Abstract
Patients with CKD are at high risk for adverse safety events because of the complexity of their care and impaired renal function. Using data from our observational study of predialysis patients with CKD enrolled in the Safe Kidney Care study, we estimated the baseline frequency of adverse safety events and determined to what extent these events co-occur. We examined patient-reported adverse safety incidents (class I) and actionable safety findings (class II), conditioned on participant use of drugs that might cause such an event, and we used association analysis as a data-mining technique to identify co-occurrences of these events. Of 267 participants, 185 (69.3%) had at least one class I or II event, 102 (38.2%) had more than one event, and 48 (18.0%) had at least one event from both classes. The adjusted conditional rates of class I and class II events ranged from 2.9 to 57.6 per 100 patients and from 2.2 to 8.3 per 100 patients, respectively. The most common conditional class I and II events were patient-reported hypoglycemia and hyperkalemia (serum potassium>5.5 mEq/L), respectively. Reporting of hypoglycemia (in patients with diabetes) and falling or severe dizziness (in patients without diabetes) were most frequently paired with other adverse safety events. We conclude that adverse safety events are common and varied in CKD, with frequent association between disparate events. Further work is needed to define the CKD “safety phenotype” and identify patients at highest risk for adverse safety events.
Keywords: chronic kidney disease, patient safety, data mining
Providing safe medical care is a national priority, and substantial effort has been devoted to reducing medical errors and adverse safety events.1–3 Predialysis patients with CKD have enhanced susceptibility to harm related to health care.4,5 Several factors account for this risk, including impaired renal function and altered drug clearance, along with comorbid conditions and frequent hospitalizations.5–8 Adverse safety events probably play a role in CKD progression, and strategies to reduce them may mitigate loss or disruption of renal function in many patients with CKD.9 Although patients with CKD are often hospitalized, their care is largely delivered outside of the hospital.9 There is little information on the frequency of adverse safety events among patients with CKD in the outpatient setting.
We examined patients with CKD enrolled in the ongoing Safe Kidney Care cohort study (NCT01407367), which attempts to determine the frequency of adverse safety events pertinent to the outpatient care of patients with CKD. The Safe Kidney Care study events reported in this analysis include patient-reported adverse safety incidents (class I), which are reported hazardous events or symptoms that study participants attribute to a medication. Actionable safety findings (class II) are hazardous clinical disturbances detected at study evaluations that have the potential for correction with treatment or medication modification. We also examine which class I and class II events are associated with other events within and between classes. Given the complex interaction between the heterogeneous array of potential adverse safety events and the limitations of using regression methods with such data, we used a data-mining technique known as association analysis—commonly used in genomic studies—to examine the interrelationship between these safety findings.10–12
Results
Table 1 displays the frequency and adjusted rates of both class I and II adverse safety events among all 267 participants in the analysis cohort (unconditional), and also among participants who report use of medications from a drug class during the prior 30 days that can plausibly account for each respective event (conditional), adjusting for age, sex, African American race, diabetes, cancer, cardiovascular disease, GFR, annual household income, education level, marital status, and number of medications.
Table 1.
Event counts for conditional and nonconditional patient-reported safety incidents (class I) and actionable safety findings (class II)
| Event | Event Count (Nonconditional), n (%) | Patients (n) | Adjusted Ratea (per 100 Patients) (95% CI) | Event Count (Conditional), n (%) | Patients (n) | Adjusted Ratea (per 100 Patients) (95% CI) |
|---|---|---|---|---|---|---|
| Class I | ||||||
| Hypoglycemiab | 90 (33.7) | 267 | 33.4 (27.9 to 39.4) | 87 (58.0) | 150 | 57.6 (49.5 to 65.3) |
| Falling or severe dizzinessc | 63 (23.6) | 267 | 23.2 (18.5 to 28.7) | 61 (23.6) | 259 | 23.1 (18.3 to 28.7) |
| Hyperkalemia requiring a changed | 53 (19.9) | 267 | 19.7 (15.3 to 24.9) | 38 (18.0) | 211 | 18.1 (13.4 to 23.8) |
| Nausea, vomiting, and/or diarrheae | 42 (15.7) | 267 | 15.7 (11.8 to 20.6) | 17 (20.5) | 83 | 21.1 (13.5 to 31.5) |
| Muscle weakness or crampsf | 29 (10.9) | 267 | 10.8 (7.6 to 15.1) | 25 (12.7) | 197 | 12.7 (8.7 to 18.1) |
| Lower-extremity edemag | 22 (8.2) | 267 | 8.2 (5.5 to 12.2) | 19 (11.4) | 167 | 12.7 (8.7 to 18.1) |
| Bleedingh | 19 (7.1) | 267 | 6.0 (3.6 to 9.9) | 15 (8.2) | 183 | 6.4 (3.5 to 11.6) |
| Confusioni | 19 (7.1) | 267 | 7.0 (4.5 to 10.8) | 12 (16.7) | 72 | 16.9 (9.8 to 27.5) |
| Angioedemaj | 14 (5.2) | 267 | 5.2 (3.1 to 8.6) | 6 (4.6) | 131 | 4.6 (2.1 to 9.8) |
| Skin rashk | 8 (3.0) | 267 | 2.9 (1.4 to 5.8) | 8 (3.0) | 267 | 2.9 (1.4 to 5.8) |
| Class II | ||||||
| High hemoglobinl (>13.5 g/dl) | 60 (22.5) | 267 | 21.8 (17.2 to 27.3) | 0 (0.0) | 11 | — |
| Hyperkalemiam (potassium>5.5 mEq/L) | 21 (7.9) | 267 | 7.0 (4.4 to 11.1) | 19 (9.0) | 211 | 8.3 (5.1 to 13.2) |
| Low hemoglobinn (<10 g/dl) | 20 (7.5) | 267 | 5.1 (2.7 to 9.3) | 14 (5.5) | 256 | 4.0 (2.0 to 7.9) |
| Change in systolic BPo (>20 mmHg) | 18 (6.9) | 262p | 6.9 (4.4 to 10.6) | 17 (6.7) | 254 | 6.7 (4.2 to 10.5) |
| Low pulseq (<50 beats/min) | 15 (5.7) | 264p | 5.7 (3.4 to 9.2) | 12 (7.3) | 164 | 6.4 (3.5 to 11.6) |
| Hypoglycemiar (glucose<70 mg/dl) | 10 (3.8) | 267 | 3.7 (2.0 to 6.8) | 9 (6.0) | 150 | 8.3 (5.1 to 13.2) |
| Hypokalemias (potassium<3.5 mEq/L) | 8 (3.0) | 267 | 3.0 (1.5 to 5.9) | 7 (3.9) | 181 | 3.9 (1.9 to 7.9) |
| Hyperglycemiat (glucose>250 mg/dl) | 7 (2.6) | 267 | 2.2 (1.0 to 5.1) | 7 (2.6) | 267 | 2.2 (1.0 to 5.1) |
| Low systolic BPu (<90 mmHg) | 6 (2.3) | 264p | 2.1 (0.9 to 4.9) | 6 (2.3) | 256 | 2.2 (0.9 to 5.1) |
95% CI, 95% confidence interval.
