Abstract
BACKGROUND:
Core biopsies are valuable in obtaining sufficient tissue to ensure diagnosis of diseases in the thorax.
OBJECTIVE:
To evaluate the complication rate and the diagnostic yield in computer tomography (CT)-guided core biopsies performed in a county hospital in Sweden.
METHODS:
Medical journals, spirometry results, pathology reports and CT scans were reviewed in 463 consecutive cases, where a transthoracic core biopsy was performed between January 2005 and December 2010. Of these 380 (82%) were lung lesions, 48 (10%) were mediastinal lesions and 35 (8%) were pleural lesions.
RESULTS:
All patients underwent a chest X-ray 4 hours post-biopsy and pneumothorax was seen in 156/463 (34%) patients: 137 after lung biopsy and 17 after mediastinal biopsy. Chest tube insertion was required for 27 (17%) of these patients (6% of all core biopsies). Small intraparenchymal hemorrhages and hemoptysis were observed with subjective difficulty in one case. The diagnostic yield for the 463 patients was 212 (46%) cases of lung cancer, 188 (41%) benign lesions and 39 (8%) pulmonary metastases.
CONCLUSIONS:
A transthoracic core biopsy ensures diagnosis with a low complication rate and is suitable as an outpatient procedure. An increased risk for pneumothorax was observed when the biopsied lesion was small or when emphysema was in the path of the biopsy needle. Reduced lung function pre-biopsy or emphysema in the path of the biopsy needle increased the need for chest tube treatment of pneumothorax. A CT-guided core biopsy is safe and applicable in a county hospital.
Keywords: Core biopsies, emphysema, FEV1, hemorrhage, pneumothorax
A CT-guided transthoracic needle aspiration biopsy of the lung is a relatively safe and accurate method of diagnosing benign and malignant lesions.[1,2,3,4] Sensitivity for the diagnosis of malignancy has been reported at 90-97%.[5] The main complications are pneumothorax or intrathoracic bleeding.[6,7,8,9,10,11] Studies have shown that this technique is suitable for obtaining tissue samples of sufficient quantity and quality from which correct histological diagnosis can be made in the vast majority of cases[6,7,8,9,10,11] as well as allowing for molecular analysis of biomarkers. Accurate analysis and diagnosis are important because they are the basis upon which targeted, individualized treatment is decided.
The aim of this retrospective study was to evaluate the complication rate and diagnostic yield of core biopsies in outpatients who underwent a CT-guided core needle biopsy in our institution between January 2005 and December 2010.
Methods
Medical journals, spirometry results, pathology reports and CT scans were reviewed in 463 consecutive transthoracic core biopsies performed on 444 patients between January 2005 and December 2010 at the Dept. of Respiratory Medicine, Gävle County Hospital. Of the biopsied lesions 380 (82%) were parenchymal lung lesions, 48 (10%) were mediastinal lesions and 35 (8%) were pleural lesions.
Patient characteristics
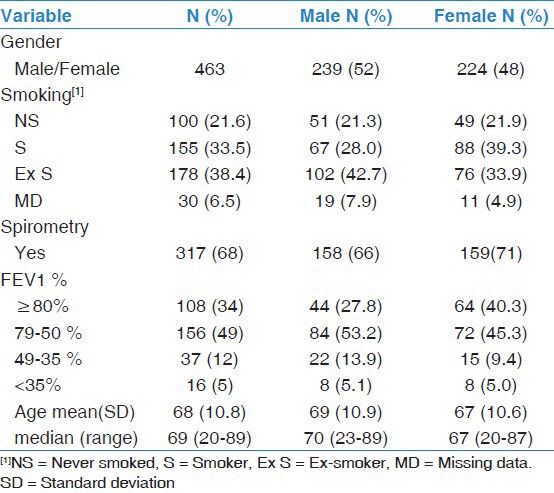
The distribution between male and female was almost equal (239/224). The female mean age was 67 years and the male mean age was 69 years. The female median age was 67 years and the male median age was 70 years. The age range for females was 20-87 years and for males was 23-89 years. About 72% of the patients were smokers or ex-smokers (had not smoked for at least 1 year). Spirometry data was available for 68% of the patients: 16 of these patients had a FEV1<35% of predicted, 15/16 underwent lung biopsy [Table 1].
Table 1.
