols. Within each diagnostic category, analysis involving Spearman rank order correlation showed that there was no significant association (P>0.05) between the score of the six- or five-gene diagnostic signatures and the time from transplantation to biopsy in patients with biopsies showing ACR, AMR, or ATI and induced with depleting or nondepleting antibodies. There was also no association between the scores of the signatures and either serum creatinine levels (six-gene signature: ACR: rs=−0.39, P=0.06; AMR: rs=−0.19, P=0.30; ATI: rs=−0.002, P=0.90; five-gene signature: ACR: rs=−0.14, P=0.50; AMR: rs=−0.07, P=0.70) or tacrolimus trough levels (six-gene signature: ACR: rs=0.14, P=0.50; AMR: rs=−0.14, P=0.50; ATI: rs=−0.02, P=0.90; five-gene signature: ACR: rs=−0.12, P=0.60; AMR: rs=−0.02, P=0.90; not shown).
Discussion
Our goal was to develop noninvasive molecular signatures in urine that differentiate common causes of acute kidney allograft dysfunction—a condition where an increase in serum creatinine suggests AR and triggers a for-cause biopsy. In this regard, physicians generally do not predict the histology of acute graft dysfunction well,3,4 and a sizable proportion of biopsies performed to confirm AR are, in fact, not AR and thus, can potentially be avoided.4
We have successfully discovered and validated urinary cell mRNA signatures for the noninvasive diagnosis of acute allograft dysfunction. The molecular signatures, comprised of multiple mRNAs and based on statistical modeling, were a better predictor of the diagnostic category than any individual mRNA or clinical parameters, such as time to biopsy, serum creatinine level, or tacrolimus trough concentration measured at the time of a for-cause biopsy. Our data indicate that, among patients who had a for-cause kidney allograft biopsy for acute allograft dysfunction, a six-gene signature differentiates AR from ATI. This signature is not only accurate, but, also, using a decision analytic method, we show that its clinical implementation would do more good than harm. Our data also indicate that, among patients with AR, a five-gene signature differentiates ACR from AMR.
Several features of our study have contributed to the development of robust noninvasive signatures. First, the three groups that we studied were well characterized, with no overlap in histologic features (Table 1). Second, our refinement of the standard RT-PCR assays allowed for absolute quantification of levels of mRNAs of interest. Third, we used a mechanistically informative mRNA panel. Fourth, we used a two-step sequential approach to differentiate the three diagnostic categories of ACR, AMR, and ATI. The relatively large number of patients with AMR is also strength of our study.
An important attribute of our signatures is that the heterogeneity in patient- and transplant-related characteristics did not undermine the ability of the signatures to differentiate AR from ATI and ACR from AMR. We also report only the cross-validated results of our signatures, potentially minimizing the upward bias of the estimate caused by model overfit. The cross-validated AUC of 0.92 for the six-gene signature distinguishing AR from ATI and the cross-validated AUC of 0.81 for the five-gene signature distinguishing ACR from AMR suggest very good discrimination. These AUCs are the expected values in an independent sample that has not been used for deriving the diagnostic signatures.
A new test can be accurate but in patient management, may or may not be useful compared with existing strategies.13 From a clinical perspective, the six-gene signature differentiating AR from ATI is probably more important than the five-gene signature distinguishing ACR from AMR. To this end, we evaluated the clinical benefit of the six-gene signature using decision curve analysis.12,14 The advantage of this approach is that it provides a quantitative estimate of the benefit of a new test compared with the existing strategy. Our proportion of samples with AR (62%) and ATI (38%) is a reasonable approximation that can be expected in consecutive biopsies done for acute allograft dysfunction.15 Thus, approximately 35%–40% of biopsies done to confirm AR, are not, in fact, AR, and can potentially be avoided. Instead of the current strategy to biopsy all to confirm AR, if the physician uses the six-gene signature, then a substantial number of biopsies can be avoided without an undue number of patients with AR experiencing delayed diagnosis. This benefit is present across a range of reasonable physician threshold probabilities to do a biopsy. Moreover, cost of the described PCR assay is approximately $300, and at our institution, the Medicare reimbursement for a kidney biopsy is approxiamtely $3000. Thus, the use of the six-gene signature for clinical decision results in substantial cost savings as well. Among patients thus identified as AR, incorporating the five-gene signature in the decision process (for example, by treating ACR based on the signature with high-dose intravenous corticosteroids and restricting biopsies only for AMR, a condition that requires complex treatment decisions, or patients with ACR who do not respond to corticosteroids) will further reduce the need for invasive biopsies.
