Abstract
Purpose
Plasma Coenzyme Q10 (CoQ10) levels are lower in septic shock (SS) patients than in healthy controls (HC). However, CoQ10 status in critically ill patients without septic shock (nSS) is unknown. Here, we investigated CoQ10 concentrations in SS and nSS patients as compared with HC.
Materials and Methods
We enrolled 36 critically ill patients and 18 HC. Plasma CoQ10 concentrations were measured and patients’ clinical and demographical data were collected.
Results
Plasma CoQ10 concentrations were lower in critically ill patients (0.50±0.36μg/ml, p<0.001), both in SS (0.37±0.25 μg/ml, p=0.002) and nSS patients (0.56±0.39, p=0.04), as compared with HC (0.79±0.19). CoQ10 levels did not differ between SS and nSS patients (p=0.13). In critically ill patients, CoQ10 levels inversely correlated with age (r=−0.40, p=0.015) and did not correlate to PaO2/FiO2, Simplified Acute Physiology Score II SAPS2, Systemic Organ Failure Assessment score or mortality. Lower CoQ10 levels were associated with lower Activities of Daily Living score after discharge (p=0.005) independent of age.
Conclusions
Decreased plasma CoQ10 levels are not specific to septic shock patients, but rather observed in a broad range of critically ill patients. In critically ill patients CoQ10 insufficiency may be associated with various conditions; age may be a risk factor.
Keywords: Plasma Coenzyme Q10, Critically ill patients, Septic shock, Sepsis, Age
Introduction
Mortality in critically ill patients is associated with multiple organ dysfunction. Despite intense investigation for many years, the precise mechanisms underlying multiple organ dysfunction syndrome still remain to be determined. Among others, mitochondrial dysfunction has been proposed as an important contributor to this devastating condition, although limited knowledge is available about the etiology of mitochondrial dysfunction in critical illness. In septic patients, mitochondrial dysfunction and decreased ATP are related to organ failure. Critically ill patients show both increased production of oxygen-free radicals leading to oxidative stress and decreased mitochondrial functional capacity in muscle, particularly in non-survivors.
Coenzyme Q10 (CoQ10, also known as ubiquinone) is an essential cofactor for the electron transport chain reactions in the mitochondria and also acts as an antioxidant. Primary and secondary CoQ10 deficiency causes mitochondrial dysfunction, which, in turn, leads to encephalopathy, myopathy, renal dysfunction and heart failure. CoQ10 insufficiency is associated with exacerbated heart failure, endothelial dysfunction and myopathy, and CoQ10 supplementation ameliorates these disease conditions. Low levels of plasma CoQ10 were associated with increased mortality in a cohort of patients affected by heart failure. These findings support the possibility that CoQ10 insufficiency may play a role in organ dysfunction in critically ill patients.
Nevertheless, until recently CoQ10 status has not been studied in critically ill patients. A recent study has reported lower plasma CoQ10 levels in 14 septic shock patients compared with 16 healthy control individuals. To date, however, CoQ10 status in non-septic shock patients with critical illness is unknown. Based on our preliminary data in mice that mild sepsis decreased plasma CoQ10 levels without causing septic shock or death (unpublished observation, M. Kaneki and M. Yamada, 2011), we hypothesized that decreased plasma CoQ10 concentration is not limited to septic shock patients, but also observed in a broad range of critically ill patients without septic shock. To test this hypothesis, we investigated plasma CoQ10 levels in critically ill patients with and without septic shock, as compared to healthy controls.
MATERIALS AND METHODS
Patients and Healthy Subjects
The protocol of this cross-sectional observational study was approved by the Partners Human Research Committee and has therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. All subjects gave their informed consent prior to their inclusion in the study.
We enrolled thirty six adult patients (age > 18 years) admitted to the surgical intensive care unit (ICU) of our tertiary-care teaching hospital from April to July 2011. Eighteen adult healthy volunteers were recruited through clinical study advertisements within Partners HealthCare System. Volunteers were studied in the morning after fasting for 8 hours or more to minimize the influence of meal. Exclusion criteria for controls were: systemic disease with or without functional limitations; known pregnancy; active smoking; use of any medication within 20 days; supplementation of CoQ10. Enrolled subjects or their healthcare proxy provided written informed consent. None of the patients or healthy control subjects took statins, which inhibit CoQ10 biosynthesis, either prior to hospital admission or during hospital stay. Septic shock was defined according to the American College of Chest Physicians/Society of Critical Care Medicine consensus conference definition: which consists of the presence of infection plus two systemic inflammatory response syndrome criteria and need of vasopressor medication for blood pressure support.
