Nitric oxide signaling
Nitric oxide (NO) is a highly diffusible, free radical signaling molecule that is produced by the endothelial NO synthase enzyme, which converts L-arginine and molecular oxygen into L-citrulline and NO [1, 2]. Nitric oxide diffuses from the endothelium to the smooth muscle where it binds with high affinity to the heme group of soluble guanylate cyclase, which in turn catalyzes the conversion of guanosine triphosphate (GTP) to cyclic guanosine monophosphate (cGMP) [3]. Nitric oxide signaling is largely paracrine, with potential endocrine effects limited by its radical nature and extremely high reactivity with other heme containing proteins such as hemoglobin and myoglobin [4]. When NO encounters oxyhemoglobin in blood or oxymyoglobin in cardiomyocites it reacts at rates near the diffusion limit to form nitrate and methemoglobin (dioxygenation reaction) [5,6]. It will also react with the deoxyhemes of these proteins to form iron-nitrosyl-complexes, which can release NO but quite inefficiently via the oxidative denitrosylation reaction [7]. These two reactions, dioxygenation and iron-nitrosylation, prevent NO from forming in the endothelium and diffusing to distant organ targets, such as the heart, intestine, kidney, brain or liver.
Despite the strict paracrine limitations imposed by this chemistry, a number of studies suggested that endocrine NO signaling is possible. The Kubes group showed that NO delivered by inhalation to cats could improve blood flow and limit inflammation in the cat intestine subjected to ischemia-reperfusion (I/R) injury [8]; Gladwin and Cannon later showed that this was possible in the human circulation [6]. Many subsequent studies have shown that inhaled NO could rescue distal organs from I/R injury and infarction. In fact, upregulation of eNOS selectively in the heart could rescue the liver from I/R injury [9]. However, free NO cannot account for these effects based on the short half life of NO in blood, on the order of 2 milliseconds or less [10].
Many investigators have examined reaction products of NO in blood, attempting to devine the mediator of endocrine NO signaling. While S-nitroso-albumin and S-nitrosohemoglobin were first proposed as endocrine NO metabolites, the levels of these species even during NO inhalation are quite low, using validated chemiluminescent detection methods [4]. Human studies with NO inhalation suggested that the NO oxidation product nitrite (NO2-) increases significantly, with arterial levels higher than venous levels, suggesting this anion could account for the effect [4, 6, 11]. Unlike authentic NO, nitrite has a half life in mammals approaching 60-minutes [12]. Infusions of nitrite in humans and animal models indicated that nitrite was a potent vasodilator and cytoprotective agent that could mimic all the observed effects of NO inhalation [13-16]. Recent studies have carefully repleted nitrite levels to those observed with NO inhalation and produced similar reductions in organ infarction volumes, confirming the role of nitrite as the endocrine effector of inhaled NO [17].
Elusive endocrine mediator of remote ischemic preconditioning
Another line of investigation suggests the existence of an endocrine mediator of organ cytoprotection during remote ischemic preconditioning (rIPC). The idea that a signal transduction exists between the local site of remote ischemia and the myocardium was demonstrated by Przyklenk et al. in the early 1990’s. They found, using a canine model, that brief episodes of ischemia and reperfusion in the circumflex coronary artery reduce the size of the myocardial infarct arising from the occlusion of the left anterior descending artery [18]. This form of myocardial protection was subsequently found to occur with “remote” ischemia and reperfusion of non-cardiac organs. Transient ischemia of a variety of tissues such as kidney, small bowel, liver, skeletal muscle and even brain induces a systemic protective effect against the subsequent extended I/R injury of the myocardium [19-21]. Such phenomenon was termed “preconditioning at a distance” [22] and appears to be highly conserved across species. Animal studies with transplanted hearts further support the role of a circulating substance or a group of transduction mediators with protective effects against I/R injury. Remote limb preconditioning of a pig that received a donor heart was able to reduce myocardial infarct size [23] and hearts excised from a rat that had been subjected to remote limb preconditioning experienced a smaller infarct size when subjected to sustained I/R on a Langendorff-apparatus [24].