Demographic information, smoking status, and spirometry results distributed by gender
This study was approved by the ethics committee of Uppsala University (2009/367).
Core biopsy procedure
Spirometry before a biopsy is recommended. Coagulation factors (platelet count, prothrombin time, activated partial thromboplastin time) were analyzed before the procedure. Low-dose aspirin was allowed but warfarin natrium was replaced with dalteparin until PK-INR was less than 1.8. Clopidogrel bisulfate was withdrawn 1 week prior to the biopsy.
The majority of the patients were biopsied on an outpatient basis. They were premedicated with one tablet Diazepam 10 mg and were observed at the clinic after the biopsy. Most patients had contrast-enhanced chest CT scans available for review before the biopsy procedure. When giving the local anesthesia (Lidocaine 10 mg/ml, 5-10 ml) before the biopsy, the needle tip did never advance through the pleura because of the risk for pneumothorax early in the procedure, which made the biopsy more difficult. Biopsies were taken with the Quick-Core Biopsy Needle Set 16G/11 cm with an inner needle of 18G/15 cm (Cook Medical, USA). The patients were instructed to breathe freely and quiet during the biopsy procedure. Needle direction was planned to have the most vertical approach to the pleura, the shortest distance to the lesion and to avoid penetrating bullae, vessels and fissures. The same inner needle was used for all the samples (usually three). The biopsies were visually inspected for confirmation that the specimen seemed adequate. No on-site cytopathologist was available. The specimens were collected on filter paper and then fixed in formalin. A single CT scan was done over the area direct after the biopsy to evaluate for any complications. All of the patients underwent chest X-ray 4 hours after the intervention to rule out pneumothorax. Two experienced radiologists performed the majority of the biopsies.
Statistical analysis
Descriptive results are presented as numbers, percentages, mean values with associated standard deviations, and medians with associated ranges. The association between complication, pneumothorax detected by X-ray after 4 hours, and risk factors and potential confounders were analyzed by logistic regression. Risk factors and potential confounders include number of pleural passages, depth of the lesion (mm), size of the lesion (mm), emphysema in the path of the biopsy needle (yes or no), result of the pathology report (benign tumor, lung cancer, metastases or lymphoma), age, and sex.
The logistic regression analysis was performed in two steps. First, univariate logistic regression was conducted where each of the independent variables was analyzed separately. Secondly, multiple logistic regression was conducted in which all the independent variables were included and analyzed simultaneously. In the development of the final model, the continuous variables “size of lesion” and “depth of lesion” were checked for linearity in logit by methods proposed by Hosmer and Lemeshow.[12] These methods include a classification of the values of measurement in millimeters of the variables in quartiles. Subsequently, these two variables of grouped values were included in the logistic regression model. An inspection of the estimated odds ratio would then reveal deviation from linearity. A second method used to check for linearity was the application of using fractional polynomials in the models. We also tested for inclusion of statistical interaction between the depth and size of the lesion. For this purpose we included a product term (depth × size) in the model. We then compared this model with a model without the product term by means of likelihood ratio tests. We also tested the statistical significance of the coefficient of the product term by a Wald test.
The result of the univariate and multiple logistic regression are presented as odds ratios (OR), 95% confidence intervals (CI), and P-values of the Wald test, testing the null hypothesis of odds ratios equal to zero. The odds ratios should be interpreted as the change in ratio of the occurrence of a complication to the absence of a complication when comparing two individuals with one unit change in one of the factors, all other factors held constant. In the statistical tests a P-value <0.05 was considered statistically significant. The calculations and analyses were made by using the statistical computer package SPSS, version 19 for Windows.
Results
Small intraparenchymal hemorrhages and hemoptysis were noticed but did not cause any subjective problems for the patients, with the exception of one patient to be discussed later.
Pneumothorax was seen on the single CT scan directly after the biopsy in 119/463 patients. Of these 14 were evacuated with a thin needle directly while still at the Department of Radiology. Eleven of these pneumothorax exceeded 20 mm. All patients underwent a chest X-ray 4 hours post-procedure. Pneumothorax was diagnosed in 156/463 (34%) patients: 137 after lung biopsy and 17 after mediastinal biopsy. Fifty-four of these patients had no pneumothorax on the single CT scan directly after the biopsy.