Clinical Data Collection and CoQ10 Measurement
At the time of blood sampling, demographic information, including age, gender, height, and body weight were recorded and body mass index (BMI) was calculated for each subject. Additionally, ventilatory parameters, data for calculation of Simplified Acute Physiology Score II (SAPS2) and Systemic Organ Failure Assessment (SOFA) score were recorded for the ICU patients. To assess patients’ functional ability to perform activities of daily living (ADL), we used the Katz index that evaluates levels of independence when performing six activities required for daily living (bathing, dressing, toileting, moving, continence, feeding) ranging from 0 (total dependence) to 6 (completely independent). We assessed the Katz ADL score by telephone interview after discharge, as previously performed in critically ill patients.
Blood samples were withdrawn into EDTA vacutainers and immediately centrifuged at 2,000 rpm at 4°C for 20 minutes. Plasma samples were stored at −80°C until analyzed for CoQ10 concentrations. Total plasma CoQ10 levels were measured by high-performance liquid chromatography with electrochemical detection as previously described at the laboratory in the Division of Pathology & Laboratory Medicine, Cincinnati Children’s Hospital Medical Center and University of Cincinnati College of Medicine, where analysis of CoQ10 is performed for both commercial and research purposes. Plasma total cholesterol concentration, which correlates with plasma CoQ10 level, was measured using a colorimetric assay (Sigma-Aldrich, St. Louis, MO).
Statistical Analysis
Data are reported as mean ± standard deviation unless otherwise indicated. We compared continuous data by independent-samples t Test and ANOVA as appropriate. Tukey’s post-hoc test was performed to adjust for multiple comparisons. Normality was assessed by the Kolmogoronov-Smirnov test. Non-normally distributed variables were compared by the Mann-Whitney U test. Categorical variables were compared by Chi-Square test or Fisher’s exact test as appropriate. To further analyze factors associated with plasma CoQ10 levels and coenzyme Q10 to total cholesterol (CoQ10/T-Chol) ratio (ADL score and age), a multivariate backwards stepwise logistic regression was used. Statistical analyses were performed using SPSS software version 18.0 (Chicago, IL). A p value <0.05 was considered statistically significant.
RESULTS