Adjusted for first principal component of a principal component analysis of study participants characteristics based on age, sex, African American race, diabetes, cancer, cardiovascular disease, GFR, annual household income, education level, marital status, and number of medications.
Insulin and all other diabetic therapies.
Renin-angiotensin-aldosterone system, potassium-sparing diuretic, and nonpotassium-sparing diuretic, calcium-channel blocker, central-acting antihypertensive, β-blocker, other antihypertensive, and antianginal.
Renin-angiotensin-aldosterone system blocker, potassium-sparing diuretic, nonsteroidal anti-inflammatory drug.
Nonsteroidal anti-inflammatory drug, colchicine, iron preparation.
Hydroxymethylglutaryl coenzyme A reductase inhibitors (statins).
Calcium-channel blocker, thiazolidinedione, other antihypertensive.
Aspirin, warfarin, or clopidogrel.
Opioid analgesic, tramadol.
Angiotensin-converting enzyme inhibitor.
Any drug.
Erythropoietic stimulating agent.
Renin-angiotensin-aldosterone system blocker, potassium-sparing diuretic, nonsteroidal anti-inflammatory drug.
No treatment with an erythropoietic stimulating agent.
Renin-angiotensin-aldosterone system blocker, β-blocker, nonpotassium-sparing and potassium-sparing diuretic, calcium-channel blocker, central-acting antihypertensive, other antihypertensive, and antianginal.
Missing values due to atrial fibrillation or inability to stand for vital sign measurements.
β-Blocker.
Insulin and all other diabetic therapies.
Nonpotassium-sparing diuretic.
No medication required.
Renin-angiotensin-aldosterone system blocker, β-blocker, nonpotassium-sparing and potassium-sparing diuretic, calcium-channel blocker, central-acting antihypertensive, other antihypertensive, and antianginal.
The most common class I event was self-reported hypoglycemia, with a higher adjusted rate conditional on use of diabetic medication within the last 30 days. Falling or severe dizziness was the second most common class I event. A high venous hemoglobin (using a value of 13.5 g/dl as a safety threshold)13–15 was the most common class II event, but when conditional on use of an erythropoietic stimulating agent, no participant had this class II event. Hyperkalemia (serum potassium>5.5 mEq/L) was the next most common class II event, which rose in rank to the most common event in this class conditional on use of a drug that can lead to hyperkalemia (e.g., renin-angiotensin-aldosterone system blocker, potassium-sparing diuretic, or nonsteroidal anti-inflammatory drug).
Table 2 lists characteristics of the 267 participants grouped by the occurrence and type of adverse safety event. One hundred eighty-five (69.3%) had at least one class I or II adverse safety event, and 48 (18.0%) had at least one adverse event in both categories. Participants with no event were more likely to be older, male, African American, and nondiabetic and to have a higher GFR but were less likely to have had cancer or cardiovascular disease. Those who did not experience any event took fewer medications.
Table 2.