Twenty-seven of the patients required treatment with chest tube (17% of those with pneumothorax and 6% of all who underwent core biopsy).
When specifically studying the patients who had undergone lung biopsies, we found 110/380 (29%) with pneumothorax on the single CT scan directly after the biopsy and 41/380 (11%) with other complications such as hemoptysis, small intraparenchymal bleeding or instant pain. The chest X-ray 4 hours post-procedure showed that 137/380 (36%) of these patients had pneumothorax, 22 (16%) needed treatment with a chest tube.
Spirometry data was available for 274/380 patients. Fifteen of these patients had FEV1<35% of predicted. The single CT scans of these 15 patients directly after the biopsy showed that 8/15 had pneumothorax and 1/15 had some other complication. The chest X-ray 4 hours post-procedure confirmed all but one of the cases of pneumothorax and no new cases had appeared. Chest tube insertion was needed after the biopsy in 4/7 cases (57%) and of those 3 had had emphysema in the path of the biopsy needle. Ten patients had both emphysema in the path of the biopsy needle and FEV1<35%. Of these three had no complications, one had pneumothorax on the single CT scan but not at the X-ray 4 hours later, one had pneumothorax on the chest X-ray taken 4 hours post-procedure and five had pneumothorax on both the single CT scan and the X-ray 4 hours later.
In total of 87 patients there was emphysema in the path of the biopsy needle. Pneumothorax was seen with single CT scan in 42.5% and the chest X-ray after 4 hours showed pneumothorax in 55% and chest tube insertion was needed in 12/48 (25%).
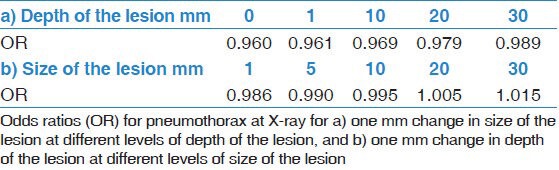
The following data were gathered on the 380 patients who had undergone a CT-guided core biopsy of the lung parenchyma: Sex, age, number of pleural passages, depth of the lesion, size of the lesion, whether or not there was emphysema in the path of the biopsy needle and the result of the pathology study (lung cancer, metastasis, benign lesion, or other). The data were analyzed to see if there was any correlation to the complication rate. We found higher odds for pneumothorax in patients with smaller lesions, deeper lesions and when there was emphysema in the path of the biopsy needle [Table 2]. The multiple regression analysis showed a significantly increased risk for pneumothorax in patients with smaller lesions and those with emphysema in the path of the biopsy needle. We also found that the size and depth of the lesion interacted. That should be interpreted as the odds ratios of one of the factors vary with the level of the other factor, that is, one factor modifies the effect of the other factor [Table 2 and 3]. The relationship between size of the lesion and pneumothorax at X-ray among patients with lesser depth of lesion is different from that among patients with deeper position of the lesion, e.g. the odds ratio (OR) of pneumothorax for one mm increase in size for lesion in pleura compared to a lesion of 30 mm depth increases from OR = 0.96 to OR 0.99.
Table 2.
Estimated odds ratios (OR), 95% confidence intervals (CI), and P-values for pneumothorax at X-ray from univariate and multivariate logistic regression
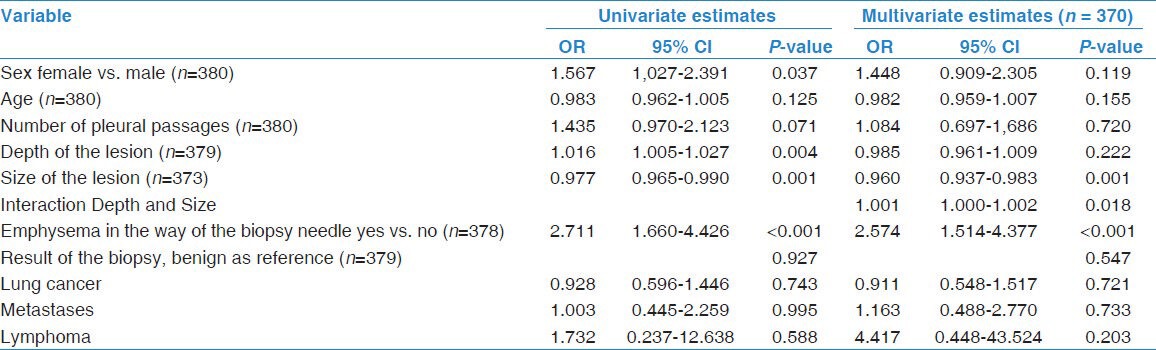
Table 3.