Plasma CoQ10 Concentrations Were Lower in Critically Ill Patients
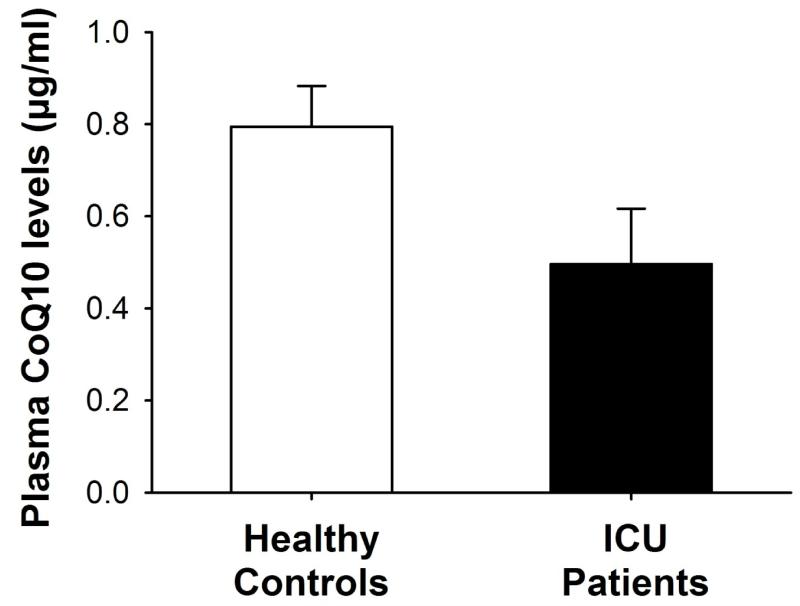
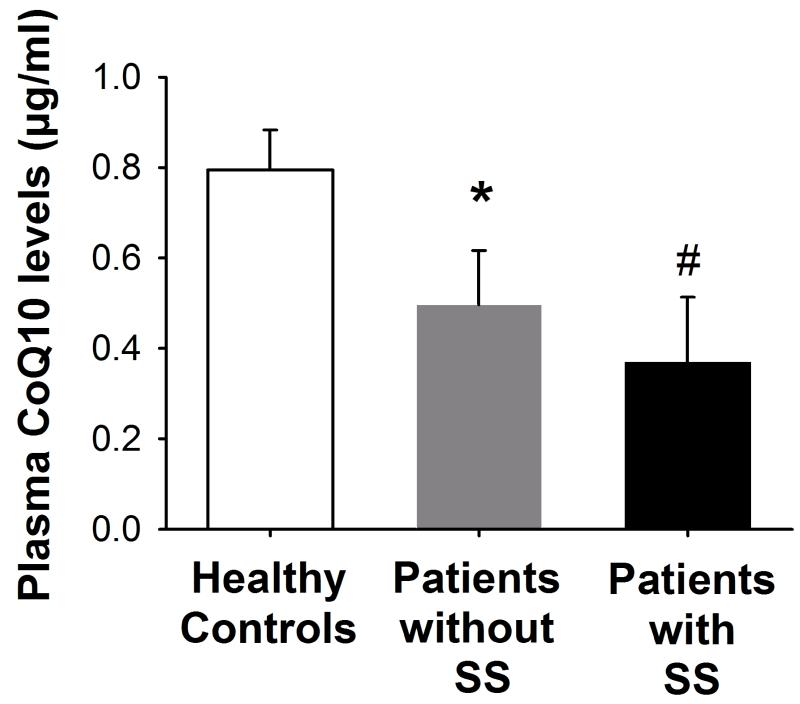
The populations of healthy controls and critically ill patients did not differ in terms of age (46 ± 9 vs. 53 ± 18 years), gender (44% vs. 25% of females), and BMI (24 ± 5 vs. 27 ± 6, p>0.05 for all). Plasma CoQ10 concentrations were significantly lower in the 36 critically ill ICU patients as compared with the 18 healthy controls (0.50 ± 0.36 vs. 0.79 ± 0.19 μg/ml, p<0.001, Figure 1). Among the 36 ICU patients, septic shock was diagnosed in 12 patients. Plasma CoQ10 concentrations were significantly lower both in patients with and without septic shock, as compared with healthy controls (p=0.002 and p=0.04, respectively, Figure 2). In contrast, no significant difference was found in plasma CoQ10 levels (p=0.13, Figure 2) as well as in age (59 ±19 vs. 50 ±17), gender (25% vs. 25%) and BMI (26 ±3 vs. 28 ±7, all p>0.05) between patients with and without septic shock. Gender was not associated with plasma CoQ10 concentrations both among patients (0.47 ± 0.33 for females vs. 0.50 ± 0.37 μg/ml for males, p=0.79) and healthy controls (0.80 ± 0.17 for females vs. 0.79 ± 0.21 μg/ml for males, p=0.95).
Fig. 1.
Plasma CoQ10 levels resulted significantly lower in the total population of critically ill patients as compared to healthy controls. *p<0.001
Fig. 2.
Plasma CoQ10 levels were significantly lower in both septic shock patients (with SS) and critically ill patients without septic shock (without SS) as compared to healthy controls. *p=0.04, #p=0.002 vs. healthy controls; p=0.002 by ANOVA; SS: septic shock
Low-density lipoprotein (LDL) and high-density lipoprotein (HDL) are the major carriers of CoQ10 in the circulation and hence plasma total cholesterol concentration correlates with plasma CoQ10 level. We, therefore, measured total cholesterol levels. Consistent with previous studies, plasma total cholesterol levels were significantly lower in ICU patients than healthy controls (111 ± 57 mg/dL vs. 187 ± 24, p<0.001). Plasma CoQ10 to total cholesterol (CoQ10/T-Chol) ratio did not differ between the two groups (4.3 ± 2.4 × 104 vs. 4.3 ± 1.0 × 104). While total cholesterol levels were lower in septic shock patients than non-septic shock patients (83 ± 15 vs. 125 ± 11, p<0.05), CoQ10/T-Chol ratio did not significantly differ between patients with and without septic shock (4.8 ± 0.9 × 104 vs. 4.1 ± 0.4 × 104, p>0.1).
