Abstract
Consumption of a high-fat and/or high-cholesterol diet can have detrimental effects on the brain. In the present study, dietary treatment with saturated fats, trans fats, or cholesterol to middle-aged Fischer 344 rats resulted in alterations to serum triglyceride and cholesterol levels, organ weights, and hippocampal morphology. Previously, we demonstrated that a 10% hydrogenated coconut oil and 2% cholesterol diet resulted in worse performance on the 12-day water radial arm maze, increased cholesterol and triglyceride levels, and decreased dendritic microtubule associated protein 2 (MAP2) staining in the hippocampus. The diets administered herein were used to examine components from the previous diet and further examine their effects on hippocampal morphology. Specifically, neuronal morphology, dendritic integrity, fatty acid metabolism, microgliosis, and blood vessel structure in the hippocampus and/or adjacent structures were explored. Our results indicate alterations to peripheral and neural systems following each of the diets.
Keywords: Saturated fat, Trans fat, Morphology, Aging, Dietary effects
Introduction
The effects of dietary lipids on brain structure and function are currently rising in interest. The brain is a lipid-rich environment with many different fatty acids and cholesterol playing important roles in membrane structure, myelination, and signaling.1 It is well known that cholesterol is produced in situ, with little to none passing the blood–brain barrier (BBB) while essential fatty acids taken up from the diet provide building blocks for basic brain structure and function.2,3 However, the precise mechanisms by which the diet influences the brain remain to be determined. Of great interest today, as rates of cognitive decline rise with the baby boomer generation aging, and obesity reaches an epidemic proportion, is the relationship between a ‘western diet’ (high levels of saturated fat, trans fat, and cholesterol) and cognitive decline. Human epidemiological studies have revealed that a diet with high levels of omega-6 and saturated fatty acids is associated with worse performance on a cognitive task4-7 whereas a lower fat diet consisting of omega-3 fatty acids has a protective effect.7 It has also been demonstrated that increased consumption of saturated fat and cholesterol is associated with increased cholesterolemia, risk of cardiovascular disease, and impaired intellectual function.8 Furthermore, deficits in the diet late in life can be the cause of, as well as worsen, cognitive decline.5,9
Animal studies have confirmed epidemiological findings that a high-fat diet is correlated with cognitive decline. In an experiment performed by Greenwood and Winocur, 1-month old rats were treated with a diet high in saturated fat or unsaturated fat (soybean oil) for 3 months. Both diets delivered high levels of fat and led to impairments on all learning and memory tasks administered; however, the saturated fat diet revealed the most significant effects.9 In another study, a diet high in saturated fat and refined sugar administered to 2-month-old rats for 2 months, 6 months, and 2 years decreased cognitive ability as measured by the Morris water maze at every time point. The decline in cognition was correlated with a significant decrease in hippocampal brain derived neurotrophic factor levels.10 Finally, a study investigating potential mechanisms for dietinduced cognitive decline demonstrated reduced hippocampal neurogenesis in young male rats following just 4 weeks on a high-fat diet as measured by BrdU levels.11 In our recent study, 16-month-old male Fischer 344 rats were treated with a ‘Sat-Fat’ diet containing 10% hydrogenated coconut oil and 2% cholesterol (by weight) and compared to a control diet containing 12% soybean oil. The‘Sat-Fat’ animals had significantly elevated cholesterol and triglyceride levels and performed worse on the water version of the radial arm maze compared to those administered an iso-caloric control diet. In addition to our findings which confirm the correlation between a high-fat diet and cognitive ability, we found a decrease in hippocampal dendritic expression of the microtubule marker, microtubule associated protein 2 (MAP2), a significant correlation between MAP2 immunoreactivity (MAP2-ir) and errors on the water radial arm maze, and altered microglial morphology.12 Our current study builds on the previous findings to evaluate circulating cholesterol/triglyceride levels and hippocampal morphology following administration of different fatty acids or cholesterol.
One of the regions playing an integral role in learning and memory, a main region affected in cognitive decline and Alzheimer’s disease, is the hippocampus.13-15 Alterations to certain brain regions naturally occur with aging including a decrease in synaptic densities, long-term potentiation/long-term depression,16,17 calcium homeostasis,18 and neurotrophic factors.17,19,20 Specific to the hippocampus, it has been shown that dendritic integrity is significantly altered with aging.21 Therefore, the initial characterization of dietary effects on hippocampal morphology in this study included observations of dendritic integrity by assessing MAP2-ir. Microtubule associated proteins are essential to structural maintenance throughout the brain.22 MAP2 is specifically expressed in the soma and dendrites of neurons,23-25 it is commonly used as a general marker for neural injury in young as well as adult rats,26,27 and it is frequently used as a marker for hypoxic-ischemic brain injury in rats.22,26,28 We have also shown in a recent study that MAP2 expression in a down syndrome mouse model is reduced in the hippocampus and correlates with memory loss.29 The present study represents an expansion of our recent experiments that revealed effects of a ‘western diet’ on cognitive performance and hippocampal morphology in middle-aged male rats. The aims of the present study were two-fold: (1) to determine which of the diets tested will give rise to the highest levels of triglycerides and cholesterol in serum, and (2) to determine whether any of the diets have damaging effects on hippocampal morphology. Here, we reveal alterations to serum triglycerides and/or cholesterol and reduction in MAP2-ir following just 8 weeks of dietary treatments (CHOL, HSO, and LARD) in the middle-aged rat.
Materials and methods
Animals and diets
Animal protocols were approved by the Medical University of South Carolina Institutional Care and Use Committee and carried out according to the regulations of the Office of Laboratory Welfare. Sixteen-month-old male Fischer 344 rats (Harlan, Indianapolis, IN, USA) were given 1 week to acclimate to the vivarium and then randomly divided into four treatment groups (n = 10 per group). Rats were fed a ‘CHOL’ diet (10% soybean oil with an added 2% pure cholesterol), ‘HSO’ diet (12% hydrogenated soybean oil), or ‘LARD’ diet (12% pure lard) for 8 weeks. The control diet consisted of 12% 2 soybean oil (‘SOY’), in order to provide the same amount of fatty acids but of different origin so that the focus was on the type of fat rather than on a dosage effect. All diets were manufactured by MP Biomedicals and included all other necessary vitamins 3 and minerals (see Table 1). The soybean oil diet delivers some saturated fatty acids and unsaturated fatty acids. The soybean oil itself contains 0.2% arachidonic acid (polyunsaturated fatty acid, omega-6), 50–54% linoleic acid (polyunsaturated fatty acid, omega-6), 10–11% palmitic acid (common saturated fatty acid), 23–27% oleic acid (monounsaturated omega-9 fatty acid), 0.9% eicosenoic acid (monounsaturated omega-9 fatty acid), 7–8% linolenic acid (omega-3 – essential fatty acid), and 3–4% stearic acid (saturated fat). However, when compared to the saturated fat diet which consisted of 12% lard, there are significant differences in the types of fat administered. The lard consists of 1–4% myristic acid (saturated fatty acid), 20–30% palmitic acid (saturated fatty acid), 1–4% palmitoleic acid (monounsaturated omega-7 fatty acid), 5–22% stearic acid (saturated fatty acid), 41–51% oleic acid (monounsaturated omega-9 fatty acid), and 0.3–1% arachidic acid (saturated fatty acid). The other diets were based on the soybean oil diet: the HSO diet is hydrogenated soybean oil which leads to increased trans fats and an increased number of saturated fats. The cholesterol diet was 10% of the soybean oil with added 2% pure cholesterol. Total body weights and food consumption were measured weekly throughout the experiment.
Table 1. Diet composition.
| Component (g) | Soybean oil | Cholesterol | Hydrogenated soybean oil | Lard |
|---|---|---|---|---|
| Corn starch | 2027 | 2027 | 2027 | 2027 |
| Casein purified high nitrogen | 1400 | 1400 | 1400 | 1400 |
| Dextrinized corn starch | 1550 | 1550 | 1550 | 1550 |
| Sucrose | 1000 | 1000 | 1000 | 1000 |
| Soybean oil | 1200 | 1000 | 1200* | 1200** |
| Alphacel non-nutritive bulk | 2355 | 2355 | 2355 | 2355 |
| AIN-93M mineral mix | 350 | 350 | 350 | 350 |
| AIN-93M vitamin mix | 100 | 100 | 100 | 100 |
| l-cystine | 18 | 18 | 18 | 18 |
| T-butyl hydroquinone | 0.08 | 0.08 | 0.08 | 0.08 |
| Cholesterol | X | 200 | X | X |
Hydrogenated.
