Abstract
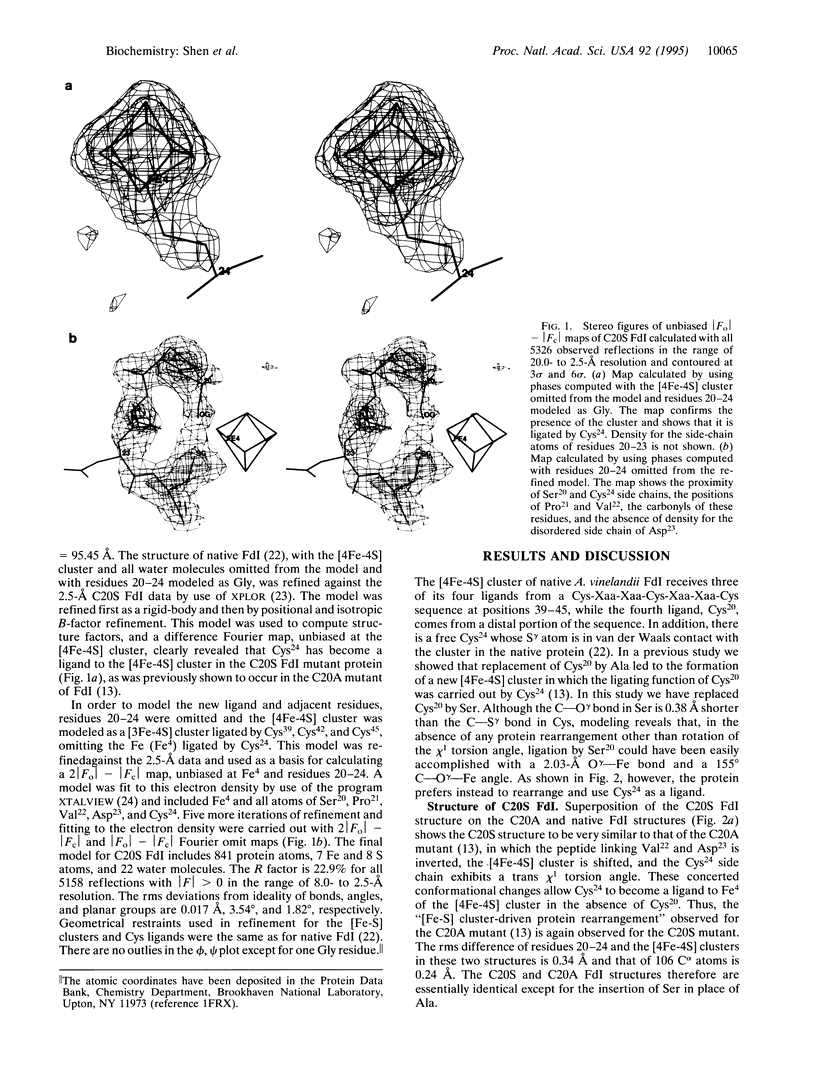
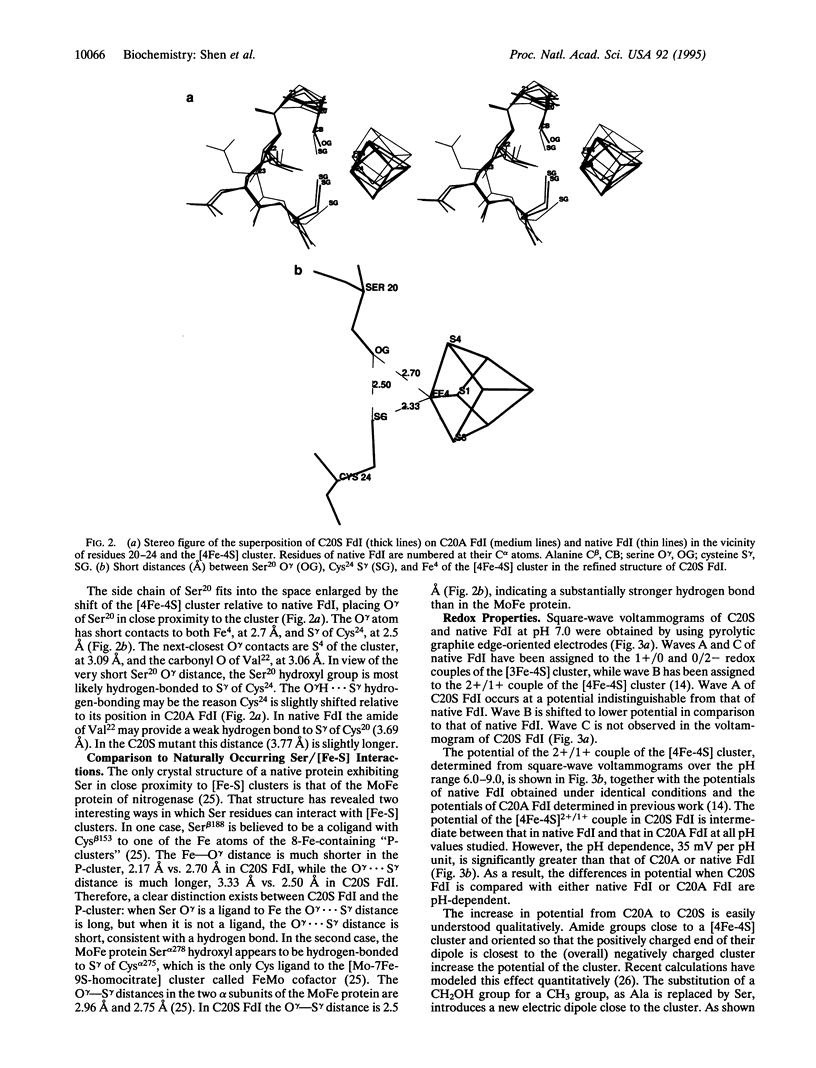
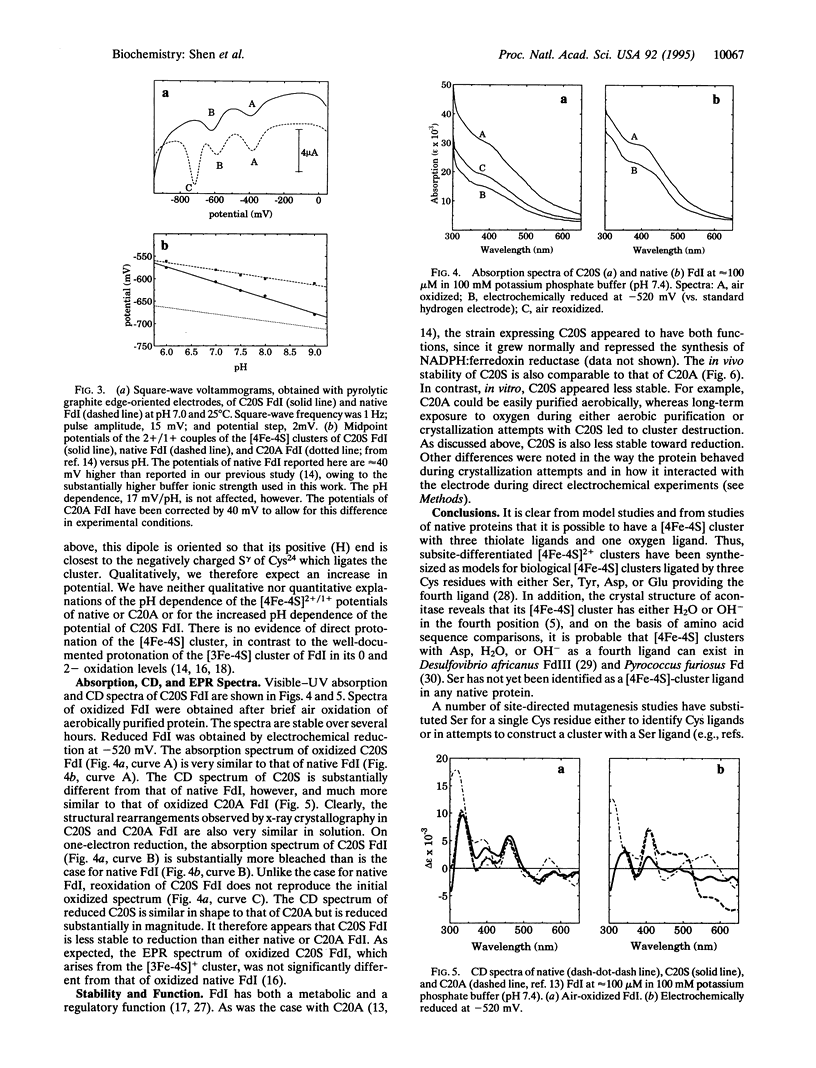
The [4Fe-4S] cluster of Azotobacter vinelandii ferredoxin I receives three of its four ligands from a Cys-Xaa-Xaa-Cys-Xaa-Xaa-Cys sequence at positions 39-45 while the fourth ligand, Cys20, is provided by a distal portion of the sequence. Previously we reported that the site-directed mutation of Cys20 to Ala (C20A protein) resulted in the formation of a new [4Fe-4S] cluster that obtained its fourth ligand from Cys24, a free cysteine in the native structure. That ligand exchange required significant protein rearrangement. Here we report the conversion of Cys20 to Ser (C20S protein), which gives the protein the opportunity either to retain the native structure and use the Ser20 O gamma as a ligand or to rearrange and use Cys24. X-ray crystallography demonstrates that the cluster does not use the Ser20 O gamma as a ligand; rather it rearranges to use Cys24. In the C20S protein the [4Fe-4S] cluster has altered stability and redox properties relative to either C20A or the native protein.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong F. A., George S. J., Cammack R., Hatchikian E. C., Thomson A. J. Electrochemical and spectroscopic characterization of the 7Fe form of ferredoxin III from Desulfovibrio africanus. Biochem J. 1989 Nov 15;264(1):265–273. doi: 10.1042/bj2640265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augier V., Asso M., Guigliarelli B., More C., Bertrand P., Santini C. L., Blasco F., Chippaux M., Giordano G. Removal of the high-potential [4Fe-4S] center of the beta-subunit from Escherichia coli nitrate reductase. Physiological, biochemical, and EPR characterization of site-directed mutated enzymes. Biochemistry. 1993 May 18;32(19):5099–5108. doi: 10.1021/bi00070a018. [DOI] [PubMed] [Google Scholar]
- Augier V., Guigliarelli B., Asso M., Bertrand P., Frixon C., Giordano G., Chippaux M., Blasco F. Site-directed mutagenesis of conserved cysteine residues within the beta subunit of Escherichia coli nitrate reductase. Physiological, biochemical, and EPR characterization of the mutated enzymes. Biochemistry. 1993 Mar 2;32(8):2013–2023. doi: 10.1021/bi00059a018. [DOI] [PubMed] [Google Scholar]