Inverse Correlation Between Plasma CoQ10 Levels and Age in Critically Ill Patients
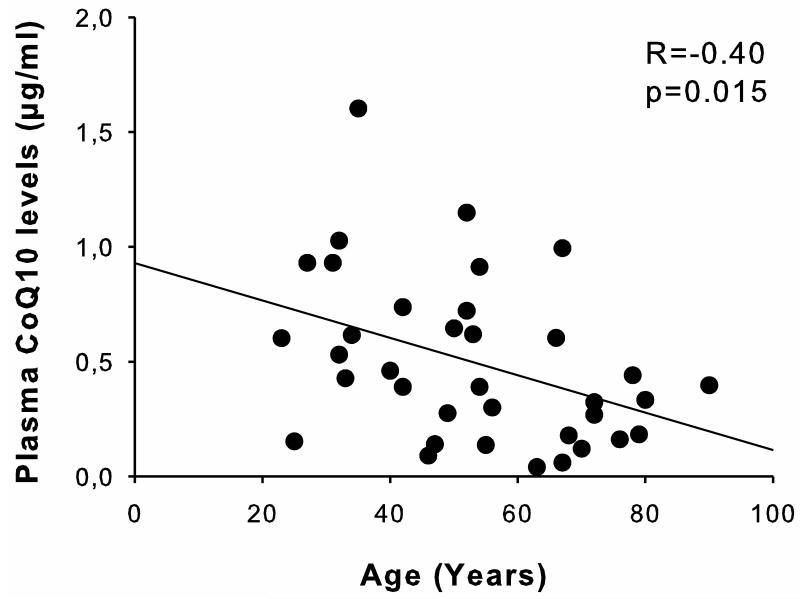
Among critically ill patients, plasma CoQ10 levels inversely correlated with age (r= −0.40, p=0.015, Figure 3). Lower levels of plasma CoQ10 (<0.4 μg/ml, the median of CoQ10 levels in the patient group) were associated with lower ADL score after discharge (p=0.005 by Mann-Whitney test, Table 1). Multivariate logistic regression analysis showed that ADL score ≤5 was associated with low levels of plasma CoQ10 independent of age (p=0.005). Similarly, CoQ10/T-Chol ratio significantly correlated with ADL score independent of age (p=0.013). In line with these findings, lower levels of plasma CoQ10 were associated with lower CoQ10/T-Chol ratio (Table 1).
Fig. 3.
Inverse correlation between CoQ10 levels and age in critically ill patients
Table 1.
Characteristics of ICU patients relative to median CoQ10 plasma level (0.4 μg/ml)
| Total ICU patients (n=36) |
Low CoQ10 (n=18) |
High CoQ10 (n=18) |
P-value | |
|---|---|---|---|---|
| Age, years | 51 ± 16 0.002 |
62 ± 16 | 45 ± 15 | |
| Females, n. (%) | 9 (25) | 5 (28) | 4 (22) | 1.00 |
| PaO2/FiO2 | 267 ± 81 0.603 |
260 ± 77 | 275 ± 87 | |
| Pneumonia, n. (%) | 14 (39) 0.305 |
9 (50) | 5 (28) | |
| CPIS | 5 (3-6) 0.171 |
5 (5-6) | 4 (3-6) | |
| ALI/ARDS, n (%) | 16 (44) 0.738 |
9 (50) | 7 (39) | |
| SOFA score, median (IQ range) | 4 (2-7) 0.356 |
5 (3-7) | 3 (1-7) | |
| Bilirubin, mg/dl | 1.0 ± 1.4 0.847 |
0.9 ± 1.2 | 1.1 ± 1.6 | |
| Platelets count, ×103/μL | 257 ±146 0.929 |
260 ±147 | 255 ±150 | |
| Creatinine, mg/dl | 1.53 ± 1.40 0.677 |
1.43 ± 1.20 | 1.63 ± 1.63 | |
| White blood cells, ×103/μL | 12.1 ± 6.6 0.605 |
12.7 ± 5.1 | 11.5 ± 7.9 | |
| Septic Shock, n. (%) | 12 (33) 0.289 |
8 (44) | 4 (22) | |
| SAPS2 | 40 ± 13 0.544 |
41 ± 11 | 39 ± 15 | |
| Survivors, n.(%) | 32 (89) | 16 (89) | 16 (89) | 1.00 |
| ADL score after discharge at home, median (IQ range) |
5 (3-6) 0.005 |
4 (2-5) | 6 (5-6) | |
| CoQ10/Cholesterol (×104) | 4.3 (2.8-5.1) <0.001 |
2.9 (1.8-3.6) | 5.1 (4.4-6.4) |
ALI/ARDS: Acute Lung Injury/Acute Respiratory Distress Syndrome; SOFA: Sequential Organ Failure Assessment; SAPS2: Simplified Acute Physiology Score; ADL: Activities of Daily Living
On the other hand, plasma CoQ10 levels were not significantly related to PaO2/FiO2, plasma creatinine concentration, white blood cell or platelet count, septic shock, SAPS2 at ICU admission, SOFA score, or mortality in the patient population (Table 1).