Lard.
Tissue collection and preparation
Rats were anesthetized deeply with isoflurane gas (Novaplus), trunk blood was collected, and the subjects were decapitated. The right hemisphere of the brain was collected for morphological experiments and was therefore blocked and post-fixed in 4% paraformaldehyde for 24 hours, followed by storage in 30% sucrose in 0.1 M phosphate buffered saline. The liver, heart, and belly fat were collected, weighed and compared between groups.
Serum total cholesterol and triglyceride levels
Serum was centrifuged and stored at −20 °C until analysis. Serum cholesterol and triglyceride levels were analyzed using the SYNCHRON® systems kit as done previously.12 CHOL reagent was utilized to measure cholesterol concentration by a timed-end-point method. In the reaction, cholesterol esterase hydrolyzes cholesterol esters to free cholesterol and fatty acids. Free cholesterol was oxidized to cholestene-3-one and hydrogen peroxide by cholesterol oxidase. Peroxidase catalyzed the reaction of hydrogen peroxide with 4-aminoantipyrine and phenol to produce a colored quineimine product. The SYNCHRON® system automatically proportioned the appropriate sample and reagent volumes into the cuvette. The ratio used was one part sample to 100 parts reagent. The system monitors the change in absorbance at 520 nm. This change in absorbance is directly proportional to the concentration of CHOL in the sample and is used to calculate and express CHOL concentration. Triglycerides GPO reagent (SYNCHRON®) was used to measure the triglyceride serum concentration by a timed-endpoint method. Triglycerides in the sample were hydrolyzed to glycerol and free fatty acids by the action of lipase. A sequence of three coupled enzymatic steps using glycerol kinase, glycerophosphate oxidase (GPO), and horseradish peroxidase causes the oxidative coupling of 3,5-dicholor-2-hydroxybenzenesulfonic acid with 4-aminoantipyrine to form a red quinoeimine dye. The SYNCHRON® system(s) automatically proportions the appropriate sample and reagent volumes into the cuvette. The ratio used is one part sample to 100 parts reagent. The system monitors the change in absorbance at 520 nm. This change in absorbance is directly proportional to the concentration of TG in 5 the sample and is utilized to calculate and express the TG concentration.
Statistical analysis
A one-way ANOVAwith Fisher’s post hoc analysis was used in order to determine whether there were significant differences in body weights, triglyceride levels, cholesterol levels, and organ weights between treatment groups.
Morphological assessment
The right hemisphere was sectioned to a thickness of 40 μm through the hippocampus on a freezing microtome (Microm HM400) and collected in 0.01 M Tris buffered saline (TBS) according to our previous protocols.30 Hematoxylin and eosin staining was performed on every 12th section in order to visualize general brain tissue and blood vessel morphology throughout the longitudinal axis of the hippocampus. Sections were mounted on slides and incubated in xylene followed by decreasing concentrations of ethanol. Slides were then incubated in Mayer’s hemalum for 4 minutes, followed by washing in tap water and incubation in eosin for 1 minute. Finally, slides were differentiated in 95% ethanol, washed in absolute ethanol and xylene, and coverslipped in Permount (Fisher Scientific, Pittsburgh, PA, USA).
Immunohistochemistry
Sections from the right hemisphere through the hippocampus (40 μm) were evaluated for dendritic morphology, fatty acid metabolism, inflammation, and blood vessel morphology with immunohistochemistry as described previously.31,32 The first section in a series was randomly picked, where after every 12th section was stained for each marker, in order to obtain a systematic random design. First, all tissues were washed in TBS (3 × 10 minutes) and then etched in a TBS: methanol: hydrogen peroxide solution with a ratio of 7:2:1 for 20 minutes. The tissue was washed again in TBS with 0.4% Triton X-100 (TBST) (3 × 10 minutes) then placed in a blocking solution of TBST and 10% serum for 1 hour. Next, sections were placed in the primary antibody solution (with serum and TBST). The following antibodies were used: MAP2 (microtubule associated protein II for dendritic morphology, Chemicon, concentration 1:1000, 3% NGS (normal goat serum)), 5-LOX (5-lipoxygenase, Cayman Chemical, concentration 1:1000, 3% NGS), OX-6 (RT1B class II monomorphic for activated microglia, Serotec, concentration 1:1000, 1% NGS), and Glut1 (glucose transporter I for blood vessel morphology, Chemicon, concentration 1:1000, 3% NGS). Specifically, 5-LOX is a catalyst for the oxidation of amino acids at the fifth position which will produce 5-hydroperoxyeicosatetraenoic acid which is converted to leukotriene A4.33 It has been demonstrated that inflammation is augmented by the lipid peroxidation produced by 5-LOX which also increases neuronal death.34
Sections were incubated in the primary antibody solutions for 48 hours at 4 °C, after which they were washed in TBS (3 × 10 minutes) and incubated in the secondary antibody solution (antibody, 3% NGS, TBS) for 1 hour at room temperature. The secondary antibody for MAP2, Glut1, and 5-LOX was biotinylated goat anti-rabbit IgG (Vector), and OX-6 used biotinylated horse anti-mouse IgG (Vector). Sections were washed in TBS (3 × 10 minutes) then placed in the Elite ABC reagent (Vector) for 1 hour. Sections were washed again, incubated with 3,3′-diaminobenzidine (DAB; 0.02%) and developed in nickel ammonium sulfate (Fisher Scientific; 25 mM), DAB (0.02%), and hydrogen peroxide (0.01%) in TBS. Finally, sections were washed, mounted on subbed slides, air dried overnight, dehydrated, coverslipped with Permount (Fisher Scientific,) and examined with a light microscope (Nikon Optiphot). Staining controls consisted of omission of the primary antibody. When the words ‘immunoreactive’ or ‘−positive’ are used in the text, this always refers to ‘like immunoreactivity’, since no direct evidence for location of molecules can be obtained with indirect immunohistochemical techniques. Sections from all groups were incubated in the same bath to avoid group inter-variability in staining.
Area of loss (AOL) measurements
Quantitation of the area covered by MAP2 staining loss in the hippocampus was performed on every 12th section through the brain region according to our previously described routine protocol.35 In short, MAP2 stained sections of the dorsal hippocampus were digitized in gray scale using a 10× lens, and the areas that exhibited a lack of staining were circled using a densitometry tool. All samples were digitized in the same time frame to ensure a constant light intensity across all groups. The NIH image software (Scion Image) was used to measure the AOL of MAP2 staining in all groups. This NIH image software measures gray scale values within the range of 0–256, with 0 representing white and 256, black. Measurements were performed blinded and the values from the images were averaged to obtain one value per animal.
Results
Weight and food consumption
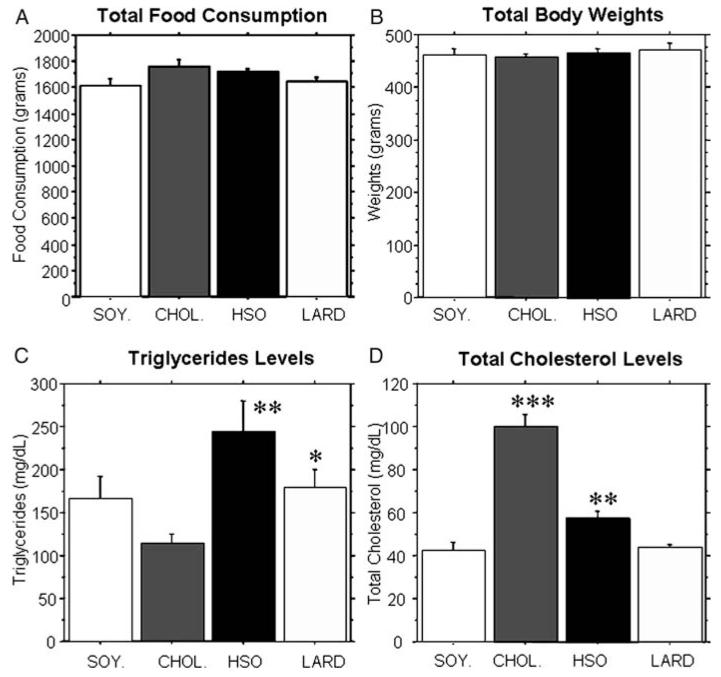
The animals were randomly divided into four groups (SOY., CHOL., HSO, LARD) and were administered their diets for 8 weeks. During this time period, no significant differences in food consumption per cage (with two animals per cage) were found between any of the groups (Fig. 1A). Data presented was an average for all 8 weeks with SEM shown and n = 10 for all groups except the HSO group which had an n = 9. The diets were designed to be isocaloric and there were no significant differences between the groups in terms of overall body weights (Fig. 1B).