The finding that a reperfusion period of the remote preconditioned organ is required after the brief ischemia suggests that the reperfusion period may be needed to “washout” a humoral factor generated by the preconditioning ischemia, which is then transported to the heart [21]. Many experimental studies have attempted to identify the nature of the endocrine mediators circulating in the blood stream which conveys the preconditioning signal from the remote organ to the heart [25-27]. However the actual identity of the humoral mediator remains unknown.
NO and cardioprotection
There is a large body of literature describing the protective properties of NO as an element of the cytoprotective factor, despite the limitations of endocrine movement to a remote site. Endogenous NOS-derived NO appears to play a pivotal role in mediating the protective effect of hindlimb rIPC in reducing liver damage and this is abrogated by treatments with the NO scavenger cPTIO and inhibited in the endothelial NO synthase knockout mouse [28]. Tokuno et al. [20] have implicated iNOS activation as a trigger for delayed rIPC of the heart using cerebral ischemia as preconditioning stimulus. The cardioprotective effect was seen 24 hours later and was absent in iNOS knockout mice. Further studies demonstrated that NO is necessary for the development of ischemia-induced delayed protection against both myocardial stunning and myocardial infarction [29]. While it is clear that NO synthase and NO appears to participate in the process of rIPC, the mechanism for NO transport to a distant site in this process, and the very nature of the “endocrine” rIPC mediator has remained a mystery.
Nitrite as endocrine mediator of rIPC
In the current issue of Circulation Research, Rassaf and colleagues investigate the mechanism of remote ischemic preconditioning (rIPC) and explore the possible identity of the circulating “endocrine” mediator [30]. They first find in human studies that, similar to the case with inhaled NO exposure, the levels of plasma nitrite increase after shear mediated eNOS activation during brachial artery occlusion and release (reactive hyperemia). This is caused by eNOS activation with NO formation and oxidation to the more stable nitrite. They confirm this by blocking the high flow shear associated with reactive hyperemia by using partial 50% compression of the brachial artery following ischemia. This is a very creative control, allowing for regional ischemia without the shear-induced activation of eNOS and formation of intravascular nitrite.
They then do remote ischemia preconditioning (rIPC) studies in the legs of mice and show that nitrite levels increase. Inhibition of NO with PTIO or in eNOS KO mice, prevents the rise in nitrite and rIPC effects on myocardial infarction. This association is mechanistically confirmed by infusions of nitrite to match elevated levels observed with rIPC. Finally, they infuse human plasma with and without rIPC into the isolated heart model of IR and show that elevations in nitrite (removed with acidified sulfanilamide and repleted) account for effects. Overall, the studies are highly translational and utilize creative methodologies to test a major pathway in biology, the process and effector of remote ischemic preconditioning.
Myoglobin as nitrite reductase
While these studies suggest that nitrite forms during rIPC and travels in the plasma to the heart, how is it then converted in the heart back into cytoprotective NO? During ischemia nitrite is reduced to NO and N2O3 by different nitrite reductase enzyme systems [31, 32].Mitochondrial NO and S-nitrosothiols formed from nitrite dynamically and reversibly inhibit complex I during reperfusion, which limits ROS formation from complex I and III [33, 34]. This ultimately prevents the opening of the mitochondrial permeability transition pore and the release of cytochrome c. It has recently been shown that the site of nitrosation is on Cys 39 of the ND3 subunit of complex I [34]. A number of enzymes are required to convert nitrite into NO during organ ischemia. For example, in the heart, deoxygenated myoglobin acts as a functional nitrite reductase [35] (Figure 1). Nitrite-dependent NO formation is significantly decreased in myoglobin deficient hearts [36] and nitrite administration reduces myocardial infarction with abrogated effects in the myoglobin knockout mice [37]. In the current study Rassaf and colleagues show that the effect of rIPC is inhibited in the myoglobin knock-out mouse, providing further support that the endocrine mediator of this effect is nitrite, which is produced in the extremity, travels in blood to the heart, where it is reduced by myoglobin to produce NO.
Figure 1.
