Abstract
Worldwide, the heterosexual route is the prevalent mode of HIV-1 transmission, and the female reproductive tract accounts for approximately 40% of all HIV-1 transmissions. HIV-1 infection in the female reproductive tract involves three major events: entry through the mucosal epithelium, productive infection in subepithelial mononuclear cells, and delivery to lymph nodes to initiate systemic infection. Here, we provide a focused review of the interaction between HIV-1 and mucosal epithelial cells, lymphocytes, macrophages, and dendritic cells in female genital mucosa. Increased understanding of these interactions could illuminate new approaches for interdicting HIV-1 heterosexual transmission.
Keywords: Dendritic cells, entry, epithelial cells, lymphocytes, macrophages, replication
Introduction
Human immunodeficiency virus type 1 (HIV-1) enters the host in virtually all transmissions, excluding those acquired parenterally, through the mucosal surfaces of either the genital tract or the gastrointestinal tract.1-3 However, despite remarkable scientific progress during the past three decades, an effective HIV-1 vaccine and strategies to prevent HIV-1 transmission remain elusive, underscoring the need for increased understanding of HIV-1 mucosal transmission. Indeed, many critical issues have not been fully elucidated, including the identification of the initial HIV-1 target cells, parameters of local viral propagation, pathways of viral dissemination, and the mechanism of R5 selection.
Worldwide, the heterosexual route is the prevalent mode of HIV-1 transmission,1,2 with the risk of transmission 0.03–0.5% per coital act.4-6 Pertinent to this review, the female reproductive tract accounts for approximately 40% of all HIV-1 transmissions.3 As in other routes of mucosal infection, HIV-1 infection in the female reproductive tract involves three major events: entry through the mucosal epithelium, productive infection in subepithelial mononuclear cells, and delivery to lymph nodes to initiate systemic infection. Recent studies, as previously reviewed,3,7-21 have expanded but not completely defined the immediate events that follow HIV-1 exposure in the genital mucosa. Here, we provide a focused review of the interactions between HIV-1 and key mucosal cells, including epithelial cells, lymphocytes, macrophages, and dendritic cells (DCs), in the female reproductive tract in heterosexual transmission. A summary of these interactions is listed in Table 1. In addition to our discussion of these events, we emphasize several unresolved issues that warrant future investigation, including the role of macrophages in HIV-1 dissemination and in latency.
Table 1.
Interactions between HIV-1 and mucosal epithelial cells, lymphocytes, macrophages, and dendritic cells (DCs) in female reproductive tract
| Mucosa | Type of epithelium | Mucosal cells | Interaction between HIV-1 and mucosal cells |
|---|---|---|---|
| Vagina | Squamous, non-keratinized | Epithelial cells | aTranscytosis44,45 |
| Myeloid DCs | bCapture virus31, trans-infection23 | ||
| Langerhans’ cells | bCapture virus24,62, trans-infection24 | ||
| Lymphocytes | bEntry23,24, breplication23,25 | ||
| Macrophages | bEntry23,25, breplication23,25 | ||
| Ectocervix | Squamous, non-keratinized | Epithelial cells | aTranscytosis44,45 |
| bImpairing barrier functions46 | |||
| aTransmitting virus to CD4 + T cells49,50 | |||
| a,bReplication43,50 | |||
| Myeloid DCs | bEntry23 | ||
| Langerhans’ cells | No report | ||
| Lymphocytes | bEntry23, breplication38–40 | ||
| Macrophages | bEntry23, breplication23,42 | ||
| Endocervix | Columnar, single layer | Epithelial cells | a,bTranscytosis44,45 |
| bImpairing barrier functions46 | |||
| aTransmitting HIV-1 to CD4+ T cells50 | |||
| a,bReplication43,50,54 | |||
| Myeloid DCs | No report | ||
| Lymphocytes | breplication38–40 | ||
| Macrophages | b,creplication28 | ||
| Uterus | Columnar, single layer | Epithelial cells | aTranscytosis44,45 |
| bImpairing barrier functions46 | |||
| a,bTransmitting virus to CD4+ T cells47,48 | |||
| a,bReplication43,47,48,51 | |||
| Myeloid DCs | No report | ||
| Lymphocytes | c,dReplication43 | ||
| Macrophages | c,dReplication43 | ||
| Fallopian tube | Columnar, single layer | Epithelial cells | a,bReplication51 |
| Myeloid DCs | No report | ||
| Lymphocytes | c,dReplication43 | ||
| Macrophages | c,dReplication43 |
Results generated from genital tissue-derived cell line cells.
Results generated from primary genital cells.
The cells responsible for HIV-1 replication were not identified, but the lymphocytes and/or macrophages were potentially permissive for HIV-1 replication.
Results generated from isolated mixed cell cultures and vibratome sections.
HIV-1 entry through genital mucosal epithelium
The first event in mucosal HIV-1 infection is translocation of the virus across the epithelium. Among persons at risk for HIV-1 infection, disruption of cervicovaginal mucosa induced by trauma or infection-associated mucosal inflammation, ulceration, and erosions provides HIV-1 access to lymphoid cells in the subepithelial lamina propria (Figs 1 and 2), resulting in higher rates of HIV-1 transmission.22 In the absence of mucosal disruption due to trauma or infection, possible cellular routes of HIV-1 entry into the mononuclear cell-rich lamina propria include epithelial cells, lymphocytes, Langerhans’ cells, myeloid DCs, and macrophages (Figs 1 and 2).23-25
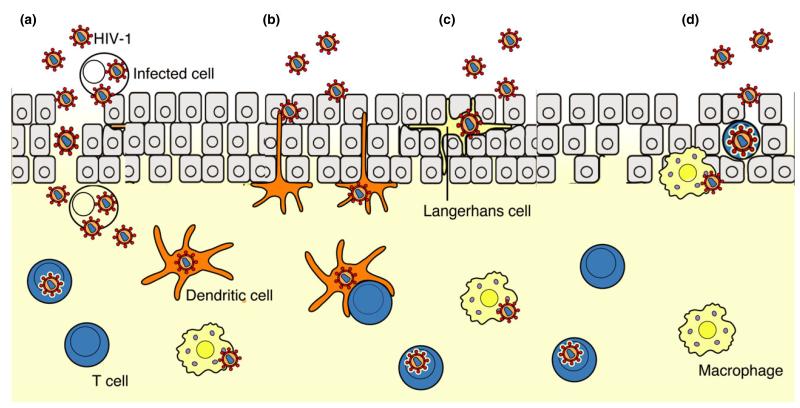
Fig. 1.
Pathways of HIV-1 entry in the vaginal and ectocervical mucosae. HIV-1 enters the vaginal and ectocervical mucosae by four potential routes. (a) Disruption of vaginal and ectocervical mucosae induced by trauma or infection-associated mucosal inflammation, ulceration, and erosion provides cell-free and cell-associated HIV-1 direct access to target cells in the stratified squamous epithelium, the lamina propria, and local lymphatic and blood vessels (vessels not shown). (b) Dendritic cells at the epithelial/lamina propria interface capture virus, migrate into the lamina propria or further to the draining lymph nodes, and trans-infect target mononuclear cells. (c) Langerhans’ cells may take up virions that enter the porous stratified epithelium. (d) HIV-1 infects directly CD4+ T cells and macrophages within the squamous epithelium. [Modified from ref. 104.]