- Beinert H. Recent developments in the field of iron-sulfur proteins. FASEB J. 1990 May;4(8):2483–2491. doi: 10.1096/fasebj.4.8.2185975. [DOI] [PubMed] [Google Scholar]
- Cheng H., Xia B., Reed G. H., Markley J. L. Optical, EPR, and 1H NMR spectroscopy of serine-ligated [2Fe-2S] ferredoxins produced by site-directed mutagenesis of cysteine residues in recombinant Anabaena 7120 vegetative ferredoxin. Biochemistry. 1994 Mar 22;33(11):3155–3164. doi: 10.1021/bi00177a003. [DOI] [PubMed] [Google Scholar]
- Conover R. C., Kowal A. T., Fu W. G., Park J. B., Aono S., Adams M. W., Johnson M. K. Spectroscopic characterization of the novel iron-sulfur cluster in Pyrococcus furiosus ferredoxin. J Biol Chem. 1990 May 25;265(15):8533–8541. [PubMed] [Google Scholar]
- Cunningham R. P., Asahara H., Bank J. F., Scholes C. P., Salerno J. C., Surerus K., Münck E., McCracken J., Peisach J., Emptage M. H. Endonuclease III is an iron-sulfur protein. Biochemistry. 1989 May 16;28(10):4450–4455. doi: 10.1021/bi00436a049. [DOI] [PubMed] [Google Scholar]
- Fujinaga J., Gaillard J., Meyer J. Mutated forms of a [2Fe-2S] ferredoxin with serine ligands to the iron-sulfur cluster. Biochem Biophys Res Commun. 1993 Jul 15;194(1):104–111. doi: 10.1006/bbrc.1993.1791. [DOI] [PubMed] [Google Scholar]
- George S. J., Armstrong F. A., Hatchikian E. C., Thomson A. J. Electrochemical and spectroscopic characterization of the conversion of the 7Fe into the 8Fe form of ferredoxin III from Desulfovibrio africanus. Identification of a [4Fe-4S] cluster with one non-cysteine ligand. Biochem J. 1989 Nov 15;264(1):275–284. doi: 10.1042/bj2640275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowski R., Hofmeister A. E., Buckel W. Bacterial L-serine dehydratases: a new family of enzymes containing iron-sulfur clusters. Trends Biochem Sci. 1993 Aug;18(8):297–300. doi: 10.1016/0968-0004(93)90040-t. [DOI] [PubMed] [Google Scholar]
