Abstract
Background:
Breast cancer (BC) is usually diagnosed in late stages in countries with limited resources. Early detection of BC is likely to improve the outcome of the disease for women in these areas.
Objective:
The aim of this study was to understand the possible personal, economic, and systems barriers to BC screening in a sample of Egyptian women.
Materials and Methods:
A cross-sectional study was conducted in family health centers representing the seven districts of Alexandria governorate, Egypt. A total of 612 women were randomly selected from the chosen centers.
Results:
In this sample of Egyptian women, the most frequently identified potential barriers to BC screening were the following: 81.8% would not seek care until they were ill, 77% were unwilling to have a mammogram until it was recommended by the doctor, 71.4% blamed the, lack of privacy, 69.2% thought that medical checkups were not worthwhile, and 64.6% blamed the cost of services. The study further revealed that women of lower education, women in the lower income category, women who did not do paid work, those who had poor knowledge of the risks of BC, and women with no family history of BC were more likely to perceive different screening barriers compared with their counterparts.
Conclusion:
Many potential personal, economic, and health system barriers were identified. Addressing these barriers by increasing the awareness of BC and dealing with the misconceptions that the women have can help the policy makers to design more culturally relevant strategies to motivate women to utilize screening services.
Keywords: Breast cancer, barriers, Egypt, screening
INTRODUCTION
Breast cancer (BC) is a major health problem for the patients and the community. The incidence, mortality, and survival rates for BC vary across the world.[1]
In Egypt, BC is the most prevalent cancer among women, representing 19% of total cancer cases. Currently, it is the leading cancer among Egyptian females, and its incidence is projected to increase by 1-2% every year. The disease is usually diagnosed at an advanced stage among the Egyptian women.[2,3]
Research has shown that distribution of BC is within the younger age group of Egyptian patients, the majority of cases occurring between 30-60 years of age. The median age at diagnosis is 49 years, one decade younger than the corresponding age in Europe and north America.[4]
Reduction in mortality from BC depends to a large extent on interventions aimed at early detection and treatment, including breast self-examination, clinical breast examination, and mammography.[5] Lack of early detection programs is the primary reason for the escalation of the mortality rate from BC in developing countries.[6]
Mammography is the only screening tool proven to reduce BC mortality,[7] with evidence that adherence to yearly screening mammography can reduce BC mortality by 22-35% for women aged 50 years or older.[8]
In Egypt, although facilities for mammographic screening are available in the major governmental and private hospitals, there is no nationwide systematic program for breast screening at the present time.
Various factors have been reported to have an effect on screening rates, including women's socioeconomic status, their knowledge of risk factors, and having a family history of BC.[9] Barriers to BC screening have been identified in a variety of populations[10,11,12] and grouped under three main categories: personal, economic, and health care service barriers.[13,14,15]
To our knowledge, no studies have been conducted on the possible barriers to BC screening among Egyptian females. Therefore, this study aimed at understanding the possible barriers to BC screening in a sample of Egyptian females.
MATERIALS AND METHODS
Sampling and sample size
Using a cross-sectional design, the study was conducted from January-June 2011, at seven family health centers affiliated to the ministry of health, representing the seven districts of Alexandria governorate, Egypt. These centers were selected randomly from a list of centers working under the newly introduced Family Health Model, which provides low-cost services for families and women from different socioeconomic strata.
According to Open Epi, Version 2, taking a level of precision of 4%, confidence interval of 95% and unknown prevalence of 50%, the total required sample size was to be at least 608 women. To compensate for missing data, 630 females were randomly selected to participate in the study. This number was proportionally allocated to the seven selected centers. The final sample size was 612 females (2.8% refused to participate in the study). Females aged 40 years or older who had never sought BC screening services before and had never been diagnosed with BC represented the study population. The women in the sample were chosen by systematically selecting every fourth woman.
Data collection
After reviewing the available literature, a structured interview questionnaire in three parts was developed. The first part provided sociodemographic characteristics and obstetric history, the second included knowledge about BC risk factors, and a list of personal, economic, and health services barriers toward BC screening formed the third part. The respondents were asked to indicate if they agreed, disagreed, or were not sure of the statements listed. Trained female data collectors interviewed the respondents.