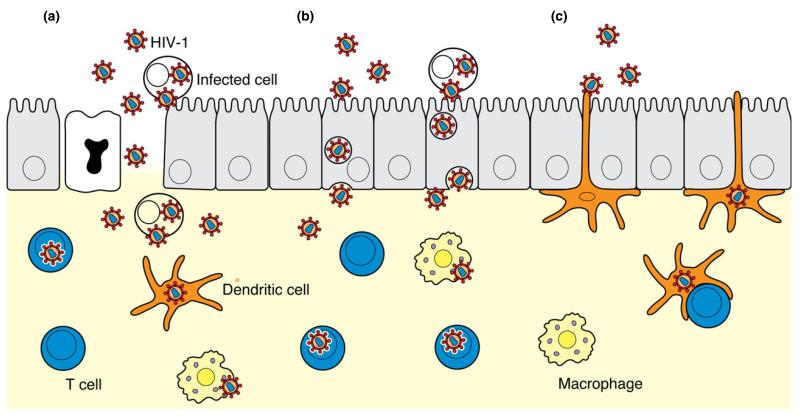
Fig. 2.
Pathways of HIV-1 entry in the mucosae of the endocervix, uterus, and possibly Fallopian tubes. HIV-1 enters the columnar epithelium by three potential routes. (a) Disruption of the mucosae of the endocervix, uterus, and Fallopian tubes induced by trauma or sexually transmitted infection provides cell-free and cell-associated HIV-1 direct access to target cells in the lamina propria and to local lymphatic and blood vessels (vessels not shown). (b) Columnar epithelial cells endocytose cell-free and cell-associated viruses and transcytose virus across epithelium into the lamina propria. (c) Dendritic cells in the lamina propria extend processes between epithelial cells, capture virus, migrate into the lamina propria or to the lymph nodes, and trans-infect target mononuclear cells. [Modified from ref. 104].
Human immunodeficiency virus-1 entry in the female reproductive tract may occur via vagina, ectocervix, endocervix, uterus, and possibly Fallopian tubes, regions with distinct epithelial architecture (Table 1). The epithelium of the endocervix, uterus, and Fallopian tubes, like that of the gut, is a single layer of polarized, columnar epithelial cells with tight junctions, dividing the epithelium into apical and basolateral domains (Fig. 2).2,26 In contrast, the epithelium of the vagina and ectocervix is composed of multilayered, pluristratified epithelial cells that do not have a polarized plasma membrane or tight junctions (Fig. 1).2,6,26,27 These distinct features may promote different translocation processes in different regions of the female genital tract (Figs 1 and 2). In the vaginal and ectocervical mucosae, the absence of epithelial cell tight junctions likely permits CD4+ T cell, macrophage, and DC migration into the vaginal and ectocervical epithelium, where the cells may take up HIV-1 that has entered the ‘leaky’ epithelium. In this regard, we have shown that vaginal macrophages25 and myeloid DCs (manuscript submitted) take up HIV-1 in a vaginal mucosa explant model. We also have shown that vaginal DCs transport virus across the vaginal mucosa (manuscript submitted). Moreover, using vaginal and ectocervical mononuclear leukocytes isolated from human vaginal and ectocervical tissues provided by healthy women undergoing reconstructive pelvic surgery, we have demonstrated that vaginal and ectocervical macrophages, myeloid DCs, and lymphocytes take up HIV-1.23 In elegant studies, Hladik, et al.24 induced vaginal blisters to show that intraepithelial CD4+ T cells and Langerhans’ cells translocate HIV-1 across the vaginal epithelium. As in the intestinal mucosa,26,28-32 transcytosis and translocation of HIV-1 by epithelial cells or surface-penetrating DCs are the likely cellular routes by which HIV-1 enters the lamina propria of the human endocervical mucosa.
Transcytosis of virions through squamous epithelial cells has been suggested,3 but classic transcytosis occurs in polarized, columnar epithelial cells and not non-polarized, pluristratified squamous epithelium.26 Thus, the recent identification of the neonatal Fc receptor (FcRn) in the basal layer of human vaginal epithelium33,34 and ectocervical epithelium34 and in the columnar epithelial cells that line the human endocervix35 and uterus,33 as well as the penile urethra,34 underscores the potential role of this receptor in HIV-1 entry in genital mucosa. The FcRn substantially enhances HIV-1 transcytosis across an endometrial epithelial cell monolayer in the presence of both an acidic environment and Env-specific IgG.34 These data indicate that FcRn-mediated transcytosis could contribute significantly to male-to-female and male-to-male transmission, because genital and rectal secretions from infected persons contain Env-specific antibodies.35-37
Productive infection in subepithelial mononuclear cells
After translocation across the mucosal epithelium, HIV-1 encounters potential target cells in the dense lymphocyte and macrophage populations of the lamina propria (Table 1). Lamina propria CD4+ T lymphocytes in human and macaque vaginal and cervical mucosae are early target cells for HIV-1 and SIV and support viral replication.23,24,38-41 Vaginal, as well as ectocervical, macrophages also have been shown to support HIV-1 replication.23,25,42 Macrophages from vaginal and ectocervical mucosa were similar in their capacity to support infection, as were lymphocytes from vaginal and ectocervical mucosa. However, when lymphocytes were compared to macrophages from the same mucosal compartment, lymphocytes supported more robust replication than the macrophages.23 Furthermore, isolated mixed cell cultures and vibratome sections from human uterus, Fallopian tube, ectocervix, and cervix are permissive to HIV-1.43 Although DCs alone do not support HIV-1 replication, DC-T-cell conjugates support high levels of viral replication.
Epithelial cells in HIV-1 mucosal infection of the female reproductive tract
Genital epithelial cells have been shown to support transcytosis of cell-free virus across epithelium in primary human endocervical epithelial cells and genital mucosa-derived cell lines, including VK2/E6E7, Endo1/E6E7, Ect1/E6E7, and HEC1A that are derived from vaginal, endocervical, ectocervical, and endometrial tissue, respectively.44,45 Nazli et al.46 reported that primary endometrial and cervical epithelial cells interact directly with HIV-1 via gp120, inducing upregulation of an array of pro-inflammatory cytokines. Pro-inflammatory cytokines such as TNF-α in turn lead to the impairment of barrier functions, promoting HIV-1 entry across the epithelium and access to lymphoid cells in lamina propria. Furthermore, human primary uterine epithelial cells,47 uterine epithelial cell lines,48 and ectocervical epithelial cell line Ect1/E6E749 capture and transmit HIV-1 to CD4+ T cells. In contrast, Micsenyi et al.50 reported that ectocervical (Ect1/E6E) and endocervical (End1/E6E7) epithelial cell line cells are capable of transmitting HIV-1 to CD4+ T cells only when de novo HIV-1 is produced within the epithelial cells.
