Abstract
Craniofacial soft tissue reconstruction may be required following trauma, tumor resection, and to repair congenital deformities. Recent advances in the field of tissue engineering have significantly widened the reconstructive armamentarium of the surgeon. The successful identification and combination of tissue engineering, scaffold, progenitor cells, and physiologic signaling molecules has enabled the surgeon to design, recreate the missing tissue in its near natural form. This has resolved the issues like graft rejection, wound dehiscence, or poor vascularity. Successfully reconstructed tissue through soft tissue engineering protocols would help surgeon to restore the form and function of the lost tissue in its originality. This manuscript intends to provide a glimpse of the basic principle of tissue engineering, contemporary, and future direction of this field as applied to craniofacial surgery.
Keywords: Craniofacial, ex vivo produced oral mucosa equivalent, reconstruction, soft tissue, tissue engineering
INTRODUCTION/BASIC PRINCIPLES OF TISSUE ENGINEERING
Tissue engineering is defined as “the reconstitution of tissues and organs, in vitro, for use as model systems in basic and applied research or for use as grafts to replace damaged, or diseased body parts or body functions.”[1] In the field of Oral and Maxillofacial Surgery, this is applied through the reconstructive efforts needed for the treatment of trauma, tumor resection, and to repair congenital deformities.
Maxillofacial surgeon's main challenge comes from having a limited supply of well-suited local tissues that are needed to approximate lost bone, muscle, nerves, skin, and mucosa. Local regional and distant flap harvests have been utilized in an attempt to increase the complexity of available tissues, with improved outcomes. Even though the availability of tissues allows the closure of a defect and obturation of dead space, the function and esthetic goals are not yet ideally met. Custom design of engineered soft tissues will be the next step for improving reconstruction of complex defects, such as the lips which will be referred to throughout this paper.
Tissue engineering is based on a triad: The scaffold (such as collagen, bone, or synthetic matrix); cells, (osteoblasts, chondroblasts, fibroblasts); and physiologic signaling molecules (growth factors, morphogenes, adhesins).[2] This is all predicated on the establishment, within 2-4 days, of a blood supply to adequately perfuse the tissue to maintain cell viability and function [Figure 1].[3]
Figure 1.
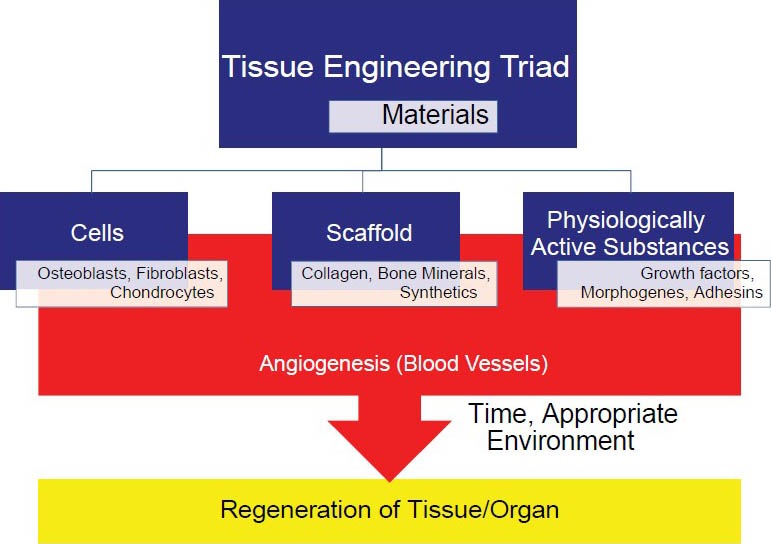
The triad of tissue regeneration; scaffolds, cells, and signaling molecules
There has been keen interest in hard tissue scaffold development, and many materials such as polycaprolactone (PCL) have been in use to replace bone and cartilage.[4,5] Recently, we have seen successes with these materials in the bronchial tree[6] and with porous polyethylene used on the maxillofacial reconstruction, such as the orbital floor.[7] Advancement of signaling molecule purification also has increased our armamentarium with material such as the bone morphogenic protein,[8,9] allowing closure of defects, and more clinically favorable outcomes than demineralized bone alone.[10] Unfortunately, the relative efforts for engineering of complex soft tissue development have not been as robust.