Our preamplification protocol for the PCR assay allows for measurement of several mRNAs in a small quantity of cDNA.8 The turnaround time for the PCR assay is about 6 hours—the same time needed for a provisional read on biopsies but at a fraction of the cost of biopsies, which is especially important in the current health care cost-conscious environment.
Other biomarkers have been evaluated for the diagnosis of acute allograft dysfunction. In a study of 182 consecutive kidney transplant recipients, urinary neutrophil gelatinase-associated lipocalin protein levels were higher in 9 patients with biopsy-proven AR compared with 35 patients with other causes of AKI. However, clinical criteria rather than biopsy were used to define AKI. Moreover, creatinine levels were also different between patients with AR and AKI.16 In a recent study, peripheral blood mononuclear cell levels of IL-6 protein differentiated 29 patients with rejection (12 patients with ACR, 7 patients with AMR, and 10 patients with borderline) from 35 patients with no rejection (6 patients with ATI, 20 patients with chronic damage, and 9 patients with others) with an AUC of 0.79 in a training cohort and AUC of 0.85 in the validation cohort. However, there were very few ATI, an important masquerade of AR. Moreover, IL-6 levels did not differentiate ACR from AMR.17 In a study of 21 ACR and 8 AMR patients, urinary protein levels of endothelial protein c receptor differentiated ACR from AMR with an AUC of 0.875. This study, however, did not include patients with ATI.18
There are a number of limitations in our study design. We do not know the temporal relation between the signature and the diagnostic outcome. We know neither the longitudinal trajectory of the signature nor the level in stable transplant patients with normal allograft function. However, because our goal was to develop signatures for allograft dysfunction, these weaknesses do not hinder its use. Another limitation is that we did not profile prospectively the urine of every patient with acute allograft dysfunction who required a biopsy to confirm AR. However, our study cohort represents the vast majority of transplant recipients who present with acute allograft dysfunction.15 External validation with the use of an independent dataset (a dataset not involved in model development) would be a robust way of showing the generalizability of our findings. Also, the study design was not conducive to determine whether the signature functions as not only a diagnostic biomarker but also, a treatment response-predictive biomarker.
An important consideration in evaluating the clinical use of the signatures developed in this study is the impact of infection on the diagnostic accuracy of the signatures. The findings from the recent Clinical Trials of Transplantation-04 (CTOT-04) study revealed that, although bacterial urinary tract infection, blood infection, and cytomegalovirus infection do not impact the diagnostic accuracy of the signature, BK virus infection does impact the signature.10 We, therefore, suggest that the clinical decision to biopsy could be made independent of the presence of urinary tract infection, blood infection, and cytomegalovirus, but the signature would not obviate a biopsy in the presence of BK virus infection.
Urinary cell mRNA profiles have been recently validated in a multicenter trial (CTOT-04 study) as robust biomarkers of ACR.10 A three-gene signature of 18S rRNA-normalized measures of CD3ε, IP-10, and 18S rRNA distinguished biopsies showing ACR from biopsies not showing rejection, and the cross-validated estimate of the AUC was 0.83 by bootstrap resampling. However, there were only nine AMR biopsy-matched urine samples in the multicenter trial, which precluded an analysis of the use of the three-gene signature in diagnosing AMR. Unfortunately, we did not measure IP-10 mRNA in the current study, because the diagnostic accuracy of the three-gene signature was not known to us when we designed the 26-member mRNA panel. Hence, in the current investigation, we were unable to compare the performance of the signatures developed in this study with the signature developed in the multicenter trial. We do note that the two transcripts measured in both studies—CD3ε mRNA and 18S rRNA—are significantly associated with ACR biopsy diagnosis in both studies.
In conclusion, we have discovered and validated urinary cell mRNA-based signatures for the differential diagnosis of acute dysfunction of kidney allografts. If validated in an independent dataset, the signatures can be incorporated in clinical decisions for managing kidney transplant recipients with acute allograft dysfunction, potentially avoiding a substantial number of biopsies.