Characteristics of study participants based on the occurrence of class I and II adverse safety events
| Demographic Variable | Eventsa | Class I and Class II Events | Class I but No Class II Events | Class II but No Class I Events | Total |
|---|---|---|---|---|---|
| All participants, n (row %) | 82 (30.7) | 48 (18.0) | 106 (39.7) | 31 (11.6) | 267 (100.0) |
| Age | |||||
| Mean±SD (yr) | 65.7±12.6 | 65.7±12.6 | 65.7±12.6 | 65.7±12.6 | 65.7±12.6 |
| <65 yr, n (%) | 32 (39.0) | 23 (47.9) | 48 (45.3) | 15 (48.4) | 118 (44.2) |
| ≥65 yr, n (%) | 50 (61.0) | 25 (52.1) | 58 (54.7) | 16 (51.6) | 149 (55.8) |
| Sex, n (%) | |||||
| Female | 21 (25.6) | 18 (37.5) | 26 (24.5) | 10 (32.3) | 75 (28.1) |
| Male | 61 (74.4) | 30 (62.5) | 80 (75.5) | 21 (67.7) | 192 (71.9) |
| African American, n (%) | |||||
| No | 23 (28.1) | 17 (35.4) | 35 (33.0) | 10 (32.3) | 85 (31.8) |
| Yes | 59 (72.0) | 31 (64.6) | 71 (67.0) | 21 (67.7) | 182 (68.2) |
| Diabetes, n (%) | |||||
| No | 49 (59.8) | 10 (20.8) | 28 (26.4) | 11 (35.5) | 98 (36.7) |
| Yes | 33 (40.2) | 38 (79.2) | 78 (73.6) | 20 (64.5) | 169 (63.3) |
| Cancer, n (%) | |||||
| No | 69 (84.1) | 38 (79.2) | 81 (76.4) | 25 (80.7) | 213 (79.8) |
| Yes | 13 (15.9) | 10 (20.8) | 25 (23.6) | 6 (19.3) | 54 (20.2) |
| Cardiovascular disease, n (%) | |||||
| No | 54 (65.9) | 17 (35.4) | 35 (33.0) | 10 (32.3) | 116 (43.4) |
| Yes | 28 (34.1) | 31 (64.6) | 71 (67.0) | 21 (67.7) | 151 (56.6) |
| GFR | |||||
| Mean±SD (ml/min per 1.73 m2) | 44.4±12.0 | 36.6±13.8 | 42.1±15.6 | 43.1±13.6 | 41.9±14.2 |
| ≥45 ml/min per 1.73 m2, n (%) | 38 (46.3) | 14 (29.2) | 46 (43.4) | 14 (45.2) | 112 (42.0) |
| <45 ml/min per 1.73 m2, n (%) | 44 (53.7) | 34 (70.8) | 60 (56.6) | 17 (54.8) | 155 (58.0) |
| Annual household income, n (%) | |||||
| ≤$50,000b | 58 (70.7) | 36 (75.0) | 75 (70.8) | 26 (83.9) | 195 (73.0) |
| >$50,000 | 24 (29.3) | 12 (25.0) | 31 (29.2) | 5 (16.1) | 72 (27.0) |
| Education, n (%) | |||||
| No high school diploma | 43 (52.44) | 32 (66.67) | 48 (45.28) | 14 (45.16) | 137 (51.31) |
| High school or greater | 39 (47.56) | 16 (33.33) | 58 (54.72) | 17 (54.84) | 130 (48.69) |
| Currently married, n (%) | |||||
| No | 56 (68.29) | 25 (52.08) | 54 (50.94) | 21 (67.74) | 156 (58.43) |
| Yes | 26 (31.71) | 23 (47.92) | 52 (49.06) | 10 (32.26) | 111 (41.57) |
| Hypertension, n (%) | |||||
| No | 3 (3.66) | 1 (2.08) | 6 (5.66) | 0 (0.00) | 10 (3.75) |
| Yes | 79 (96.34) | 47 (97.92) | 100 (94.34) | 31 (100.00) | 257 (96.25) |
| Median medications (interquartile range) (n)c | |||||
| 10 (7–14) | 14 (12–17) | 14 (11–19) | 11 (9–15) | 13 (9–17) |
All percentages are column percentages unless otherwise indicated.
All detected events are conditional on use of a drug from a class listed in Table 1.
Includes participants who declined to answer.
Number of medication components.
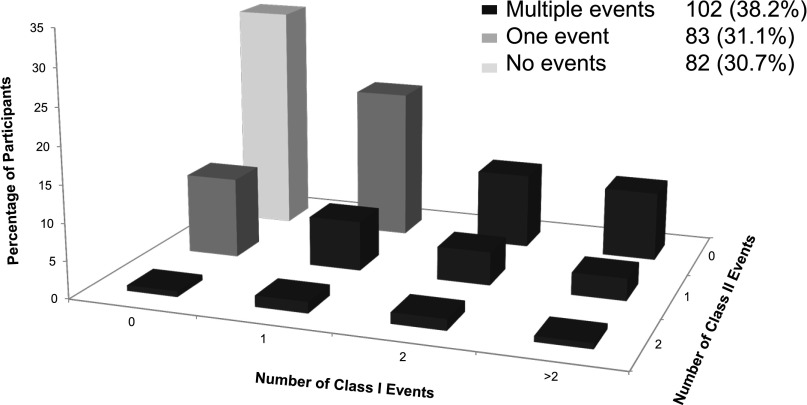
Figure 1 shows the distribution of study participants based on the number of conditional events they experienced in each category. Eighty-three (31.1%) participants experienced only one class I or II event, and 102 (38.2%) had multiple events from one or both classes. More study participants reported multiple class I events than class II events, with 78 (29.2%) and 12 (4.5%) for each class, respectively; 6 (2.3%) participants had two or more events with at least one event in both classes.
Figure 1.
Study population distributed by the occurrence and frequency of class I and II adverse safety events showing that multiple events are common.
Table 3 displays the results of the association analysis of adverse safety events among the 102 study participants who had the co-occurrence of two adverse safety events from the at-risk population of those with at least one event (n=185) and depicts several association analysis parameters. Support is a measure of how many participants experience the event pair expressed as a percentage of the whole sample. The occurrence of one event (antecedent) is used to predict another (consequent) event. Confidence is the frequency of co-occurrence divided by the occurrence of the designated antecedent in the sample and measures how much more likely it is for a consequent event to occur when the antecedent has occurred. The lift ratio is the ratio of the confidence to the expected confidence, where the latter is defined as the frequency of participants experiencing the consequent event (with or without the antecedent) as a percentage of the total sample of participants. Table 3 displays co-occurring event pairs observed at least twice in the sample, a lift ratio >1, and a P value <0.05. Associations between class I events were the most common, with the highest frequency of co-occurrence (and support) between self-reported confusion and falling or severe dizziness. The class I event pair with the highest lift ratio was a report of bleeding with angioedema, followed by the paired report of nausea, vomiting, or diarrhea with rash. There are no significant associations solely among the class 2 events. For class I and class II events examined together, self-reported hypoglycemia had the most numerous co-occurrences with laboratory-based hypoglycemia. There was no trend in the distribution of associated events by stage 3a versus stage 3b-V CKD groups.