The basic building block of all reconstruction starts with the cell. Within the last decade, we have had many options for cell supply, through in vitro growth of autogenous cells, embryonic stem cell, induced pluripotent stem cells, and purified allografts.[11] In order to develop tissues of skin and mucosa, the cells must be of ectodermal origin, specifically keratinocytes.[12,13] Recent advances in cell culturing have developed protocols to growth keratinocytes, necessary for skin and mucosa, without the use of serum, irradiated feeder layers, and pituitary extract; thus paving the way for their use in clinical applications.[14] Numerous types of scaffolds made of synthetic or natural materials such as decellularized, freeze-dried dermis (AlloDerm®) has been used for years with predictable results,[2,3,5,15] for simple soft tissue reconstruction. In contrast, success has not been ideal for reconstruction of complex soft tissue structures composed of epithelium, dermis, and muscle that require a robust vascular supply, such as the lips. Therefore, although conceptually the basic triad of tissue engineering has been met for many tissue constructs, still more complex tissues present unique challenges.
All significant grafts eventually require vascularization for viability via imbibition, inosculation, and vascular ingrowth for small grafts, or may carry their own intrinsic blood supply. This may be via pedicle or by free tissue transfer, and reanastomosis of vessels for large complex soft tissue grafts. These procedures are limited by the available tissue size, shape, and function as well as provider expertise to harvest and perform microvascular suturing of the involved vessels.[16,17] In an ideal world, our future “designer” grafts will include an engineered scaffold of the proper size and shape with tissue specific to the deficient area, with appropriate cells carrying robust signaling molecules to allow active integration into the host. These grafts will be fully vascularized to allow rapid integration, removal of toxic waste, and predictable survival. Lastly, the ultimate goal albeit and most difficult is to provide function, including sensation (sensory) and movement (motor), approximating the lost tissue being reconstructed. These challenges are largely seen in lip reconstruction cases.
CONTEMPORARY STATE OF THE ART SURGERY FOR THE LIP AND SOFT TISSUE DEFECTS
The lips are complex structures made of skin, vermilion with transition at the white line, muscle, nerves, subcutaneous bulk, and intraoral mucosa. Their complexity is three-fold; esthetics, function, and sensation. It is an intricate organ including the Cupid's bow, supported by musculature and neural innervation for competency and speech, and has an underlying bony support for esthetics and projection. With this many dimensions for one structure, it is not surprising to note that there has not been an ideal reconstructive method to replace the lips.[16,17] Current reconstructive techniques include advancement flaps such as Karapandzic flap, nasolabial flaps, lip switch (Abbey or Abbey-Estlander flap).[18,19] The disadvantages are mainly microstomia with local flaps and primary closure. For very large defects, vascularized free tissue transfers are preferred which allow reliable closure of defects, replacement of bulk, and viability of tissue.[16,17] Their disadvantages include scarring, poor color matching, lack of function and sensation, and donor site morbidity.[17]
To address the current lack of availability of appropriate reconstructive tissue, surgeons have attempted to replace the tissue directly by using allogeneic face transplants, first done by French maxillofacial surgeon Devauchelle, and recently by American maxillofacial surgeon Edward Rodriguez.[20] However, these reconstructions require life-long immunosuppression, and as with any allogeneic transplants, they may not be readily available. Regardless of technique used, the reconstruction cannot be truly personalized to the patient. A marked advancement in this area of reconstruction would be the development of autogenous functional facial units such as the lips.
ISSUES TO BE ADDRESSED IN SOFT TISSUE RECONSTRUCTION
One of the main issues that still need to be addressed for engineering soft tissue is the vascularity, which remain as the Achilles heel in reconstruction. This was originally addressed with small grafts through the physiologic processes of imbibition, inosculation or neovascularization. Some improved results have been achieved by modulation of existing tissue by growth factor or cytokine signaling, delivery of endothelial, and mesenchymal progenitor cells to form a self-assembled network, delivery of vascular-inductive engineered materials, or through controlled methods to directly incorporate vessel conduits into engineered tissue.[21] Further advances in microvascular surgery have now allowed free flap tissue transfer with immediate blood supply, thus circumventing the unpredictability of the period of vascular ingrowth.[22]
After the tissue becomes incorporated into the host, further hurdles remain in place. Most flaps, especially free tissue transfers and grafts are essentially denervated, and although they provide bulk, they have no motor function or sensation. Attempts to provide neuronal support have had very poor reliability and outcomes.[23] Connection of existing innervation using microvascular flaps to line the pectoralis major and reconstructing the lips makes little sense as this will animate tissue that was not made to function at the recipient site.[24] Adding neuronal channels in the hope of new neuronal growth rarely works and may have unwanted results of paresthesia, dysethesia, and neuroma formation.[25] So although we have accepted that reconstruction may replace bulk and close defects, our current state of the art reconstructions lacks the ability to replace functionally complex native soft tissues. In our lab, we are presently developing a protocol to tissue engineer autogenous functional facial units such as the lips.