- Haile D. J., Rouault T. A., Harford J. B., Kennedy M. C., Blondin G. A., Beinert H., Klausner R. D. Cellular regulation of the iron-responsive element binding protein: disassembly of the cubane iron-sulfur cluster results in high-affinity RNA binding. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11735–11739. doi: 10.1073/pnas.89.24.11735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo E., Demple B. An iron-sulfur center essential for transcriptional activation by the redox-sensing SoxR protein. EMBO J. 1994 Jan 1;13(1):138–146. doi: 10.1002/j.1460-2075.1994.tb06243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J. B., Rees D. C. Perspectives on non-heme iron protein chemistry. Adv Protein Chem. 1991;42:199–280. doi: 10.1016/s0065-3233(08)60537-9. [DOI] [PubMed] [Google Scholar]
- Iismaa S. E., Vázquez A. E., Jensen G. M., Stephens P. J., Butt J. N., Armstrong F. A., Burgess B. K. Site-directed mutagenesis of Azotobacter vinelandii ferredoxin I. Changes in [4Fe-4S] cluster reduction potential and reactivity. J Biol Chem. 1991 Nov 15;266(32):21563–21571. [PubMed] [Google Scholar]
- Jensen G. M., Warshel A., Stephens P. J. Calculation of the redox potentials of iron-sulfur proteins: the 2-/3-couple of [Fe4S*4Cys4] clusters in Peptococcus aerogenes ferredoxin, Azotobacter vinelandii ferredoxin I, and Chromatium vinosum high-potential iron protein. Biochemistry. 1994 Sep 13;33(36):10911–10924. doi: 10.1021/bi00202a010. [DOI] [PubMed] [Google Scholar]
- Karsten W. E., Viola R. E. Identification of an essential cysteine in the reaction catalyzed by aspartate-beta-semialdehyde dehydrogenase from Escherichia coli. Biochim Biophys Acta. 1992 May 22;1121(1-2):234–238. doi: 10.1016/0167-4838(92)90360-p. [DOI] [PubMed] [Google Scholar]
- Kennedy M. C., Mende-Mueller L., Blondin G. A., Beinert H. Purification and characterization of cytosolic aconitase from beef liver and its relationship to the iron-responsive element binding protein. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11730–11734. doi: 10.1073/pnas.89.24.11730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Rees D. C. Structural models for the metal centers in the nitrogenase molybdenum-iron protein. Science. 1992 Sep 18;257(5077):1677–1682. doi: 10.1126/science.1529354. [DOI] [PubMed] [Google Scholar]
