Abstract
PURPOSE:
Novel composite meshes routinely used in laparoscopic hernia repair reportedly lead to fewer and less dense visceral adhesions and may provide a viable alternative in thoracic surgery as well.
METHODS:
A total of 15 adult domestic pigs underwent full thickness chest wall resection and reconstruction with Parietene (polypropylene composite; PTE, n = 5), Parietex (polyester composite; PTX, n = 5) or Bard (purely polypropylene, n = 5) mesh. After an observation period of 90 days all animals were sacrificed, intrathoracic adhesions classified via thoracoscopy (VATS), meshes explanted and peak peal strength required for lung/mesh separation recorded.
RESULTS:
Adhesions assessed through VATS-exploration were strongest in the PTX-Group while PTE and BM showed comparable results. Tensiometric analyses of peak peal strength confirmed lower values in BM than for PTE and PTX. Both composite materials showed good overall bioincorporation with post-surgical perigraft-fibrosis being strongest in BM.
CONCLUSION:
We consider composite grafts a suitable alternative for chest wall reconstruction. They are characterized by good overall biointegration and limited perigraft-fibrosis, thus potentially facilitating redo-procedures, even though a hydrophilic coating per se does not appear to prevent intrathoracic adhesion formation.
Keywords: Composite grafts, chest wall resection, chest wall reconstruction
Indications for full thickness chest wall resection and repair include primary tumors like chondro-or osteosarcoma, extensive metastatic disease with direct invasion, post-traumatic or postsurgical alteration and herniation, infection and radiation-induced necrosis.[1,2,3,4,5,6]
A wide range of synthetic, biological, autologous and even bioartificial materials are available, and mostly chosen on the basis of the surgeon's experiences and preferences.[1,2,3,4,5,6,7,8]
Reported disadvantages of synthetic materials include secondary wound infections in upto 6% of cases, graft dehiscence, seroma formation, insufficient tensile strength, long-term deterioration of lung elasticity and respiratory failure.[6,9] Even though reliance on autologous grafts reportedly improves overall outcome, it tends to significantly lengthen duration of procedure due to mobilization of muscle flaps.[8] Bioartifical materials may overcome most of the aforementioned shortcomings, but are still to be considered as largely experimental at this stage.[7]
Ideally, a material for chest wall replacement should be characterized by good tensile strength, elasticity, ease of use, ready availability and high biocompatibility. Additionally, optimal bio-incorporation would induce few to no postsurgical adhesions, thus facilitating eventual redo-procedures.
Novel composite polypropylene (Parietene ®, Covidien, Mansfield MA, USA) and polyester (Parietex ®, Covidien, Mansfield MA, USA) meshes implanted in laparoscopic hernia repair reportedly lead to fewer and less dense visceral adhesions.[10,11] Additionally, macroporous meshes often are preferred because large pores permit infiltration of macrophages and allow rapid fibroplasia and angiogenesis, with reduced infiltration and growth of bacteria.[12,13] A potential drawback of macroporous meshes is an increased risk of visceral adhesions to the site of the repair as a result of fibrin deposition.[12,13] As shown for an intra-abdominal model, in a clean environment antiadhesive coatings reduce adhesion formation to macroporous meshes and they may even be suitable for implantation in infected surgical sites.[14] As no published experience for full thickness chest wall resection and reconstruction with these meshes exists, we sought to examine their ingrowth pattern and bioincorporation as compared to uncoated polypropylene. Conventional polypropylene meshes are widely adopted due to ease of use and low cost. They are still generally accepted as gold standard in chest wall reconstruction as they typically provide semi-rigid fixation and good skeletal support when sutured under tension. These materials are also used for their good in-growth and pliability[15] and thus were deemed as ideal control in our study.
Methods
We obtained approval from our local Committee for Animal Care and adhered to the guidelines on animal experimentation. The experiments were conducted on 15 adult domestic pigs (Deutsche Landschweine; W&P Agrarhandels GmbH Oberheldrungen Thüringen, Germany) weighing between 40-60kg. After premedication with azaperon (2mg/kg (Stresnil®; Jansen Cilag, Neuss, Germany)) and atropine (0.05 mg/kg (Atropinsulfat®; B. Braun, Melsungen, Germany)) general anaesthesia was induced with ketamine 10% (0.2 ml/kg (Ketanest ® S; Pfizer, Berlin, Germany)) and xylazine 2% (0,1ml/kg (Rompun ®; Bayer, Leverkusen, Germany)) and up kept with fentanil (0.005 mg/kg (Fentanyl; Janssen Cilag, Germany)) midazolam (0,05mg/kg (Dormicum ®; Roche, Germany) and isofluorane (Isofluran; Baxter, Germany)). All surgical procedures were performed under endotracheal intubation and mechanical ventilation (Siemens servo 900c ventilator, Siemens-Elema, Solna, Sweden) with an inspirational oxygen fraction (FiO2) of 50% after complete muscular relaxation (pancuronium bromide (0.1 mg/kg). Lactated Ringer΄s solution was infused at a rate of 20 ml/kg/h.