Questions were on some sociodemographic characteristics, including marital status, education, and work status of the women and their family's monthly income. Income was classified into three groups: less than 500 Egyptian pounds (EP)/month (1 US$ = ~6.2 EP), 500-1000 EP/month, and more than 1000 EP/month. Education was categorized as follows: illiterate or ability to just read and write; primary education, secondary education, and higher education.
The study was approved by the Ethical Review Committee of Alexandria Regional Center for Women Health and Development (ARC). Before being interviewed, informed consent was obtained from every woman after being informed of the purpose of the study. They were assured of privacy and confidentiality of individual information provided, informed of the average time the interview would take, and the respondent's right to refuse to participate or to withdraw at any time.
Data analysis
All data analyses were performed using Statistical Package for the Social Sciences (SPSS) version 16.0 (SPSS Inc., Chicago IL, USA). A total knowledge score for BC risk factors was calculated. The knowledge score measured on 0-1 scale for each item, giving one point for each correct response, whereas incorrect or unknown responses received zero points (the total for all items was 11). The overall knowledge score was dichotomized as ‘satisfactory knowledge’ or ‘poor knowledge’ by an arbitrary cutoff level of seven, which was the median score of the distribution.
In addition, the total BC screening barriers' score was calculated by adding all the personal, economic, and health system barriers amounting to 19 items. This score was measured on 0-2 scale for each item, and the total for all items was amounted to 38. The overall barrier score was created by allocating two points for each response that was in agreement with a certain barrier, zero point for disagreement, whereas one point for undefined answers. The total barrier score then was divided into tertiles: low (≤12), intermediate (13-25), and high levels score (26-38). Cronbach's alpha for this index was 0.81, which confirmed that the items held well as a scale score. The total barrier score was then cross-tabulated against some demographic variables to test for associating factors with the BC screening barriers.
The association between barrier items and some demographics was tested using Chi-square test. A conventional P value of ≤ 0.05 was considered as cutoff for significance.
RESULTS
Sample characteristics
Regarding the demographic characteristics of the participants, the ages of the women ranged from 40 to 69 years, with a mean (± SD (standard deviation)) of 49 years (±2.2). Only 28.8% were illiterate or could just read and write, whereas 37.6% of women had secondary education. The majority of women were currently married (81.9%) and 55.9% of them did paid work. Only 12% of the participants reported a family history of BC. Almost 57% of the women belonged to the lower income category (less than 500 EP/month; 1 US$ = ~6.2 EP).
Knowledge regarding BC risk factors
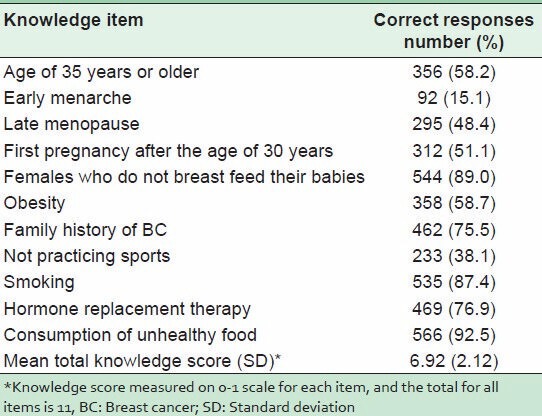
Knowledge of the sampled women regarding BC risk factors is listed in Table 1. The majority of women correctly indicated that women who consumed unhealthy foods (92.5%), who did not breast feed their babies (89%), who smoked regularly (87%), who took hormonal replacement therapy (76.9%), and who had a positive family history of BC (75.5%) were more liable to get BC. The mean knowledge score of participants (± SD) was 6.92 (±2.12). Approximately 60% of the sampled women were classified as having poor scores, and the remainder was classified as having satisfactory knowledge of BC risk (data not shown).
Table 1.
Knowledge of BC risk factors
BC screening barriers
Data in Table 2 show that multiple barriers to BC screening were identified by the sample. When asked about the personal barriers they might encounter, 81.8% of the respondents declared that they would not go to the doctor unless they were ill and 69.2% reported that physical checkups were not worthwhile. Fear of exposure to radiation and of pain was perceived as personal barriers by one in four participants.
Table 2.