Figure 1.
Total food consumption, body weights, triglycerides, and cholesterol levels. Animals were weighed weekly and food consumption per cage (2 animals per cage) was measured weekly. (A, B) No significant differences in total food consumption or total body weights were found between the four dietary groups. (C) The hydrogenated soybean oil group and the lard group had significantly higher triglycerides compared to the cholesterol group (P < 0.0019 and P < 0.0127, respectively). (D) The cholesterol group had significantly higher cholesterol levels compared to all other groups (P < 0.0001). Additionally, the hydrogenated soybean oil group had significantly higher levels compared to the saturated fat and control groups (P < 0.007).
Triglyceride and cholesterol levels
Serum triglyceride levels and total cholesterol levels were compared between groups. Animals that received the HSO diet and LARD diet exhibited significantly elevated triglycerides (P < 0.0019 and P < 0.0127, respectively) compared to the CHOL. group (Fig. 1C). Animals that received the CHOL. diet and HSO diet had significantly elevated serum cholesterol levels (P < 0.0001 and P < 0.007, respectively) compared to the other groups (Fig. 1D). Our data demonstrated that after only 8 weeks on these diets, there were significant elevations in triglycerides and/or cholesterol.
Body composition
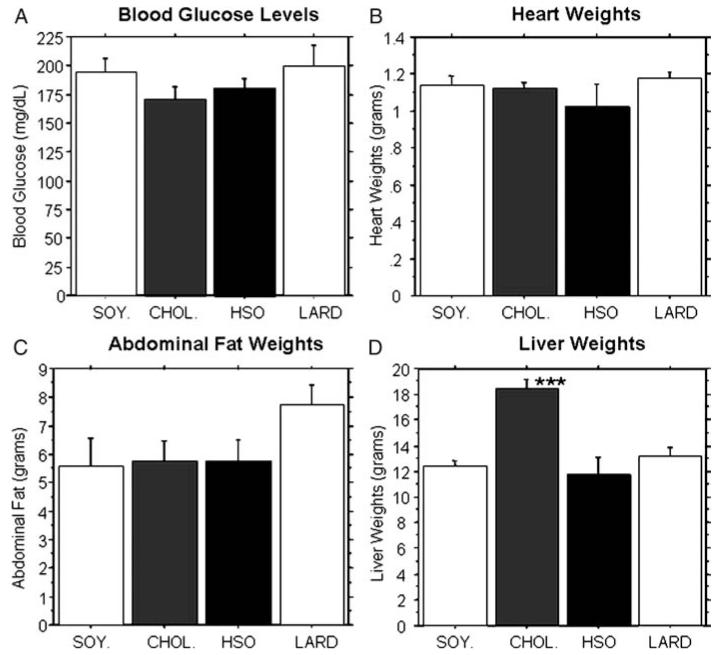
No significant differences in blood glucose levels (Fig. 2A), heart weights (Fig. 2B) or abdominal fat weights (Fig. 2C) were found between the four dietary treatment groups. However, the LARD diet gave rise to an elevation in abdominal fat weight compared to all other groups, even though an overall ANOVA did not reveal statistically significant differences between any groups for this measure. The CHOL. group exhibited significantly elevated liver weights compared to all other groups (P < 0.0001, Fig. 2D). While all three experimental diets increased serum triglyceride and/or cholesterol levels, only the CHOL. diet had a significant effect on liver weight, at least after 8 weeks on the diet.
Figure 2.
Blood glucose levels and organ weights. At the time of sacrifice, blood, hearts, abdominal fat pads, and livers were collected for analysis. There were no significant differences found between the groups for blood glucose levels (A), heart weights (B), or abdominal fat pads (C). However, the cholesterol fed animals had significantly elevated liver weights compared to all other groups (D) (P < 0.0001).
Routine histology of hippocampus
Hematoxylin-Eosin is a routine stain in which all basophilic and eosinophilic cell components are stained. All layers of the hippocampal formation were visible throughout the CA1/CA2/CA3 (see Figs. 3 and 4A-D). These results suggest that although there is a treatment effect as evidenced by MAP2 immunostaining (described below), the loss does not represent an actual neuronal cell death within the affected area but instead a reduction in MAP2 expression and/or dendritic degeneration.
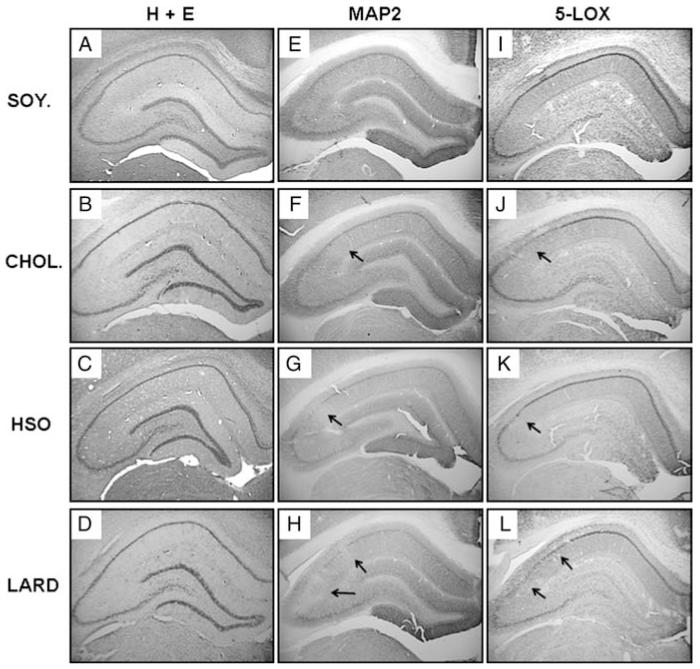
Figure 3.
Immunohistochemistical analysis of the hippocampus. H + E staining (A–D) did not reveal any major differences between groups. However, dietary treatment altered MAP2 expression (E–H) and 5-LOX expression (I–L). Representative results showing areas with reduced immunoreactivity for MAP2 and 5-LOX from the right hemisphere are presented for the control soybean oil diet (A, E, I), cholesterol diet (B, F, J), hydrogenated soybean oil diet (C, G, K), and saturated fat lard diet (D, H, L). Arrows mark areas of loss. Magnification = 2×.
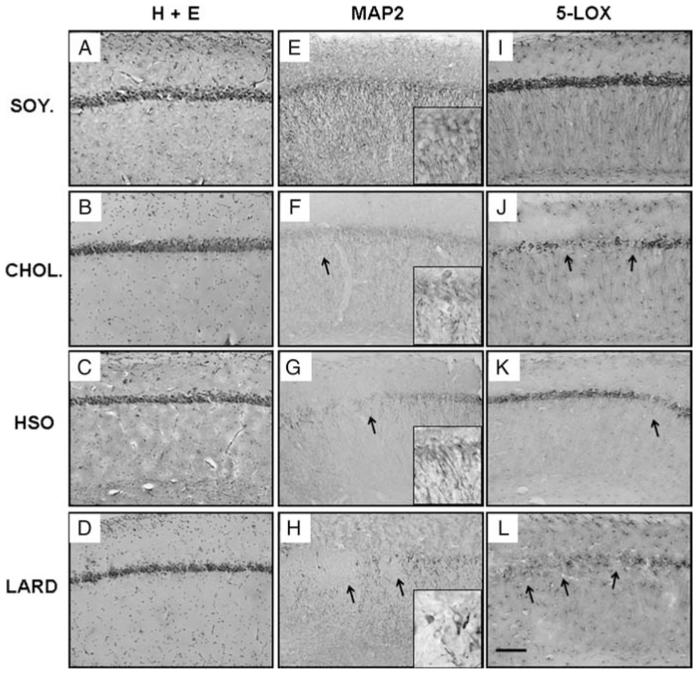
Figure 4.