In left lateral decubitus a 15 cm lateral thoracotomy in the 5th intercostal space was performed. After resection of 4 rib segments on a length of 10cm, we reconstructed the resulting defect with either composite (Parietene ® (n = 5) PTE, Parietex ® (n = 5) PTX; Covidien, Mansfield MA, USA) or conventional polypropylene (Bard ® Mesh (n = 5) BM, C. R. Bard, Germany) synthetic meshes [Table 1]. The utilized composite meshes are based on a macroporous three-dimensional polyester (Parietex) or polypropylene structure (Parietene) unilaterally coated with a hydrophilic porcine collagen/ polyethylene glycol/ glycerol compound while an uncoated polypropylene mesh (Bard) was used as control. In all animals an optimal implantation was feasible through conventional techniques, relying on running sutures with non absorbable stitches. A temporarily placed chest tube was removed once soft tissue reconstruction and dermal closure were achieved.
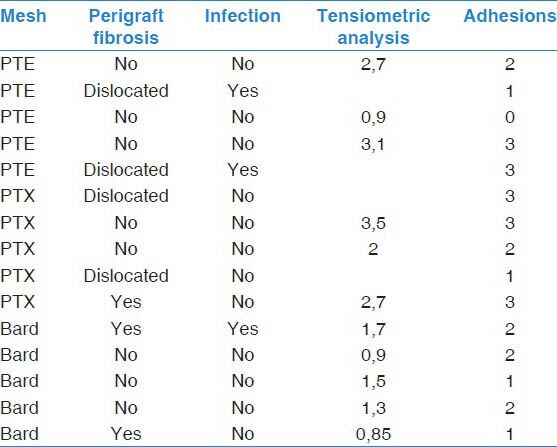
Table 1.
Characteristics of explanted mesh (PTE = Parietene Composite, PTX = Parietex Composite) with macroscopically observable perigraft fibrosis, signs of local infection, tensiometric analysis (peak peal strength required for lung/mesh separation expressed in N) and degree of adhesion formation observed on VAS (from 0 = no adhesion to 4, maximum adhesion)
Postoperative routine antipyretic treatment (ASS 100 mg/d) was administered for the first 10 days and all animals survived our planned observation period of 90 days. Thereafter all animals were sacrificed through a lethal injection of embutramide (75 mg/kg (T61®, Intervet GmbH, Schwabenheim, Germany)) and underwent a video-assisted assessment of intrathoracic adhesions. Encountered adhesions were classified from 0 to IV based on the Zühlke-classification:[16] 0 = no adhesions, I = filiform strands and thin adhesions, II = beginning vascularization with thin and thick strands, III = thick strands and widespread adhesions, IV = dense and widespread adhesions. To this purpose, the thoracic cavity was accessed through a 2 cm incision located 8-10 cm under our original thoracotomy and the field of view was inspected through a 30° DOV telescope (Olympus; Hamburg, Germany). Subsequently, the original thoracotomy was re-accessed and a total of 3 × 2 × 2 cm3 portions of each mesh were excised. Under tensiometric control (PCE FG200, PCE Arnsberg, Germany) respective mesh areas were harvested from the surrounding tissue and the required force of pull was recorded in Newton. For each single specimen mean values were calculated using a standard software solution (Excel, Microsoft, Redmond WA, USA).
Results
No critical events were encountered during our surgical procedures and continuous pulse oximetry confirmed oxygen saturation levels >95% at any time. All animals survived the entire observation period of 90 days without encountering major adverse events, including clinical signs of flail chest. On explantation, either partial or complete patch dislocations were observed in 4 animals (n = 2 PTX, n = 2 PTE) with additional macroscopic signs of local infection (mainly pus) in 3 of our 15 animals (n = 2 PTE, n = 1 BM). Interestingly, no animal had telltale symptoms of septicaemia or even discernable evidence of chest wall infection like swelling, hyperthermia or tenderness. Furthermore not a single case of postoperative bleeding, seroma formation or secondary lung erosion was encountered.