In the healthy control group, CoQ10 levels and age did not significantly correlate (p=0.1).
DISCUSSION
Here, we show that plasma CoQ10 concentrations are lower in critically ill patients compared with healthy control individuals, regardless of the presence of septic shock. In the patient group, plasma CoQ10 levels correlated with age; lower CoQ10 levels were associated with low ADL score after discharge.
Plasma CoQ10 concentrations among healthy volunteers in this study are quite similar to those of healthy adults in previous studies. Miles M.V. et al. have reported that the 95% reference range and average of plasma CoQ10 concentrations were 0.43 – 1.53 μg/ml and 0.90 ± 0.28 μg/ml [mean ± SD], respectively, in 106 self-reported healthy adults. In our study, more than half of the ICU patients had plasma CoQ10 levels lower than the lower 95% reference range limit reported by Miles et al.
In a recent study, plasma CoQ10 levels were significantly lower in 14 septic shock patients compared with 16 healthy controls. Similar to these findings, septic shock patients exhibited low CoQ10 levels in our study. More importantly, our results reveal that in a general ICU population CoQ10 levels are lower than healthy controls, irrespective of the diagnosis of septic shock. Low CoQ10 levels are not limited to septic shock patients, but rather widely observed in patients admitted to an intensive care unit. Moreover, circulating levels of CoQ10 did not differ significantly between septic shock (n=12) and non-septic shock (n=24) patients. It remains unknown which factors are responsible for low CoQ10 levels in critical illness.
Approximately 60% of plasma CoQ10 is associated with LDL and 25% with HDL. Hence, plasma CoQ10 and total cholesterol concentrations correlate with each other. The 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase-mediated mevalonate pathway plays a key role in the biosynthesis of CoQ10 as well as cholesterol. Statins, inhibitors of HMG-CoA reductase, decrease plasma concentration of not only cholesterol but also CoQ10, while CoQ10/T-Chol ratio is not altered by statins. Reduced cholesterol biosynthesis has been proposed as a player in the pathogenesis of hypocholesterolemia in critically ill patients, although the exact mechanisms remain to be determined. Our data showed that both plasma CoQ10 and total cholesterol levels were lower in the ICU patients in comparison with the healthy controls, while CoQ10/T-Chol ratio did not differ between the two groups. Taken together, one can speculate that reduced activity of the mevalonate pathway and subsequent decrease in cholesterol and COQ10 biosynthesis may underlie the combination of reduced levels of plasma CoQ10 and total cholesterol with unaltered CoQ10/T-Chol ratio in critically ill patients.
Although the majority of CoQ10 in the human body is produced endogenously, it has been estimated that approximately 20-25% of plasma CoQ10 is derived from dietary sources. Thus, feeding status and intestinal absorption also influence plasma CoQ10 levels. Based on the unaltered CoQ10/T-Chol ratio, however, our data do not support the notion that decreased CoQ10 intake, which supposedly lowers CoQ10/T-Chol ratio, is the major determinant of the reduced plasma CoQ10 levels in the ICU patients. It should be noted that our data do not exclude the possibility that it could contribute to the reduced CoQ10 levels as an aggravating factor.
Recent studies showed that plasma CoQ10 levels were significantly lower in septic shock patients and after cardiac arrest as compared to healthy controls. However, in these studies the authors did not provide data of CoQ10/LDL ratio or CoQ10/T-Chol ratio. Hence, it is not known whether CoQ10/LDL or CoQ10/T-Chol ratio was altered in these patients’ populations.