Genital epithelial cell permissiveness to HIV-1 infection is controversial. Human primary epithelial cells isolated from uterus,43,47,51 ectocervix,44 cervix,43,51 Fallopian tube,52 as well as human uterine (RL95-2, HEC1A, and ECC1),48 ectocervical (Ect1/E6E7) and endocervical (End1/E6E7) epithelial cell line cells,50 support HIV-1 replication. Human cervix-derived epithelial cell line ME180 also can be productively infected by cell-associated HIV-1 and remains infected for 8 months, suggesting that cervical epithelial cells support HIV infection.52-54 In sharp contrast, others have reported that isolated human primary ectocervical and endocervical epithelial cells do not support productive HIV-1 infection by cell-free or cell-associated HIV-1.42,55 As noted by the authors, the non-permissiveness of cervical primary epithelial cells to HIV-1 in these studies is likely related to the absence of surface expression of HIV-1 receptor and co-receptors.56 The absence of HIV-1 receptor and co-receptors on isolated cervical primary epithelial cells may be due to the enzyme used to isolate the cells. Dispase, for example, has been shown to cleave CD4 from lymphocytes after 1-hr incubation, whereas collagenase has no effect after 3-hr incubation.56
Dendritic cells in HIV-1 mucosal infection of female reproductive tract
Studies of monocyte-derived DCs (MoDCs), blood DCs, Langerhans’ cells, and myeloid DCs in the simian immunodeficiency virus (SIV)/rhesus macaque non-human primate model have provided many insights into the critical role of DCs in HIV-1 transmission.7,57-60 However, DCs in different tissues and mucosal compartments display distinct phenotypes and functionality, precluding the simple extrapolation of findings to DCs in the female reproductive tract. Study of the interactions between HIV-1 and human mucosal DCs in the female reproductive tract has been hindered by the limited availability of human female genital tissue and the difficulty in isolating mucosal cells.
In the macaque model, SIV inoculated into the macaque vagina enters the mucosa within 60 min through intraepithelial DCs and can be detected in draining lymph nodes within 18 hr.61 The presence of DC-SIGN on genital and gut DCs61 may facilitate DC transport of virus in the mucosa. Although Langerhans’ cells do not express DC-SIGN or CCR5, they may participate in early HIV-1 uptake, as shown in macaques inoculated intravaginally with SIV.59 The location of Langerhans’ cells in the upper layer of the stratified epithelium positions these cells for the uptake of free virions that have penetrated this region of the squamous epithelial barrier.
Mucosal DCs consist of myeloid DCs, plasmacytoid DCs, and Langerhans’ cells. In human studies, attention has focused on Langerhans’ cells, which have been shown to take up HIV-1 in vaginal epithelial sheets,24,62 human skin explants,63 and epidermal cells isolated from human skin.64 Recently, we showed that human intestinal lamina propria myeloid DCs rapidly take up HIV-1, transport the virus through the mucosa, and transmit virus in trans to peripheral blood and intestinal lymphocytes.31 In addition, DC-SIGN+ cells from human rectal mucosa have been shown to bind and transfer HIV-1 to peripheral blood CD4+ T cells.65 In female genital mucosa, myeloid DCs and Langerhans’ cells have been shown to take up and/or transfer HIV-1 to CD4+ T cells.23,24 Vaginal mucosa contains more than 10-fold less plasmacytoid DCs, compared to myeloid DCs (manuscript submitted). Hladik and colleagues24 showed that HIV-1 rapidly penetrates intraepithelial CD1a+ Langerhans’ cells, which reside in the genital tract epithelium. Among vaginal and ectocervical mononuclear cells, DCs are the first cells to take up HIV-1.23 As early as 15 min after virus inoculation, 5.1% of vaginal myeloid DCs and 1.7% of ectocervical myeloid DCs contain virus. In contrast, vaginal and ectocervical macrophages first contain detectable HIV-1 at 2 hr.23 Notably, myeloid DCs in human vaginal lamina propria differ phenotypically from MoDCs, take up HIV-1 up to 10-fold more virus than MoDCs, transport HIV-1 through the mucosa, and then trans-infect vaginal and peripheral blood target cells (manuscript submitted).
Lymphocytes in HIV-1 mucosal infection of female reproductive mucosa
The identification of CD4+ T cells in the human vaginal epithelium and documentation of HIV-1 penetration into lymphocytes in the epithelial sheets obtained by suction blister24 suggest that CD4+ T cells may be involved in HIV-1 entry into vaginal mucosa. In suspension culture of mucosal mononuclear cells, vaginal and ectocervical lymphocytes take up HIV-1.23 These cells first displayed detectable HIV-1 uptake at 2 hr, much later compared with DC uptake of the virus.23
In human and macaque gastrointestinal mucosa, most attention has focused on the small intestine, where lamina propria CD4+ T cells are prominent HIV-1 and SIV target cells and undergo profound depletion shortly after infection.66-72 In human vaginal mucosa, CD3+ T cells are scattered throughout the lamina propria and infrequently at the basal region of the squamous epithelium. Lymphocytes also are detected at the edges of dermal papillae.25 HIV-1 has been detected in CD4+ T cells in vaginal epithelium in a modified organ culture system by electron microscopy.24 We also have shown that vaginal, as well as ectocervical, lymphocytes support HIV-1 replication.23,25 Similarly, CD4+ T cells in macaque vaginal mucosa support HIV-1 replication and are rapidly depleted following intravenous inoculation of SIV.41 In human and macaque cervical mucosa, lamina propria CD4+ T lymphocytes also are early target cells for HIV-1 and SIV and support viral replication.23,38-40 Howell et al.43 reported that isolated mixed cell cultures and vibratome sections from human uterus, Fallopian tubes, ectocervix, and cervix are permissive to HIV-1, but the cells responsible for HIV-1 replication were not identified.
Macrophages in HIV-1 mucosal infection of female reproductive mucosa
Monocytes and macrophages play a fundamental role in the transmission, establishment, pathogenesis, and persistence of HIV-1 infection.73-75 Evidence that macrophages participate in the transmission of HIV-1 derives from the identification of HIV-1-infected tissue macrophages in the body fluids that transmit HIV-1, including semen, cervical fluid, and colostrum/early breast milk.76-80 Among suspension of mucosal mononuclear cells, vaginal and ectocervical macrophages capture HIV-1 and first display detectable virus uptake at 2 hr.23 In a vaginal explant model, HIV-1 was inoculated onto the apical surface of explanted vaginal mucosa, and 30 min later, the explants were harvested, sectioned, stained, and analyzed by confocal microscopy for macrophages that contain virus. HIV-1 virions were identified in macrophages,25 indicating that HIV-1 translocates across the epithelium in vaginal mucosa and then is taken up by subepithelial macrophages.
In vaginal mucosa, macrophages are detected in the lamina propria, occasionally in the basal region of the squamous epithelium and at the edges of dermal papillae.25 In cervical mucosa, CD14-/68-positive macrophages are restricted to subepithelial mucosa.42 Ectocervical subepithelial macrophages have been shown to be among the first cells to be infected in ectocervical explants.42 In addition, using isolated vaginal and ectocervical mononuclear cells23 or purified vaginal macrophages,25 we have shown that both vaginal and ectocervical macrophages support HIV-1 replication.