Table 3.
Association analysis of study participants who had two or more class I or II adverse safety events (n=102) from the at-risk sample of participants with at least one event (n=185)
| Event (a) | Count (a) | Event (b) | Count (b) | Co-occurrence (n) | Support (%) | Confidence (a→b) (%) | Confidence (b→a) (%) | Lift Ratio | P Value | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patients | Patients with GFR<45 ml/min per 1.73 m2 | Patients with GFR≥45 ml/min per 1.73 m2 | |||||||||
| Class I only | |||||||||||
| Falling or severe dizziness | 61 | Confusion | 12 | 9 | 3 | 6 | 4.9 | 14.8 | 75.0 | 2.3 | 0.001 |
| Muscle weakness or cramps | 25 | Edema | 19 | 7 | 6 | 1 | 3.8 | 28.0 | 36.8 | 2.7 | 0.002 |
| Muscle weakness or cramps | 25 | Nausea, vomiting, and/or diarrhea | 17 | 6 | 4 | 2 | 3.2 | 24.0 | 35.3 | 2.6 | 0.01 |
| Muscle weakness or cramps | 25 | Confusion | 12 | 4 | 0 | 4 | 2.2 | 16.0 | 33.3 | 2.5 | 0.04 |
| Nausea, vomiting and/or diarrhea | 17 | Rash | 8 | 3 | 3 | 0 | 1.6 | 17.7 | 37.5 | 4.1 | 0.01 |
| Bleeding | 15 | Angioedema | 6 | 2 | 1 | 1 | 1.1 | 13.3 | 33.3 | 4.1 | 0.02 |
| Rash | 8 | Confusion | 12 | 2 | 1 | 1 | 1.1 | 25.0 | 16.7 | 3.9 | 0.03 |
| Class II only | |||||||||||
| None | |||||||||||
| Class I and class II | |||||||||||
| Hypoglycemia (class I) | 87 | Hypoglycemia (class II) | 9 | 8 | 2 | 6 | 4.3 | 9.2 | 88.9 | 1.9 | 0.01 |
| Hypoglycemia (class I) | 87 | Hyperglycemia (class II) | 7 | 6 | 1 | 5 | 3.2 | 6.9 | 85.7 | 1.8 | 0.04 |
| Muscle weakness or cramps (class I) | 25 | Hypokalemia (class II) | 7 | 4 | 2 | 2 | 2.2 | 16.0 | 57.1 | 4.2 | <0.001 |
| Edema (class I) | 19 | Low hemoglobin (class II) | 14 | 4 | 3 | 1 | 2.2 | 21.1 | 28.6 | 2.8 | 0.02 |
| Confusion (class I) | 12 | Hypokalemia (class II) | 7 | 2 | 0 | 2 | 1.1 | 16.7 | 28.6 | 4.4 | 0.02 |
Support: participants with a co-occurrence of the named events divided by the at-risk sample with at least one event (n=185). Confidence: frequency of participants with co-occurrence of events (a and b) divided by the frequency of participants with the designated antecedent event. Expected confidence: frequency of participants with the consequent event divided by the at-risk sample (not shown). Lift ratio: confidence divided by the expected confidence for the at-risk sample (note: lift ratio is identical regardless of the choice of antecedent and consequent designation in a pair).
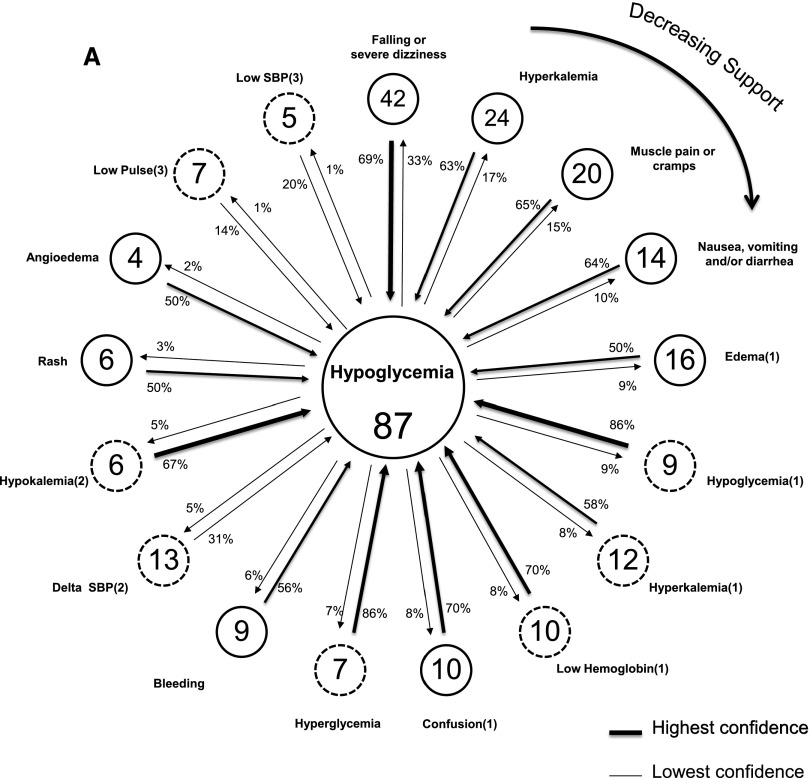
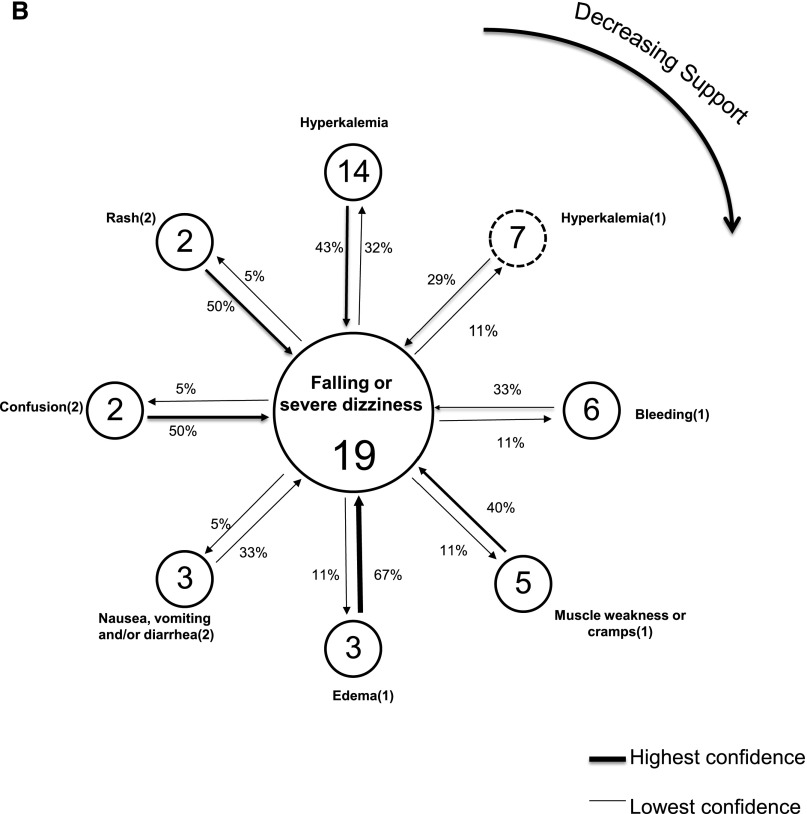
Figure 2A displays a link graph depicting the inter-relationship of adverse safety events that co-occur with the most common (index) event of class I hypoglycemia among diabetic study participants with at least one event (n=136). No patients without diabetes reported hypoglycemia. Figure 2B displays the corresponding link graph in patients without diabetes who had at least one adverse safety event (n=49) and the events’ co-occurring with the index event of class I falling or severe dizziness. In both figures, peripheral nodes represent the events that co-occur with the index, centrally located, event. The pairings with the highest support are in the 12 o’clock position and decline in a clockwise fashion. Class I and class II nodes have solid and dashed borders, respectively, with the frequency of participants with each event noted in the node. Both directions of the link between index and peripherally distributed events are shown with a designated arrow and the associated confidence. The boldness of the arrow depicts the strength of confidence.
Figure 2.