IN VITRO TISSUE DEVELOPMENT OF AN EX VIVO PRODUCED ORAL MUCOSA EQUIVALENT (EVPOME)
Through recent advancements in in vitro tissue development we now can create mucosa, skin, bone, and even cartilage.[26,27,28,29] The creation of soft tissue constructs with several different cell populations is a more complex process. We have, in vitro, developed such a construct composed of mucosa and skin, a mucocutaneous construct, that is based on technique previously reported by Izumi, et al.[1,29,30] At the center of our current research in the reconstruction of lips, it has revolved around the use of an EVPOME, created by the Feinberg, Marcelo, Izumi group at the University of Michigan.[31] The EVPOME construct is based on the patients’ own autogenous cells, which are then purified and cultivated along with skin matrix such as an acellular freeze-dried dermis scaffolding for attachment and growth of cells [Figure 2]. The scaffold and cells are immersed within a specific chemically-defined cell culture media ideal for keratinocyte growth with the necessary signaling molecules (calcium) to push their development along the desired path.[14,32]
Figure 2.
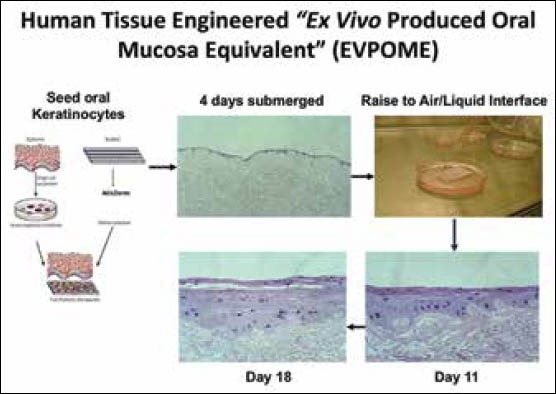
Steps in in vitro tissue development of an ex vivo produced oral mucosa equivalent
These ex vivo produced oral mucosal constructs are then implanted and further allowed to mature in situ. This tissue is autologous allowing personalized construct formation without immune suppression or rejection. Similar technologies are now being employed for replacement of bone, skin, and cartilage. EVPOME allows us to create a scaffold of the appropriate size, seed it with the appropriate cells, keratinocytes, required for specific tissues, and their growth in an appropriate culture medium. When adequate expansion has occurred the engineered EVPOME can be transferred to an autogenous host allowing continued differentiation and vascularization. Immediate viability is maintained by imbibition and inosculation owing to their thin cross-sectional area. Most recently, EVPOME has been used successfully in a clinical trial in Japan[14] and in the USA in a Food and Drug Administration (FDA)-approved phase I clinical trial[33] involving intraoral defects. This year, EVPOME will be used in a larger Phase II and Phase I/II clinical trials to increase intraorally keratinized tissue for placement of endosseous dental implants. This research has recently been expanded in an effort to fabricate mucocutaneous constructs representing more complex composite tissue structures containing distinct cell transition borders [Figure 3] that will ultimately be required for creating boundaries between different cell populations such as is seen in lip, that is, the skin-vermillion-oral mucosa.[30]
Figure 3.