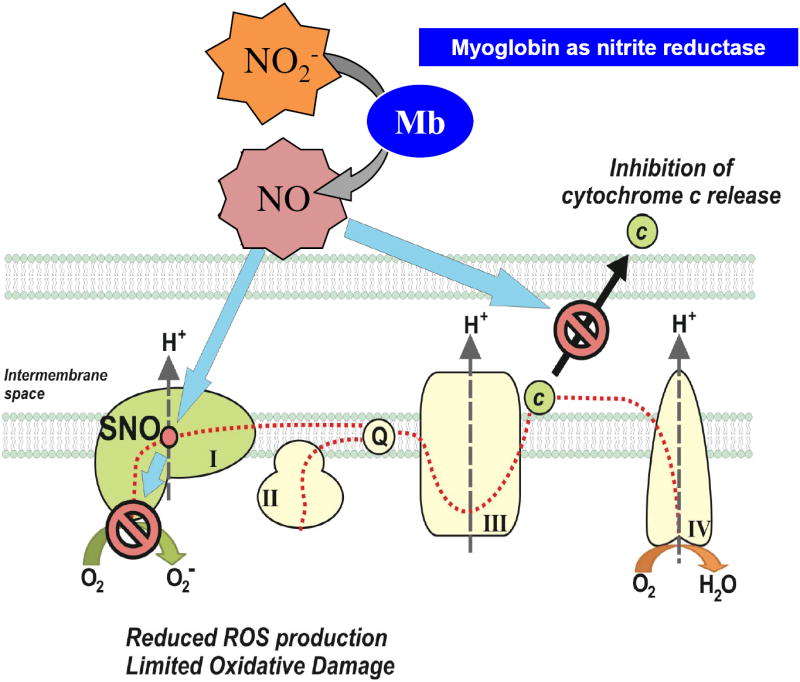
Mechanisms of nitrite-mediated cytoprotection. In the cardiomyocytes nitrite is reduced to NO by reactions with deoxy-myoglobin and then can react with and inhibit complex I of the mitochondrial electron transport chain. This inhibition is reversible and occurs immediately during reperfusion to limit reactive oxygen species formation and to prevent the release of cytochrome c. Figure adapted from Bueno et al, “Nitrite signaling in pulmonary hypertension: mechanisms of bioactivation, signaling, and therapeutics.” Antioxidants & redox signaling, 2013. 18(14): p. 1797-809.
Conclusion
A potential limitation of the current study is the reliance on mouse models of myocardial infarction to test the cytoprotective effects of nitrite. A recent clinical trial was presented at the 2013 American Heart Association meetings investigating the therapeutic effects of nitrite in ST-elevation myocardial infarction (STEMI) showed that sodium nitrite administered prior reperfusion does not reduce infarct size [38]. Evaluation of the full results of this trial will be required to understand the dose, timing, plasma nitrite levels achieved and fidelity of the study design. However, these results are likely to raise questions as to the relevance of findings from mouse models of ischemia-reperfusion injury to human disease.
In summary, this study provides compelling evidence that limb ischemia causes metabolic vasodilation that leads to increased blood flow and shear-force on the endothelium of conductance blood vessels to activates eNOS. Activated eNOS produces NO which is oxidized in plasma to nitrite. Nitrite then circulates as the endocrine mediator of rIPC and travels to the heart. Finally, when the heart is subjected to ischemia the nitrite is then reduced by deoxymyoglobin to form NO in the cardiomyocyte, limiting cellular injury and infarction.
Acknowledgments
SOURCES OF FUNDING
Dr. Gladwin receives research support from NIH grants RO1HL098032, RO1HL096973, P01HL103455, T32 HL110849 and the Institute for Transfusion Medicine and Hemophilia Center of Western Pennsylvania. Dr. Corti is supported by a fellowship from the Ri.MED Foundation.
Footnotes
DISCLOSURES
Dr. Gladwin is listed as co-inventor on an NIH government patent for the use of nitrite salts in cardiovascular diseases. Dr. Gladwin consults with Aires Pharmaceuticals on the development of a phase II proof of concept trial using inhaled nitrite for pulmonary arterial hypertension.