Macrophages in local spread of HIV-1
Productively infected tissue macrophages also likely contribute to the local spread of virus through the transfer of HIV-1 to other target cells. In this connection, HIV-1-infected macrophages have been shown to transmit virus to CD4+ lymphocytes by fusing with the T cell81 and through the formation of transient viral synapses between viral Gag and Env on the macrophage and CD4 on the T cell.82,83 Macrophage transfer of HIV-1 to T cells may serve to augment CD4+ T-cell infection and subsequent depletion.84 In addition, the macrophage mannose-binding receptor appears to be capable of capturing and transferring virus to T cells through a non-fusigenic mechanism.85 Notably, Vpx-dependent viral amplification in macrophages at sites of virus inoculation appears to be a prerequisite for the efficient dissemination of virus in tissue sites.86 Also, the ability of HIV-1-infected monocytes to cross the blood–brain barrier likely contributes to the delivery of HIV-1 to the brain, leading to microglial infection.87 Similarly, blood monocytes are the exclusive source of intestinal macrophages,88 promoting the delivery of systemically infected monocytes to the intestinal mucosa.
Emerging role for macrophages in HIV-1 persistence
During highly active antiretroviral therapy (HAART), latent HIV-1 infection in memory CD4+ T cells is a major obstacle to curative therapy. These latent-infected lymphocytes are considered the primary reservoir for latent/persistent HIV-1 infection.89-94 In addition, HIV-1-infected macrophages have gained appreciation as another important population of latently infected cells.93,95-98 In patients receiving HAART, HIV-1 proviral DNA has been detected in blood monocytes as well as in activated and resting CD4+ T cells, but viral DNA decays more slowly over time in monocytes than in T cells. CD16+ monocytes appear to constitute a continuing source of viral persistence during HAART.99 Furthermore, sequence evolution and phylogenetic analysis have shown that monocytes, not lymphocytes, are the source of viral replication during and after prolonged HAART,96 implicating mononuclear phagocytes as an important source of latent virus. Consistent with human studies, macrophages have been identified as the principal reservoir of mononuclear cell in macaques infected with SIV/HIV-1 chimeric virus.100 Therefore, macrophages are well suited to serve as reservoir cells in HIV-1 infection due to their remarkable longevity, resistance to HIV-1 cytopathic effects, and ability to evade host immune responses. In this regard, we have reported that intestinal macrophages, unlike monocyte-derived macrophages, are resistant to HIV-1 infection and can carry integrated but transcriptionally silent HIV-1,101-103 indicating that intestinal macrophages are a potential reservoir for latent HIV-1 during HAART. Whether female genital mucosal macrophages function as a potential reservoir for latent HIV-1 is an important topic for future study.
Concluding remarks
Available evidence reviewed here shows that both lower and upper female reproductive tracts are involved in HIV-1 heterosexual transmission (Table 1). The interactions between HIV-1 and mucosal cells in the female reproductive tract are far from being well defined and further investigation is needed. Important topics that warrant investigation include local HIV-1 propagation, pathways of viral dissemination, the mechanism of selection of transmitted/founder viruses, and the influence of the local mucosal microenvironment on each of these events. Increased understanding of the interactions between HIV-1 and mucosal cells in the female reproductive tract will provide critical new insights for the development of effective vaccine and microbicide strategies for the prevention of HIV-1 transmission.
Acknowledgments
This work was supported by National Institutes of Health Grants AI093151 and AI106395, UAB Center for AIDS Research (CFAR) and Comprehensive Cancer Center Development Core Grant (RS); and AI083127, RR-20136, DK064400, and the Research Service of the Veterans Administration (PDS).
References
- 1.Royce RA, Sena A, Cates W, Jr, Cohen MS. Sexual transmission of HIV. N Engl J Med. 1997;336:1072–1078. doi: 10.1056/NEJM199704103361507. [DOI] [PubMed] [Google Scholar]
- 2.Shattock RJ, Moore JP. Inhibiting sexual transmission of HIV-1 infection. Nat Rev Microbiol. 2003;1:25–34. doi: 10.1038/nrmicro729. [DOI] [PubMed] [Google Scholar]
- 3.Hladik F, McElrath MJ. Setting the stage: host invasion by HIV. Nat Rev Immunol. 2008;8:447–457. doi: 10.1038/nri2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boily MC, Baggaley RF, Wang L, Masse B, White RG, Hayes RJ, Alary M. Heterosexual risk of HIV-1 infection per sexual act: systematic review and meta-analysis of observational studies. Lancet Infect Dis. 2009;9:118–129. doi: 10.1016/S1473-3099(09)70021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arien KK, Jespers V, Vanham G. HIV sexual transmission and microbicides. Rev Med Virol. 2011;21:110–133. doi: 10.1002/rmv.684. [DOI] [PubMed] [Google Scholar]
- 6.Gray RH, Wawer MJ, Brookmeyer R, Sewankambo NK, Serwadda D, Wabwire-Mangen F, Lutalo T, Li X, vanCott T, Quinn TC. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet. 2001;357:1149–1153. doi: 10.1016/S0140-6736(00)04331-2. [DOI] [PubMed] [Google Scholar]
- 7.Piguet V, Steinman RM. The interaction of HIV with dendritic cells: outcomes and pathways. Trends Immunol. 2007;28:503–510. doi: 10.1016/j.it.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Southern PJ. Missing out on the biology of heterosexual HIV-1 transmission. Trends Microbiol. 2013;21:245–252. doi: 10.1016/j.tim.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Kaushic C. HIV-1 infection in the female reproductive tract: role of interactions between HIV-1 and genital epithelial cells. Am J Reprod Immunol. 2011;65:253–260. doi: 10.1111/j.1600-0897.2010.00965.x. [DOI] [PubMed] [Google Scholar]
- 10.Kaushic C, Ferreira VH, Kafka JK, Nazli A. HIV infection in the female genital tract: discrete influence of the local mucosal microenvironment. Am J Reprod Immunol. 2010;63:566–575. doi: 10.1111/j.1600-0897.2010.00843.x. [DOI] [PubMed] [Google Scholar]