Link graphs depicting associations of hypoglycemia with other adverse safety events among diabetics, and associations of falling or severe dizziness with other adverse safety events among nondiabetics. (A) Displays adverse safety events related to the index (most common) adverse safety event of reported hypoglycemia (class I) among diabetics with at least one event (n=136). Nodes reflect individual event types and display the number of event occurrences, with arrow links representing the strength of confidence between each event and the index event. Confidence estimates are displayed for the co-occurring events proximate to the arrow representing the assumed directionality, for its computation. Dashed and solid node borders are class I and II events, respectively. The highest support estimate is for the node in the 12 o’clock position, with declining support emanating in a clockwise fashion. SBP, systolic BP. (B) Displays adverse safety events related to the index (most common) adverse safety event of reported falling or severe dizziness (class I) among nondiabetics with at least one event (n=49). Nodes reflect individual event types and display number of event occurrences, with arrow links representing the strength of confidence between each event and the index event. Confidence estimates are displayed for the co-occurring events proximate to the arrow representing the assumed directionality for its computation. Dashed and solid node borders are class I and II events, respectively. The highest support estimate is for the node in the 12 o’clock position, with declining support emanating in a clockwise fashion.
The strongest support among patients with diabetes with adverse safety events was the pairing of the index event and a class I report of falling or severe dizziness (the second most common independently occurring class I event). Of the 42 patients with diabetes who reported falling or severe dizziness, there was a high degree of confidence that they would also experience hypoglycemia—69% of them reported the index event; conversely, of the 87 patients with diabetes who reported at least one episode of hypoglycemia, 33% reported falling or severe dizziness. The co-occurring pair with the second highest degree of support was a class I event of hyperkalemia with the index event. Both class II hyperglycemia and hypoglycemia had the strongest confidence relationship with the index event. If hyperglycemia (or hypoglycemia) was detected at the baseline study visit, 86% of these individuals also reported having had hypoglycemia. Additional adverse safety events with strong confidence relationships with the index event were confusion and low hemoglobin. Among the patients without diabetes depicted, class I hyperkalemia had the highest support with the index event of falling or severe dizziness. The pairing with the strongest confidence was class I edema with the index event such that two thirds of individuals with a report of edema reported the index event.
Discussion
This analysis offers insight into the frequency and inter-relationship of adverse safety events in ambulatory patients with CKD. The occurrence of these events ranged from sporadic to common but was frequent when considered in aggregate; this demonstrates the susceptibility of the CKD population to complications of medical care. The causal relationship between treatment, or medications, and the class I and II events as we define them is difficult to confirm, but by focusing our analysis on events that occur conditional on medications that could account for such events, we offer a potential causal link between treatment and adverse safety event with the risk for harmful consequences.
