Abstract
In vivo mapping of alterations in redox status is important for understanding organ specific pathology and disease. While electron paramagnetic resonance imaging (EPRI) enables spatial mapping of free radicals, it does not provide anatomic visualization of the body. Proton MRI is well suited to provide anatomical visualization. We applied EPR/NMR co-imaging instrumentation to map and monitor the redox state of living mice under normal or oxidative stress conditions induced by secondhand cigarette smoke (SHS) exposure. A hybrid co-imaging instrument, EPRI (1.2 GHz) / proton MRI (16.18 MHz), suitable for whole-body co-imaging of mice was utilized with common magnet and gradients along with dual EPR/NMR resonators that enable co-imaging without sample movement. The metabolism of the nitroxide probe, 3–carbamoyl–proxyl (3-CP), was used to map the redox state of control and SHS-exposed mice. Co-imaging allowed precise 3D mapping of radical distribution and reduction in major organs such as the heart, lungs, liver, bladder and kidneys. Reductive metabolism was markedly decreased in SHS-exposed mice and EPR/NMR co-imaging allowed quantitative assessment of this throughout the body. Thus, in vivo EPR/NMR co-imaging enables in vivo organ specific mapping of free radical metabolism and redox stress and the alterations that occur in the pathogenesis of disease.
Keywords: proton MRI, free radicals, nitroxide, cigarette smoking, EPR/NMR co-imaging
In tissues and cells, endogenous antioxidants such as reduced glutathione (GSH), NAD(P)H, and ascorbate maintain the redox status [1-3]. However, under pathophysiological conditions with oxidative stress, tissue redox state is altered which in turn affects cellular and organ function [4]. While smoking is thought to induce inflammation and oxidative stress, its precise effects on the redox state of organs within the body are not known. Therefore, measurement of tissue redox state is vital for understanding the functioning of organs under conditions of redox stress as occur with both chronic active tobacco smoking and passive tobacco smoking, referred to as secondhand smoking (SHS).
Stable paramagnetic aminoxyl radicals, commonly called nitroxides, have been widely utilized in biophysical studies as probes for measuring redox metabolism. The reactions of nitroxides in cells are largely controlled by enzymatic processes, although these reactions can also occur non-enzymatically. The main mechanism of cellular metabolism of nitroxides is the reduction to the hydroxylamine by the mitochondrial electron transport chain. Reverse reactions are also possible in the presence of oxidants [5]. Thus, nitroxides can serve as redox probes providing information regarding cellular redox state. Nitroxides provide a noninvasive method to measure the presence of reactive oxygen species (ROS) and related oxidative stress due to the effects of ROS on the concentration and metabolic rate of the nitroxides [6, 7]. Also, ROS react with endogenous antioxidants and alter the tissue redox state which in turn affects cellular and organ function [4, 8]. However, in addition to the reduction of the nitroxide to the hydroxylamine and the reoxidation of the hydroxylamine back to the nitroxide, the observed nitroxide concentration is also affected by its rate of clearance from the tissue or organ that has to be followed and distinguished from the reductive metabolism.
The techniques of electron paramagnetic resonance (EPR) spectroscopy and imaging (EPRI) have been widely used for non-invasive measurement and mapping of the distribution of paramagnetic materials. With nitroxide radical administration, EPR images have been reported for the head [9], brain [10], lung [11], heart [12], and abdomen in animal models [13]. The EPR method for redox measurements is particularly valuable because there are few other ways to follow the kinetics of redox reactions in vivo [14].
EPRI provides unique information enabling imaging of the processes of radical metabolism, tissue oxygenation and nitric oxide generation in normal physiology and disease [15-23]. However, this is often not sufficient in itself to enable organ specific localization of the paramagnetic probe in the body of a living animal. On the other hand, proton magnetic resonance imaging (PMRI) is capable of obtaining high resolution images of the anatomical structure of small animals [24]. Nitroxides, as paramagnetic probes, can function as contrast agents for PMRI at high concentrations and provide images that reflect their oxygen-dependent metabolism [25]. However, PMRI can not directly localize low submillimolar levels of paramagnetic molecules.
Over the last decade, studies from various laboratories have also shown that the combination of EPRI and PMRI can enable improved anatomical mapping of nitroxide radicals in living mice [26-28]. EPRI and PMRI both are based on similar magnetic resonance principles and since they provide complementary image information, a hybrid instrument combining both modalities has unique value in combining the strengths of both methods to correlate free radical distribution with the anatomical structure of the body. Several technical challenges have been overcome, enabling construction of dual mode EPRI and PMRI imaging instrumentation. Since requirements for static magnetic field and field gradient systems are quite different in each mode, we developed a system with gradients capable of pulsed and high current modes together with the ability of ramping the magnetic field from 0.04 to 0.4 T. An accurate and timely image co-registration was achieved [29]. This instrument intrinsically provides high quality free radical and anatomic image co-registration, enabling timely correlation of the information obtained from EPRI and PMRI. The uniqueness of this hybrid EPRI/PMRI instrument also lies in its specially designed dual EPR/NMR resonator assembly [30].
In the current study, we evaluated the ability of the hybrid EPR/NMR co-imaging system to perform in vivo measurements and mapping of radical metabolism and redox state in living mice and the effects of oxidative stress on this process. We utilized the redox probe 3–carbamoyl–proxyl (3-CP) for in vivo experiments to measure and map organ specific differences in probe distribution and metabolism and the effects of oxidative stress induced by smoking.
MATERIALS AND METHODS
Materials
The nitroxide 2,2,5,5–tetramethyl–1-pyrrolidinyloxy–3–carboxamide, also called 3–carbamoyl– proxyl (3-CP), (Aldrich Chemical Co. Milwaukee, WI) was used as spin probe.
EPR/NMR Co-imaging Instrumentation
The co-imaging instrument configuration has been described in our previous publication [29]. The system utilizes a common magnet and gradient coils. This configuration was coupled with the previously reported dual-purpose resonator assembly [30]. This design eliminates the need to move the sample relative to the magnetic field gradient coordinate system. This approach has been shown to provide intrinsically high quality co-registration of free radical maps along with the anatomy of small living animals.