Results of statistical interaction between depth of the lesion and size of the lesion
The diagnostic yield for all 463 patients was 212 cases (46%) with lung cancer, 188 cases (41%) with benign lesions and 39 cases (8%) pulmonary metastases. Other diagnoses included lymphoma, malignant mesothelioma, thymoma and solitary fibrous tumor of the pleura.
Nineteen patients were biopsied twice because of a discrepancy between the radiological findings and the pathology report (14 of the lung biopsies, 3 of the mediastinal biopsies, 2 of the pleural biopsies). Of these 8 were diagnosed with malign tumors after the second biopsy (5 of the lung, 2 of the mediastinal and 1 of the pleural biopsies).
Discussion
This study shows that a CT-guided core biopsy is applicable in a county hospital with a low complication rate and is suitable as an outpatient diagnostic procedure.
Bronchoscopy with biopsy and cytology specimens is often sufficient for securing a diagnosis but the tissue obtained in this way is considered sparse when compared with core biopsies and is often not sufficient for biomarker analysis. The tissue is the issue.
Lung cancer is the leading cause of cancer-related death worldwide.[13,14] A large field of research is ongoing for improving the outcome in lung cancer. Targeted therapies hold a considerable promise in the treatment of patients with lung cancer. The histological subtyping of non-small cell lung carcinoma in squamous cell carcinoma and lung adenocarcinoma is important because the therapeutic approaches are different. Therapy with bevacizumab and pemetrexed have a non-squamous indication.[15,16,17] In adenocarcinoma several targetable molecular alterations as EGFR mutations[18] and EML4-ALK[19] rearrangements are identified.
Bronchoscopy preceded core biopsy in 329/463 (71%) of our patients. Seventy-three percent of the patients with lesions in the lung had undergone bronchoscopy but without diagnosis because the lesion was too peripheral to sample, the lesion did not penetrate the mucous membrane or sparse, inconclusive material was obtained.
The technique for a CT-guided core biopsy of lung lesions is described by Tsai et al.[20]
When the biopsy gun fires, there is a shock wave distal and lateral to the biopsy needle tip. This vibration may cause bleeding in the parenchyma. In our patient group, the single CT scan directly after the biopsy revealed small hemorrhages and some patients had hemoptysis direct after but there were no major problem except for in one case. In that case, a 79 year-old female patient incurred a severe hemorrhage with aspiration during the biopsy procedure. She was subsequently in need of intensive care, intubated and died 10 days later. The results of her biopsy showed lung cancer. We have in retrospect analyzed her case and found that this complication could not have be anticipated.
The pneumothorax on the single CT scan taken directly after the biopsy may be a result of air leakage from the room via the needle or via damage to the lung parenchyma during the biopsy. We present our data according to the occurrence of pneumothorax without regard to its extent.
The increased risk for pneumothorax associated with the biopsy of smaller lesions has been described in earlier studies. Yeow et al.[21] published a large consecutive series of 660 patients who underwent cutting needle biopsies of the lung and found that the highest pneumothorax rate correlated with a lesion size <2 cm and a lesion depth of 0,1 to 2 cm. The risk factors for highest bleeding rate are lesion size <2 cm, lesion depth >2.1 cm, and lung lesions not associated with a pleural effusion. Yeow et al. did not find that emphysema seen on CT scan was associated with an increased risk of pneumothorax as we found in our study. In our study, we found that the positive effect of lesion size on risk of pneumothorax is decreasing with the depth of the lesion, and the depth of the lesion has only a negative effect on pneumothorax when the size is considerable.
In our study, we tested if the assumption of linearity in the logit were fulfilled regarding the continuous variables depth of lesion and size of lesion and found nothing that contradicted that assumption. Thus, we used the original measure scale (mm) in the analysis. We also conducted the analysis with cut-off values dichotomizing the scale of the two variables. The results of this analysis showed lower odds for pneumothorax with increasing size of lesion and lower odds for pneumothorax with lesser depth of lesion, which is in agreement with the results in the study of Yeow.[21] Size of lesion was a significant factor but depth of lesion was not a statistically significant factor. Furthermore, the interaction term was not statistically significant. We decided to keep the factors size of lesion and depth of the lesion in the continuous scale so as not to loose information.