Immunohistochemistical analysis of the hippocampus. Higher magnification of the results from the right hemisphere are shown for the control soybean oil diet (A, E, I), cholesterol diet (B, F, J), hydrogenated soybean oil diet (C, G, K), and saturated fat lard diet (D, H, L). Panels A–D reveal H + E staining. Antibodies used include MAP2 (E-H) and 5-LOX (I-L). Arrows mark regions of loss or lowered expression. Magnification = 20 × , scale bar = 100 μm.
Dietary treatment groups revealed alterations in dendritic integrity
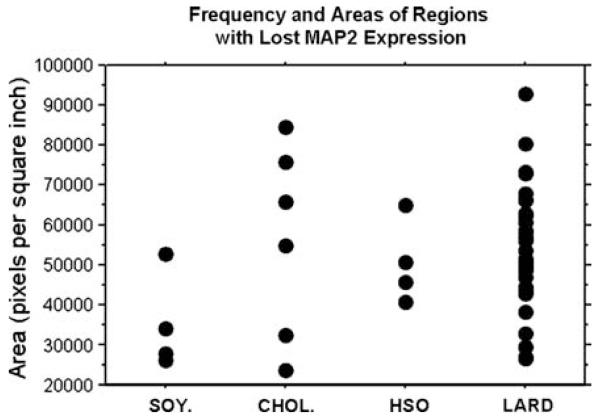
Immunohistochemistry for the neuronal marker MAP2 was performed on serial sections (every 12th section) through the hippocampus of the right hemisphere. Fig. 3E-H and Fig. 4E-H reveal the varying MAP2-ir for each dietary treatment. The SOY. group exhibited the greatest overall MAP2-ir while the CHOL., HSO, and LARD groups exhibited greatly reduced overall MAP2 staining in comparison. The hippocampus for each group also had focal areas with complete loss of immunoreactivity. The LARD group displayed the greatest number and areas of loss, as shown in Fig. 4H (arrows indicate regions without MAP2-ir) and Fig. 5. These focal areas of lost expression were mostly found in the lateral CA1 region close to the bordering CA2/CA3 regions; the loss excluded bordering regions suggesting a regional specificity. Some neurons exhibited a complete lack of MAP2-ir in both cell bodies and neurites, even though routine staining revealed that the CA1 pyramidal neurons were still present, suggesting a targeted protein expression loss rather than cell death per se. Fig. 4F-H and their insets represent higher magnification micrographs of the immunoreactive neurons and their dendritic tree that appeared shortened, swollen, and sparse, contrary to the robust apical dendritic trees observed in soybean oil controls. Because the focal loss was restricted to certain areas of the CA1-3, and including both neurites and cell bodies, quantification of MAP2 was carried out using AOL measurements. Fig. 5 is a bivariate scattergram charting the frequency as well as the areas of these regions (expressed as pixels per square inch) with lost MAP2 expression per dietary group. Because a greater frequency of AOLs were observed in the LARD, HSO, and CHOL groups compared to controls, these three groups were collapsed and overall MAP2 immunostaining was found to be reduced in these groups (not shown), compared to overall MAP2 AOL observed in the control group (approaching significance, P = 0.0512). The graph in Fig. 5 demonstrates that the LARD group has the greatest frequency and the greatest area covered by these regions.
Figure 5.
Frequency and area of regions with lost MAP2 expression. The number of regions with lost MAP2 expression were counted throughout the serial hippocampal sections. Densitometry was also used to measure the areas displaying lost expression. Area is measured in pixels per square inch. The scattergram reveals the ‘LARD’ group with the greatest number of regions with lost MAP2 expression as well as the greatest area covered by these regions.
Neuro-inflammatory markers
In order to examine possible biological mechanisms for the observed MAP2 loss in the CA1, we stained for two different neuroinflammatory markers: 5-LOX, an enzyme whose action is to convert essential fatty acids to leukotrienes, and the MHC Class II marker OX-6, which labels activated microglial cells. In Figs 3I-L and 4I-L, a decrease in 5-LOX immunoreactivity for the treatment groups (CHOL, HSO, and LARD) as compared to the SOY group is shown. Again, the pattern of loss correlates with that seen for MAP2 immunohistochemistry. The LARD group displayed the greatest number and areas of loss, as shown in Fig. 4L. The CHOL and HSO groups also displayed focal areas of loss as shown in a higher magnification (Fig. 4J and K). However, the most striking result was the overall lack of 5-LOX expression especially in glial cells of stratum oriens and radiatum in the HSO animals (Figs 3K and 4K). The CHOL and LARD animals revealed a loss of 5-LOX expression in the pyramidal cells and dendrites, but the astrocytes throughout the hippocampus were still immunoreactive. On the other hand, the HSO tissue exhibited an overall loss of 5-LOX immunoreactivity in the pyramidal cells, dendrites, and surrounding astrocytes.
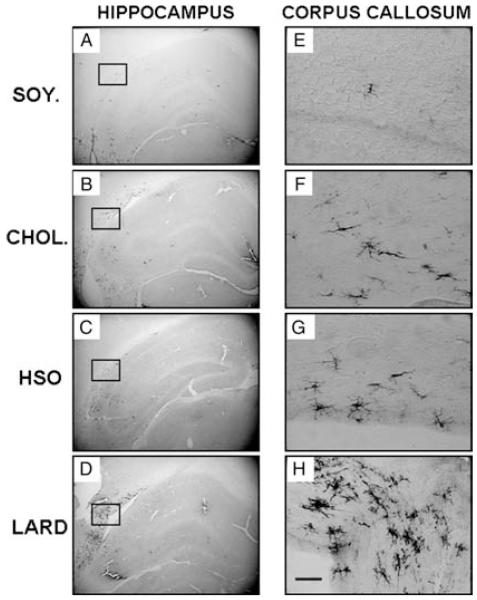
There was an overall, low level of activated microglia present throughout the hippocampus for all groups (Fig. 6). Although aging gives rise to a slow but progressive increase in OX-6 immunolabeling,30 normal aging in itself does not give rise to a significant activation of microglia in gray matter of the hippocampus, as evidenced by the SOY control group in the present study. However, CHOL, LARD, and HSO diets gave rise to increased OX-6 expression in the white matter compared to the SOY-treated group. This was especially true for the LARD-treated group, as can be seen in Fig. 6D + H. The clustering of activated microglial cells, identified by both their staining with OX-6 and their morphological appearance, was especially prominent in the white matter adjacent to the CA2/CA3 border. The location and the significant increase in OX-6-immunoreactive glial cells definitely suggested an involvement of this cell type in detrimental effects of the high-fat diets observed herein, in previous work from our group12 and others.36-38
Figure 6.
Inflammation as measured by OX-6 expression. Inflammation was measured in the hippocampus using the antibody to MHC class II macrophages (OX-6). OX-6 expression was not significantly altered in the whole hippocampus as all animals had a low expression (A: control soybean oil diet; B: cholesterol diet; C: hydrogenated soybean oil diet; and D: saturated fat lard diet). However, a closer look at the corpus callosum (E–H) revealed increased expression especially in the saturated fat animals. This area is located above those shown in previous results to have decreased expression (MAP2 and 5-LOX). Magnification = 40 × , scale bar = 200 μm (E–H).