Smoke Exposure and Preparation of Mice for in vivo EPR/NMR Co-imaging
All animal procedures were performed in accordance with the regulations of the Institutional Animal Care and Use Committee at The Ohio State University, and conformed to the Guide for the Care and Use of Laboratory Animals. C57BL/6J male mice were purchased from Jackson Laboratory and housed at 23 ± 1 0C, at 55% relative humidity, with 12:12-h light-dark cycle, and water ad libitum. After two weeks of acclimatization, mice were used to conduct experimental protocols. Co-imaging protocols were performed on normal control mice or mice exposed SHS for a period of 48 weeks. The initial series of studies in control mice were performed on male mice (n=7) of about 16 weeks of age.
Whole body SHS or air exposure was done in a 0.44 m3 Hinners-type exposure chamber, maintained at 21±1°C and 40±5% relative humidity. Sidestream smoke (SS) was generated by burning conditioned (48h in 60% humidity) Kentucky 3R4F reference cigarettes using a Teague smoking machine (TE-10; Teague Enterprises, California) with standardized 35 ml puffs of 2 sec duration, once every min, for a total of eight puffs per cigarette as described in detail [31]. After dilution and aging in a conditioning chamber (2 min), the SS smoke produced between puffs was drawn into the exposure chambers. To simulate SHS, the SS smoke was reinforced every 58 sec with a 2 sec puff of mainstream smoke (representing 11% of the overall smoke mixture) [32, 33] Mice were exposed to a single concentration of SHS with 400 mg/m3 of total suspended particulate matter for a total one hour/day (in addition to 2 fresh air breaks of 10 minute each), 6 days/week for 48 weeks. Air exposed, mice that were age-matched to the SHS-exposed mice, were used as controls (n = 4, for each group).
Prior to performing our imaging protocol, mice were anesthetized with ketamine/xylazine (55/15 mg/kg) and cannulated through the jugular vein. Afterward, the cannulated mouse was placed inside a polymer tube (OD 32 mm and ID 28 mm) opened at both ends and received a constant flow of isoflurane gas. Respiration and EKG monitoring with a SA instruments monitoring system model 1025 (Stony Brook, NY) were used to follow the metabolic stability and level of sedation. Once the mouse reached a steady physiological state with stable respiratory and heart rate, a volume of 0.3 to 0.4 ml of 300 mM nitroxide 3-CP was injected intravenously via the jugular vein with a constant rate of 0.02 ml/min. During the entire data collection period, the mice were residing inside the resonator. After the 3-CP loading, full 3D mapping of free radicals in each mouse was acquired using EPRI every 8 minutes for approximately 64 minutes. After EPRI showed a large decrease in the intensity signal (at least 5-fold) from the initial value, the system was switched to 0.38 T for MRI. A full 3D gradient echo sequence was used to acquire the proton distribution of the living mouse. EPRI was performed at 1.2 GHz and proton MRI at 16.18 MHz.
EPRI and Proton MRI Data Acquisition and Image Reconstruction
EPRI and proton MRI data were acquired on the co-imaging system that utilizes a 0.38 T Resonex electromagnet (5000/Paradigm; Resonex Corp.) equipped with a set of specially designed gradient coils (Tesla Engineering, gap 14 cm, gradient strength = 1 mT/m/A), a set of shim coils (24 active channels), and a specially constructed EPRI/PMRI dual-resonator containing a single loop multigap EPR resonator and solenoidal NMR coil (ID = 42 mm, resonance frequency = 16.18 MHz).
EPRI was performed at 1.2 GHz. The low field peak of the 3-CP triplet spectrum was used for imaging. After the 3-CP loading, a full 3D mapping of the distribution of the paramagnetic probe in each mouse was acquired using EPRI every 8 minutes for approximately 64 min. The imaging experiments were carried out using a magnetic field gradient of 7 G/cm for the axial view plane (x and z direction) and 2.5 G/cm along the long axis (y axis). A total of 256 projections were acquired. The field of view (FOV) and sweep window were 40 x 40 x 110 mm and 28 G, respectively. The measured projections were corrected for hyperfine artifacts and then deconvoluted with a corresponding zero-gradient projection. The 3D image data (128 × 128 × 128 pixels) were reconstructed using laboratory developed software. A 10 % cutoff threshold was used to remove background noise in the EPR image data before quantitative analysis. This value was determined from a compromise of maximum suppression of background noise without inducing image dropout.
After the EPRI data acquisition sequence, the system was switched to higher field for proton MRI at 16.18 MHz. 3D anatomical PMRI images for both control and SHS-exposed mice were collected. A 3D gradient echo pulse sequence (MR Solutions, Inc.) was used to acquire the 3D PMRI images of the mice in vivo (TE/TR = 13/100 ms, FOV = 40 x 40 mm, FOV in slice selection direction = 110 mm, matrix size = 128 x 128 x 128, slab thickness = 100 mm, flip angle = 45°, image orientation = axial, ECG gating = OFF, number of averages = 1, scan time = 27 min 18 sec).
Merging EPR and MR images and Data Analysis
The reconstructed volumes obtained from both modalities were superimposed. Through this method, we obtained 7-8 such fused volumes acquired in 8 min intervals for each mouse showing the free radical distribution correlated anatomically with the whole body 3D PMRI. A precise slicing of the fused volume was done and certain slices were selected and analyzed manually contouring a specific organ such as, heart, liver, bladder, kidneys etc. The mean value I (t) of the intensity signal of the free radical distribution inside the contoured organ was extracted and plotted against the time (t) for the EPRI acquisition starting from the end of the loading. The results were fitted with a simple model of an exponential decay I(t) = I0e–t/τ + N and an added constant offset. The model then was used to determine the half life (t1/2 = τ · ln(2)) of the free radical inside each of the organs. The volumes were constructed, fused, and measured using in-house software developed using Matlab (Mathworks, Natick, MA) and the ROI mean value was extracted using a program for visualization of medical images, MRIcro (University of South Carolina).