Frequency of BC screening barriers among the sampled women
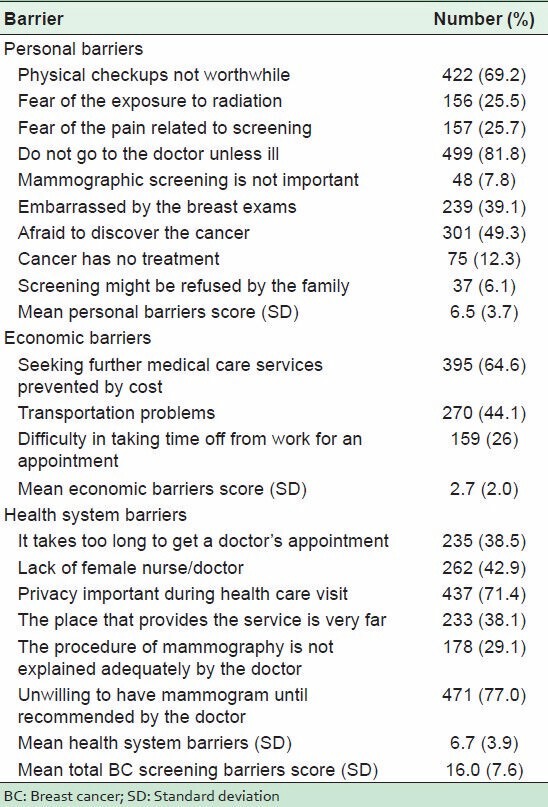
Concerning the economic barriers, 64.6% of the participants indicated that cost was a barrier to seeking further medical care.
On the question of barriers in the health system, 77% of the women surveyed indicated that they were unwilling to have a mammogram until it was recommended by the doctor. One of the frequently identified potential barriers in the health system was the lack of privacy in the screening facilities as reported by 71.4%. The absence of a female doctor/nurse to perform the screening, difficulty in getting a doctor's appointment, and distance to the screening facility were anticipated as barriers in the health system by nearly equal proportions of participants (42.9, 38.5, and 38.1%, respectively).
Factors associated with BC screening barriers
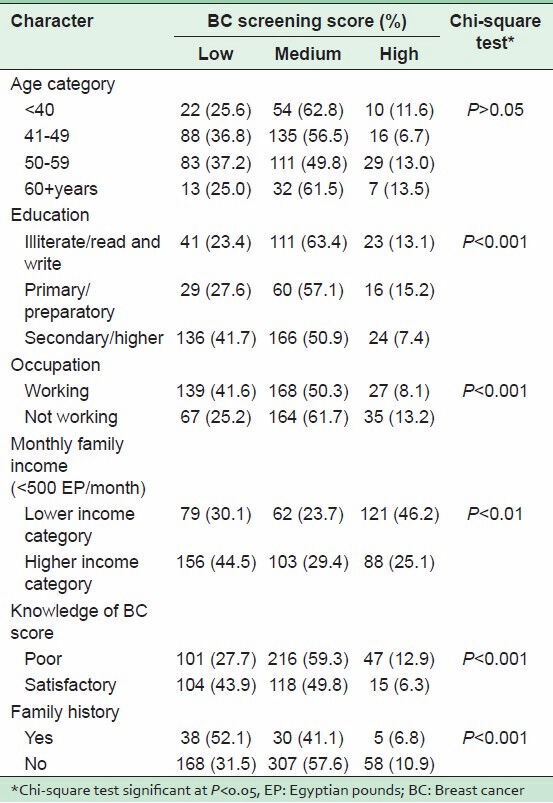
Factors associated with BC screening score of the women are provided in Table 3. With regard to age, no significant differences were detected between the women of different age groups with respect to their scores on barriers to BC screening. Women with secondary or higher education had significantly fewer screening barriers than either illiterate or those with primary education (7.4, 13.1, 15.2%, respectively; P < 0.001). Generally, women with satisfactory BC knowledge score indicated significantly lower barriers score than those with poor knowledge (43.9 and 27.7%, respectively; P < 0.001). Moreover, those with a family history of BC significantly had a lower total barrier score than their counterparts (52.1 versus 31.5%; P < 0.001).
Table 3.