Concise Methods
Study Cohorts
All kidney graft recipients provided written informed consent to participate in the study, and our Institutional Review Board approved the study. The clinical and research activities that we report are consistent with the Principles of the Declaration of Istanbul as outlined in the Declaration of Istanbul on Organ Trafficking and Transplant Tourism. A single pathologist (S.V.S.), with no prior information about the urinary cell gene expression results, evaluated the biopsy specimens and categorized them using the Banff 2007 update of the Banff 1997 classification.19 There were 26 ACR (interstitial inflammation and tubulitis with minimal microcirculatory inflammation and absence of peritubular capillary C4d staining), 26 AMR (microcirculatory inflammation and presence of peritubular capillary C4d staining with minimal interstitial inflammation and tubulitis), and 32 ATI (attenuation or loss of brush border or necrosis and sloughing of tubular epithelium with or without isometric vacuolization and no interstitial inflammation, tubulitis, or microcirculatory inflammation and absence of peritubular capillary C4d staining) patients. Among 26 patients with AMR, 22 patients had results regarding circulating anti-HLA donor-specific antibodies (DSAs) available, and all 22 patients were positive for DSAs. The remaining four patients did not have results available for circulating anti-HLA DSAs and hence, should be categorized as suspicious for AMR based on Banff classification.
Quantification of mRNAs
Details for the absolute quantification of mRNAs using preamplification-enhanced real-time quantitative PCR assays are provided in Supplemental Appendix.
Statistical Analyses
We used a two-step approach to develop our diagnostic signatures. In both steps, we first calculated the AUC for each mRNA measure to differentiate the two diagnostic categories. We then used quadratic discriminant function analysis to develop a linear combination of variables that best predicted the diagnostic outcome. The linear combination of variables yielded a discriminant score that constituted the diagnostic signature. We did 10-fold cross-validation to internally validate our diagnostic signatures. The predicted probability for each patient from the cross-validation was then used in decision curve analysis to quantify the clinical benefit of the diagnostic signature in terms of the number of unnecessary biopsies that can be avoided in the diagnosis of AR. We used JMP 10.0.2 software (SAS Institute, Inc., Cary, NC) for discriminant analysis and Stata 11.2 software (StataCorp., College Station, TX) for decision curve analysis. Further details about the statistical analyses are provided in the Supplemental Appendix.
Disclosures
None.
Supplementary Material
Acknowledgments
This work was supported, in part, by an award from the Assistance Publique-Hôpitaux de Paris and Institut Fédératif de Recherche en Néphrologie et Transplantation (IFRNT), France (to M.M.), Qatar National Research Foundation Award NPRP 08-503-3-11 (to B.N. and M.S.), National Institutes of Health Grants 2R37-AI051652 (to M.S.) and K08-DK087824 (to T.M.), and Weill Cornell Medical College Clinical and Translational Science Center Award UL1TR000457.
Parts of the information reported in this article were presented as an abstract at the American Transplant Congress 2012, June 2–6, 2012, Boston, MA; and the American Society of Nephrology Kidney Week 2013, November 5–10, 2013, Atlanta, GA.
The research reported in this article is in partial fulfillment of the Clinical and Translational Science Center’s Graduate Program (K30) in Clinical and Translational Investigation for T.M.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013080900/-/DCSupplemental.