There is no consensus on standards to gauge safety in CKD, and there are few tools with which to detect adverse safety events in the outpatient setting, where most care is delivered. Our study is unique in reporting on two important dimensions of adverse safety events: (1) patient-reported experiences that are hazardous or are attributed to their treatment and (2) hazardous clinical findings that are potentially addressable with treatment modification. Along with reporting the frequency of such events in an ambulatory CKD population, we identify notable linkages between apparently disparate incidents. Our findings show varying results across key demographic groups, with fewer events in elderly and in African American patients. Although there may be recall bias in elderly patients’ reporting of class I events, the lower rate of adverse safety events in this group is sustained with class II events, which are ascertained in-center and are not subject to such a bias. Similarly, the lower rate of events reported among African American patients is consistent with our previous finding of fewer medication errors among African Americans with CKD. We previously hypothesized that this was related to higher serum creatinine values among blacks relative to whites at any level of GFR, which serves as a prompt for more cautious care.16
Prior studies of safety in CKD used large administrative data sets4,17 or clinical trial data to examine the frequency of selected laboratory disturbances,18–21 but these analyses could not examine patient-reported events or be used for more extensive clinical assessments. Additional studies have also looked at the prevalence of medication errors in CKD,22–29 but most have not examined the link between medication use and adverse safety events. This study provides a more patient-centered evaluation of safety in CKD and the conditionality of two key dimensions of adverse safety events on medication use and how events in these distinct dimensions potentially inter-relate.
The results of this descriptive study should be interpreted with the study limitations in mind. The design of the Safe Kidney Care study and the means by which adverse safety events are measured has the potential of biased detection because the instruments used are based on expected adverse consequences of treatments common in CKD, but they may overlook unexpected safety incidents in the disease population. Nevertheless, the study identifies unrecognized connections between detected adverse safety events, some of which require further exploration as to the causal link. The relatively small sample size limits the robustness of rate estimates, especially for adverse safety events that are relatively uncommon. Although more precise estimates of the frequency of such events can be obtained from larger administrative or population-based samples, there is a tradeoff with the degree of detail obtained from a study on the scale of the Safe Kidney Care cohort. The relatively modest sample size limits the ability to demonstrate statistically significant associations among a heterogeneous array of adverse safety events within individuals; however, association analysis, while infrequently used in chronic disease epidemiology,30–32 provides a novel way to offer preliminary evidence of such relationships. Finally, because these data are cross-sectional, we are unable to show any causative relationships in our analysis, although future longitudinal analyses from the Safe Kidney Care study will assist in delineating causal pathways.
Determining the range and frequency of adverse safety events in the CKD population, as well as the relationship these events have with each other, confirms the assertion that patients with CKD are at risk for harm from their management along with that anticipated from the biology of their disease. The findings of this study represent a first step in defining a safety “phenotype” in CKD, and mapping the safety phenotype in CKD offers new opportunities to modify treatment approaches and alter the high rate of adverse outcomes common in CKD.
Concise Methods
Study Population
The analysis includes participants of the Safe Kidney Care study, which is an ongoing observational study designed to track the frequency of disease-specific adverse safety events longitudinally in predialysis CKD (ClinicalTrials.gov NCT01407367). Inclusion in the Safe Kidney Care cohort requires participants to be age 21 years or older, with two measures of GFR—obtained using the abbreviated Modification of Diet in Renal Disease equation33—that are <60 ml/min per 1.73 m2 at least 90 days apart, and both obtained <18 months before enrollment. Patients who are expected to initiate dialysis or die in the 12 months are excluded. A total of 108 eligible patients were first enrolled into phase I of the cohort, which pilots a medical alert device designed to increase the recognition of CKD. Enrollment then commenced for phase II, based on the same inclusion and exclusion criteria cited, and included participants who underwent identical baseline evaluations and longitudinal follow-up but were not given the medical alert device. This report includes data from the baseline visit of all study participants from phase I and phase II enrolled in the study before June 18, 2013. The study was approved by the University of Maryland, Baltimore, Institutional Review Board and the Baltimore Veterans Affairs Research and Development R&D committees.
Study Safety Assessments
This descriptive analysis includes two categories of adverse safety events determined at baseline assessment. Patient-reported adverse safety incidents (class I) are ascertained from a baseline self-reported safety event questionnaire developed for the Safe Kidney Care study, which asks participants whether, over the prior 12 months, they experienced hypoglycemia or hyperkalemia requiring treatment or change in therapy or diet, along with other adverse safety incidents that they attribute to an administered medication or treatment. The potential incidents, in addition to hypoglycemia and hyperkalemia cited on the self-reported safety event questionnaire, include falling or severe dizziness; bleeding; angioedema; confusion or inability to think clearly; nausea, vomiting, and/or diarrhea; new or worsening ankle swelling; muscle weakness or muscle cramps; and skin rash (see Supplemental Appendix). Our definition for self-reported hypoglycemia was based on a composite of whether a participant answered “yes” to question 1a or 1b or both, and for falling and severe dizziness whether participant answered “yes” to question 2a or 2b on the questionnaire. Actionable safety findings (class II) are potentially hazardous abnormalities in baseline laboratory and vital sign parameters associated with concomitant treatment with a drug known to cause such a disturbance, and with the potential to be corrected with discontinuation or modification in the dose of the suspect agent or some other treatment (e.g., low pulse in the setting of a β-blocker).
Phlebotomy is performed at baseline visit after a fast of 6 hours or more and provides measurements of serum glucose, potassium, venous hemoglobin, and creatinine. The baseline vital sign assessment includes seated BP and pulse obtained after a 5-minute quiet period and a repeat measurement of BP and pulse after standing for 2 minutes (Omron HEM-907XL; Wake Forest, IL).