RESULTS
EPR/NMR Co-imaging Studies in Living Mice
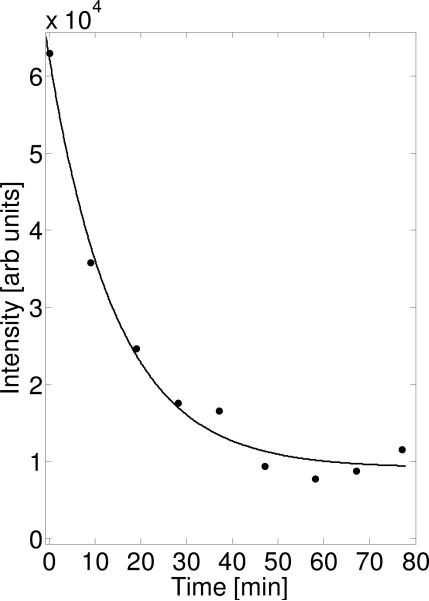
Following in vivo intravenous administration, the EPR detectable nitroxide 3-CP was rapidly reduced to the corresponding EPR silent hydroxylamine (Fig. 1). We measured and followed the kinetics of this process in the whole body of the mouse both with simple spectroscopy and 3D EPRI. The EPR spectrum of the whole body, in the absence of any applied field gradient, was recorded before every 3D data collection was started. The double integral value of the zero gradient spectrum is plotted at each point in time, as shown in figure 2. From the figure, it is clearly seen that the nitroxide signal intensity decreased and exhibited an exponential decay with half life of ~ 10 min. These data should reflect the total rate of radical reduction throughout the body. As seen in this graph, the signal does not decay to zero, as would be expected, but asymptotically approaches some fixed value. This implies an accumulation of 3-CP in some tissue or organ where it is stable. While this spectroscopy data is useful, it does not provide any insights regarding localized differences in distribution or metabolism of the radical probe in the body. Once the magnetic field gradients are applied and 3D EPRI performed followed by the proton MRI, which allows anatomic registration and organ specific contouring of the 2D image slices, differences in probe distribution and clearance can be identified.
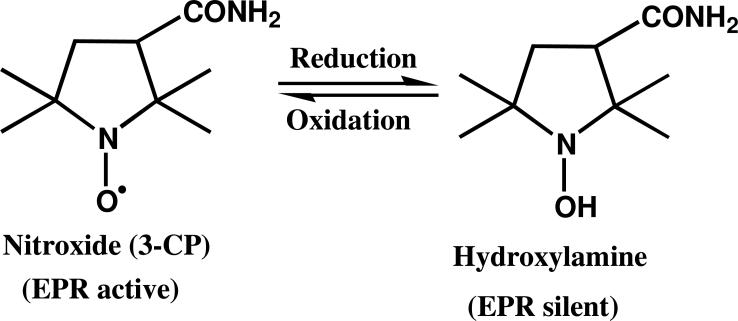
FIG. 1.
Molecular structure of the paramagnetic redox probe 2,2,5,5–tetramethyl–1-pyrrolidinyloxy–3–carboxamide, also called 3–carbamoyl–proxyl (3-CP) and its corresponding EPR silent hydroxylamine formed on metabolic reduction.
FIG. 2.
Time-dependent decay of the EPR signal intensity of the 3-CP nitroxide probe in the whole body of a control mouse. Data determined from the double integral of the zero-gradient spectrum acquired before each of the 3D EPRI acquisitions. The equation used for fitting was an exponential decay, y(t) = y0 ·e-t/ + N. The fitting parameters were: t1/2=10.2 min, y0=5.3 × 104 arbitrary (arb) units, N=9176 arb units.
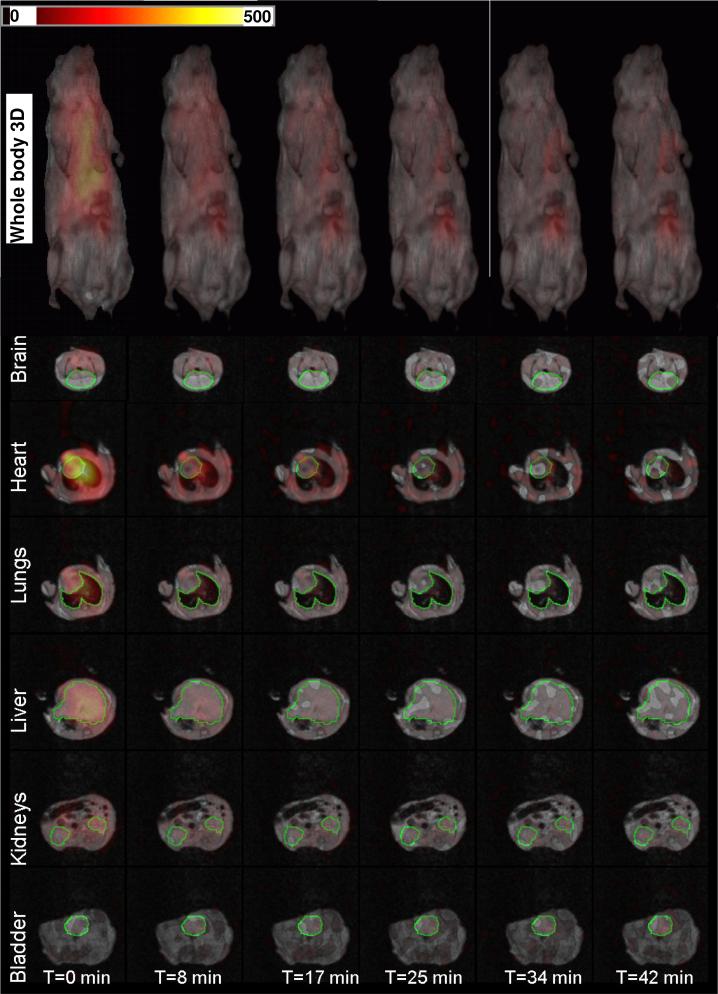
3D EPR image projection data were collected at 0.04 T and used to reconstruct the nitroxide image within the mouse. After EPRI data collection, the magnetic field was increased to 0.38 T and PMRI data collection carried out. In the top row of figure 3, we show a time course of 3D renderings of the EPR/NMR co-imaging data. Inside the full 3D proton MRI images depicted in gray scale, which provide the anatomic visualization, the full 3D distribution of the nitroxide is shown in color scale. From the color scale images, one can see the nitroxide probe decaying in time (Figure. 3, top row). This figure contains a complete set of information for all the voxels. The images show that the nitroxide probe is distributed in various organs in the mouse in differing concentrations and with temporal differences in clearance. In the initial image, it is seen that highest levels of the probe are present in the heart and lowest levels are in the brain, where very little if any probe is present. These low levels in the brain are likely due to lack of passage through the blood-brain barrier. In order to quantify the nitroxide distribution, slices were selected through different organs of the mouse. The selected slices for the brain, heart, lung, liver, kidneys, and bladder are displayed in Figure 3. The time-dependent changes in the intensity of each voxel or set of voxels can be determined and the clearance rate determined for each organ.