On VATS-assessment, the strongest adhesions were observed in our PTX-Group (median 3, range 1-3), while PTE (median 2, range 0-3) and BM (median 2, range 1-2) showed comparable results. Post-surgical perigraft-fibrosis, as documented prior to explantation, was strongest in BM, with 2 out of 5 meshes presenting signs of massive fibrosis. Both brands of composite grafts were characterized by little to no fibrotic reaction with surrounding tissue.
Tensiometric analyses of peak peal strength required for lung/ mesh separation were partially contradictory, being lowest in BM (mean 1.25N, range 0.85-1.7) and increasing for both PTE (mean 2.2N, range 0.9-3.1) and PTX (mean 2.7N, range 0.9-3.1). All findings are summarized in Table 1.
Discussion
Even though a wide range of synthetic, biological, autologous and even bioartificial materials is available for chest wall repair, the choice of optimal replacement graft remains difficult.[2,3,5,6,7] Ideally, the adopted material should have high tensile strength, elasticity, longevity, ease of use, high biocompatibility and low affinity to bacterial adhesion. A good secondary incorporation into adjacent tissue could limit calcification and fibrosis, thus reducing adhesion formation between chest wall and lung and possibly facilitating redo-procedures. Even though extensive adhesions may potentially prove to be beneficial by preventing pneumothorax formation, they may engender secondary complications like long-term onset of restrictive lung disease[6] and render redo-procedures far more challenging.
Especially in paediatric patients bio-artificial grafts with intrinsic growth potential may constitute a viable alternative in the near future. Unfortunately, routine clinical implementation is not yet foreseeable.[7] Procedures with muscle transfer techniques (Musculus latissimus dorsi, Musculus rectus abdominis) lead to overall good clinical results due to reliance on autologous materials, but tend to significantly lengthen overall operating time and often require implementation of microvascular techniques.[17] Even though biological materials may prove a viable alternative, at present no real long-term data is available.[6]
Currently, the most widespread meshes are made of synthetic materials like polypropylene or polytetrafluoroethylene (Gore-Tex®). Reported side effects range from secondary infection, to dehiscence and seroma formation.[3,18] Additionally, reported complications for chest wall reconstruction with synthetic materials include respiratory failure due to altered lung elasticity, long-term onset of restrictive lung disease, chronic pain, haemorrhage and secondary lung erosion.[6]
Novel composite materials like polyester (Parietex ®, Covidien, Mansfield MA, USA) or polypropylene (Parietene ® composite, Covidien, Mansfield MA, USA) meshes with hydrophilic coating have been successfully introduced in laparoscopic hernia repair.[10,11,19] A macroporous three-dimensional polyester knit structure supposedly facilitates tissue ingrowth and contemporarily a hydrophilic porcine collagen/ polyethylene glycol/ glycerol-coating may lead to fewer post-surgical adhesions,[11] even though hydrophilic coating does not completely prevent adhesion formation.[20] Similar observations were made for ePTFE-grafts with two distinct surfaces (DualMesh ®, W. L. Gore, Flagstaff AZ, USA), whose mechanical properties promote unilateral bioincorporation and contralaterally supposedly impede adhesion formation.[21] Even though macrophages absorb the collagen layer of a Parietex ® composite mesh by 30 days and are no longer present afterwards, they are replaced by fibroblasts, which possibly explain an increased adhesion formation yet absence of visceral adhesions up to 30 days after implantation.[22] This remarkable property is not yet completely understood, but has been confirmed by other work groups.[22] Interestingly, adhesions assessed through VATS-exploration were strongest in our PTX-Group while PTE and BM showed comparable results. Even though we expected less and fewer adhesions in composite materials, tensiometric analyses of peak peal strength confirmed this initial impression with lower values in BM than for PTE and PTX. On a side note, VATS-exploration and pleurolysis appeared to be easiest in animals that received composite materials, but these impressions were not quantifiable. This may suggest good overall bioincorporation of both composite materials with post-surgical perigraft-fibrosis being strongest in BM as well. On the other hand we could not conclusively demonstrate an inhibiting effect of hydrophilic coating on adhesion formation, which is in line with observations made by other groups in laparoscopic models.[20]
Other previously described benefits of composite meshes include decreased infection risks.[12,14] If a mesh is used in a contaminated environment, consensus exists that a biological collagen mesh or a synthetic macroporous, monofilament mesh may be advantageous.[12,23] Macroporous Parietene ® Composite meshes had a low risk of infection in an animal model,[14] which was explained by large pores allowing admission of macrophages, fibroplasia and angiogenesis, which in turn improves the ability to clear infection.[14] In this series coated materials did not appear to prevent infection, even though the PTX-Group was the only one without infected grafts on explantation.