Of note, we found a significant correlation between CoQ10 levels and age in the patient group. The association between CoQ10 levels and age did not result statistically significant in the healthy control group (p=0.1), but we cannot exclude the possibility that the sample size (n=18) might be too small to find such an association. It should be noted that the pre-existing postulate of age-related decline in circulating plasma CoQ10 has not been supported by previous data. To date, no studies found age-related CoQ10 decline in normal adult cohorts. Similar to our findings in critically ill patients, a previous study by McMurray et al. showed that heart failure patients with lower serum CoQ10 levels are older and have more advanced heart failure, although CoQ10 levels in healthy controls were not measured. Despite differences in patient population characteristics between the study by McMurray and ours, such as disease (heart failure vs. critically ill condition) and age (73 ± 7 vs. 53 ± 18 years), it is tempting to speculate that (a) mechanism(s) by which low CoQ10 levels are linked to age might exist, particularly under systemic disease conditions. Considering the relatively small number of the enrolled patients (n=36), the relationship between CoQ10 and age needs to be further confirmed by large-scale clinical studies.
We found a significant association between CoQ10 and ADL score in the patient population after controlling for the confounding effect of age, suggesting that there might be an independent association between plasma CoQ10 levels during ICU stay and independence after discharge at home. In addition to plasma CoQ10 levels, CoQ10/T-Chol ratio significantly correlated with ADL independent of age. CoQ10 supplementation has been shown to improve ADL score, neuromuscular function, and/or long-term outcome in other patient populations such as heart failure and Parkinson’s disease. Although a confirmation in larger cohort of ICU patients is needed, these results raise the possibility that CoQ10 supplementation may be beneficial and improve the clinical outcome of critically ill patients.
It is an open question whether plasma CoQ10 concentration or CoQ10/T-Chol ratio is more clinically relevant as a biomarker of CoQ10 insufficiency. Previous studies in healthy individuals and diabetic patients indicate that plasma CoQ10, but not CoQ10/T-Chol, correlates with CoQ10 content in platelets and circulating white blood cells. It is conceivable that plasma CoQ10 rather than CoQ10/T-Chol ratio may be a better biomarker of CoQ10 insufficiency. Further studies are, however, required to clarify whether plasma CoQ10 is an appropriate indicator of intracellular CoQ10 content and CoQ10 status in organs in critical illness.
In this study, we could not demonstrate a significant association between CoQ10 levels and the common indicators of severity of illness in the patient population. It is possible that the lack of statistically significant association may be attributable to the small sample size and the heterogeneity in the patient population. Larger studies including both medical and surgical ICU patients may help clarify whether low CoQ10 levels have a real impact on organ and/or mitochondrial dysfunction. Alternatively, a clinical trial to evaluate the effects of CoQ10 supplementation on organ and mitochondrial function in ICU patients with low CoQ10 levels may be necessary to clarify CoQ10 insufficiency in critically ill patients.
CONCLUSIONS
In this cross-sectional observational study, reduced plasma CoQ10 levels were observed in a mixed population of critically ill patients both with and without septic shock, as compared with healthy controls. Older patients were more likely to have lower CoQ10 levels. Taken together, our data provide a rationale for further observational and intervention clinical studies to define CoQ10 insufficiency and evaluate the safety and efficacy of CoQ10 supplementation in critically ill patients.
Acknowledgements
We are grateful to Dr. J.P. Cobb for organizing this research team. This study was supported in part by grants from Shriners Hospitals for Children to M.K.
Footnotes
Competing Interests: The authors have no conflict of interest to declare.