References
- 1.Palmer RM, Ashton DS, Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988;333(6174):664–6. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- 2.Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327(6122):524–6. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 3.Gladwin MT. Deconstructing endothelial dysfunction: soluble guanylyl cyclase oxidation and the NO resistance syndrome. The Journal of clinical investigation. 2006;116(9):2330–2. doi: 10.1172/JCI29807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gladwin MT, Ognibene FP, Pannell LK, Nichols JS, Pease-Fye ME, Shelhamer JH, Schechter AN. Relative role of heme nitrosylation and beta-cysteine 93 nitrosation in the transport and metabolism of nitric oxide by hemoglobin in the human circulation. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(18):9943–8. doi: 10.1073/pnas.180155397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raat NJ, Tabima DM, Specht PA, Tejero J, Champion HC, Kim-Shapiro DB, Baust J, Mik EG, Hildesheim M, Stasch JP, Becker EM, Truebel H, Gladwin MT. Direct sGC activation bypasses NO scavenging reactions of intravascular free oxy-hemoglobin and limits vasoconstriction. Antioxidants & redox signaling. 2013;19(18):2232–43. doi: 10.1089/ars.2013.5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cannon RO, Schechter AN, Panza JA, Ognibene FP, Pease-Fye ME, Waclawiw MA, Shelhamer JH, Gladwin MT. Effects of inhaled nitric oxide on regional blood flow are consistent with intravascular nitric oxide delivery. The Journal of clinical investigation. 2001;108(2):279–87. doi: 10.1172/JCI12761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Basu S, Grubina R, Huang J, Conradie J, Huang Z, Jeffers A, Jiang A, He X, Azarov I, Seibert R, Mehta A, Patel R, King SB, Hogg N, Ghosh A, Gladwin MT, Kim-Shapiro DB. Catalytic generation of N2O3 by the concerted nitrite reductase and anhydrase activity of hemoglobin. Nature chemical biology. 2007;3(12):785–94. doi: 10.1038/nchembio.2007.46. [DOI] [PubMed] [Google Scholar]
- 8.Fox-Robichaud A, Payne D, Hasan SU, Ostrovsky L, Fairhead T, Reinhardt P, Kubes P. Inhaled NO as a viable antiadhesive therapy for ischemia/reperfusion injury of distal microvascular beds. The Journal of clinical investigation. 1998;101(11):2497–505. doi: 10.1172/JCI2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elrod JW, Calvert JW, Gundewar S, Bryan NS, Lefer DJ. Nitric oxide promotes distant organ protection: evidence for an endocrine role of nitric oxide. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(32):11430–5. doi: 10.1073/pnas.0800700105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu X, Miller MJ, Joshi MS, Sadowska-Krowicka H, Clark DA, Lancaster JR. Diffusion-limited reaction of free nitric oxide with erythrocytes. The Journal of biological chemistry. 1998;273(30):18709–13. doi: 10.1074/jbc.273.30.18709. [DOI] [PubMed] [Google Scholar]
- 11.Gladwin MT, Shelhamer JH, Schechter AN, Pease-Fye ME, Waclawiw MA, Panza JA, Ognibene FP, Cannon RO. Role of circulating nitrite and S-nitrosohemoglobin in the regulation of regional blood flow in humans. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(21):11482–7. doi: 10.1073/pnas.97.21.11482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dejam A, Hunter CJ, Tremonti C, Pluta RM, Hon YY, Grimes G, Partovi K, Pelletier MM, Oldfield EH, Cannon RO, Schechter AN, Gladwin MT. Nitrite infusion in humans and nonhuman primates: endocrine effects, pharmacokinetics, and tolerance formation. Circulation. 2007;116(16):1821–31. doi: 10.1161/CIRCULATIONAHA.107.712133. [DOI] [PubMed] [Google Scholar]