FIG. 3.
Renderings of the superimposed 3D EPRI and 3D proton MRI. The color map is for the EPR intensity of the 3-CP nitroxide probe distribution. Slice selection through different organs of the animal showing the EPR intensity for the 3-CP nitroxide probe signal in that specific slice. The green contour lines select regions of interest (ROI) which were used for calculations of the average EPR intensity distribution of the probe.
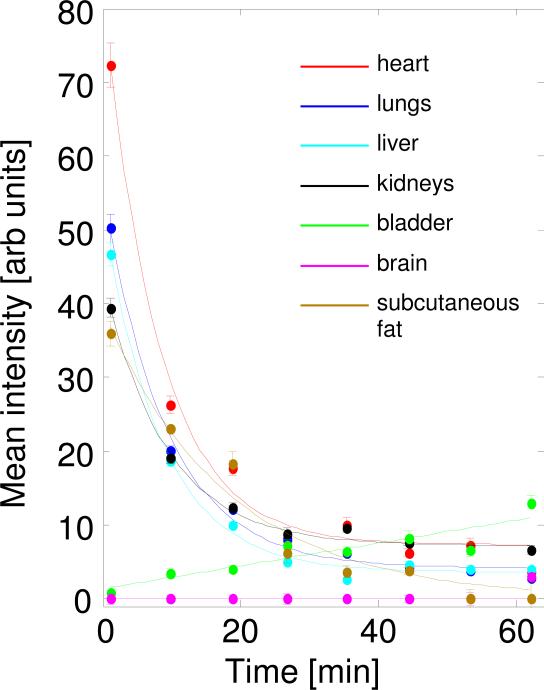
By contouring individual organs on representative slices (green lines on Figure 3), we followed the nitroxide probe distribution over time for the major organs. Quantitative information about the reduction rate in each of these organs under normal physiological conditions in young mice (16 weeks old) was collected (Figure 4; Table 1). The single-exponential fit of the signal decay was performed and the decay half-life was extracted for each organ-slice. The single-exponential fit model of signal decay showed a good fit. In table 1, the parameters extracted from the fit of the data from figure 4 are shown for various organs of healthy young mice. Over time, decay of the probe signal is seen to be most rapid in the hearts and slowest in the kidneys and lungs, while signal in the bladder increased linearly over time due to renal clearence. For the measured parameters, t1/2 demonstrated only modest differences for heart, lungs, liver, and kidneys in the range of 5.5±0.4 min, with a significant difference in the initial intensity values (65 and 32 arb units) between heart and kidneys. The bladder is unique in showing a time-dependent linear increase in nitroxide concentration and having the highest concentration of nitroxide at the end of the experiment. The data from the brain region show low accumulation of nitroxide, indicating that the 3-CP did not significantly cross the blood-brain barrier. Areas with a relatively low blood supply, i.e. subcutaneous tissues on the periphery of the mouse body, showed low initial accumulation of the probe and had longer t1/2 values for the decay, reaching up to 22 min (Table 1). These peripheral tissues with their large volume affect the decay of the EPR spectrum from the whole body, explaining the fact that total whole body nitroxide decay is slower than the decay in many organs analyzed separately.
FIG. 4.
Plot of the average signal intensity values over time of the 3-CP paramagnetic probe. Measured from given ROI values as shown and contoured in Fig. 3. Slices through several organs were contoured and the corresponding fits reflect the reduction rate in these different tissues.
Table 1.
Reduction of nitroxide in each of the selected organs shown in Fig. 4.
| Organ | y0 | t1/2 [min] |
|---|---|---|
| Heart | 65.0 | 5.1 |
| Lungs | 45.4 | 5.9 |
| Liver | 43.0 | 5.4 |
| Kidneys | 31.9 | 5.9 |
| Subcutaneous tissues | 39.0 | 22.2 |
| Organ | p1 | p2 |
| Bladder* | 0.2 | 1.7 |
| Brain* | 0 | 0 |
The equation used for fitting an exponential decay (y(t) = y0 .e-t/ + N).
linear model has been used (y(t) = p1.t + p2).
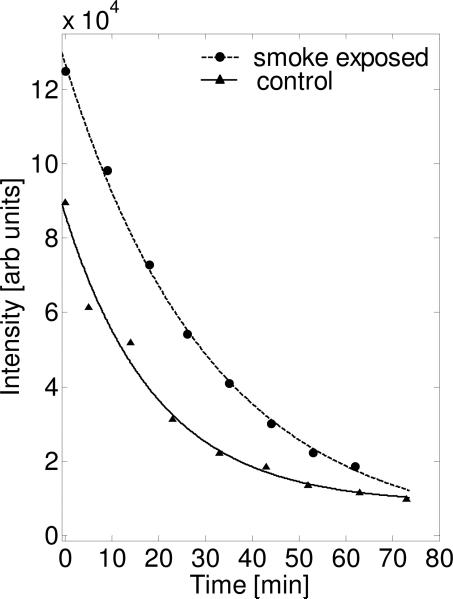
As shown in figures 5 and 6, we followed the kinetics of 3-CP reduction in the whole body of SHS-exposed and air-exposed age matched control mice with spectroscopy and 3D EPRI. The EPR spectrum of the whole body was recorded before every 3D data collection was started and the double integral value of the intensity over magnetic field is plotted at each point in time (Fig. 5). It was clearly seen that the nitroxide signal decreased much more slowly in SHS-exposed mice than in the age matched controls with exponential decay fitting demonstrating half-lives of 21.8 vs 13.6 min. This data reflects the total rate of radical reduction throughout the body.
FIG. 5.
Time-dependent decay of the signal intensity of the 3-CP nitroxide probe in the whole body of chronic SHS-exposed and air-exposed age matched control mice. Data were determined from the double integral of the zero-gradient spectrum acquired before each of the 3D EPRI acquisitions. A much slower rate of reduction of the EPR signal is seen in SHS-exposed mouse. The fitting parameters for the control mouse were: t1/2=13.6 min, y0=8.9 × 104 arb units, N=17346 arb units and for the SHS-exposed mouse were: t1/2=21.8 min, y0=13.1 × 104 arb units, N=19412 arb units.