Author Contributions: MK and EAB were responsible for all aspects of this investigation. MK, EAB, UHS, AC and LB contributed to the study design. AC, LB, AK, RP and MY carried out primary data collection and substantial portion of the data analysis. EAB, AC and LB were responsible for statistical analysis. All authors were involved in the manuscript preparation and approved the final version. The authors declare they have full control of all primary data and they agree to allow the journal to review their data if requested.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270:2957–2963. doi: 10.1001/jama.270.24.2957. [DOI] [PubMed] [Google Scholar]
- 2.Kozlov AV, Bahrami S, Calzia E, et al. Mitochondrial dysfunction and biogenesis: do ICU patients die from mitochondrial failure? Ann Intensive Care. 2011:1–41. doi: 10.1186/2110-5820-1-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brealey D, Brand M, Hargreaves I, et al. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet. 2002;360:219–223. doi: 10.1016/S0140-6736(02)09459-X. [DOI] [PubMed] [Google Scholar]
- 4.Carre JE, Orban JC, Re L, et al. Survival in critical illness is associated with early activation of mitochondrial biogenesis. Am J Respir Crit Care Med. 2010;182:745–751. doi: 10.1164/rccm.201003-0326OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goodyear-Bruch C, Pierce JD. Oxidative stress in critically ill patients. Am J Crit Care. 2002;11:543–551. quiz 552-543. [PubMed] [Google Scholar]
- 6.Quinzii CM, Hirano M. Primary and secondary CoQ(10) deficiencies in humans. Biofactors. 2011;37:361–365. doi: 10.1002/biof.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Langsjoen PH, Vadhanavikit S, Folkers K. Response of patients in classes III and IV of cardiomyopathy to therapy in a blind and crossover trial with coenzyme Q10. Proc Natl Acad Sci U S A. 1985;82:4240–4244. doi: 10.1073/pnas.82.12.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tiano L, Belardinelli R, Carnevali P, et al. Effect of coenzyme Q10 administration on endothelial function and extracellular superoxide dismutase in patients with ischaemic heart disease: a double-blind, randomized controlled study. Eur Heart J. 2007;28:2249–2255. doi: 10.1093/eurheartj/ehm267. [DOI] [PubMed] [Google Scholar]
- 9.Dai YL, Luk TH, Yiu KH, et al. Reversal of mitochondrial dysfunction by coenzyme Q10 supplement improves endothelial function in patients with ischaemic left ventricular systolic dysfunction: a randomized controlled trial. Atherosclerosis. 2011;216:395–401. doi: 10.1016/j.atherosclerosis.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Abe K, Matsuo Y, Kadekawa J, et al. Effect of coenzyme Q10 in patients with mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes (MELAS): evaluation by noninvasive tissue oximetry. J Neurol Sci. 1999;162:65–68. doi: 10.1016/s0022-510x(98)00296-2. [DOI] [PubMed] [Google Scholar]
- 11.McMurray JJ, Dunselman P, Wedel H, et al. Coenzyme Q10, rosuvastatin, and clinical outcomes in heart failure: a pre-specified substudy of CORONA (controlled rosuvastatin multinational study in heart failure) J Am Coll Cardiol. 2010;56:1196–1204. doi: 10.1016/j.jacc.2010.02.075. [DOI] [PubMed] [Google Scholar]
- 12.Donnino MW, Cocchi MN, Salciccioli JD, et al. Coenzyme Q10 levels are low and may be associated with the inflammatory cascade in septic shock. Crit Care. 2011;15:R189. doi: 10.1186/cc10343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brealey DA, Singer M, Terblanche M. Potential metabolic consequences of statins in sepsis. Crit Care Med. 2011;39:1514–1520. doi: 10.1097/CCM.0b013e31820eb74f. [DOI] [PubMed] [Google Scholar]
- 14.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–874. [PubMed] [Google Scholar]
- 15.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 16.Katz S, Downs TD, Cash HR, et al. Progress in development of the index of ADL. Gerontologist. 1970;10:20–30. doi: 10.1093/geront/10.1_part_1.20. [DOI] [PubMed] [Google Scholar]
- 17.Delannoy B, Floccard B, Thiolliere F, et al. Six-month outcome in acute kidney injury requiring renal replacement therapy in the ICU: a multicentre prospective study. Intensive Care Med. 2009;35:1907–1915. doi: 10.1007/s00134-009-1588-z. [DOI] [PubMed] [Google Scholar]