- Kuo C. F., McRee D. E., Fisher C. L., O'Handley S. F., Cunningham R. P., Tainer J. A. Atomic structure of the DNA repair [4Fe-4S] enzyme endonuclease III. Science. 1992 Oct 16;258(5081):434–440. doi: 10.1126/science.1411536. [DOI] [PubMed] [Google Scholar]
- Makaroff C. A., Paluh J. L., Zalkin H. Mutagenesis of ligands to the [4 Fe-4S] center of Bacillus subtilis glutamine phosphoribosylpyrophosphate amidotransferase. J Biol Chem. 1986 Aug 25;261(24):11416–11423. [PubMed] [Google Scholar]
- Martin A. E., Burgess B. K., Iismaa S. E., Smartt C. T., Jacobson M. R., Dean D. R. Construction and characterization of an Azotobacter vinelandii strain with mutations in the genes encoding flavodoxin and ferredoxin I. J Bacteriol. 1989 Jun;171(6):3162–3167. doi: 10.1128/jb.171.6.3162-3167.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín A. E., Burgess B. K., Stout C. D., Cash V. L., Dean D. R., Jensen G. M., Stephens P. J. Site-directed mutagenesis of Azotobacter vinelandii ferredoxin I: [Fe-S] cluster-driven protein rearrangement. Proc Natl Acad Sci U S A. 1990 Jan;87(2):598–602. doi: 10.1073/pnas.87.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May H. D., Dean D. R., Newton W. E. Altered nitrogenase MoFe proteins from Azotobacter vinelandii. Analysis of MoFe proteins having amino acid substitutions for the conserved cysteine residues within the beta-subunit. Biochem J. 1991 Jul 15;277(Pt 2):457–464. doi: 10.1042/bj2770457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen B., Jollie D. R., Stout C. D., Diller T. C., Armstrong F. A., Gorst C. M., La Mar G. N., Stephens P. J., Burgess B. K. Azotobacter vinelandii ferredoxin I. Alteration of individual surface charges and the [4FE-4S]2+/+ cluster reduction potential. J Biol Chem. 1994 Mar 18;269(11):8564–8575. [PubMed] [Google Scholar]
- Shen B., Martin L. L., Butt J. N., Armstrong F. A., Stout C. D., Jensen G. M., Stephens P. J., La Mar G. N., Gorst C. M., Burgess B. K. Azotobacter vinelandii ferredoxin I. Aspartate 15 facilitates proton transfer to the reduced [3Fe-4S] cluster. J Biol Chem. 1993 Dec 5;268(34):25928–25939. [PubMed] [Google Scholar]