- 11.Howell AL, Asin SN, Yeaman GR, Wira CR. HIV-1 infection of the female reproductive tract. Curr HIV/AIDS Rep. 2005;2:35–38. doi: 10.1007/s11904-996-0007-0. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez-Garcia M, Patel MV, Wira CR. Innate and adaptive anti-HIV immune responses in the female reproductive tract. J Reprod Immunol. 2013;97:74–84. doi: 10.1016/j.jri.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Merbah M, Introini A, Fitzgerald W, Grivel JC, Lisco A, Vanpouille C, Margolis L. Cervico-vaginal tissue ex vivo as a model to study early events in HIV-1 infection. Am J Reprod Immunol. 2011;65:268–278. doi: 10.1111/j.1600-0897.2010.00967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen R, Smith PD. Mucosal correlates of protection in HIV-1-exposed sero-negative persons. Am J Reprod Immunol. 2014 doi: 10.1111/aji.12202. DOI:10.1111/aji.12202. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blish CA, Baeten JM. Hormonal contraception and HIV-1 transmission. Am J Reprod Immunol. 2011;65:302–307. doi: 10.1111/j.1600-0897.2010.00930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doncel GF, Joseph T, Thurman AR. Role of semen in HIV-1 transmission: inhibitor or facilitator? Am J Reprod Immunol. 2011;65:292–301. doi: 10.1111/j.1600-0897.2010.00931.x. [DOI] [PubMed] [Google Scholar]
- 17.Fahey JV, Bodwell JE, Hickey DK, Ghosh M, Muia MN, Wira CR. New approaches to making the microenvironment of the female reproductive tract hostile to HIV. Am J Reprod Immunol. 2011;65:334–343. doi: 10.1111/j.1600-0897.2010.00949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ganor Y, Bomsel M. HIV-1 transmission in the male genital tract. Am J Reprod Immunol. 2011;65:284–291. doi: 10.1111/j.1600-0897.2010.00933.x. [DOI] [PubMed] [Google Scholar]
- 19.Mayer KH, Venkatesh KK. Interactions of HIV, other sexually transmitted diseases, and genital tract inflammation facilitating local pathogen transmission and acquisition. Am J Reprod Immunol. 2011;65:308–316. doi: 10.1111/j.1600-0897.2010.00942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shacklett BL, Greenblatt RM. Immune responses to HIV in the female reproductive tract, immunologic parallels with the gastrointestinal tract, and research implications. Am J Reprod Immunol. 2011;65:230–241. doi: 10.1111/j.1600-0897.2010.00948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wira CR, Patel MV, Ghosh M, Mukura L, Fahey JV. Innate immunity in the human female reproductive tract: endocrine regulation of endogenous antimicrobial protection against HIV and other sexually transmitted infections. Am J Reprod Immunol. 2011;65:196–211. doi: 10.1111/j.1600-0897.2011.00970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Telzak EE, Chiasson MA, Bevier PJ, Stoneburner RL, Castro KG, Jaffe HW. Hiv-1 Seroconversion in patients with and without genital ulcer disease - a prospective-study. Ann Intern Med. 1993;119:1181–1186. doi: 10.7326/0003-4819-119-12-199312150-00005. [DOI] [PubMed] [Google Scholar]
- 23.Shen R, Richter HE, Smith PD. Early HIV-1 target cells in human vaginal and ectocervical mucosa. Am J Reprod Immunol. 2011;65:261–267. doi: 10.1111/j.1600-0897.2010.00939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hladik F, Sakchalathorn P, Ballweber L, Lentz G, Fialkow M, Eschenbach D, McElrath MJ. Initial events in establishing vaginal entry and infection by human immunodeficiency virus type-1. Immunity. 2007;26:257–270. doi: 10.1016/j.immuni.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen R, Richter HE, Clements RH, Novak L, Huff K, Bimczok D, Sankaran-Walters S, Dandekar S, Clapham PR, Smythies LE, Smith PD. Macrophages in vaginal but not intestinal mucosa are monocyte-like and permissive to human immunodeficiency virus type 1 infection. J Virol. 2009;83:3258–3267. doi: 10.1128/JVI.01796-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bomsel M, Alfsen A. Entry of viruses through the epithelial barrier: pathogenic trickery. Nat Rev Mol Cell Biol. 2003;4:57–68. doi: 10.1038/nrm1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vittinghoff E, Douglas J, Judson F, McKirnan D, MacQueen K, Buchbinder SP. Per-contact risk of human immunodeficiency virus transmission between male sexual partners. Am J Epidemiol. 1999;150:306–311. doi: 10.1093/oxfordjournals.aje.a010003. [DOI] [PubMed] [Google Scholar]
- 28.Meng G, Wei X, Wu X, Sellers MT, Decker JM, Moldoveanu Z, Orenstein JM, Graham MF, Kappes JC, Mestecky J, Shaw GM, Smith PD. Primary intestinal epithelial cells selectively transfer R5 HIV-1 to CCR5 + cells. Nat Med. 2002;8:150–156. doi: 10.1038/nm0202-150. [DOI] [PubMed] [Google Scholar]
- 29.Bomsel M. Transcytosis of infectious human immunodeficiency virus across a tight human epithelial cell line barrier. Nat Med. 1997;3:42–47. doi: 10.1038/nm0197-42. [DOI] [PubMed] [Google Scholar]
- 30.Shen R, Drelichman ER, Bimczok D, Ochsenbauer C, Kappes JC, Cannon JA, Tudor D, Bomsel M, Smythies LE, Smith PD. GP41-specific antibody blocks cell-free HIV type 1 transcytosis through human rectal mucosa and model colonic epithelium. J Immunol. 2010;184:3648–3655. doi: 10.4049/jimmunol.0903346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shen R, Smythies LE, Clements RH, Novak L, Smith PD. Dendritic cells transmit HIV-1 through human small intestinal mucosa. J Leukoc Biol. 2010;87:663–670. doi: 10.1189/jlb.0909605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cavarelli M, Foglieni C, Rescigno M, Scarlatti G. R5 HIV-1 envelope attracts dendritic cells to cross the human intestinal epithelium and sample luminal virions via engagement of the CCR5. EMBO Mol Med. 2013;5:776–794. doi: 10.1002/emmm.201202232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Z, Palaniyandi S, Zeng R, Tuo W, Roopenian DC, Zhu X. Transfer of IgG in the female genital tract by MHC class I-related neonatal Fc receptor (FcRn) confers protective immunity to vaginal infection. Proc Natl Acad Sci U S A. 2011;108:4388–4393. doi: 10.1073/pnas.1012861108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gupta S, Gach JS, Becerra JC, Phan TB, Pudney J, Moldoveanu Z, Joseph SB, Landucci G, Supnet MJ, Ping LH, Corti D, Moldt B, Hel Z, Lanzavecchia A, Ruprecht RM, Burton DR, Mestecky J, Anderson DJ, Forthal DN. The Neonatal Fc receptor (FcRn) enhances human immunodeficiency virus type 1 (HIV-1) transcytosis across epithelial cells. PLoS Pathog. 2013;9:e1003776. doi: 10.1371/journal.ppat.1003776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raux M, Finkielsztejn L, Salmon-Ceron D, Bouchez H, Excler JL, Dulioust E, Grouin JM, Sicard D, Blondeau C. IgG subclass distribution in serum and various mucosal fluids of HIV type 1-infected subjects. AIDS Res Hum Retroviruses. 2000;16:583–594. doi: 10.1089/088922200309007. [DOI] [PubMed] [Google Scholar]