- 18.Miles MV, Horn PS, Tang PH, et al. Age-related changes in plasma coenzyme Q10 concentrations and redox state in apparently healthy children and adults. Clin Chim Acta. 2004;347:139–144. doi: 10.1016/j.cccn.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 19.Tang PH, Miles MV, Steele P, et al. Anticoagulant effects on plasma coenzyme Q(10) estimated by HPLC with coulometric detection. Clin Chim Acta. 2002;318:127–131. doi: 10.1016/s0009-8981(02)00003-7. [DOI] [PubMed] [Google Scholar]
- 20.Ernster L, Dallner G. Biochemical, physiological and medical aspects of ubiquinone function. Biochim Biophys Acta. 1995;1271:195–204. doi: 10.1016/0925-4439(95)00028-3. [DOI] [PubMed] [Google Scholar]
- 21.Lagendijk J, Ubbink JB, Vermaak WJ. Measurement of the ratio between the reduced and oxidized forms of coenzyme Q10 in human plasma as a possible marker of oxidative stress. J Lipid Res. 1996;37:67–75. [PubMed] [Google Scholar]
- 22.Alleva R, Tomasetti M, Bompadre S, et al. Oxidation of LDL and their subfractions: kinetic aspects and CoQ10 content. Mol Aspects Med. 1997;18(Suppl):S105–112. doi: 10.1016/s0098-2997(97)00039-3. [DOI] [PubMed] [Google Scholar]
- 23.Gordon BR, Parker TS, Levine DM, et al. Relationship of hypolipidemia to cytokine concentrations and outcomes in critically ill surgical patients. Crit Care Med. 2001;29:1563–1568. doi: 10.1097/00003246-200108000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Kaikkonen J, Nyyssonen K, Tuomainen TP, et al. Determinants of plasma coenzyme Q10 in humans. FEBS Lett. 1999;443:163–166. doi: 10.1016/s0014-5793(98)01712-8. [DOI] [PubMed] [Google Scholar]
- 25.Avis HJ, Hargreaves IP, Ruiter JP, et al. Rosuvastatin lowers coenzyme Q10 levels, but not mitochondrial adenosine triphosphate synthesis, in children with familial hypercholesterolemia. J Pediatr. 2011;158:458–462. doi: 10.1016/j.jpeds.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 26.Vyroubal P, Chiarla C, Giovannini I, et al. Hypocholesterolemia in clinically serious conditions--review. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2008;152:181–189. doi: 10.5507/bp.2008.029. [DOI] [PubMed] [Google Scholar]
- 27.Weber C, Bysted A, Holmer G. Coenzyme Q10 in the diet--daily intake and relative bioavailability. Mol Aspects Med. 1997;18(Suppl):S251–254. doi: 10.1016/s0098-2997(97)00003-4. [DOI] [PubMed] [Google Scholar]
- 28.Cocchi MN, Giberson B, Berg K, et al. Coenzyme Q10 levels are low and associated with increased mortality in post-cardiac arrest patients. Resuscitation. 2012;83:991–995. doi: 10.1016/j.resuscitation.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miles MV, Horn PS, Morrison JA, et al. Plasma coenzyme Q10 reference intervals, but not redox status, are affected by gender and race in self-reported healthy adults. Clin Chim Acta. 2003;332:123–132. doi: 10.1016/s0009-8981(03)00137-2. [DOI] [PubMed] [Google Scholar]
- 30.Belardinelli R, Mucaj A, Lacalaprice F, et al. Coenzyme Q10 and exercise training in chronic heart failure. Eur Heart J. 2006;27:2675–2681. doi: 10.1093/eurheartj/ehl158. [DOI] [PubMed] [Google Scholar]
- 31.Liu J, Wang L, Zhan SY, et al. Coenzyme Q10 for Parkinson’s disease. Cochrane Database Syst Rev. 2011;12:CD008150. doi: 10.1002/14651858.CD008150.pub2. [DOI] [PubMed] [Google Scholar]
- 32.Niklowitz P, Menke T, Andler W, et al. Simultaneous analysis of coenzyme Q10 in plasma, erythrocytes and platelets: comparison of the antioxidant level in blood cells and their environment in healthy children and after oral supplementation in adults. Clin Chim Acta. 2004;342:219–226. doi: 10.1016/j.cccn.2003.12.020. [DOI] [PubMed] [Google Scholar]
- 33.Niklowitz P, Sonnenschein A, Janetzky B, et al. Enrichment of coenzyme Q10 in plasma and blood cells: defense against oxidative damage. Int J Biol Sci. 2007;3:257–262. doi: 10.7150/ijbs.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.El-ghoroury EA, Raslan HM, Badawy EA, et al. Malondialdehyde and coenzyme Q10 in platelets and serum in type 2 diabetes mellitus: correlation with glycemic control. Blood Coagul Fibrinolysis. 2009;20:248–251. doi: 10.1097/mbc.0b013e3283254549. [DOI] [PubMed] [Google Scholar]