The importance of the lesion size may be explained by the difficulty of maneuvering the needle into a small lesion thus extending the time for the procedure.
In our material, 463 patients, chest tube insertion was required in 27 of the 156 (17%) with pneumothorax. Twelve of those had emphysema in the path of the biopsy needle. The routine is to avoid emphysematous lung when possible.
The incidence of pneumothorax in our material is almost the same as in earlier publications but more common than in the study done by Yeow[21] who presented 23% pneumothorax (155/660) and 1% (9/155) needing chest tube. Saji et al.[22] reported an incidence of 26.6% pneumothorax (77/289) and 53% (41/77) needing a chest tube.
Symptoms rather than the size of the pneumothorax served as a guide for when chest tube was needed. Spontaneous resorption is the rule and a larger pneumothorax needs a longer time to be reabsorbed. The chest tube was kept in place until air leakage ceased. The patients were informed prior to the procedure of the risks of delayed pneumothorax. In our material, one patient returned the day after the biopsy with a pneumothorax and in need of a chest tube.
The recommendation is that all patients should have recent spirometry before needle biopsy. Patients with FEV1<35% predicted should not undergo needle biopsy without further assessment by a multidisciplinary team.[23] In our material 7/15 (47%) of the patients who underwent lung biopsy with FEV1<35% predicted got pneumothorax and 57% of those needed a chest tube. Our study supports the recommendation. A retrospective study as this makes it difficult to find all spirometry data and the examinations may be done but the data are missing as they were not consistently saved.
Acknowledgments
We would like to thank Staffan Gullsby MD, formerly radiologist at the Department of Radiology Gävle County Hospital, for his valuable initiatives in the early stages of this study.
This study was supported by grants from Centre for Research and Development, Uppsala University/County Council of Gävleborg and Gävle Cancer Fund, Sweden.
Footnotes
Source of Support: Centre for Research and Development, Uppsala University/County Council of Gävleborg and Gävle Cancer Fund, Sweden.
Conflict of Interest: None declared.
References
- 1.Laurent F, Latrabe V, Vergier B, Michel P. Percutaneous CT guided biopsy of the lung: Comparison between aspiration and automated cutting needles using a coaxial technique. Cardiovasc Intervent Radiol. 2000;23:266–72. doi: 10.1007/s002700010067. [DOI] [PubMed] [Google Scholar]
- 2.Hayashi N, Sakai T, Kitagawa M, Kimoto T, Inagaki R, Ishii Y, et al. CT-guided biopsy of pulmonary nodules less than 3 cm: Usefulness of the spring-operated core biopsy needle and frozen section pathologic diagnosis. AJR Am J Roentgenol. 1998;170:329–31. doi: 10.2214/ajr.170.2.9456939. [DOI] [PubMed] [Google Scholar]
- 3.Laurent F, Latrabe V, Vergier B, Montaudon M, Vernejoux JM, Dubrez J. CT-guided transthoracic needle biopsy of pulmonary nodules smaller than 20 mm: Results with an automated 20-gauge coaxial cutting needle. Clin Radiol. 2000;55:281–7. doi: 10.1053/crad.1999.0368. [DOI] [PubMed] [Google Scholar]
- 4.Tsukada H, Satou T, Iwashima A, Souma T. Diagnostic accuracy of CT guided automated needle biopsy of lung nodules. AJR Am J Roentgenol. 2000;175:239–43. doi: 10.2214/ajr.175.1.1750239. [DOI] [PubMed] [Google Scholar]
- 5.Westcott JL. Percutaneous transthoracic needle biopsy. Radiology. 1988;169:593–601. doi: 10.1148/radiology.169.3.3055026. [DOI] [PubMed] [Google Scholar]
- 6.Lucidarme O, Howarth N, Finet JF, Grenier PA. Intrapulmonary lesions: Percutaneous automated biopsy with a detachable, 18-gauge, coaxial cutting needle. Radiology. 1998;207:759–65. doi: 10.1148/radiology.207.3.9609901. [DOI] [PubMed] [Google Scholar]