- 36.Wright PF, Kozlowski PA, Rybczyk GK, Goepfert P, Staats HF, VanCott TC, Trabattoni D, Sannella E, Mestecky J. Detection of mucosal antibodies in HIV type 1-infected individuals. AIDS Res Hum Retroviruses. 2002;18:1291–1300. doi: 10.1089/088922202320886334. [DOI] [PubMed] [Google Scholar]
- 37.Yates NL, Stacey AR, Nolen TL, Vandergrift NA, Moody MA, Montefiori DC, Weinhold KJ, Blattner WA, Borrow P, Shattock R, Cohen MS, Haynes BF, Tomaras GD. HIV-1 gp41 envelope IgA is frequently elicited after transmission but has an initial short response half-life. Mucosal Immunol. 2013;6:692–703. doi: 10.1038/mi.2012.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Collins KB, Patterson BK, Naus GJ, Landers DV, Gupta P. Development of an in vitro organ culture model to study transmission of HIV-1 in the female genital tract. Nat Med. 2000;6:475–479. doi: 10.1038/74743. [DOI] [PubMed] [Google Scholar]
- 39.Gupta P, Collins KB, Ratner D, Watkins S, Naus GJ, Landers DV, Patterson BK. Memory CD4(+) T cells are the earliest detectable human immunodeficiency virus type 1 (HIV-1)-infected cells in the female genital mucosal tissue during HIV-1 transmission in an organ culture system. J Virol. 2002;76:9868–9876. doi: 10.1128/JVI.76.19.9868-9876.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Z, Schuler T, Zupancic M, Wietgrefe S, Staskus KA, Reimann KA, Reinhart TA, Rogan M, Cavert W, Miller CJ, Veazey RS, Notermans D, Little S, Danner SA, Richman DD, Havlir D, Wong J, Jordan HL, Schacker TW, Racz P, Tenner-Racz K, Letvin NL, Wolinsky S, Haase AT. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science. 1999;286:1353–1357. doi: 10.1126/science.286.5443.1353. [DOI] [PubMed] [Google Scholar]
- 41.Veazey RS, Marx PA, Lackner AA. Vaginal CD4+ T cells express high levels of CCR5 and are rapidly depleted in simian immunodeficiency virus infection. J Infect Dis. 2003;187:769–776. doi: 10.1086/368386. [DOI] [PubMed] [Google Scholar]
- 42.Greenhead P, Hayes P, Watts PS, Laing KG, Griffin GE, Shattock RJ. Parameters of human immunodeficiency virus infection of human cervical tissue and inhibition by vaginal virucides. J Virol. 2000;74:5577–5586. doi: 10.1128/jvi.74.12.5577-5586.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Howell AL, Edkins RD, Rier SE, Yeaman GR, Stern JE, Fanger MW, Wira CR. Human immunodeficiency virus type 1 infection of cells and tissues from the upper and lower human female reproductive tract. J Virol. 1997;71:3498–3506. doi: 10.1128/jvi.71.5.3498-3506.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stoddard E, Cannon G, Ni H, Kariko K, Capodici J, Malamud D, Weissman D. gp340 expressed on human genital epithelia binds HIV-1 envelope protein and facilitates viral transmission. J Immunol. 2007;179:3126–3132. doi: 10.4049/jimmunol.179.5.3126. [DOI] [PubMed] [Google Scholar]
- 45.Stoddard E, Ni H, Cannon G, Zhou C, Kallenbach N, Malamud D, Weissman D. gp340 promotes transcytosis of human immunodeficiency virus type 1 in genital tract-derived cell lines and primary endocervical tissue. J Virol. 2009;83:8596–8603. doi: 10.1128/JVI.00744-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nazli A, Chan O, Dobson-Belaire WN, Ouellet M, Tremblay MJ, Gray-Owen SD, Arsenault AL, Kaushic C. Exposure to HIV-1 directly impairs mucosal epithelial barrier integrity allowing microbial translocation. PLoS Pathog. 2010;6:e1000852. doi: 10.1371/journal.ppat.1000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Asin SN, Fanger MW, Wildt-Perinic D, Ware PL, Wira CR, Howell AL. Transmission of HIV-1 by primary human uterine epithelial cells and stromal fibroblasts. J Infect Dis. 2004;190:236–245. doi: 10.1086/421910. [DOI] [PubMed] [Google Scholar]
- 48.Asin SN, Wildt-Perinic D, Mason SI, Howell AL, Wira CR, Fanger MW. Human immunodeficiency virus type 1 infection of human uterine epithelial cells: viral shedding and cell contact-mediated infectivity. J Infect Dis. 2003;187:1522–1533. doi: 10.1086/374782. [DOI] [PubMed] [Google Scholar]
- 49.Wu Z, Chen Z, Phillips DM. Human genital epithelial cells capture cell-free human immunodeficiency virus type 1 and transmit the virus to CD4 + cells: implications for mechanisms of sexual transmission. J Infect Dis. 2003;188:1473–1482. doi: 10.1086/379248. [DOI] [PubMed] [Google Scholar]
- 50.Micsenyi AM, Zony C, Alvarez RA, Durham ND, Chen BK, Klotman ME. Postintegration HIV-1 infection of cervical epithelial cells mediates contact-dependent productive infection of T cells. J Infect Dis. 2013;208:1756–1767. doi: 10.1093/infdis/jit362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yeaman GR, White HD, Howell A, Prabhala R, Wira CR. The mucosal immune system in the human female reproductive tract: potential insights into the heterosexual transmission of HIV. AIDS Res Hum Retroviruses. 1998;14(Suppl 1):S57–62. [PubMed] [Google Scholar]
- 52.Phillips DM, Zacharopoulos VR, Tan X, Pearce-Pratt R. Mechanisms of sexual transmission of HIV: does HIV infect intact epithelia? Trends Microbiol. 1994;2:454–458. doi: 10.1016/0966-842x(94)90804-4. [DOI] [PubMed] [Google Scholar]
- 53.Tan X, Pearce-Pratt R, Phillips DM. Productive infection of a cervical epithelial cell line with human immunodeficiency virus: implications for sexual transmission. J Virol. 1993;67:6447–6452. doi: 10.1128/jvi.67.11.6447-6452.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tan X, Phillips DM. CAT-transfected epithelial cells provide evidence for a CD4 independent pathway of HIV infection. J Reprod Immunol. 1998;41:307–319. doi: 10.1016/s0165-0378(98)00067-9. [DOI] [PubMed] [Google Scholar]
- 55.Dezzutti CS, Guenthner PC, Cummins JE, Jr, Cabrera T, Marshall JH, Dillberger A, Lal RB. Cervical and prostate primary epithelial cells are not productively infected but sequester human immunodeficiency virus type 1. J Infect Dis. 2001;183:1204–1213. doi: 10.1086/319676. [DOI] [PubMed] [Google Scholar]
- 56.Abuzakouk M, Feighery C, O’Farrelly C. Collagenase and Dispase enzymes disrupt lymphocyte surface molecules. J Immunol Methods. 1996;194:211–216. doi: 10.1016/0022-1759(96)00038-5. [DOI] [PubMed] [Google Scholar]
- 57.Wu L, KewalRamani VN. Dendritic-cell interactions with HIV: infection and viral dissemination. Nat Rev Immunol. 2006;6:859–868. doi: 10.1038/nri1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Teleshova N, Frank I, Pope M. Immunodeficiency virus exploitation of dendritic cells in the early steps of infection. J Leukoc Biol. 2003;74:683–690. doi: 10.1189/jlb.0403178. [DOI] [PubMed] [Google Scholar]