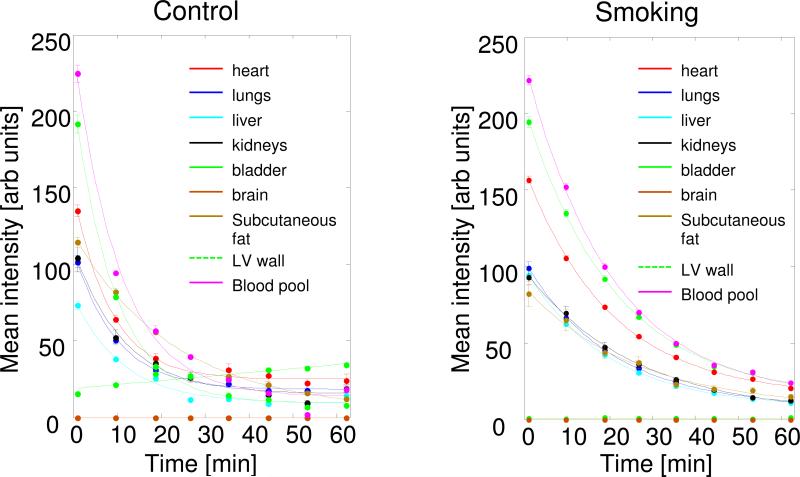
FIG. 6.
Plot of the average signal intensity values over time of the 3-CP paramagnetic probe for different organs in: left panel, air-exposed (control) mouse and right panel, SHS-exposed (smoking) mouse. Measured from given ROI values contoured in a manner as shown in Fig. 3. Slices through several organs were contoured and the corresponding fits reflect the reduction rate in these different tissues or compartments.
In Figure 6, data from air-exposed age-matched control and SHS-exposed mice are shown. Table 2 shows the parameters extracted from the fit using the models given for various slices/organs. Compared to the age-matched air exposed controls, SHS-exposed mice showed about 2-fold decrease of 3-CP reduction rate in all the organs measured with the kidneys showing slightly slower decay with t1/2 of 18.3 min than heart, lungs (14.3), liver, and blood which demonstrated t1/2 of about 14 min. Initial accumulation values show some differences between SHS-exposed organs compared to corresponding controls with higher values in the SHS-exposed mice, consistent with the slower reduction and longer half-life. For the bladder, we observed a gradual linear increase in the signal intensity of the paramagnetic probe in control mice with little increase in SHS-exposed mice, suggesting decreased renal clearance. In general, young control mice (Fig. 4; Table 1) showed shorter t1/2 in all measured areas compared to the older (48 weeks) mice, used as control of SHS exposure experiment (Fig. 6 left panel; Table 2). Overall, these applications demonstrate that the hybrid EPR/NMR co-imaging instrument is effective in measuring and mapping alterations in the radical metabolism and redox state in living mice and can detect alterations that occur with redox stress and disease.
Table 2.
Reduction of nitroxide in each of the selected organs shown in Fig. 6.
| Normal Control | Smoke exposed | |||
|---|---|---|---|---|
| Organ | y0 | t1/2 [min] | y0 | t1/2 [min] |
| Heart | 110.4 | 6.0 | 141.9 | 14.3 |
| Lungs | 83.2 | 6.8 | 90.8 | 14.3 |
| Liver | 63.9 | 7.8 | 86.6 | 13.7 |
| Kidneys | 88.1 | 8.0 | 91.0 | 18.3 |
| Blood | 209.1 | 7.4 | 209 | 13.9 |
| LV wall | 182.4 | 6.5 | 180.9 | 14.3 |
| Subcutaneous fat | 116.5 | 25.1 | 79.5 | 23.0 |
The equation used for fitting was an exponential decay (y(t) = y0 .e-t/ + N).
*linear model has been used (y(t) = p1.t + p2).
DISCUSSION
EPRI can be performed utilizing magnetic field gradients [34] in a manner similar to NMR imaging. EPRI, however, has technical difficulties that make it more challenging to achieve. The linewidths of EPR signals are 3 orders of magnitude larger than that of NMR signals and hence EPRI requires 100-1000 fold more powerful gradients. The paramagnetic centers to be studied are present at sub-millimolar levels compared to more than 100 molar water protons utilized in NMR imaging. In spite of these challenges, EPRI has become an important tool in many branches of science.
To date, most in vivo EPR spectroscopy and imaging applications have been performed using CW EPR spectroscopy. While the use of pulsed EPR for in vivo studies is being explored by a few groups, due to the very short relaxation times of most paramagnetic probes, as well as, issues of system dead-times longer than the probe relaxation times, pulsed EPR applications have mostly been limited to a few narrow-line EPR probes like trityls or isotope-substituted nitroxides with narrow line widths [35]. Therefore, CW EPR remains the most versatile and broadly applicable approach for direct in vivo measurement of a variety of important paramagnetic probes as needed for the study of the tissue redox biology in normal physiology and disease.
Since the first biological EPR images were reported [34], many applications of EPRI to studies of living systems have been performed [13, 23, 36-40]. However, broad application of EPRI to obtain high quality images of lossy biological samples is limited by several factors, including suitable resonators, sensitivity, and speed of acquisition. Over the last decade, we and others have developed instrumentation enabling high-resolution 3D spatial imaging of free radicals in biological tissues and in vivo animals [17, 41]. Implementation of these prior EPR innovations into the current co-imaging system has made it possible to perform in vivo EPRI in small animals such as mice or in isolated perfused organs [42].
Although great progress has been made in EPRI techniques, this imaging modality has the fundamental limitation that the detection is limited to the distributed paramagnetic material. Obtaining necessary anatomic information to match EPRI with organ locations within the body can be done by combining EPRI with other suitable imaging modalities. Proton MRI is well suited to provide this anatomic imaging with good resolution. In principle, paramagnetic materials including nitroxides can be mapped based on the contrast they confer in proton MRI; however, the sensitivity of this approach is very limited [25, 43]. While acquisition of EPR and NMR images in separate but nearby instruments can be performed, approaches to register these independent image sets are required. The combination of EPRI and proton MRI of phantoms and small animals using independent equipment for both modalities has been reported [27, 28]. In these studies, radical probes were used as fiducial markers visible in both modalities and as infusion agents. The accuracy of the EPR/NMR 2D image fusion technique using nitroxyl or trityl fiducial markers was tested and found to provide reliable co-registration after software alignment of the markers. However, even perfect co-registration of the markers can not provide unambiguous evidence of paramagnetic probe location in particular organs due to animal movement in relation to the markers. While special fixtures can be used to minimize animal movement during acquisition and animal transfer from instrument to instrument, this approach can only be utilized for images focused on relatively stiff regions which can be reliably immobilized in the holder like extremities or head. To eliminate the problem of sample repositioning, the approach of utilizing the same instrument with fixed magnetic field and common gradient set for both modalities has been introduced [29].