Factors associated with barriers to BC screening score
DISCUSSION
As BC is usually diagnosed in late stages in countries with limited resources,[16] efforts aimed at detecting it in the earlier stages are likely to improve the outcome of the disease for women in these areas.[17] Globally, efforts to increase screening have met with moderate success.[1] The Breast Health Global Initiative recommends that women aged 50-69 should have mammographic screening every 2 years even in countries with limited resources.[18]
Among the current group of Egyptian women, who had never sought a BC screening service, many personal, economic, and health care barriers to the screening were identified.
Not seeking medical advice unless one is ill, followed by the women's beliefs that physical checkups were not worthwhile were the most common personal barriers revealed by the present participants. In their study among women from rural Egypt, Younes et al., stated that many Egyptian women suffered in silence, endured much pain and discomfort before they would admit to being ill, and would mostly only seek treatment when their symptoms became severe.[19]
Unsurprisingly, a significant proportion of the women in the present study reported they were afraid of discovering that they had cancer, and embarrassment by the screening was a personal barrier. Generally, there are many personal obstacles for women to access prevention services. The fear of discovering cancer, embarrassment, and fear of the screening procedure were among the most commonly reported personal/cultural barriers to using the screening services.[14,20] Spirituality and religion have been identified as major determinants of fear and fatalism with regard to BC in previous research.[20] Personal barriers can be overcome by promoting health seeking behavior and educating the public on the importance of early detection of cancer with a message that empowers women to take charge of their own health.
The unwillingness to have a mammogram unless recommended by a physician was the most prevalent health system barrier in this study, followed by the lack of privacy in health facilities. The role of health care providers in recommending mammogram was important for the current participants. This is also acknowledged as a motivation for compliance by women to breast screening in other studies.[21,22,23] It underlines the importance of ensuring that the health system staff endeavors to promote positive health seeking behavior and change negative attitudes and behaviors.
The lack of privacy in the health facilities that provide screening remains one of the most important barriers in the health care service perceived by the sampled women. This is an indication of the importance of privacy in health facilities for this group of women when dealing with breast screening. The individual's rights to confidentiality and privacy are highly valued, widely endorsed, and routinely violated.[24] Ensuring privacy is of fundamental importance to the further enhancement of screening programs. A doctor's recommendation for screening, privacy, and feelings of uneasiness are, fortunately, issues that can be addressed within the healthcare system by in-service staff training on communication skills, respect for women's dignity, and counseling techniques.
From the present study, the high cost of screening and transportation problems seem to be the most important economic barriers cited by women. Cost has undoubtedly and generally been a major barrier in seeking appropriate health care and screening service in particular.[23,25,26,27] It is recommended that providing BC screening free-of-charge would help to eliminate some of the obstacles to screening mentioned here.
Regarding factors affecting BC screening, the current study revealed that women with lower education, lower income, and those without paid work (factors reflecting lower socioeconomic status), those with poor knowledge of the risks of BC, and women with no family history of BC seemed more likely to perceive different kinds of barriers to screening compared with their counterparts.
The low status of women prevents them from recognizing and voicing their concerns about their health needs. Poor women are likely to have many other competing priorities related to survival that puts health screening low on their scale of priorities.[15] Empowering women and raising their awareness is very important to remove these barriers.
Studies have shown that women with lower levels of education may have the least knowledge about BC and screening for it. They also exhibited the most negative attitudes regarding mammograms. These women were more concerned about the fact that mammography was embarrassing, painful, and harmful than women with higher education.[9,23] Unfortunately, it is well documented that women with low income and low education tend to present with more advanced disease and had a higher mortality rate than those with higher income and more education.[23,28,29]
Overall, knowledge of the participants in the present study and of the risk factors of BC was generally poor. This may reflect low public awareness and understanding of the disease. Poor knowledge of the current group of women regarding the risk of BC was one of the underlying reasons for not seeking cancer screening service. Past studies have reported the lack of knowledge of BC as the factor that inhibited mammographic screening.[27,30,31]
Interestingly, the present study revealed that women who reported a family history of BC perceived fewer barriers to breast screening than their counterparts. They seemed to be more able to overcome fears of the disease and some of the barriers. The findings of the association between family history of BC and breast screening have not been consistent. Family history has had both positive and negative effects on the rates of breast screening. Women who deem themselves to be at a moderate risk were more likely to get a mammogram,[12,13] whereas women with a low or high risk may avoid these screenings tests either because of the lack of the awareness of screening or due to the fear of results.[10,11,12]
We acknowledge that the present study has some limitations. Taking the sample of women from the health facilities may reflect certain characteristics in this group of women who may have better health seeking behavior than other women. Therefore, conducting this study in the community would give a better representation of the barriers to breast screening among Egyptian women in general. Moreover, the current sample represents women who had never sought BC screening service. Accordingly, a comparative study in which such a group is compared with women who had previous screening will give a better idea of what are the obstacles to breast screening. Future qualitative analysis to investigate the barriers to screening perceived by Egyptian women will shed more light on the results.