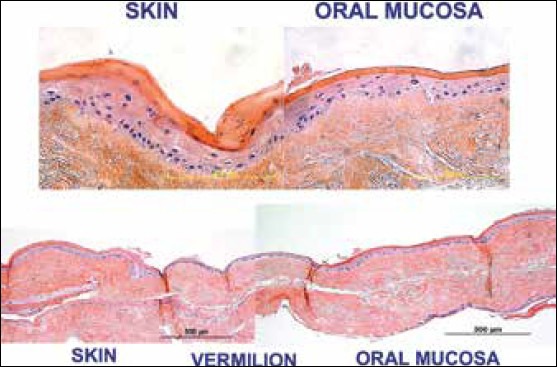
The histological representation of skin, oral mucosa, and the transitional area; the vermilion border of lips
These engineered tissues are grown in vitro, and would continue to develop and mature in situ after grafting with the development of their own microcapillary system. Present attempts at engineering of complex soft tissues in vitro is presently deficient in inclusion of the muscular construct which is a necessary requirement for replacement of the orbicularis oris muscle of the lip. Our current approach to address this issue is to use mucocutaneous constructs fabricated in vitro to create prelaminated muscle flaps in preparation for future multilaminar en bloc tissue transfer.
IN SITU DEVELOPMENT OF COMPLEX SOFT TISSUE STRUCTURES
Prefabricated prelaminated microvascular flaps for tissue transfer is an innovative approach to address function, esthetics, and perfusion. Prefabricated tissue flap transfer can involve autogenous grafts to the site, such as using rib grafts to reconstruct the ear.[34] If more complex reconstruction is required, prefabricated tissues can be combined with known flap methods to increase bulk, vascular supply, and the tissue types transferred.[35] This allows individualized structure and nonimmune reactive grafts, but has numerous difficulties. Most prefabricated prelaminated microvascular flaps are allowed to mature in situ beneath the skin in a buried position not easily visible to observation[35,36] [Figure 4]. Thus, there is no way to judge when a graft is mature enough for transfer short of surgical exploration of the buried graft and Doppler exam. This has inherent morbidity and mortality. It also requires prolonged growth time. We are, however, developing devices such as Raman and reflectance spectroscopy that may allow noninvasive measurement of graft viability/perfusion through native skin.[37] Successful in situ maturation and transfer has been seen in the reconstruction of the ear and bronchial tree.[6,34] For more complex tissue such as the lip there is presently no construct that can provide all required tissues (skin/mucosa, dermis/muscle, and nerves), nor even the complex transitions required at the superficial level, for proper esthetics.
Figure 4.
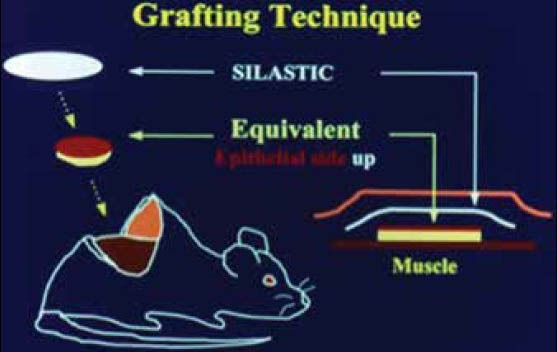
Images showing the stages of in situ development of complex soft tissue structures
COMBINATION APPROACHES USING IN VITRO AND IN SITU METHODS
Currently, our research involves growth of EVPOME in vitro with maturation in situ over a well-suited graft site, the muscle. This has been demonstrated using the rat latissimus dorsi muscle.[38] The mucocutaneous portion is derived from in vitro constructed tissue graft and the muscle and/or bone from the host native tissue. The in vitro grown tissue is then placed over a known vascularized structure such as the latissimus dorsi muscle to create a prelaminated musculocutaneous flap, allowed to mature and then transferred to the area to be reconstructed, the donor site or lips. This can be done via rotational or free tissue transfer with vascular pedicle. We also have furthered our advancement by creating a functional stoma to recreate the oral opening or mouth with functional musculature that can contract as the orbicularis oris complex does [Figure 5]. The mature trilaminar flap is then harvested and placed in the defect site allowing more complex reconstruction than present surgical techniques to simply replacing bulk and lip seal. Currently, our main research focus is volitional control of the orbicularis oris equivalent musculature of the latissimus dorsi muscle, allowing function, as well as creating sensate flaps [Figure 6].
Figure 5.