In the present work, we have used this recently developed hybrid EPR/NMR co-imaging system that is free from the above limitations. The common magnet eliminates the need for transfer of the sample, minimizing the transition time from EPRI to proton MRI measurements. Instead, the magnetic field is set for different values for L-band EPR and 16.18 MHz proton MRI; the same magnet and gradient system were used for both EPRI and proton MRI. This provides EPRI and proton MRI image data sets that are intrinsically co-registered. Since EPRI and proton MRI are performed at different fields and at different times, spatially separated EPRI and PMRI resonators were used. To exclude sample repositioning from resonator to resonator, a special moving stage was designed to slide resonators around a stationary sample. This setup minimizes image distortions due to animal movement and excludes the need for additional computational post-processing and alignment of markers to achieve co-registration of these EPR and NMR images. Thus, the major practical advantage is that the visualized object is stationary in both working modes of the system. This allows complex experiments with unstable physiological preparations on instrumented animals or perfused organ studies where moving of the samples is either very difficult or even not at all possible.
Overall, this instrument enables time efficient acquisition of EPR image data along with proton MR images that enable anatomical image registration. The feasibility of performing this type of analysis using our instrument is shown. Following systemic intravenous administration of 3-CP, we are able to map the distribution and decay rates of a given paramagnetic probe throughout the body of mice. This technique with the use of nitroxide probes was found to be a powerful tool for determining quantitative information regarding in vivo redox metabolism and the effects of oxidative stress on this process.
The current study assessed the effect of SHS exposure on organ specific tissue redox status of mice. EPR/NMR co-imaging made it possible to visualize and quantify the effect of SHS exposure on the redox metabolism in different organs. From EPR spectroscopy (Fig. 5) it is clearly seen that the reduction of nitroxide in SHS-exposed mice is attenuated compared with control and age-matched animals. Thus, overall redox tissue environment became less reductive with chronic SHS exposure. Three dimensional CW EPRI-based images were used to spatially resolve the rate of nitroxide reduction by monitoring the time-dependent change of nitroxide concentration. The 2D slices from the 3D resultant concentration map were superimposed with corresponding slices from 3D MR images and allowed organ specific assessment of concentration change and its rates (Fig. 6). Combined images demonstrated that initial organ uptake of the probe was different for different organs. Highest levels were in the heart and in the blood pool. No significant nitroxide concentration was observed in the brain, indicating that 3-CP did not penetrate the blood-brain barrier. Rates of decay also varied and were much slower in subcutaneous tissues than in major organs. In both young control mice and the older SHS-exposed and air-exposed mice, the 3-CP probe was retained longer by peripheral tissues, and this may be due to its much slower reduction in subcutaneous tissues and fat. The nitroxide concentration maps show that most of the nitroxide is reduced to the diamagnetic form; however, a smaller portion is excreted through the urinary tract and accumulates in the bladder.
SHS exposure attenuated the rate of nitroxide reduction in all organs. The shift in reduction rate was slightly different in organs with the biggest decrease observed in heart and kidneys. Thus, EPR/NMR co-imaging noninvasively demonstrates that SHS affected the redox metabolism of all organs. Overall it is shown that EPR/NMR co-imaging can visualize and map differences in radical metabolism and redox state induced by oxidant stress.
SUMMARY AND CONCLUSIONS
We utilized a hybrid EPR/NMR co-imaging instrument that uses EPRI to detect the distribution of paramagnetic materials in combination with the power of proton MRI to provide high resolution three dimensional mapping of the anatomical structures of the body. This approach has the advantage that the sample is not moved between two different systems. It utilizes the same set of field gradients for both EPRI and PMRI that are controlled either in continuous mode for EPRI or pulsed mode for proton MRI, in the same magnet system that can be set to the field required for either mode. The resonator assembly holds the sample in place and allows either resonator to slide over the sample, eliminating the need for spatial markers and image postprocessing to achieve co-registration. This instrumentation enables in vivo 3D EPR/NMR co-imaging in small living animals in a time suitable for physiological investigations.
Co-imaging allowed 3D mapping of the spatial distribution and metabolism of the nitroxide probe 3-CP in living mice. Reductive metabolism was markedly decreased in SHS-exposed mice and EPR/NMR co-imaging enabled quantitation of this throughout the body. Thus, in vivo EPR/NMR co-imaging enabled in vivo organ specific mapping of free radical metabolism and redox stress and the alterations that occur in the pathogenesis of disease. Future advances including incorporation of fast scan EPR acquisition and dual tuned EPR/NMR resonators [44] will enable more rapid image acquisition and even interleaved dual-mode acquisition further, enhancing the power of this approach of combined magnetic resonance imaging for the detection of radical metabolism and its role in physiological function and disease.
EPR/NMR co-imaging detected the distribution of a nitroxide probe in living mice.
3D mapping of the in vivo distribution and metabolism of the probe was performed.
Organ specific differences of in probe uptake, metabolism and clearance were imaged.
Reductive metabolism was markedly decreased in smoking-exposed mice.
EPR/NMR co-imaging enabled imaging and mapping of this smoking-induced redox stress.