Our results, including secondary infection rate and overall clinical outcome, were generally in line with previously published data. Most publications focus on abdominal hernia repair and at this point only a very limited experience with composite materials for chest wall resection exists.[24] Despite being limited by a small sample size and mostly observational data, this study confirms a good overall biointegration and limited perigraft-fibrosis for both composite meshes. Clear cut benefits of novel materials over conventional grafts could not be described and should be further investigated.
Conclusion
At this point we consider composite grafts a viable alternative for chest wall reconstruction. These materials appear to be characterized by good overall biointegration and limited perigraft-fibrosis, thus potentially facilitating redo-procedures. Diverging from intraperitoneal findings by other groups, we could not confirm that a hydrophilic coating per se does prevent either intrathoracic adhesion formation or postsurgical infection. Further investigation is warranted.
Footnotes
Source of Support: We would like to thank Mr. Christian Finke for his kind support
Conflict of Interest: This work was supported by a research grant provided by Covidien, Mansfield MA, USA
References
- 1.Gonfiotti A, Santini PF, Campanacci D, Innocenti M, Ferrarello S, Caldarella A, et al. Malignant primary chest-wall tumours: Techniques of reconstruction and survival. Eur J Cardiothorac Surg. 2010;38:39–45. doi: 10.1016/j.ejcts.2009.12.046. [DOI] [PubMed] [Google Scholar]
- 2.Rocco G, Fazioli F, Scognamiglio F, Parisi V, La Manna C, La Rocca A, et al. The combination of multiple materials in the Creation of an artificial anterior chest cage after extensive demolition for recurrent chondrosarcoma. J Thorac Cardiovasc Surg. 2007;133:1112–4. doi: 10.1016/j.jtcvs.2006.11.045. [DOI] [PubMed] [Google Scholar]
- 3.Arnold PG, Pairolero PC. Chest-wall reconstruction: An account of 500 consecutive patients. Plast Reconstr Surg. 1996;98:804–10. doi: 10.1097/00006534-199610000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Blasberg JD, Donington JS. Infections and radiation injuries involving the chest wall. Thorac Surg Clin. 2010;20:487–94. doi: 10.1016/j.thorsurg.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Mansoour KA, Thourani VH, Losken A, Reeves JG, Miller JI, Jr, Carlson GW, et al. Chest wall resections and reconstruction: A 25-year experience. Ann Thorac Surg. 2002;73:1725–6. doi: 10.1016/s0003-4975(02)03527-0. [DOI] [PubMed] [Google Scholar]
- 6.Wiegmann B, Zardo P, Dickgreber N, Länger F, Fegbeutel C, Haverich A, et al. Biological materials in chest wall reconstruction: Initial experience with the Peri-Guard Repair Patch((R)) Eur J Cardiothorac Surg. 2010;37:602–5. doi: 10.1016/j.ejcts.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 7.Ge PS, Imai TA, Aboulian A, Van Natta TL. The use of human acellular dermal matrix for chest wall reconstruction. Ann Thorac Surg. 2010;90:1799–804. doi: 10.1016/j.athoracsur.2010.07.080. [DOI] [PubMed] [Google Scholar]
- 8.Iblher N, Penna V, Momeni A, Padron NT, Stark GB. The extended pectoralis major flap for reconstruction of the upper posterior chest wall and axilla. J Thorac Cardiovasc Surg. 2008;136:790–1. doi: 10.1016/j.jtcvs.2007.11.066. [DOI] [PubMed] [Google Scholar]
- 9.Qin X, Tang H, Xu Z, Zhao X, Sun Y, Gong Z, et al. Chest Wall reconstruction with two types of biodegradable polymer prostheses in dogs. Eur J Cardiothorac Surg. 2008;34:870–4. doi: 10.1016/j.ejcts.2008.06.038. [DOI] [PubMed] [Google Scholar]