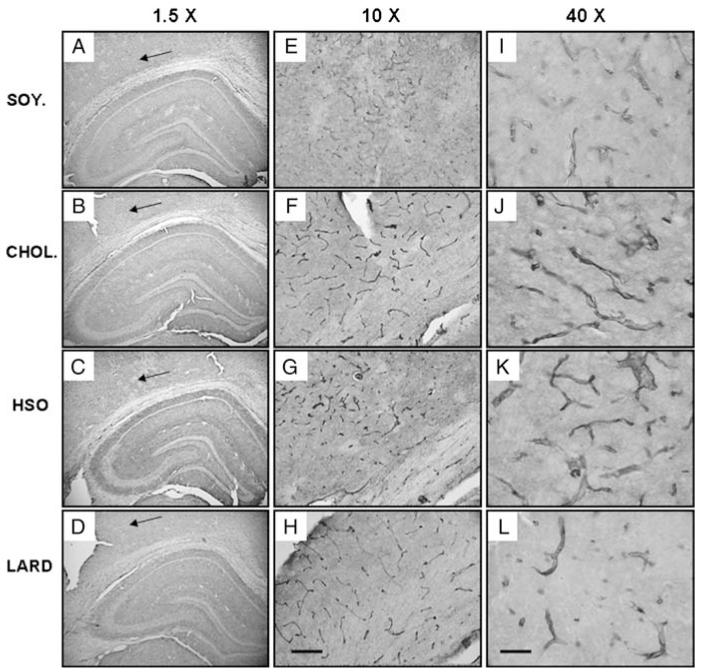
Effect on vascularization
Lastly, we evaluated blood vessel morphology with an antibody to the glucose transporter 1, Glut1. Gross overall examination of blood vessel morphology did not reveal any significant differences in vascularization of the hippocampal gray matter between the diet groups. However, the white matter adjacent to the CA1 and cortex revealed altered microvessel morphology which correlated with regions close to the focal MAP2 loss depicted in Figs 3 and 4. The blood vessels (shown in a larger magnification, Fig. 7E-L) revealed greater intensity of Glut1 immunoreactivity in the CHOL, HSO, and LARD groups with thicker walls and possible initial cuffing.
Figure 7.
Blood vessel structure as measured by Glut-1 expression. (A–D) illustrates the whole hippocampus while (E–H) and (I–L) are a higher magnification of what is found at the arrows. The area of interest (arrows) correlates with areas shown previously with a loss of MAP2 expression, 5-LOX expression, and increased inflammation in the corpus callosum. Here, we see altered blood vessel structure in the treatment groups (cholesterol: B, F, J; hydrogenated soybean oil: C, G, K; and saturated fat: D, H, L) compared to control (soybean oil: A, E, I). Magnification = 10 × , scale bar = 50 μm (E–H). Magnification = 40 × , scale bar = 200 μm (I–L).
Discussion
In the present study, dietary treatment with saturated fats, trans fats, and cholesterol to middle-aged Fischer 344 rats resulted in alterations to serum triglyceride and cholesterol levels, organ weights, and hippocampal morphology. Previously, we demonstrated that a 10% hydrogenated coconut oil and 2% cholesterol diet resulted in worse performance on the 12-day water radial arm maze, increased cholesterol and triglyceride levels, and a decrease in dendritic MAP2 staining in the hippocampus.12 The diets administered in the current study were used to examine components from the previous diet and their effects on hippocampal morphology, circulating triglycerides, and cholesterol levels. The hippocampus was chosen as a main target due to its known role in learning and memory and its implications in aging-related diseases such as Alzheimer’s disease.13-15 This study has focused on a small window of time (8 weeks of diet treatment) in order to observe relatively early effects of the diets.
Rats were housed two animals per cage and isocaloric diets were administered, resulting in similar body weights for all four dietary groups. No significant differences were observed for food consumption either; therefore, we believe there were no significant differences in palatability. This was consistent with previous studies from our lab and others in which a trans fat diet or saturated fat diet was administered and did not result in significant weight gain.12,39-41 Many studies have used high-fat diets of varying composition to induce obesity or metabolic syndrome;42-46 our study did not use a high enough percentage of fat for this to occur as many other factors from these conditions could confound the results.
On the other hand, alterations to serum triglyceride and cholesterol levels were remarkable. Most notable was the HSO group which had significantly elevated triglyceride and cholesterol levels. It has been demonstrated in humans that consumption of trans fats (hydrogenated oils) can lead to an increase in total cholesterol and low-density lipoprotein (LDL) cholesterol.47 There are controversial results regarding the effects on lowering high-density lipoprotein cholesterol and raising triglyceride levels, but it is clear that there is an association between trans fat consumption and risk for coronary heart disease from epidemiological data.48-50 Animal studies on trans fats have also produced varying results: a study in hamsters demonstrated a neutral effect on LDL receptor activity and plasma LDL concentration whereas a study in male green monkeys demonstrated increased abdominal fat deposition and increased plasma insulin levels.51 Metabolic alterations induced by an unhealthy diet, especially to insulin levels, have significant effects on overall health leading to type 2 diabetes, heart disease, or stroke.52 Finally, a study in Wistar rats demonstrated increased serum and hepatic triacylglycerol levels for trans fat and saturated fat-treated animals, with saturated fat animals demonstrating the greatest increase.53 To our knowledge, we are the first to reveal alterations in triglyceride and total cholesterol levels for the middle-aged rat fed a trans fat diet (HSO). The specific effects of the HSO diet on triglycerides and cholesterol levels may be due to issues of metabolism. Trans fats (hydrogenated oils) are a man-made fat produced to increase the shelf life of foods. Metabolism of this irregular fat could be altered, even in the brain; it has been previously shown that adipocyte metabolism is affected for trans fatty acids which leads to a reduced uptake of triglycerides.48 These findings have significant relevance for future studies, since elevated triglyceride levels are known to be strongly associated with Type II diabetes, cardiovascular disease, and stroke.54
While some of the data on trans fatty acids are controversial, most likely due to differences in fatty acid composition, length of treatment, and different outcome measures, saturated fats have clearly demonstrated a negative impact on lipid profiles and increased risk for cardiovascular disease in humans49 and animals.53 Our data are consistent with current literature53,55,56 as the saturated fat animals exhibited significantly elevated triglyceride levels as well as increased abdominal fat weights. Lastly, animals fed the cholesterol diet had remarkably high cholesterol levels and significantly elevated liver weights compared to all other animals. Previous studies have outlined the synthesis and pathways for cholesterol production and breakdown, revealing very different courses for the brain and periphery.57 The results obtained in the present study are consistent with our previous findings in which cholesterol was added to the diet (‘western’ diet) and resulted in elevated circulating cholesterol levels.12
Our main goal in this study was to determine which aspect of the ‘western diet’ has the most damaging effect on health. With simply analyzing serum cholesterol and triglycerides as well as organ weights, we have demonstrated each factor of the diet (hydrogenated fat, saturated fat, and cholesterol) to have varying effects on organ weights and lipid profiles. After establishing these effects, we evaluated several markers in the hippocampus. Considerable results were observed with the MAP2 and 5-LOX antibodies (described below) and indicated an effect of the diet on protein expression in pyramidal neurons.
The most intriguing results were found for dendritic integrity using the microtubule associated protein marker, MAP2. All groups revealed areas within the hippocampus with reduced MAP2-ir (Figs 3 and 4E-H), but the LARD group exhibited both larger areas of loss and increased frequency of these areas compared to other groups, as outlined in Fig. 5. This is consistent with previous data that have shown reduced MAP2-positive dendrites and alterations in dendritic morphology due to aging.21 However, the treatment diets (CHOL, HSO, and LARD) generated a greater reduction in MAP2-ir compared to the control diet, both in terms of frequency and actual areas with loss of MAP2 immunostaining. The most significant loss of MAP2-ir was produced by the LARD diet which caused the largest area of damage and noticeably altered dendritic morphology: swellings and a choppy appearance throughout the remaining dendrites (see inset for Fig. 4H). To our knowledge, this is the first report of diet alone resulting in an exaggerated loss of MAP2-ir in the middle-aged rat.