ACKNOWLEDGMENTS
We thank Dr. David Johnson for his useful input to various problems we encountered during data analysis and processing. This work was supported by a gift from Lorillard Tobacco Company and NIH Research Grants EB00890, HL63744, HL65608, and HL38324.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Hansen RE, Roth D, Winther JR. Quantifying the global cellular thiol-disulfide status. Proc. Natl. Acad. Sci. U. S. A. 2009;106:422–427. doi: 10.1073/pnas.0812149106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ceconi C, Bernocchi P, Boraso A, Cargnoni A, Pepi P, Curello S, Ferrari R. New insights on myocardial pyridine nucleotides and thiol redox state in ischemia and reperfusion damage. Cardiovasc. Res. 2000;47:586–594. doi: 10.1016/s0008-6363(00)00104-8. [DOI] [PubMed] [Google Scholar]
- 3.Chen Q, Espey MG, Sun AY, Lee JH, Krishna MC, Shacter E, Choyke PL, Pooput C, Kirk KL, Buettner GR, Levine M. Ascorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluid in vivo. Proc. Natl. Acad. Sci. U. S. A. 2007;104:8749–8754. doi: 10.1073/pnas.0702854104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zweier JL, Talukder MA. The role of oxidants and free radicals in reperfusion injury. Cardiovasc. Res. 2006;70:181–190. doi: 10.1016/j.cardiores.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 5.Gallez B, Bacic G, Goda F, Jiang J, O'Hara JA, Dunn JF, Swartz HM. Use of nitroxides for assessing perfusion, oxygenation, and viability of tissues: in vivo EPR and MRI studies. Magn. Reson. Med. 1996;35:97–106. doi: 10.1002/mrm.1910350113. [DOI] [PubMed] [Google Scholar]
- 6.Miura Y, Hamada A, Utsumi H. In vivo ESR studies of antioxidant activity on free radical reaction in living mice under oxidative stress. Free Radic. Res. 1995;22:209–214. doi: 10.3109/10715769509147540. [DOI] [PubMed] [Google Scholar]
- 7.Valgimigli L, Pedulli GF, Paolini M. Measurement of oxidative stress by EPR radical-probe technique. Free Radic. Biol. Med. 2001;31:708–716. doi: 10.1016/s0891-5849(01)00490-7. [DOI] [PubMed] [Google Scholar]
- 8.Franco R, Schoneveld OJ, Pappa A, Panayiotidis MI. The central role of glutathione in the pathophysiology of human diseases. Arch. Physiol. Biochem. 2007;113:234–258. doi: 10.1080/13813450701661198. [DOI] [PubMed] [Google Scholar]
- 9.Ishida S, Matsumoto S, Yokoyama H, Mori N, Kumashiro H, Tsuchihashi N, Ogata T, Yamada M, Ono M, Kitajima T, et al. An ESR-CT imaging of the head of a living rat receiving an administration of a nitroxide radical. Magn. Reson. Imaging. 1992;10:109–114. doi: 10.1016/0730-725x(92)90379-e. [DOI] [PubMed] [Google Scholar]
- 10.Hiramatsu M, Oikawa K, Noda H, Mori A, Ogata T, Kamada H. Free radical imaging by electron spin resonance computed tomography in rat brain. Brain Res. 1995;697:44–47. doi: 10.1016/0006-8993(95)00759-j. [DOI] [PubMed] [Google Scholar]
- 11.Takeshita K, Utsumi H, Hamada A. ESR measurement of radical clearance in lung of whole mouse. Biochem. Biophys. Res. Commun. 1991;177:874–880. doi: 10.1016/0006-291x(91)91871-9. [DOI] [PubMed] [Google Scholar]
- 12.Kuppusamy P, Wang P, Zweier JL. Three-Dimensional Spatial EPR Imaging of the Rat Heart. Magn. Reson. Med. 1995;34:99–105. doi: 10.1002/mrm.1910340115. [DOI] [PubMed] [Google Scholar]
- 13.Quaresima V, Alecci M, Ferrari M, Sotgiu A. Whole rat electron paramagnetic resonance imaging of a nitroxide free radical by a radio frequency (280 MHz) spectrometer. Biochem. Biophys. Res. Commun. 1992;183:829–835. doi: 10.1016/0006-291x(92)90558-3. [DOI] [PubMed] [Google Scholar]
- 14.Swartz HM, Khan N, Khramtsov VV. Use of electron paramagnetic resonance spectroscopy to evaluate the redox state in vivo. Antioxid. Redox Signal. 2007;9:1757–1771. doi: 10.1089/ars.2007.1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halpern HJ, Peric M, Yu C, Barth ED, Chandramouli GV, Makinen MW, Rosen GM. In vivo spin-label murine pharmacodynamics using low-frequency electron paramagnetic resonance imaging. Biophys. J. 1996;71:403–409. doi: 10.1016/S0006-3495(96)79241-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halpern HJ, Yu C, Peric M, Barth ED, Karczmar GS, River JN, Grdina DJ, Teicher BA. Measurement of differences in pO2 in response to perfluorocarbon/carbogen in FSa and NFSa murine fibrosarcomas with low-frequency electron paramagnetic resonance oximetry. Radiat. Res. 1996;145:610–618. [PubMed] [Google Scholar]
- 17.Kuppusamy P, Chzhan M, Samouilov A, Wang P, Zweier JL. Mapping the spin-density and lineshape distribution of free radicals using 4D spectral-spatial EPR imaging. J. Magn. Reson. B. 1995;107:116–125. doi: 10.1006/jmrb.1995.1067. [DOI] [PubMed] [Google Scholar]
- 18.Kuppusamy P, Chzhan M, Wang P, Zweier JL. Three-dimensional gated EPR imaging of the beating heart: time-resolved measurements of free radical distribution during the cardiac contractile cycle. Magn. Reson. Med. 1996;35:323–328. doi: 10.1002/mrm.1910350309. [DOI] [PubMed] [Google Scholar]
- 19.Kuppusamy P, Shankar RA, Zweier JL. In vivo measurement of arterial and venous oxygenation in the rat using 3D spectral-spatial electron paramagnetic resonance imaging. Phys. Med. Biol. 1998;43:1837–1844. doi: 10.1088/0031-9155/43/7/003. [DOI] [PubMed] [Google Scholar]
- 20.Lurie DJ. Commentary: electron spin resonance imaging studies of biological systems. Br. J. Radiol. 1996;69:983–984. doi: 10.1259/0007-1285-69-827-983. [DOI] [PubMed] [Google Scholar]
- 21.Matsumoto K, Hyodo F, Matsumoto A, Koretsky AP, Sowers AL, Mitchell JB, Krishna MC. High-resolution mapping of tumor redox status by magnetic resonance imaging using nitroxides as redox-sensitive contrast agents. Clin. Cancer Res. 2006;12:2455–2462. doi: 10.1158/1078-0432.CCR-05-2747. [DOI] [PubMed] [Google Scholar]