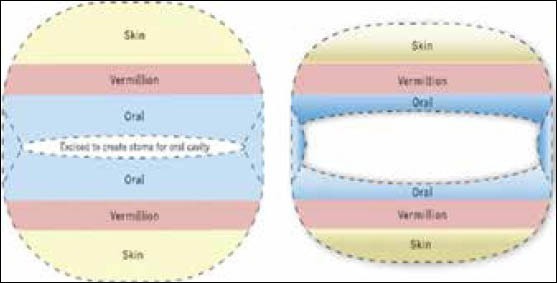
Combination approaches using in vitro and in situ methods for reconstruction of soft tissue plan
Figure 6.
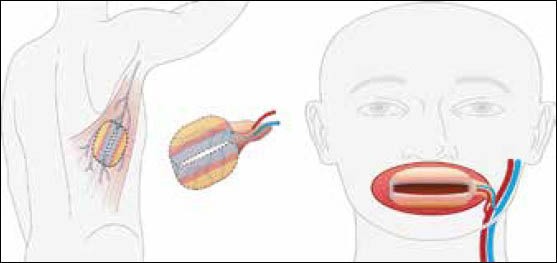
Plan for reconstruction mimicking missing oral tissues
In addition, our approach to the lips to create a functional sphincter can be applied to other areas outside the craniomaxillofacial region, such as external and internal anal sphincters. Functional difficulties still remain, as the anal sphincter has a continuous basal tone, and relaxes when in function which is opposite from the lip which functions on contraction. The anal sphincter is also different in that it involves autonomic control of the smooth musculature. Although early in development, the utility and practicality of multilaminar, nonimmunogenic, prevascularized tissues is encouraging, in offering viable alternatives to the immunosuppression seen with allogeneic facial transplants.
CONCLUSIONS
The future of reconstructive therapy is to develop designer flaps to provide the appropriate structure, tissue type, function, and sensation that will replace the lost tissue, with acceptable esthetics. Ideally a fully functional, complex, vascularized multilaminar tissue would be made in vitro, matured in situ, and then transferred to the defect site resulting in a more esthetic and functional reconstruction. These customized designer prefabricated prelaminated flaps will carry their own intrinsic vascular and neuronal supply. This is likely to be accomplished through a marriage of traditional tissue grafting such as the latissimus dorsi muscle flap, and an overlay of a construct composed of epithelia and a dermal equivalent that would be used for complex soft tissue fabrication.
Footnotes
Source of Support: National Institute of Health R01 DE013417 (SEF) and Dept. of Defense/US Army Medical Research Acquisition Activity, Alliance for Regenerative Medicine, W81XWH.08.2.0034 (SEF)
Conflict of Interest: No.
REFERENCES
- 1.Izumi K, Song J, Feinberg SE. Development of a tissue-engineered human oral mucosa: From the bench to the bed side. Cells Tissues Organs. 2004;176:134–52. doi: 10.1159/000075034. [DOI] [PubMed] [Google Scholar]
- 2.Payne KF, Balasundaram I, Deb S, Di Silvio L, Fan KF. Tissue engineering technology and its possible applications in oral and maxillofacial surgery. Br J Oral Maxillofac Surg. 2013 doi: 10.1016/j.bjoms.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Beiguera S, Ribatti D. Endothelialization approaches for viable engineered tissues. Angiogenesis. 2013;16:1–14. doi: 10.1007/s10456-012-9307-8. [DOI] [PubMed] [Google Scholar]
- 4.Williams JM, Adewunmi A, Schek RM, Flanagan CL, Krescbach PH, Feinberg SE, et al. Bone tissue engineering using polycaprolactone scaffolds fabricated via selective laser sintering. Biomaterials. 2005;26:4817–27. doi: 10.1016/j.biomaterials.2004.11.057. [DOI] [PubMed] [Google Scholar]
- 5.Bagheri S, Bell B, Khan H. Tissue Engineering. Ch 9. St. Louis, Missouri: Elsevier Saunders; 2011. Current therapy in oral and maxillofacial surgery. ISBN: 978-1-4160-2527-6. [Google Scholar]
- 6.Zopf D, Hollister S, Ohye RG, Green GE. Bioresorbable airway splint created with a three-dimensional printer. N Engl J Med. 2013;368:2043–5. doi: 10.1056/NEJMc1206319. [DOI] [PubMed] [Google Scholar]
- 7.Gunarajah DR, Samman N. Biomaterials for repair of orbital floor blowout fractures: A systematic review. J Oral Maxillofacial Surg. 2013;71:550–70. doi: 10.1016/j.joms.2012.10.029. [DOI] [PubMed] [Google Scholar]