References
- 1.Knechtle SJ, Pirsch JD: Early course of the patient with a kidney transplant. In: Kidney Transplantation: Principles and Practice, 6th Ed., edited by Morris PJ, Knechtle SJ, Philadelphia, Saunders, 2008, pp 210–219 [Google Scholar]
- 2.Wilkinson A: The “first quarter”: The first three months after transplant. In: Handbook of Kidney Transplantation, 5th Ed., edited by Danovitch GM, Philadelphia, Lippincott Willimas & Wilkins, 2010, pp 198–216 [Google Scholar]
- 3.Al-Awwa IA, Hariharan S, First MR: Importance of allograft biopsy in renal transplant recipients: Correlation between clinical and histological diagnosis. Am J Kidney Dis 31[Suppl 1]: S15–S18, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Pascual M, Vallhonrat H, Cosimi AB, Tolkoff-Rubin N, Colvin RB, Delmonico FL, Ko DS, Schoenfeld DA, Williams WW, Jr.: The clinical usefulness of the renal allograft biopsy in the cyclosporine era: A prospective study. Transplantation 67: 737–741, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Furness PN, Taub N, Convergence of European Renal Transplant Pathology Assessment Procedures (CERTPAP) Project : International variation in the interpretation of renal transplant biopsies: Report of the CERTPAP Project. Kidney Int 60: 1998–2012, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Williams WW, Taheri D, Tolkoff-Rubin N, Colvin RB: Clinical role of the renal transplant biopsy. Nat Rev Nephrol 8: 110–121, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li B, Hartono C, Ding R, Sharma VK, Ramaswamy R, Qian B, Serur D, Mouradian J, Schwartz JE, Suthanthiran M: Noninvasive diagnosis of renal-allograft rejection by measurement of messenger RNA for perforin and granzyme B in urine. N Engl J Med 344: 947–954, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Muthukumar T, Dadhania D, Ding R, Snopkowski C, Naqvi R, Lee JB, Hartono C, Li B, Sharma VK, Seshan SV, Kapur S, Hancock WW, Schwartz JE, Suthanthiran M: Messenger RNA for FOXP3 in the urine of renal-allograft recipients. N Engl J Med 353: 2342–2351, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Anglicheau D, Muthukumar T, Hummel A, Ding R, Sharma VK, Dadhania D, Seshan SV, Schwartz JE, Suthanthiran M: Discovery and validation of a molecular signature for the noninvasive diagnosis of human renal allograft fibrosis. Transplantation 93: 1136–1146, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suthanthiran M, Schwartz JE, Ding R, Abecassis M, Dadhania D, Samstein B, Knechtle SJ, Friedewald J, Becker YT, Sharma VK, Williams NM, Chang CS, Hoang C, Muthukumar T, August P, Keslar KS, Fairchild RL, Hricik DE, Heeger PS, Han L, Liu J, Riggs M, Ikle DN, Bridges ND, Shaked A, Clinical Trials in Organ Transplantation 04 (CTOT-04) Study Investigators : Urinary-cell mRNA profile and acute cellular rejection in kidney allografts. N Engl J Med 369: 20–31, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hair JF, Anderson RE, Tatham RL, Black WC, editors: Multivariate Analysis, Upper Saddle River, NJ, Prentice Hall, 1998 [Google Scholar]
- 12.Vickers AJ, Elkin EB: Decision curve analysis: A novel method for evaluating prediction models. Med Decis Making 26: 565–574, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vickers AJ: Decision analysis for the evaluation of diagnostic tests, prediction models and molecular markers. Am Stat 62: 314–320, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steyerberg EW, Vickers AJ: Decision curve analysis: A discussion. Med Decis Making 28: 146–149, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kon SP, Templar J, Dodd SM, Rudge CJ, Raftery MJ: Diagnostic contribution of renal allograft biopsies at various intervals after transplantation. Transplantation 63: 547–550, 1997 [DOI] [PubMed] [Google Scholar]
- 16.Heyne N, Kemmner S, Schneider C, Nadalin S, Königsrainer A, Häring HU: Urinary neutrophil gelatinase-associated lipocalin accurately detects acute allograft rejection among other causes of acute kidney injury in renal allograft recipients. Transplantation 93: 1252–1257, 2012 [DOI] [PubMed] [Google Scholar]
- 17.De Serres SA, Mfarrej BG, Grafals M, Riella LV, Magee CN, Yeung MY, Dyer C, Ahmad U, Chandraker A, Najafian N: Derivation and validation of a cytokine-based assay to screen for acute rejection in renal transplant recipients. Clin J Am Soc Nephrol 7: 1018–1025, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lattenist L, Kers J, Claessen N, ten Berge IJ, Bemelman FJ, Florquin S, Roelofs JJ: Renal and urinary levels of endothelial protein C receptor correlate with acute renal allograft rejection. PLoS One 8: e64994, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M, Halloran PF, Baldwin W, Banfi G, Collins AB, Cosio F, David DS, Drachenberg C, Einecke G, Fogo AB, Gibson IW, Glotz D, Iskandar SS, Kraus E, Lerut E, Mannon RB, Mihatsch M, Nankivell BJ, Nickeleit V, Papadimitriou JC, Randhawa P, Regele H, Renaudin K, Roberts I, Seron D, Smith RN, Valente M: Banff 07 classification of renal allograft pathology: Updates and future directions. Am J Transplant 8: 753–760, 2008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.