Interestingly, we found similar effects including areas of loss using the antibody to 5-LOX, an enzyme that catalyzes the reaction of fatty acids to leukotrienes.58 There is a marked loss of 5-LOX immunoreactive cells in the pyramidal layer of the CA1 (Figs 3 and 4I-L) particularly in the LARD group which is consistent with our findings for the MAP2 antibody discussed above. Equally notable is the results for HSO fed animals which exhibit a loss of 5-LOX expression throughout the hippocampus. The LARD and CHOL groups had a specific, reduced immunoreactivity in the cell bodies and dendrites, but their astrocytes (particularly in the stratum lacunosum and stratum moleculare) remained intact. On the other hand, the HSO group exhibited a major reduction in 5-LOX-positive astrocytes in these areas. Previous work has demonstrated that a diet rich in trans fats can be detrimental to synthesis of polyunsaturated fatty acids59 which can be very damaging to brain health since polyunsaturated fatty acids are essential to brain function and development.60 Therefore, it is possible that the reduced 5-LOX activity may result from reduced fatty acid availability. This is an interesting result and the biological mechanism and consequences of the observed reduction in 5-LOX immunoreactivity will be examined in future studies.
One of the mechanisms by which high-fat diets may exert their effects is via activation of pro-inflammatory cytokines and concomitant increased activation of microglial cells.12,37 Here, the marker for MHC Class II macrophages, OX-6, revealed an increased expression of activated microglia particularly in the white matter (corpus callosum) adjacent to the hippocampal CA3 region. The greatest expression was found bordering areas with focal loss of MAP2 and 5-LOX immunoreactivity. It was intriguing to find increased activated microglia in the adjacent white matter rather than within the hippocampus. However, this is consistent with previous findings in which an ischemic event resulted in increased ramified microglia at the border zone of the insult rather than the core of the insult, especially in adjacent white matter.61-63 This further points to a possible role of ischemia. Animals treated with the saturated fat diet had the greatest loss in MAP2 and 5-LOX immunoreactivity and the greatest frequency of these areas with reduced expression. They also had the highest expression of OX-6 positive microglia in the bordering white matter (corpus callosum). Furthermore, the control animals had the smallest areas and lowest frequency for MAP2 and 5-LOX reduced immunoreactivity. They also had very low levels of inflammation in the hippocampus as well as the adjacent white matter. These results further support our hypothesis that the damage done to the hippocampus via dietary fats is inflammation mediated.
Finally, we analyzed blood vessel structure using the antibody to Glut1, since one of the most prominent results of high-fat diets is an increase in incidence of cardiovascular disease.64 We observed Glut1 positive blood vessels in the cortex and white matter in the area adjacent to late CA1 and early CA3 of the hippocampus, corresponding to the area where increased OX-6 immunoreactive microglial cells were found. Upon closer observation of the treatment groups, it was apparent that the blood vessel walls were thickened and contained higher immunoreactivity to the Glut1 marker, suggesting a reactive morphological alteration of the blood vessel wall in this region. As a marker of microvessels, Glut1 also serves as a measure for BBB integrity. It has been hypothesized that an increase in Glut1 expression may be correlated with hypoxia, BBB disruption, or blood–cerebrospinal fluid barrier disruption, and may actually be a compensatory response to meet energy demands by the cell.65 Further, Glut1 expression has been described as a measure of how much glucose enters endothelial cells. A previous study demonstrated hypoxia and high glucose concentration increased Glut1 expression in neural stem cells.66 In this study, the dietary treatments increased Glut1 expression, with CHOL and HSO exhibiting the greatest effects, suggesting either a direct effect of the dietary components on the blood vessel wall, or a compensatory increase in response to brain tissue damage.
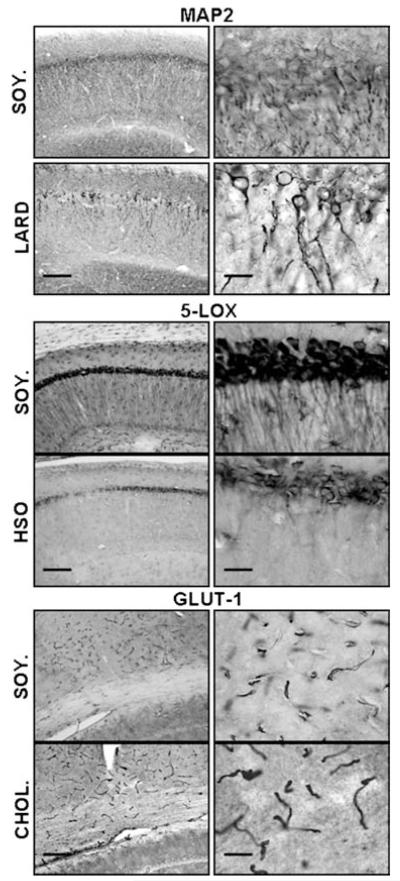
In summary, a diet rich in lard (saturated fat) increased circulating triglyceride levels, increased abdominal fat weight, reduced MAP2 and 5-LOX immunoreactivity in the hippocampus, and increased microglial activation in the corpus callosum. The HSO diet (trans fats) increased cholesterol and triglyceride levels, moderately reduced MAP2-ir, and reduced 5-LOX immunoreactivity throughout the hippocampus. Finally, the CHOL diet significantly increased cholesterol levels, moderately reduced MAP2 and 5-LOX immunoreactivity in the hippocampus, and moderately increased microglial activation in the corpus callosum. A summary of the major morphological findings from each of the diets is presented in Fig. 8. Our study provides the first evidence of differential effects of saturated fats vs. hydrogenated fats vs. cholesterol on hippocampal morphology and lipid profiles in the middle-aged rat. We have shown that each of these dietary components alone result in damage to the brain, and therefore we believe the ‘western diet’ which combines all of these factors, can be even more detrimental to the brain. Our findings also suggest that the middle-aged rat may provide a unique model for studies on dietary composition and brain health. The biological mechanisms for these effects may relate to a combination of vascular and inflammatory processes, and these mechanisms will be explored in future long-term studies.
Figure 8.
Summary of findings. Highlights of results found in this study are shown including: differences found in MAP2-ir between LARD and control (SOY.), differences found in 5-LOX immunoreactivity between HSO and control, and differences found in Glut-1 immunoreactivity between CHOL and control. Magnification = 10 × , scale bar = 50 μm (left); magnification = 40 × , scale bar = 200 μm (right).
Acknowledgements
Thanks are due to Ms Claudia Umphlet and Mr Alfred Moore for their expert technical assistance.