- 8.Costello BJ, Shah G, Kumata P, Sfeir CS. Regenerative medicine for craniomaxillofacial surgery. Oral Maxillofac Surg Clin North Am. 2010;22:33–42. doi: 10.1016/j.coms.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 9.Garrison KR, Shemilt I, Donell S, Ryder JJ, Mugford M, Harvey I, et al. Bone morphogenetic protein (BMP) for fracture healing in adults. Cochrane Database Syst Rev. 2010;6:CD006950. doi: 10.1002/14651858.CD006950.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo J, Li C, Zhang Q, Wu G, Deacon SA, Chen J, et al. Secondary bone grafting for alveolar cleft in children with cleft lip or cleft lip and palate. Cochrane Database Syst Rev. 2011;6:CD008050. doi: 10.1002/14651858.CD008050.pub2. [DOI] [PubMed] [Google Scholar]
- 11.Schinzel RT, Ahfeldt T, Lau FH, Lee YK, Cowley A, Shen T, et al. Efficient Culturing and Genetic Manipulation of Human Pluripotent Stem Cells. PLoS One. 2011;6:e27495. doi: 10.1371/journal.pone.0027495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phillips TJ. New skin for old: Developments in bilogocal skin substitutes. Arch Dermatol. 1998;134:344–9. doi: 10.1001/archderm.134.3.344. [DOI] [PubMed] [Google Scholar]
- 13.Bilousova G, Rop DR. Generation of functional multipotent keratinocytes from mouse induced pluripotent stem cells. Methods Mol Biol. 2013;961:337–50. doi: 10.1007/978-1-62703-227-8_22. [DOI] [PubMed] [Google Scholar]
- 14.Izumi K, Feinberg SE, Iida A, Yoshizawa M. Intraoral grafting of an ex vivo produced oral mucosa equivalent: A preliminary report. Int J Oral Maxillofac Surg. 2003;32:188–97. doi: 10.1054/ijom.2002.0365. [DOI] [PubMed] [Google Scholar]
- 15.Kriegbaum U, Mildenberger M, Mueller-Richter A, Klammert U, Kuebler AC, Reuther T. Tissue engineering of human oral mucosa on different scaffolds: In vitro experiments as a basis for clinical applications. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114:S190–8. doi: 10.1016/j.oooo.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 16.Fernandes R, Clemow J. Outcomes of total or near total lip reconstruction with microvascular tissue transfer. J Oral Maxillofac Surg. 2012;70:2899–906. doi: 10.1016/j.joms.2012.02.035. [DOI] [PubMed] [Google Scholar]
- 17.Lubek JE, Ord RA. Lip Reconstruction. Oral Maxillofac Surg Clin North Am. 2013;25:203–14. doi: 10.1016/j.coms.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Fries R. Advantages of a basic concept in lip reconstruction after tumour resection. J Oral Maxillofac Surg. 1973;1:13–8. doi: 10.1016/s0301-0503(73)80007-4. [DOI] [PubMed] [Google Scholar]
- 19.Baker S. Reconstruction of The Lips. Ch 17. Philadelphia, PA: Elsevier Mosby; 2007. Local flaps in facial reconstruction. ISBN: 9780323036849. [Google Scholar]
- 20.Dorafshar AH, Bojovic B, Christy MR, Borsuk DE, Iliff NT, Brown EN, et al. Total face, double jaw, and tongue transplantation: An evolutionary concept. Plast Reconstr Surg. 2013;131:241–51. doi: 10.1097/PRS.0b013e3182789d38. [DOI] [PubMed] [Google Scholar]
- 21.Pashuck T, Stevens MM. Designing regenerative biomaterial therapies for the clinic. biomaterials. Sci Transl Med. 2012;4:160sr4. doi: 10.1126/scitranslmed.3002717. [DOI] [PubMed] [Google Scholar]
- 22.Cusano A, Fernandes R. Technology in microvascular surgery. Oral Maxillofac Surg Clin North Am. 2010;22:73–90. doi: 10.1016/j.coms.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Kerrebijn JD, Freeman JL. Facial nerve reconstruction: Outcome and failures. J Otolaryngol. 1998;27:183–6. [PubMed] [Google Scholar]