- 59.Spira AI, Marx PA, Patterson BK, Mahoney J, Koup RA, Wolinsky SM, Ho DD. Cellular targets of infection and route of viral dissemination after an intravaginal inoculation of simian immunodeficiency virus into rhesus macaques. J Exp Med. 1996;183:215–225. doi: 10.1084/jem.183.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hu J, Gardner MB, Miller CJ. Simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and infects intraepithelial dendritic cells. J Virol. 2000;74:6087–6095. doi: 10.1128/jvi.74.13.6087-6095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jameson B, Baribaud F, Pohlmann S, Ghavimi D, Mortari F, Doms RW, Iwasaki A. Expression of DC-SIGN by dendritic cells of intestinal and genital mucosae in humans and rhesus macaques. J Virol. 2002;76:1866–1875. doi: 10.1128/JVI.76.4.1866-1875.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kawamura T, Gulden FO, Sugaya M, McNamara DT, Borris DL, Lederman MM, Orenstein JM, Zimmerman PA, Blauvelt A. R5 HIV productively infects Langerhans cells, and infection levels are regulated by compound CCR5 polymorphisms. Proc Natl Acad Sci USA. 2003;100:8401–8406. doi: 10.1073/pnas.1432450100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reece JC, Handley AJ, Anstee EJ, Morrison WA, Crowe SM, Cameron PU. HIV-1 selection by epidermal dendritic cells during transmission across human skin. J Exp Med. 1998;187:1623–1631. doi: 10.1084/jem.187.10.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Blauvelt A, Glushakova S, Margolis LB. HIV-infected human Langerhans cells transmit infection to human lymphoid tissue ex vivo. AIDS (London, England) 2000;14:647–651. doi: 10.1097/00002030-200004140-00003. [DOI] [PubMed] [Google Scholar]
- 65.Gurney KB, Elliott J, Nassanian H, Song C, Soilleux E, McGowan I, Anton PA, Lee B. Binding and transfer of human immunodeficiency virus by DC-SIGN+ cells in human rectal mucosa. J Virol. 2005;79:5762–5773. doi: 10.1128/JVI.79.9.5762-5773.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brenchley JM, Schacker TW, Ruff LE, Price DA, Taylor JH, Beilman GJ, Nguyen PL, Khoruts A, Larson M, Haase AT, Douek DC. CD4(+) T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200:749–759. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guadalupe M, Reay E, Sankaran S, Prindiville T, Flamm J, McNeil A, Dandekar S. Severe CD4 + T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J Virol. 2003;77:11708–11717. doi: 10.1128/JVI.77.21.11708-11717.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li Q, Duan L, Estes JD, Ma ZM, Rourke T, Wang Y, Reilly C, Carlis J, Miller CJ, Haase AT. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature. 2005;434:1148–1152. doi: 10.1038/nature03513. [DOI] [PubMed] [Google Scholar]
- 69.Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. Massive infection and loss of memory CD4 + T cells in multiple tissues during acute SIV infection. Nature. 2005;434:1093–1097. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- 70.Mehandru S, Poles MA, Tenner-Racz K, Horowitz A, Hurley A, Hogan C, Boden D, Racz P, Markowitz M. Primary HIV-1 infection is associated with preferential depletion of CD4(+) T lymphocytes from effector sites in the gastrointestinal tract. J Exp Med. 2004;200:761–770. doi: 10.1084/jem.20041196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Veazey RS, DeMaria M, Chalifoux LV, Shvetz DE, Pauley DR, Knight HL, Rosenzweig M, Johnson RP, Desrosiers RC, Lackner AA. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 72.Veazey RS, Tham IC, Mansfield KG, DeMaria M, Forand AE, Shvetz DE, Chalifoux LV, Sehgal PK, Lackner AA. Identifying the target cell in primary simian immunodeficiency virus (SIV) infection: highly activated memory CD4(+) T cells are rapidly eliminated in early SIV infection in vivo. J Virol. 2000;74:57–64. doi: 10.1128/jvi.74.1.57-64.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kedzierska K, Crowe SM, Turville S, Cunningham AL. The influence of cytokines, chemokines and their receptors on HIV-1 replication in monocytes and macrophages. Rev Med Virol. 2003;13:39–56. doi: 10.1002/rmv.369. [DOI] [PubMed] [Google Scholar]
- 74.Gorry PR, Churchill M, Crowe SM, Cunningham AL, Gabuzda D. Pathogenesis of macrophage tropic HIV-1. Curr HIV Res. 2005;3:53–60. doi: 10.2174/1570162052772951. [DOI] [PubMed] [Google Scholar]
- 75.Carter CA, Ehrlich LS. Cell biology of HIV-1 infection of macrophages. Annu Rev Microbiol. 2008;62:425–443. doi: 10.1146/annurev.micro.62.081307.162758. [DOI] [PubMed] [Google Scholar]
- 76.Pudney J, Anderson D. Orchitis and human immunodeficiency virus type 1 infected cells in reproductive tissues from men with the acquired immune deficiency syndrome. Am J Pathol. 1991;139:149–160. [PMC free article] [PubMed] [Google Scholar]
- 77.Quayle AJ, Xu C, Mayer KH, Anderson DJ. T lymphocytes and macrophages, but not motile spermatozoa, are a significant source of human immunodeficiency virus in semen. J Infect Dis. 1997;176:960–968. doi: 10.1086/516541. [DOI] [PubMed] [Google Scholar]
- 78.Phillips DM, Tan X, Perotti ME, Zacharopoulos VR. Mechanism of monocyte-macrophage-mediated transmission of HIV. AIDS Res Hum Retroviruses. 1998;14(Suppl 1):S67–S70. [PubMed] [Google Scholar]
- 79.Crowe SM, Sonza S. HIV-1 can be recovered from a variety of cells including peripheral blood monocytes of patients receiving highly active antiretroviral therapy: a further obstacle to eradication. J Leukoc Biol. 2000;68:345–350. [PubMed] [Google Scholar]
- 80.Satomi M, Shimizu M, Shinya E, Watari E, Owaki A, Hidaka C, Ichikawa M, Takeshita T, Takahashi H. Transmission of macrophage-tropic HIV-1 by breast-milk macrophages via DC-SIGN. J Infect Dis. 2005;191:174–181. doi: 10.1086/426829. [DOI] [PubMed] [Google Scholar]
- 81.Crowe SM, Mills J, Kirihara J, Boothman J, Marshall JA, McGrath MS. Full-length recombinant CD4 and recombinant gp120 inhibit fusion between HIV infected macrophages and uninfected CD4-expressing T-lymphoblastoid cells. AIDS Res Hum Retroviruses. 1990;6:1031–1037. doi: 10.1089/aid.1990.6.1031. [DOI] [PubMed] [Google Scholar]
- 82.Groot F, Welsch S, Sattentau QJ. Efficient HIV-1 transmission from macrophages to T cells across transient virological synapses. Blood. 2008;111:4660–4663. doi: 10.1182/blood-2007-12-130070. [DOI] [PubMed] [Google Scholar]