References
- 1.Bourre JM. Biochemistry of brain lipids (especially fatty acids). In situ synthesis and exogenous origin during development. Various aspects of nutritional effects. Reprod Nutr Dev. 1982;22(1B):179–91. [PubMed] [Google Scholar]
- 2.Vance JE, Hayashi H, Karten B. Cholesterol homeostasis in neurons and glial cells. Semin Cell Dev Biol. 2005;16(2):193–212. doi: 10.1016/j.semcdb.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Vance JE, Karten B, Hayashi H. Lipid dynamics in neurons. Biochem Soc Trans. 2006;34(Pt 3):399–403. doi: 10.1042/BST0340399. [DOI] [PubMed] [Google Scholar]
- 4.Morris MC, Evans DA, Bienias JL, et al. Dietary fat intake and 6-year cognitive change in an older biracial community population. Neurology. 2004;62(9):1573–9. doi: 10.1212/01.wnl.0000123250.82849.b6. [DOI] [PubMed] [Google Scholar]
- 5.Ortega RM, Requejo AM, Andres P, et al. Dietary intake and cognitive function in a group of elderly people. Am J Clin Nutr. 1997;66(4):803–9. doi: 10.1093/ajcn/66.4.803. [DOI] [PubMed] [Google Scholar]
- 6.Kalmijn S, Launer LJ, Ott A, et al. Dietary fat intake and the risk of incident dementia in the Rotterdam Study. Ann Neurol. 1997;42(5):776–82. doi: 10.1002/ana.410420514. [DOI] [PubMed] [Google Scholar]
- 7.Kalmijn S, Feskens EJ, Launer LJ, et al. Polyunsaturated fatty acids, antioxidants, and cognitive function in very old men. Am J Epidemiol. 1997;145(1):33–41. doi: 10.1093/oxfordjournals.aje.a009029. [DOI] [PubMed] [Google Scholar]
- 8.Requejo AM, Ortega RM, Robles F, et al. Influence of nutrition on cognitive function in a group of elderly, independently living people. Eur J Clin Nutr. 2003;57(Suppl 1):S54–7. doi: 10.1038/sj.ejcn.1601816. [DOI] [PubMed] [Google Scholar]
- 9.Winocur G, Greenwood CE. Studies of the effects of high fat diets on cognitive function in a rat model. Neurobiol Aging. 2005;26(Suppl 1):46–9. doi: 10.1016/j.neurobiolaging.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Molteni R, Wu A, Vaynman S, et al. Exercise reverses the harmful effects of consumption of a high-fat diet on synaptic and behavioral plasticity associated to the action of brain-derived neurotrophic factor. Neuroscience. 2004;123(2):429–40. doi: 10.1016/j.neuroscience.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 11.Lindqvist A, Mohapel P, Bouter B, et al. High-fat diet impairs hippocampal neurogenesis in male rats. Eur J Neurol. 2006;13(12):1385–8. doi: 10.1111/j.1468-1331.2006.01500.x. [DOI] [PubMed] [Google Scholar]
- 12.Granholm AC, Bimonte-Nelson HA, Moore AB, et al. Effects of a saturated fat and high cholesterol diet on memory and hippocampal morphology in the middle-aged rat. J Alzheimers Dis. 2008;14(2):133–45. doi: 10.3233/jad-2008-14202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gallagher M, Nicolle MM. Animal models of normal aging: relationship between cognitive decline and markers in hippocampal circuitry. Behav Brain Res. 1993;57(2):155–62. doi: 10.1016/0166-4328(93)90131-9. [DOI] [PubMed] [Google Scholar]
- 14.Billard JM. Ageing, hippocampal synaptic activity and magnesium. Magnes Res. 2006;19(3):199–215. [PubMed] [Google Scholar]
- 15.Miller DB, O’Callaghan JP. Aging, stress and the hippocampus. Ageing Res Rev. 2005;4(2):123–40. doi: 10.1016/j.arr.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Burke SN, Barnes CA. Neural plasticity in the ageing brain. Nat Rev Neurosci. 2006;7(1):30–40. doi: 10.1038/nrn1809. [DOI] [PubMed] [Google Scholar]
- 17.Mattson MP, Magnus T. Ageing and neuronal vulnerability. Nat Rev Neurosci. 2006;7(4):278–94. doi: 10.1038/nrn1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toescu EC, Verkhratsky A, Landfield PW. Ca2+ regulation and gene expression in normal brain aging. Trends Neurosci. 2004;27(10):614–20. doi: 10.1016/j.tins.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 19.Hattiangady B, Rao MS, Shetty GA, et al. Brain-derived neurotrophic factor, phosphorylated cyclic AMP response element binding protein and neuropeptide Y decline as early as middle age in the dentate gyrus and CA1 and CA3 subfields of the hippocampus. Exp Neurol. 2005;195(2):353–71. doi: 10.1016/j.expneurol.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 20.Gooney M, Messaoudi E, Maher FO, et al. BDNF-induced LTP in dentate gyrus is impaired with age: analysis of changes in cell signaling events. Neurobiol Aging. 2004;25(10):1323–31. doi: 10.1016/j.neurobiolaging.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Di Stefano G, Casoli T, Fattoretti P, et al. Distribution of map2 in hippocampus and cerebellum of young and old rats by quantitative immunohistochemistry. J Histochem Cytochem. 2001;49(8):1065–6. doi: 10.1177/002215540104900818. [DOI] [PubMed] [Google Scholar]
- 22.Dawson DA, Hallenbeck JM. Acute focal ischemia-induced alterations in MAP2 immunostaining: description of temporal changes and utilization as a marker for volumetric assessment of acute brain injury. J Cereb Blood Flow Metab. 1996;16(1):170–4. doi: 10.1097/00004647-199601000-00020. [DOI] [PubMed] [Google Scholar]
- 23.Matus A, Bernhardt R, Hugh-Jones T. High molecular weight microtubule-associated proteins are preferentially associated with dendritic microtubules in brain. Proc Natl Acad Sci USA. 1981;78(5):3010–4. doi: 10.1073/pnas.78.5.3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morales M, Fifkova E. Distribution of MAP2 in dendritic spines and its colocalization with actin. An immunogold electron-microscope study. Cell Tissue Res. 1989;256(3):447–56. doi: 10.1007/BF00225592. [DOI] [PubMed] [Google Scholar]
- 25.Tucker RP. The roles of microtubule-associated proteins in brain morphogenesis: a review. Brain Res Rev. 1990;15(2):101–20. doi: 10.1016/0165-0173(90)90013-e. [DOI] [PubMed] [Google Scholar]
- 26.Malinak C, Silverstein FS. Hypoxic-ischemic injury acutely disrupts microtubule-associated protein 2 immunostaining in neonatal rat brain. Biol Neonate. 1996;69(4):257–67. doi: 10.1159/000244319. [DOI] [PubMed] [Google Scholar]
- 27.Zhu C, Wang X, Hagberg H, et al. Correlation between caspase-3 activation and three different markers of DNA damage in neonatal cerebral hypoxia-ischemia. J Neurochem. 2000;75(2):819–29. doi: 10.1046/j.1471-4159.2000.0750819.x. [DOI] [PubMed] [Google Scholar]
- 28.Li Y, Jiang N, Powers C, et al. Neuronal damage and plasticity identified by microtubule-associated protein 2, growth-associated protein 43, and cyclin D1 immunoreactivity after focal cerebral ischemia in rats. Stroke. 1998;29(9):1972–80. doi: 10.1161/01.str.29.9.1972. discussion 1980-1. [DOI] [PubMed] [Google Scholar]
- 29.Lockrow J, Prakasam A, Huang P, et al. Cholinergic degeneration and memory loss delayed by vitamin E in a down syndrome mouse model. Exp Neurol. 2009;216(2):278–89. doi: 10.1016/j.expneurol.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Willis LM, Small BJ, Bickford PC, et al. Dietary blueberry supplementation affects growth but not vascularization of neural transplants. J Cereb Blood Flow Metab. 2008;28(6):1150–64. doi: 10.1038/jcbfm.2008.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Backman C, Rose GM, Hoffer BJ, et al. Systemic administration of a nerve growth factor conjugate reverses age-related cognitive dysfunction and prevents cholinergic neuron atrophy. J Neurosci. 1996;16(17):5437–42. doi: 10.1523/JNEUROSCI.16-17-05437.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Granholm AC, Sanders L, Seo H, et al. Estrogen alters amyloid precursor protein as well as dendritic and cholinergic markers in a mouse model of Down syndrome. Hippocampus. 2003;13(8):905–14. doi: 10.1002/hipo.10130. [DOI] [PubMed] [Google Scholar]