- 22.Matsumoto K, Subramanian S, Devasahayam N, Aravalluvan T, Murugesan R, Cook JA, Mitchell JB, Krishna MC. Electron paramagnetic resonance imaging of tumor hypoxia: enhanced spatial and temporal resolution for in vivo pO2 determination. Magn. Reson. Med. 2006;55:1157–1163. doi: 10.1002/mrm.20872. [DOI] [PubMed] [Google Scholar]
- 23.Zweier JL, Kuppusamy P. Electron paramagnetic resonance measurements of free radicals in the intact beating heart: a technique for detection and characterization of free radicals in whole biological tissues. Proc. Natl. Acad. Sci. U. S. A. 1988;85:5703–5707. doi: 10.1073/pnas.85.15.5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andrew ER. N.m.r. imaging of intact biological systems. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1980;289:471–481. doi: 10.1098/rstb.1980.0065. [DOI] [PubMed] [Google Scholar]
- 25.Swartz HM, Chen K, Pals M, Sentjurc M, Morse PD., 2nd Hypoxia-sensitive NMR contrast agents. Magn. Reson. Med. 1986;3:169–174. doi: 10.1002/mrm.1910030126. [DOI] [PubMed] [Google Scholar]
- 26.Di Giuseppe S, Placidi G, Sotgiu A. New experimental apparatus for multimodal resonance imaging: initial EPRI and NMRI experimental results. Phys. Med. Biol. 2001;46:1003–1016. doi: 10.1088/0031-9155/46/4/307. [DOI] [PubMed] [Google Scholar]
- 27.He G, Deng Y, Li H, Kuppusamy P, Zweier JL. EPR/NMR co-imaging for anatomic registration of free-radical images. Magn. Reson. Med. 2002;47:571–578. doi: 10.1002/mrm.10077. [DOI] [PubMed] [Google Scholar]
- 28.Hyodo F, Yasukawa K, Yamada K, Utsumi H. Spatially resolved time-course studies of free radical reactions with an EPRI/MRI fusion technique. Magn. Reson. Med. 2006;56:938–943. doi: 10.1002/mrm.21019. [DOI] [PubMed] [Google Scholar]
- 29.Caia GL, Samouilov A, Kesselring E, Petryakov S, Wasowicz T, Zweier JL. Development of a hybrid EPR/NMR coimaging system. Magn. Reson. Med. 2007;58:156–166. doi: 10.1002/mrm.21205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petryakov S, Samouilov A, Kesselring E, Wasowicz T, Caia GL, Zweier JL. Single loop multi-gap resonator for whole body EPR imaging of mice at 1.2 GHz. J. Magn. Reson. 2007;188:68–73. doi: 10.1016/j.jmr.2007.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Teague SV, Pinkerton KE, Goldsmith M, Gebremichael A, Chang S, Jenkins RA, Moneyhun JH. Sidestream cigarette smoke generation and expsure system for environmental tobacco smoke studies. Inhalation Toxicology. 1994;6:79–93. [Google Scholar]
- 32.Gairola CG. Animal models of secondhand smoking. In: Wang XL, Scott DA, editors. Molecular mechanisms of tobacco-induced diseaeses. Nova Science; New York: 2006. pp. 121–132. [Google Scholar]
- 33.Woodruff PG, Ellwanger A, Solon M, Cambier CJ, Pinkerton KE, Koth LL. Alveolar macrophage recruitment and activation by chronic second hand smoke exposure in mice. COPD. 2009;6:86–94. doi: 10.1080/15412550902751738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berliner LJ, Fujii H. Magnetic resonance imaging of biological specimens by electron paramagnetic resonance of nitroxide spin labels. Science. 1985;227:517–519. doi: 10.1126/science.2981437. [DOI] [PubMed] [Google Scholar]
- 35.Hyodo F, Matsumoto S, Devasahayam N, Dharmaraj C, Subramanian S, Mitchell JB, Krishna MC. Pulsed EPR imaging of nitroxides in mice. J. Magn. Reson. 2009;197:181–185. doi: 10.1016/j.jmr.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berliner LJ, Fujii H, Wan XM, Lukiewicz SJ. Feasibility study of imaging a living murine tumor by electron paramagnetic resonance. Magn. Reson. Med. 1987;4:380–384. doi: 10.1002/mrm.1910040410. [DOI] [PubMed] [Google Scholar]
- 37.Halpern HJ, Spencer DP, Polen JV, Bowman MK, Nelson AC, Dowey EM, Teicher BA. Imaging radio frequency electron spin resonance spectometer with high resolution and sensitivity for in vivo mesurements. Review of Scientific Instruments. 1989;60:1040–1050. [Google Scholar]
- 38.Woods RK, Dobrucki JW, Glockner JF, Morse PD, 2nd, Swartz HM. Spectal-spatial ESR imaging as a method of non-invasive biological oximetry. J. Mag. Reson. 1989;85:50–59. [Google Scholar]
- 39.Alecci M, Colacicchi S, Indovina PL, Momo F, Pavone P, Sotgiu A. Three-dimensional in vivo ESR imaging in rats. Magn. Reson. Imaging. 1990;8:59–63. doi: 10.1016/0730-725x(90)90213-l. [DOI] [PubMed] [Google Scholar]
- 40.Dobrucki JW, Sutherland RM, Swartz HM. Nonperturbing test for cytotoxicity in isolated cells and spheroids, using electron paramagnetic resonance. Magn. Reson. Med. 1991;19:42–55. doi: 10.1002/mrm.1910190105. [DOI] [PubMed] [Google Scholar]
- 41.Kuppusamy P, Chzhan M, Zweier JL. Development and optimization of three-dimensional spatial EPR imaging for biological organs and tissues. J. Magn. Reson. B. 1995;106:122–130. doi: 10.1006/jmrb.1995.1022. [DOI] [PubMed] [Google Scholar]
- 42.Caia G, Sun Z, Yang FC, Petryakov S, Velayutham M, Samouilov A, Zweier JL. EPR/NMR co-imaging of in vivi redox status in mice. Free Radic. Biol. Med. 2009;47:S55. [Google Scholar]
- 43.Hyodo F, Murugesan R, Matsumoto K, Hyodo E, Subramanian S, Mitchell JB, Krishna MC. Monitoring redox-sensitive paramagnetic contrast agent by EPRI, OMRI and MRI. J. Magn. Reson. 2008;190:105–112. doi: 10.1016/j.jmr.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Petryakov S, Samouilov A, Kesselring E, Caia GL, Sun Z, Zweier JL. Dual frequency resonator for 1.2 GHz EPR/16.2 MHz NMR co-imaging. J. Magn. Reson. 2010;205:1–8. doi: 10.1016/j.jmr.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]