CONCLUSIONS
Many important BC screening barriers have been identified among this group of Egyptian women. Women's perception of these barriers was associated with some sociodemographic characteristics. Identifying barriers to breast screening in the local community will help to remove those obstacles and design more culturally relevant strategies to increase the utilization of breast screening service and to ensure adequate breast care of these women.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Althuis MD, Dozier JM, Anderson WF, Devesa SS, Brinton LA. Global trends in breast cancer incidence and mortality 1973-1997. Int J Epidemiol. 2005;34:405–12. doi: 10.1093/ije/dyh414. [DOI] [PubMed] [Google Scholar]
- 2.Elatar I. Cancer registration, NCI Egypt, 2001. Cairo: National Cancer Institute; 2002. [Google Scholar]
- 3.Boulos S, Gadallah M, Neguib S, Essam E, Youssef A, Costa A, et al. Breast screening in the emerging world: High prevalence of breast cancer in Cairo. Breast. 2005;14:340–6. doi: 10.1016/j.breast.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Elattar I, Ali-Eldin N, Moneer M, Elbasmy A. Cancer registry. Cairo: National Cancer Institute of Egypt; 2002-2003. [Google Scholar]
- 5.Sadler GR, Dhanjal SK, Shah NB, Ko CM, Anghel M. Asian India women: Knowledge, attitudes and behaviours toward breast cancer early detection. Public Health Nurs. 2001;18:357–63. doi: 10.1046/j.1525-1446.2001.00357.x. [DOI] [PubMed] [Google Scholar]
- 6.Pinotti JA, Barros AC, Hegg R, Zeferino LC. Breast cancer control program in developing countries. Breast Dis. 1995;8:243–50. [PubMed] [Google Scholar]
- 7.Greif J. Mammographic screening for breast cancer: An invited review of the benefits and costs. Breast. 2010;19:268–72. doi: 10.1016/j.breast.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 8.Elmore JG, Armstrong K, Lehman CD. Screening for breast cancer. J Am Med Assoc. 2005;293:1245–56. doi: 10.1001/jama.293.10.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zapka JG, Stoddard AM, Costanze JE, Greene HL. Breast cancer screening by mammography: Utilization and associated factors. Am J Public Health. 1989;79:1499–502. doi: 10.2105/ajph.79.11.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lerman C, Schwartz M. Adherence and psychological adjustment among women at high risk for breast cancer. Breast Cancer Res Treat. 1993;28:145–55. doi: 10.1007/BF00666427. [DOI] [PubMed] [Google Scholar]
- 11.Kash KM, Holland JC, Halper MS, Miller DG. Psychological distress and surveillance behaviors of women with a family history of breast cancer. J Natl Cancer Inst. 1992;84:24–30. doi: 10.1093/jnci/84.1.24. [DOI] [PubMed] [Google Scholar]
- 12.McCaul KD, Branstetter AD, Schroeder DM, Glasgow RE. What is the relationship between breast cancer risk and mammography screening. A meta-analytic review? Health Psychol. 1996;15:423–9. doi: 10.1037//0278-6133.15.6.423. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez ME, Palmer RC, Leong-Wu CA. Repeat mammography screening among low-income and minority women: A qualitative study. Cancer Control. 2005;12:77–83. doi: 10.1177/1073274805012004S11. [DOI] [PubMed] [Google Scholar]
- 14.Thompson HS, Littles M, Jacob S, Coker C. Post treatment breast cancer surveillance and follow-up care experiences of breast cancer survivors of African descent: An exploratory qualitative study. Cancer Nurs. 2006;29:478–87. doi: 10.1097/00002820-200611000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Ahmed NU, 1, Fort JG, Elzey JD, Belay Y. Empowering factors for regular mammography screening in under-served populations: Pilot survey results in Tennessee. Ethn Dis. 2005;15:387–94. [PubMed] [Google Scholar]