- 33.Rouzer CA, Matsumoto T, Samuelsson B. Single protein from human leukocytes possesses 5-lipoxygenase and leukotriene A4 synthase activities. Proc Natl Acad Sci USA. 1986;83(4):857–61. doi: 10.1073/pnas.83.4.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Genovese T, Mazzon E, Rossi A, et al. Involvement of 5-lipoxygenase in spinal cord injury. J Neuroimmunol. 2005;166(1-2):55–64. doi: 10.1016/j.jneuroim.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 35.Boger HA, Middaugh LD, Huang P, et al. A partial GDNF depletion leads to earlier age-related deterioration of motor function and tyrosine hydroxylase expression in the substantia nigra. Exp Neurol. 2006;202(2):336–47. doi: 10.1016/j.expneurol.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 36.Mulder M, Blokland A, van den Berg DJ, et al. Apolipoprotein E protects against neuropathology induced by a high-fat diet and maintains the integrity of the blood-brain barrier during aging. Lab Invest. 2001;81(7):953–60. doi: 10.1038/labinvest.3780307. [DOI] [PubMed] [Google Scholar]
- 37.Thirumangalakudi L, Prakasam A, Zhang R, et al. High cholesterol-induced neuroinflammation and amyloid precursor protein processing correlate with loss of working memory in mice. J Neurochem. 2008;106(1):475–85. doi: 10.1111/j.1471-4159.2008.05415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hwang IK, Kim IY, Kim YN, et al. Comparative study on high fat diet-induced 4-hydroxy-2E-nonenal adducts in the hippocampal CA1 region of C57BL/6N and C3H/HeN mice. Neurochem Res. 2009;34(5):964–72. doi: 10.1007/s11064-008-9846-y. [DOI] [PubMed] [Google Scholar]
- 39.Pisani LP, Oyama LM, Bueno AA, et al. Hydrogenated fat intake during pregnancy and lactation modifies serum lipid profile and adipokine mRNA in 21-day-old rats. Nutrition. 2008;24(3):255–61. doi: 10.1016/j.nut.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 40.Hanis T, Zidek V, Sachova J, et al. Effects of dietary trans-fatty acids on reproductive performance of Wistar rats. Br J Nutr. 1989;61(3):519–29. doi: 10.1079/bjn19890140. [DOI] [PubMed] [Google Scholar]
- 41.Pereira N, Monteiro F, Abraham ME. Influence of dietary fats on weight gain in albino rats. Indian J Physiol Pharmacol. 2005;49(2):206–12. [PubMed] [Google Scholar]
- 42.Lin S, Thomas TC, Storlien LH, et al. Development of high fat diet-induced obesity and leptin resistance in C57Bl/6J mice. Int J Obes Relat Metab Disord. 2000;24(5):639–46. doi: 10.1038/sj.ijo.0801209. [DOI] [PubMed] [Google Scholar]
- 43.Tamashiro KL, Terrillion CE, Hyun J, et al. Prenatal stress or high-fat diet increases susceptibility to diet-induced obesity in rat offspring. Diabetes. 2009;58(5):1116–25. doi: 10.2337/db08-1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuo LE, Czarnecka M, Kitlinska JB, et al. Chronic stress, combined with a high-fat/high-sugar diet, shifts sympathetic signaling toward neuropeptide Y and leads to obesity and the metabolic syndrome. Ann NY Acad Sci. 2008;1148:232–7. doi: 10.1196/annals.1410.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tozuka Y, Wada E, Wada K. Diet-induced obesity in female mice leads to peroxidized lipid accumulations and impairment of hippocampal neurogenesis during the early life of their off-spring. FASEB J. 2009;23(6):1920–34. doi: 10.1096/fj.08-124784. [DOI] [PubMed] [Google Scholar]
- 46.Bartolomucci A, Cabassi A, Govoni P, et al. Metabolic consequences and vulnerability to diet-induced obesity in male mice under chronic social stress. PLoS ONE. 2009;4(1):e4331. doi: 10.1371/journal.pone.0004331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Willett WC. Trans fatty acids and cardiovascular disease-epidemiological data. Atheroscler Suppl. 2006;7(2):5–8. doi: 10.1016/j.atherosclerosissup.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 48.Mozaffarian D, Katan MB, Ascherio A, et al. Trans fatty acids and cardiovascular disease. N Engl J Med. 2006;354(15):1601–13. doi: 10.1056/NEJMra054035. [DOI] [PubMed] [Google Scholar]
- 49.Feldman EB, Kris-Etherton PM, Kritchevsky D, et al. American Society for Clinical Nutrition and American Institute of Nutrition Position paper on trans fatty acids. ASCN/AIN task force on trans fatty acids. Am J Clin Nutr. 1996;63(5):663–70. doi: 10.1093/ajcn/63.5.663. [DOI] [PubMed] [Google Scholar]
- 50.Khosla P, Hayes KC. Dietary trans-monounsaturated fatty acids negatively impact plasma lipids in humans: critical review of the evidence. J Am Coll Nutr. 1996;15(4):325–39. doi: 10.1080/07315724.1996.10718607. [DOI] [PubMed] [Google Scholar]
- 51.Kavanagh K, Jones KL, Sawyer J, et al. Trans fat diet induces abdominal obesity and changes in insulin sensitivity in monkeys. Obesity (Silver Spring) 2007;15(7):1675–84. doi: 10.1038/oby.2007.200. [DOI] [PubMed] [Google Scholar]
- 52.Navab M, Gharavi N, Watson AD. Inflammation and metabolic disorders. Curr Opin Clin Nutr Metab Care. 2008;11(4):459–64. doi: 10.1097/MCO.0b013e32830460c2. [DOI] [PubMed] [Google Scholar]
- 53.Colandre ME, Diez RS, Bernal CA. Metabolic effects of trans fatty acids on an experimental dietary model. Br J Nutr. 2003;89(5):631–9. doi: 10.1079/BJN2003834. [DOI] [PubMed] [Google Scholar]
- 54.Horrocks LA, Yeo YK. Health benefits of docosahexaenoic acid (DHA) Pharmacol Res. 1999;40(3):211–25. doi: 10.1006/phrs.1999.0495. [DOI] [PubMed] [Google Scholar]
- 55.Lai HC, Lasekan JB, Yang H, et al. In vivo determination of triglyceride secretion using radioactive glycerol in rats fed different dietary saturated fats. Lipids. 1991;26(10):824–30. doi: 10.1007/BF02536164. [DOI] [PubMed] [Google Scholar]
- 56.Ney DM, Lai HC, Lasekan JB, et al. Interrelationship of plasma triglycerides and HDL size and composition in rats fed different dietary saturated fats. J Nutr. 1991;121(9):1311–22. doi: 10.1093/jn/121.9.1311. [DOI] [PubMed] [Google Scholar]
- 57.Dietschy JM, Turley SD. Cholesterol metabolism in the brain. Curr Opin Lipidol. 2001;12(2):105–12. doi: 10.1097/00041433-200104000-00003. [DOI] [PubMed] [Google Scholar]
- 58.Manev H, Uz T, Sugaya K, et al. Putative role of neuronal 5-lipoxygenase in an aging brain. FASEB J. 2000;14(10):1464–9. doi: 10.1096/fj.14.10.1464. [DOI] [PubMed] [Google Scholar]
- 59.Koletzko B. Trans fatty acids may impair biosynthesis of long-chain polyunsaturates and growth in man. Acta Paediatr. 1992;81(4):302–6. doi: 10.1111/j.1651-2227.1992.tb12230.x. [DOI] [PubMed] [Google Scholar]
- 60.Innis SM. Essential fatty acids in growth and development. Prog Lipid Res. 1991;30(1):39–103. doi: 10.1016/0163-7827(91)90006-q. [DOI] [PubMed] [Google Scholar]
- 61.Bellander BM, Bendel O, Von Euler G, et al. Activation of microglial cells and complement following traumatic injury in rat entorhinal-hippocampal slice cultures. J Neurotrauma. 2004;21(5):605–15. doi: 10.1089/089771504774129937. [DOI] [PubMed] [Google Scholar]
- 62.Roggendorf W, Strupp S, Paulus W. Distribution and characterization of microglia/macrophages in human brain tumors. Acta Neuropathol. 1996;92(3):288–93. doi: 10.1007/s004010050520. [DOI] [PubMed] [Google Scholar]
- 63.Zhao BQ, Ikeda Y, Ihara H, et al. Essential role of endogenous tissue plasminogen activator through matrix metalloproteinase 9 induction and expression on heparin-produced cerebral hemorrhage after cerebral ischemia in mice. Blood. 2004;103(7):2610–6. doi: 10.1182/blood-2003-03-0835. [DOI] [PubMed] [Google Scholar]
- 64.Ascherio A, Rimm EB, Giovannucci EL, et al. Dietary fat and risk of coronary heart disease in men: cohort follow up study in the United States. BMJ. 1996;313(7049):84–90. doi: 10.1136/bmj.313.7049.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nordal RA, Wong CS. Intercellular adhesion molecule-1 and blood-spinal cord barrier disruption in central nervous system radiation injury. J Neuropathol Exp Neurol. 2004;63(5):474–83. doi: 10.1093/jnen/63.5.474. [DOI] [PubMed] [Google Scholar]
- 66.Maurer MH, Geomor HK, Burgers HF, et al. Adult neural stem cells express glucose transporters GLUT1 and GLUT3 and regulate GLUT3 expression. FEBS Lett. 2006;580(18):4430–4. doi: 10.1016/j.febslet.2006.07.012. [DOI] [PubMed] [Google Scholar]