Covariates
Additional factors included demographic characteristics (age, sex, African American race, diabetes, cancer, cardiovascular disease, GFR, annual household income, education level, marital status, and number of medications). Medication use was recorded for up to 30 days before the baseline study assessment. The number of medications recorded reflected all participants’ drug entries. Combination products are listed as individual constituents unless the drug is available only as a combination. Baseline renal function was measured using the serum creatinine obtained at the study visit; the abbreviated Modification of Diet in Renal Disease equation was used to calculate the estimated GFR.
Statistical Analyses
Study participants were classified as having a class I and class II adverse safety event, both unconditionally in all participants and conditionally according to whether participants were prescribed a drug from a class that could potentially cause such a complication. All analyses subsequent to those conducted for descriptive purposes used the conditional determination and the restricted sample at risk for each respective class I and II adverse safety event. Categorical variables are reported with number and percentage of total number. Continuous variables are reported with mean and SD or median and interquartile range. All analyses were done using SAS software, version 9.3, and SAS Enterprise Miner, version 12.1 (SAS Institute, Inc., Cary, NC).
Because of the small counts of several events, we used principal component analysis to calculate corresponding adjusted rates. This statistical technique converts a set of observed variables into a set of uncorrelated variables called principal components, wherein the first several principal components can explain most of the variation in the original variables.34,35 Therefore, we used the first principal component, which accounts for the largest variation in the original patient characteristic variables, to adjust for multiple patient characteristics in the logistic regression models used to report adjusted rates.
We used association analysis to examine the inter-relationships between the heterogeneous array of events reported both within and between class I and II adverse safety events. This data-mining technique is commonly applied to complex data, such as those found in genomics, but is less commonly used in chronic disease epidemiology,30–32 to identify genetic polymorphisms that have a significant association with disease manifestations.10–12 A similar method, market basket analysis, is used with large retail marketing data sets to delineate shopping patterns and predict purchasing behavior.36 Using the association node in SAS Enterprise Miner, we were able to determine all single-dimensional affinities that occur among the conditional adverse safety events identified in our data set.37
Association analyses do not typically involve statistical hypothesis testing; however, co-occurrence can be expressed in contingency tables, allowing for the calculation of chi-squared statistics and P values.38 Because all associations in single-dimensional association analysis can be bi-directional, we treated both factors in a co-occurrence as the antecedent event and display both confidence values. We also derived link graphs from the association analysis39 exhibiting the relationship between the index adverse event among patients with diabetes and patients without diabetes, which were class I reports of hypoglycemia and falling or severe dizziness, respectively.
Disclosures
None.
Supplementary Material
Acknowledgments
The work included in this paper was supported by National Institute of Diabetes and Digestive and Kidney Diseases (R01-DK084017) (J.S.G., M.Z., C.W., J.C.F.). This work was supported by the University of Maryland Clinical Translational Science Institute and the University of Maryland General Clinical Research Center.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013090921/-/DCSupplemental.
References
- 1.Leape L, Berwick D, Clancy C, Conway J, Gluck P, Guest J, Lawrence D, Morath J, O’Leary D, O’Neill P, Pinakiewicz D, Isaac T, Lucian Leape Institute at the National Patient Safety Foundation : Transforming healthcare: A safety imperative. Qual Saf Health Care 18: 424–428, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Leape LL, Berwick DM: Five years after To Err Is Human: What have we learned? JAMA 293: 2384–2390, 2005 [DOI] [PubMed] [Google Scholar]
- 3.McCannon CJ, Hackbarth AD, Griffin FA: Miles to go: An introduction to the 5 million lives campaign. Jt Comm J Qual Patient Saf 33: 477–484, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Seliger SL, Zhan M, Hsu VD, Walker LD, Fink JC: Chronic kidney disease adversely influences patient safety. J Am Soc Nephrol 19: 2414–2419, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chapin E, Zhan M, Hsu VD, Seliger SL, Walker LD, Fink JC: Adverse safety events in chronic kidney disease: The frequency of “multiple hits”. Clin J Am Soc Nephrol 5: 95–101, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fink JC, Chertow GM: Medication errors in chronic kidney disease: One piece in the patient safety puzzle. Kidney Int 76: 1123–1125, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Nitsch D, Nonyane BAS, Smeeth L, Bulpitt CJ, Roderick PJ, Fletcher A: CKD and hospitalization in the elderly: A community-based cohort study in the United Kingdom. Am J Kidney Dis 57: 664–672, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Fink JC, Brown J, Hsu VD, Seliger SL, Walker L, Zhan M: CKD as an underrecognized threat to patient safety. Am J Kidney Dis 53: 681–688, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Q, Chen YP: Mining frequent patterns for AMP-activated protein kinase regulation on skeletal muscle. BMC Bioinformatics 7: 394, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morgan XC, Ni S, Miranker DP, Iyer VR: Predicting combinatorial binding of transcription factors to regulatory elements in the human genome by association rule mining. BMC Bioinformatics 8: 445, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Creighton C, Hanash S: Mining gene expression databases for association rules. Bioinformatics 19: 79–86, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, de Zeeuw D, Eckardt KU, Feyzi JM, Ivanovich