- 83.Gousset K, Ablan SD, Coren LV, Ono A, Soheilian F, Nagashima K, Ott DE, Freed EO. Real-time visualization of HIV-1 GAG trafficking in infected macrophages. PLoS Pathog. 2008;4:e1000015. doi: 10.1371/journal.ppat.1000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Garaci E, Aquaro S, Lapenta C, Amendola A, Spada M, Covaceuszach S, Perno CF, Belardelli F. Anti-nerve growth factor Ab abrogates macrophage-mediated HIV-1 infection and depletion of CD4+ T lymphocytes in hu-SCID mice. Proc Natl Acad Sci USA. 2003;100:8927–8932. doi: 10.1073/pnas.1332627100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pollicita M, Schols D, Aquaro S, Peumans WJ, Van Damme EJ, Perno CF, Balzarini J. Carbohydrate-binding agents (CBAs) inhibit HIV-1 infection in human primary monocyte-derived macrophages (MDMs) and efficiently prevent MDM-directed viral capture and subsequent transmission to CD4+ T lymphocytes. Virology. 2008;370:382–391. doi: 10.1016/j.virol.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 86.Hirsch VM, Sharkey ME, Brown CR, Brichacek B, Goldstein S, Wakefield J, Byrum R, Elkins WR, Hahn BH, Lifson JD, Stevenson M. Vpx is required for dissemination and pathogenesis of SIVSM PBj: evidence of macrophage-dependent viral amplification. Nat Med. 1998;4:1401–1408. doi: 10.1038/3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wahl SM, Allen JB, McCartney-Francis N, Morganti-Kossmann MC, Kossmann T, Ellingsworth L, Mai UEH, Mergenhagen SE, Orenstein JM. Macrophage- and astrocyte-derived transforming growth factor b as a mediator of central nervous system dysfunction in acquired immune deficiency syndrome. J Exp Med. 1991;173:981–991. doi: 10.1084/jem.173.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Smythies LE, Maheshwari A, Clements RH, Eckhoff D, Novak L, Mosteller-Barnum M, Sellers M, Smith PD. Mucosal IL-8 and TGF-b recruit blood monocytes: evidence for cross-talk between the lamina propria stroma and myeloid cells. J Leukoc Biol. 2006;80:492–499. doi: 10.1189/jlb.1005566. [DOI] [PubMed] [Google Scholar]
- 89.Schnittman SM. The reservoir for HIV-1 in human peripheral blood is a T cell that maintains expression of CD4. Science. 1989;245:305–308. doi: 10.1126/science.2665081. [DOI] [PubMed] [Google Scholar]
- 90.Bagasra O, Wright SD, Seshamma T, Oakes JW, Pomerantz RJ. Cd14 is involved in control of human-immunodeficiency-virus type-1 expression in latently infected-cells by lipopolysaccharide. Proc Natl Acad Sci USA. 1992;89:6285–6289. doi: 10.1073/pnas.89.14.6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bukrinsky MI, Sharova N, Dempsey MP, Stanwick TL, Bukrinskaya AG, Haggerty S, Stevenson M. Active nuclear import of human immunodeficiency virus type 1 preintegration complexes. Proc Natl Acad Sci U S A. 1992;89:6580–6584. doi: 10.1073/pnas.89.14.6580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chun TW, Davey RT, Ostrowski M, Justement JS, Engel D, Mullins JI, Fauci AS. Relationship between pre-existing viral reservoirs and the re-emergence of plasma viremia after discontinuation of highly active anti-retroviral therapy. Nat Med. 2000;6:757–761. doi: 10.1038/77481. [DOI] [PubMed] [Google Scholar]
- 93.Chun TW, Carruth L, Finzi D, Shen X, DiGiuseppe JA, Taylor H, Hermankova M, Chadwick K, Margolick J, Quinn TC, Kuo YH, Brookmeyer R, Zeiger MA, Barditch-Crovo P, Siliciano RF. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature. 1997;387:183–188. doi: 10.1038/387183a0. [DOI] [PubMed] [Google Scholar]
- 94.Bukrinsky MI, Stanwick TL, Dempsey MP, Stevenson M. Quiescent T lymphocytes as an inducible virus reservoir in HIV-1 infection. Science. 1991;254:423–427. doi: 10.1126/science.1925601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Embretson J, Zupancic M, Beneke J, Till M, Wolinsky S, Ribas JL, Burke A, Haase AT. Analysis of human immunodeficiency virus-infected tissues by amplification and in situ hybridization reveals latent and permissive infections at single-cell resolution. Proc Natl Acad Sci USA. 1993;90:357–361. doi: 10.1073/pnas.90.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhu T, Muthui D, Holte S, Nickle D, Feng F, Brodie S, Hwangbo Y, Mullins JI, Corey L. Evidence for human immunodeficiency virus type 1 replication in vivo in CD14(+) monocytes and its potential role as a source of virus in patients on highly active antiretroviral therapy. J Virol. 2002;76:707–716. doi: 10.1128/JVI.76.2.707-716.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Embretson J, Zupancic M, Ribas JL, Burke A, Racz P, Tenner-Racz K, Haase AT. Massive covert infection of helper T lymphocytes and macrophages by HIV during the incubation period of AIDS. Nature. 1993;362:359–362. doi: 10.1038/362359a0. [DOI] [PubMed] [Google Scholar]
- 98.Zhu T. HIV-1 in peripheral blood monocytes: an underrated viral source. J Antimicrob Chemother. 2002;50:309–311. doi: 10.1093/jac/dkf143. [DOI] [PubMed] [Google Scholar]
- 99.Ellery PJ, Tippett E, Chiu YL, Paukovics G, Cameron PU, Solomon A, Lewin SR, Gorry PR, Jaworowski A, Greene WC, Sonza S, Crowe SM. The CD16 + monocyte subset is more permissive to infection and preferentially harbors HIV-1 in vivo. J Immunol. 2007;178:6581–6589. doi: 10.4049/jimmunol.178.10.6581. [DOI] [PubMed] [Google Scholar]
- 100.Igarashi T, Brown CR, Endo Y, Buckler-White A, Plishka R, Bischofberger N, Hirsch V, Martin MA. Macrophage are the principal reservoir and sustain high virus loads in rhesus macaques after the depletion of CD4 + T cells by a highly pathogenic simian immunodeficiency virus/HIV type 1 chimera (SHIV): implications for HIV-1 infections of humans. Proc Natl Acad Sci USA. 2001;98:658–663. doi: 10.1073/pnas.021551798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li L, Meng G, Graham MF, Shaw GM, Smith PD. Intestinal macrophages display reduced permissiveness to human immunodeficiency virus 1 and decreased surface CCR5. Gastroenterology. 1999;116:1043–1053. doi: 10.1016/s0016-5085(99)70007-7. [DOI] [PubMed] [Google Scholar]
- 102.Meng G, Sellers M, Mosteller-Barnum M, Rogers T, Shaw G, Smith P. Lamina propria lymphocytes, not macrophages, express CCR5 and CXCR4 and are the likely target cell for human immunodeficiency virus type 1 in the intestinal mucosa. J Infect Dis. 2000;182:785–791. doi: 10.1086/315790. [DOI] [PubMed] [Google Scholar]
- 103.Shen R, Meng G, Ochsenbauer C, Clapham PR, Grams J, Novak L, Kappes JC, Smythies LE, Smith PD. Stromal down-regulation of macrophage CD4/CCR5 expression and NF-kappaB activation mediates HIV-1 non-permissiveness in intestinal macrophages. PLoS Pathog. 2011;7:e1002060. doi: 10.1371/journal.ppat.1002060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Estes MK, Dandekar S, Smith PD. Chapter 25, viral infections. In: Smith PD, MacDonald TT, Blumberg RS, editors. Principles of Mucosal Immunology. Garland Science; New York: 2013. pp. 377–396. [Google Scholar]