- 16.Marchick J, Henson DE. Correlations between access to mammography and breast cancer stage at diagnosis. Cancer. 2005;103:1571e80. doi: 10.1002/cncr.20915. [DOI] [PubMed] [Google Scholar]
- 17.Andersen MR, 1, Smith R, Meischke H, Bowen D, Urban N. Breast cancer worry and mammography use by women with and without a family history in population-based sample. Cancer Epidemiol Biomarkers Prev. 2003;12:314–20. [PubMed] [Google Scholar]
- 18.Yip CH, Anderson BO. The Breast Health Global Initiative: Clinical practice guidelines for management of breast cancer in low- and middle-income countries. Expert Rev Anticancer Ther. 2007;7:1095–104. doi: 10.1586/14737140.7.8.1095. [DOI] [PubMed] [Google Scholar]
- 19.Younis N, Khattab H, Zurayk H, El-Moelhy M, Amin M, Farag A. A community study of gynaecological and related morbidities in rural Egypt. Stud Fam Plann. 1993;24:175–86. [PubMed] [Google Scholar]
- 20.Flynn P, Betancour H, Tucker J, Garberoglio C, Riggs M. Culture, emotions, and breast self- examinations among culturally diverse women. Presented at the Annual Conference for the American Psychological Association; August, 2007; San Francisco, California. [Google Scholar]
- 21.Champion V, Menon U. Predicting mammography and breast-self examination in African American women. Cancer Nurs. 1997;20:315–22. doi: 10.1097/00002820-199710000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Alexandraki I, Mooradian AD. Barriers related to mammography use for breast cancer screening among minority women. J Natl Med Assoc. 2010;102:206–18. doi: 10.1016/s0027-9684(15)30527-7. [DOI] [PubMed] [Google Scholar]
- 23.Davis TC, Arnold C, Berkel HJ, Nandy I, Jackson RH, Glass J. Knowledge and attitude on screening mammography among low-literate, low-income women. Cancer. 1996;78:1912–20. doi: 10.1002/(sici)1097-0142(19961101)78:9<1912::aid-cncr11>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 24.PATH. Ensuring Privacy and Confidentiality in Reproductive Health Services: A Training Module and Guide. Washington, D.C: PATH; 2003. [Google Scholar]
- 25.Fatimi Z, Avan I. Demographic, Socio-economic and environmental determinants of utilization of antenatal care in rural setting of Sindh Pakistan. J Pak Med Assoc. 2002;52:138–42. [PubMed] [Google Scholar]
- 26.Rani M, Bonu S. Rural Indian women's care seeking behavior and choice of provider for gynecological symptoms. Stud Fam Plan. 2003;34:173–85. doi: 10.1111/j.1728-4465.2003.00173.x. [DOI] [PubMed] [Google Scholar]
- 27.Paskett ED, Tatum C, Rushing J, Michielutte R, Bell R, Foley KL, et al. Racial differences in knowledge, attitudes, and cancer screening practices among a triracial rural population. Cancer. 2004;101:2650–9. doi: 10.1002/cncr.20671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peek ME, Han JH. Disparities in screening mammography. Current status, interventions and implications. J Gen Intern Med. 2004;19:184–94. doi: 10.1111/j.1525-1497.2004.30254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silver Wallace L, Gupta R. Predictors of screening for breast and colorectal cancer among middle-aged women. Fam Med. 2003;35:349–54. [PubMed] [Google Scholar]
- 30.Sim HL, Seah M, Tan S. Breast cancer knowledge and screening practices: A survey of 1,000 Asian women. Singapore Med J. 2009;50:137–44. [PubMed] [Google Scholar]
- 31.Weir R, Day P, Ali W. Risk factors for breast cancer in women. NZHTA Report. 2007:10. [Google Scholar]