- Smith J. L., Zaluzec E. J., Wery J. P., Niu L., Switzer R. L., Zalkin H., Satow Y. Structure of the allosteric regulatory enzyme of purine biosynthesis. Science. 1994 Jun 3;264(5164):1427–1433. doi: 10.1126/science.8197456. [DOI] [PubMed] [Google Scholar]
- Stephens P. J., Jensen G. M., Devlin F. J., Morgan T. V., Stout C. D., Martin A. E., Burgess B. K. Circular dichroism and magnetic circular dichroism of Azotobacter vinelandii ferredoxin I. Biochemistry. 1991 Apr 2;30(13):3200–3209. doi: 10.1021/bi00227a007. [DOI] [PubMed] [Google Scholar]
- Stout C. D. Refinement of the 7 Fe ferredoxin from Azotobacter vinelandii at 1.9 A resolution. J Mol Biol. 1989 Feb 5;205(3):545–555. doi: 10.1016/0022-2836(89)90225-8. [DOI] [PubMed] [Google Scholar]
- Thomson A. J. Does ferredoxin I (Azotobacter) represent a novel class of DNA-binding proteins that regulate gene expression in response to cellular iron(II)? FEBS Lett. 1991 Jul 22;285(2):230–236. doi: 10.1016/0014-5793(91)80807-f. [DOI] [PubMed] [Google Scholar]
- Vázquez A., Shen B., Negaard K., Iismaa S., Burgess B. Overexpression of ferredoxin I in Azotobacter vinelandii. Protein Expr Purif. 1994 Feb;5(1):96–102. doi: 10.1006/prep.1994.1014. [DOI] [PubMed] [Google Scholar]
- Werth M. T., Cecchini G., Manodori A., Ackrell B. A., Schröder I., Gunsalus R. P., Johnson M. K. Site-directed mutagenesis of conserved cysteine residues in Escherichia coli fumarate reductase: modification of the spectroscopic and electrochemical properties of the [2Fe-2S] cluster. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8965–8969. doi: 10.1073/pnas.87.22.8965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werth M. T., Sices H., Cecchini G., Schröder I., Lasage S., Gunsalus R. P., Johnson M. K. Evidence for non-cysteinyl coordination of the [2Fe-2S] cluster in Escherichia coli succinate dehydrogenase. FEBS Lett. 1992 Mar 24;299(1):1–4. doi: 10.1016/0014-5793(92)80086-v. [DOI] [PubMed] [Google Scholar]