- 7.Anderson JM, Murchison J, Patel D. CT-guided lung biopsy: Factors influencing diagnostic yield and complication rate. Clin Radiol. 2003;58:791–7. doi: 10.1016/s0009-9260(03)00221-6. [DOI] [PubMed] [Google Scholar]
- 8.Loubeyre P, Copercini M, Dietrich PY. Percutaneous CT-guided multisampling core needle biopsy of thoracic lesions. AJR Am J Roentgenol. 2005;185:1294–8. doi: 10.2214/AJR.04.1344. [DOI] [PubMed] [Google Scholar]
- 9.Heyer CM, Reichelt S, Peters SA, Walther JW, Muller KM, Nicolas V. Computed tomography-navigated transthoracic core biopsy of pulmonary lesions: Which factors affect diagnostic yield and complication rates? Acad Radiol. 2008;15:1017–26. doi: 10.1016/j.acra.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 10.Richardson CM, Pointon KS, Manhire AR, Macfarlane JT. Percutaneous lung biopsies: A survey of UK practice based on 5444 biopsies. Br J Radiol. 2002;75:731–5. doi: 10.1259/bjr.75.897.750731. [DOI] [PubMed] [Google Scholar]
- 11.Tomiyama N, Yasuhara Y, Nakajima Y, Adachi S, Arai Y, Kusumoto M, et al. CT-guided needle biopsy of lung lesions: A survey of severe complication based on 9783 biopsies in Japan. Eur J Radiol. 2006;59:60–4. doi: 10.1016/j.ejrad.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Hosmer DW, Lemeshow S. 2nd ed. New York: John Wiley and Sons; 2000. Applied logistic regression. [Google Scholar]
- 13.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global Cancer Statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 14.Ferlay J, Parkin DM, Steliarova — Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer. 2010;46:765–81. doi: 10.1016/j.ejca.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 15.Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2452–550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 16.Reck M, von Pawel J, Zatloukal P, Ramlau R, Gorbounova V, Hirsh V, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small cell lung cancer: AVAiL. J Clin Oncol. 2009;27:1227–34. doi: 10.1200/JCO.2007.14.5466. [DOI] [PubMed] [Google Scholar]
- 17.Scagliotti G, Brodowicz T, Shepherd FA, Zielinski C, Vansteenkiste J, Manegold C, et al. Treatment-by-histology interaction analyses in three phase III trials show superiority of pemetrexed in nonsquamous non-small cell lung cancer. J Thorac Oncol. 2011;6:64–70. doi: 10.1097/JTO.0b013e3181f7c6d4. [DOI] [PubMed] [Google Scholar]
- 18.Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefinitib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–57. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 19.Shaw AT, Yeap BY, Mino-Kenudson M, Digumarthy SR, Costa DB, Heist RS, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol. 2009;27:4247–53. doi: 10.1200/JCO.2009.22.6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsai IC, Tsai WL, Chen MC, Chang GC, Tzeng WS, Chan SW, et al. CT-Guided Core Biopsy of Lung Lesions: A Primer. AJR Am J Roentgenol. 2009;193:1228–35. doi: 10.2214/AJR.08.2113. [DOI] [PubMed] [Google Scholar]
- 21.Yeow KM, Su IH, Pan KT, Tsay PK, Lui KW, Cheung YC, et al. Risk factors of pneumothorax and bleeding: Multivariate analysis of 660 CT-guided coaxial cutting needle lung biopsies. Chest. 2004;126:748–54. doi: 10.1378/chest.126.3.748. [DOI] [PubMed] [Google Scholar]
- 22.Saji H, Nakamura H, Tsuchida T, Tsuboi M, Kawate N, Konaka C, et al. The incidence and the risk of pneumothorax and chest tube placement after percutaneous CT-guided lung biopsy: The angle of the needle trajectory is a novel predictor. Chest. 2002;121:1521–6. doi: 10.1378/chest.121.5.1521. [DOI] [PubMed] [Google Scholar]
- 23.Manhire A, Charig M, Clelland C, Gleeson F, Miller R, Moss H, et al. BTS: Guidelines for radiologically guided lung biopsy. Thorax. 2003;58:920–36. doi: 10.1136/thorax.58.11.920. [DOI] [PMC free article] [PubMed] [Google Scholar]