- 24.Celik E, Tercan M, Uzunismail A, Sağlam A. Versatility of botulinum toxin: A use in stabilization of pedicled muscle flaps. Plast Reconst Surg. 2006;117:462–7. doi: 10.1097/01.prs.0000197214.57838.9b. [DOI] [PubMed] [Google Scholar]
- 25.Wolff KD, Stiller D. Functional aspects of free muscle transplantation: Atrophy, reinnervation, and Mmtabolism. J Reconstr Microsurg. 1992;8:137–42. doi: 10.1055/s-2007-1006696. [DOI] [PubMed] [Google Scholar]
- 26.Jones MS, Messersmith PB. In Situ forming biomaterials. Oral Maxillofac Surg Clin North Am. 2002;14:29–38. doi: 10.1016/s1042-3699(02)00015-8. [DOI] [PubMed] [Google Scholar]
- 27.Spagnoli DB, Marx RE. Dental implants and the use of rhBMP-2. Oral Maxillofac Surg Clin North Am. 2011;23:347–61. doi: 10.1016/j.coms.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 28.Boyan BD, Dean DD, Lohmann CH, Niederauer GG, McMillan J, Sylvia VL, et al. Cartilage regeneration. Oral Maxillofac Surg Clin North Am. 2002;14:106–16. doi: 10.1016/s1042-3699(02)00017-1. [DOI] [PubMed] [Google Scholar]
- 29.Izumi K, Feinberg SE. Skin and oral mucosal substitutes. Oral Maxillofac Surg Clin North Am. 2002;14:61–71. doi: 10.1016/s1042-3699(02)00010-9. [DOI] [PubMed] [Google Scholar]
- 30.Peramo A, Marcelo CL, Feinberg SE. Tissue engineering of lips and muco-Cutaneous junctions: In vitro development of tissue engineered constructs of oral mucosa and skin for lip reconstruction. Tissue Eng Part C Methods. 2012;18:273–82. doi: 10.1089/ten.tec.2011.0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Izumi K, Terashi H, Marcelo CL, Feinberg SE. Development and characterization of a tissue-engineered human oral mucosa equivalent produced in a serum-free culture system. J Dent Res. 2000;79:798–805. doi: 10.1177/00220345000790030301. [DOI] [PubMed] [Google Scholar]
- 32.Izumi K, Marcelo CL, Feinberg SE. Enrichment of oral mucosa and skin keratinocyte Progenitor/stem cells. Methods Mol Biol. 2013;989:293–303. doi: 10.1007/978-1-62703-330-5_23. [DOI] [PubMed] [Google Scholar]
- 33.Izumi K, Neiva R, Feinberg SE. Intraoral grafting of a tissue engineered human oral mucosa. Oral Craniofac Tissue Eng. 2011;1:103–11. doi: 10.11607/jomi.te11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haisch A, Klaring S, Groger A, Gebert C, Sittinger M. A tissue-engineering model for the manufacture of auricular-shaped cartilage implants. Eur Arch Otorhinolaryngol. 2002;259:316–21. doi: 10.1007/s00405-002-0446-1. [DOI] [PubMed] [Google Scholar]
- 35.Orgill D, Ogawa R. Current methods of burn reconstruction. Plast Reconstr Surg. 2013;131:827–36e. doi: 10.1097/PRS.0b013e31828e2138. [DOI] [PubMed] [Google Scholar]
- 36.Cinpolat A, Bektas G, Coskunfirat OK. Complex partial nasal reconstruction using free prelaminated temporoparietal fascial flap. Microsurgery. 2013;33:56–9. doi: 10.1002/micr.22058. [DOI] [PubMed] [Google Scholar]
- 37.Khmaladze A, Ganguly A, Kuo S, Raghavan M, Kainkaryam R, Cole JH, et al. Tissue-engineered constructs of human oral mucosa examined by raman spectroscopy. Tissue Eng Part C Methods. 2013;19:299–306. doi: 10.1089/ten.tec.2012.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tan BK, Chen HC, He TM, Song IC. Flap prefabrication – the bridge between conventional flaps and tissue-engineered flaps. Ann Acad Med Singapore. 2004;33:662–6. [PubMed] [Google Scholar]