- 10.Jacob BP, Hogle NJ, Durak E, Kim T, Fowler DL. Tissue ingrowth and bowel adhesion formation in an animal comparative stydy: Polypropylene versus Proceed versus Parietex composite. Surg Endosc. 2007;21:629–33. doi: 10.1007/s00464-006-9157-9. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez R, Rodeheaver GT, Moody DL, Forseman PA, Ramshaw BJ. Resistance to adhesion formation: A comparative study of treated and untreated mesh products placed in the abdominal cavity. Hernia. 2004;8:213–9. doi: 10.1007/s10029-004-0213-x. [DOI] [PubMed] [Google Scholar]
- 12.Amid PK. Classification of biomaterials and their related complications in abdominal wall hernia surgery. Hernia. 1997;1:15–21. [Google Scholar]
- 13.Muhl T, Binnebösel M, Klinge U, Goedderz T. New objective measurement to characterize the porosity of textile implants. J Biomed Mater Res B Appl Biomater. 2008;84:176–83. doi: 10.1002/jbm.b.30859. [DOI] [PubMed] [Google Scholar]
- 14.Deerenberg EB, Mulder IM, Grotenhuis N, Ditzel M, Jeekel J, Lange JF. Experimental study on synthetic and biological mesh implantation in a contaminated environment. Br J Surg. 2012;99:1734–41. doi: 10.1002/bjs.8954. [DOI] [PubMed] [Google Scholar]
- 15.Losken A, Thourani VH, Carlson GW, Jones GE, Culbertson JH, Miller JI, et al. A reconstructive algorithm for plastic surgery following extensive chest wall resection. Br J Plast Surg. 2004;57:295–302. doi: 10.1016/j.bjps.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Zühlke HV, Lorenz EM, Straub EM, Savvas V. Pathophysiology and classification of adhesions. Langenbecks Arch Chir Suppl II Verh Dtsch Ges Chir. 1990:1009–16. [PubMed] [Google Scholar]
- 17.Netscher DT, Baumholtz MA. Chest reconstruction: I. Anterior and anterolateral chest wall and wounds affecting respiratory function. Plast reconstr Surg. 2009;124:240–52e. doi: 10.1097/PRS.0b013e3181b98c9c. [DOI] [PubMed] [Google Scholar]
- 18.Sugarbaker DJ, Jaklitsch MT, Bueno R, Richards W, Lukanich J, Mentzer SJ, et al. Prevention, early detection, and managment of complications after 328 consecutive extrapleural pneumonectomies. J Thorac Cardiovasc Surg. 2004;128:138–46. doi: 10.1016/j.jtcvs.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 19.Balique JG, Benchetrit S, Bouillot JL, Flament JB, Gouillat C, Jarsaillon P, et al. Intraperitoneal treatment of incisional and umbilical hernias using an innovative composite mesh: Four-year results of a prospective multicenter clinical trial. Hernia. 2005;9:68–74. doi: 10.1007/s10029-004-0300-z. [DOI] [PubMed] [Google Scholar]
- 20.Schug-Pass C, Sommerer F, Tannapfel A, Lippert H, Köckerling F. The use of composite meshes in laparoscopic repair of abdominal wall hernia: Are there differences in biocompatibility? Experimental results obtained in a laparoscopic porcine model. Surg Endosc. 2009;23:487–95. doi: 10.1007/s00464-008-0085-8. [DOI] [PubMed] [Google Scholar]
- 21.Harrell AG, Novitsky YW, Peindl RD, Cobb WS, Austin CE, Cristiano JA, et al. Prospective evaluation of adhesion formation and shrinkage of intra-abdominal prosthetics in a rabbit model. Am Surg. 2006;72:808–14. [PubMed] [Google Scholar]
- 22.Schreinemacher MH, Emans PJ, Gijbels MJ, Greve JW, Beets GL, Bouvy ND. Degradation of mesh coatings and intraperitoneal adhesion formation in an experimental model. Br J Surg. 2009;96:305–13. doi: 10.1002/bjs.6446. [DOI] [PubMed] [Google Scholar]
- 23.Engelsman AF, van Dam GM, van der Mei HC, Busscher HJ, Ploeg RJ. In vivo evaluation of bacterial infection involving morphologically different surgical meshes. Ann Surg. 2010;251:133–7. doi: 10.1097/SLA.0b013e3181b61d9a. [DOI] [PubMed] [Google Scholar]
- 24.Nagayasu T, Yamasaki N, Tagawa T, Tsuchiya T, Miyazaki T, Nanashima A, et al. Long-term results of chest wall reconstruction with DualMesh. Interact Cardiovasc Thorac Surg. 2010;11:581–4. doi: 10.1510/icvts.2010.242040. [DOI] [PubMed] [Google Scholar]