P, Kewalramani R, Levey AS, Lewis EF, McGill JB, McMurray JJV, Parfrey P, Parving HH, Remuzzi G, Singh AK, Solomon SD, Toto R, TREAT Investigators : A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 361: 2019–2032, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Drüeke TB, Locatelli F, Clyne N, Eckardt KU, Macdougall IC, Tsakiris D, Burger HU, Scherhag A, CREATE Investigators : Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 355: 2071–2084, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, Reddan D, CHOIR Investigators : Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 355: 2085–2098, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Diamantidis CJ, Seliger SL, Zhan M, Walker L, Rattinger GB, Hsu VD, Fink JC: A varying patient safety profile between black and nonblack adults with decreased estimated GFR. Am J Kidney Dis 60: 47–53, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Norris K, Bourgoigne J, Gassman J, Hebert L, Middleton J, Phillips RA, Randall O, Rostand S, Sherer S, Toto RD, Wright JT, Jr, Wang X, Greene T, Appel LJ, Lewis J, AASK Study Group : Cardiovascular outcomes in the African American Study of Kidney Disease and Hypertension (AASK) Trial. Am J Kidney Dis 48: 739–751, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Moen MF, Zhan M, Hsu VD, Walker LD, Einhorn LM, Seliger SL, Fink JC: Frequency of hypoglycemia and its significance in chronic kidney disease. Clin J Am Soc Nephrol 4: 1121–1127, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Einhorn LM, Zhan M, Hsu VD, Walker LD, Moen MF, Seliger SL, Weir MR, Fink JC: The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med 169: 1156–1162, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perazella MA: Drug-induced hyperkalemia: old culprits and new offenders. Am J Med 109: 307–314, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Juurlink DN, Mamdani MM, Lee DS, Kopp A, Austin PC, Laupacis A, Redelmeier DA: Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N Engl J Med 351: 543–551, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Luyckx VA: Nephrotoxicity of alternative medicine practice. Adv Chronic Kidney Dis 19: 129–141, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Luyckx VA, Naicker S: Acute kidney injury associated with the use of traditional medicines. Nat Clin Pract Nephrol 4: 664–671, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Corsonello A, Pedone C, Corica F, Mussi C, Carbonin P, Antonelli Incalzi R, Gruppo Italiano di Farmacovigilanza nell’Anziano (GIFA) Investigators : Concealed renal insufficiency and adverse drug reactions in elderly hospitalized patients. Arch Intern Med 165: 790–795, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Hug BL, Witkowski DJ, Sox CM, Keohane CA, Seger DL, Yoon C, Matheny ME, Bates DW: Occurrence of adverse, often preventable, events in community hospitals involving nephrotoxic drugs or those excreted by the kidney. Kidney Int 76: 1192–1198, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Laliberté MC, Normandeau M, Lord A, Lamarre D, Cantin I, Berbiche D, Corneille L, Prud’homme L, Lalonde L: Use of over-the-counter medications and natural products in patients with moderate and severe chronic renal insufficiency. Am J Kidney Dis 49: 245–256, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Cox ZL, McCoy AB, Matheny ME, Bhave G, Peterson NB, Siew ED, Lewis J, Danciu I, Bian A, Shintani A, Ikizler TA, Neal EB, Peterson JF: Adverse drug events during AKI and its recovery. Clin J Am Soc Nephrol 8: 1070–1078, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Korgaonkar S, Tilea A, Gillespie BW, Kiser M, Eisele G, Finkelstein F, Kotanko P, Pitt B, Saran R, Saran R: Serum potassium and outcomes in CKD: Insights from the RRI-CKD cohort study. Clin J Am Soc Nephrol 5: 762–769, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weinberg JM, Appel LJ, Bakris G, Gassman JJ, Greene T, Kendrick CA, Wang X, Lash J, Lewis JA, Pogue V, Thornley-Brown D, Phillips RA, African American Study of Hypertension and Kidney Disease Collaborative Research Group : Risk of hyperkalemia in nondiabetic patients with chronic kidney disease receiving antihypertensive therapy. Arch Intern Med 169: 1587–1594, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Wang X: Investigating the impact of Medicare Part D on the diabetes medications using Enterprise iner and survival analysis. SAS Global Forum 2010, Seattle, Washington. Available at: http://support.sas.com/resources/papers/proceedings10/TOC.html Accessed November 26, 2013 [Google Scholar]
- 31.Breault JL, Goodall CR, Fos PJ: Data mining a diabetic data warehouse. Artif Intell Med 26: 37–54, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Tian H, Brimmer DJ, Lin JM, Tumpey AJ, Reeves WC: Web usage data as a means of evaluating public health messaging and outreach. J Med Internet Res 11: e52, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levey AS, Greene T, Kusek JW, Beck GJ: A simplified equation to predict glomerular filtration rate from serum creatinine [abstract]. J Am Soc Nephrol 111: 155A, 2000 [Google Scholar]
- 34.Jolliffe IT: A note on the use of principal components in regression. J R Stat Soc Ser C Appl Stat 31: 300–303, 1982 [Google Scholar]
- 35.Brown SGA, Stone SF, Fatovich DM, Burrows SA, Holdgate A, Celenza A, Coulson A, Hartnett L, Nagree Y, Cotterell C, Isbister GK. Anaphylaxis: Clinical patterns, mediator release, and severity. J Allergy Clin Immunol. 2013. (ePub). [DOI] [PubMed]
- 36.Chen YL, Chen J, Tung C: A data mining approach for retail knowledge discovery with consideration of the effect of shelf-space adjacency on sales. Decision Support Systems and Electronic Commerce an International Journal. 42: 1503, 2006 [Google Scholar]
- 37.Xin Y, Ju S: Mining conditional hybrid-dimension association rules on the basis of multi-dimensional transaction database. Machine Learning and Cybernetics, 2003 International Conference 1: 216-221, 2003 [Google Scholar]
- 38.Alvarez SA: Chi-squared computation for association rules: Preliminary results. Computer Science Department, Boston College, Chestnut Hill, MA, Technical Report BC-CS-2003-01. 2003 [Google Scholar]
- 39.Cerrito PB: Introduction to Data Mining Using SAS Enterprise Miner; 2006, Cary, NC, SAS Institute Inc., 2006 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.