- 13.Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon RO, Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nature medicine. 2003;9(12):1498–505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 14.Webb A, Bond R, McLean P, Benjamin N, Ahluwalia A. Reduction of nitrite to nitric oxide during ischemia protects against myocardial ischemia-reperfusion damage. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(37):13683–8. doi: 10.1073/pnas.0402927101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duranski MR, Greer JJ, Dejam A, Jaganmohan S, Hogg N, Langston W, Patel RP, Yet SF, Wang X, Kevil CG, Gladwin MT, Lefer DJ. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. The Journal of clinical investigation. 2005;115(5):1232–40. doi: 10.1172/JCI22493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez FM, Shiva S, Vincent PS, Ringwood LA, Hsu LY, Hon YY, Aletras AH, Cannon RO, Gladwin MT, Arai AE. Nitrite anion provides potent cytoprotective and antiapoptotic effects as adjunctive therapy to reperfusion for acute myocardial infarction. Circulation. 2008;117(23):2986–94. doi: 10.1161/CIRCULATIONAHA.107.748814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neye N, Enigk F, Shiva S, Habazetti H, Plesnila N, Kuppe H, Gladwin MT, Kuebler WM. Inhalation of NO during myocardial ischemia reduces infarct size and improves cardiac function. Intensive care medicine. 2012;38(8):1381–91. doi: 10.1007/s00134-012-2605-1. [DOI] [PubMed] [Google Scholar]
- 18.Przyklenk K, Bauer B, Ovize M, Kloner RA, Whittaker P. Regional ischemic ‘preconditioning’ protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation. 1993;87(3):893–9. doi: 10.1161/01.cir.87.3.893. [DOI] [PubMed] [Google Scholar]
- 19.Gho BC, Schoemaker RG, van den Doel MA, Duncker DJ, Verdouw PD. Myocardial protection by brief ischemia in noncardiac tissue. Circulation. 1996;94(9):2193–200. doi: 10.1161/01.cir.94.9.2193. [DOI] [PubMed] [Google Scholar]
- 20.Tokuno S, Hinokiyama K, Tokuno K, Lowbeer C, Hansson LO, Valen G. Spontaneous ischemic events in the brain and heart adapt the hearts of severely atherosclerotic mice to ischemia. Arteriosclerosis, thrombosis, and vascular biology. 2002;22(6):995–1001. doi: 10.1161/01.atv.0000017703.87741.12. [DOI] [PubMed] [Google Scholar]
- 21.Hausenloy DJ, Yellon DM. Remote ischaemic preconditioning: underlying mechanisms and clinical application. Cardiovascular research. 2008;79(3):377–86. doi: 10.1093/cvr/cvn114. [DOI] [PubMed] [Google Scholar]
- 22.Dickson EW, Porcaro WA, Fenton RA, Heard SO, Reindhardt CP, Renzi FP, Przyklenk K. “Preconditioning at a distance” in the isolated rabbit heart. Academic emergency medicine : official journal of the Society for Academic Emergency Medicine. 2000;7(4):311–7. doi: 10.1111/j.1553-2712.2000.tb02228.x. [DOI] [PubMed] [Google Scholar]
- 23.Konstantinov IE, Li J, Cheung MM, Shimizu M, Stokoe J, Kharbanda RK, Redington AN. Remote ischemic preconditioning of the recipient reduces myocardial ischemia-reperfusion injury of the denervated donor heart via a Katp channel-dependent mechanism. Transplantation. 2005;79(12):1691–5. doi: 10.1097/01.tp.0000159137.76400.5d. [DOI] [PubMed] [Google Scholar]
- 24.Kristiansen SB, Henning SB, Kharbanda RK, Nielsen-Kudsk JE, Schmidt MR, Redington AN, Nielsen TT, Botker HE. Remote preconditioning reduces ischemic injury in the explanted heart by a KATP channel-dependent mechanism. American journal of physiology. Heart and circulatory physiology. 2005;288(3):H1252–6. doi: 10.1152/ajpheart.00207.2004. [DOI] [PubMed] [Google Scholar]
- 25.Lang SC, Elsasser A, Scheler C, Vetter S, Tiefenbacher CP, Kubler W, Katus HA, Vogt AM. Myocardial preconditioning and remote renal preconditioning--identifying a protective factor using proteomic methods? Basic research in cardiology. 2006;101(2):149–58. doi: 10.1007/s00395-005-0565-0. [DOI] [PubMed] [Google Scholar]
- 26.Serejo FC, Rodriguez LF, da Silva Tavares KC, de Carvalho AC, Nascimento JH. Cardioprotective properties of humoral factors released from rat hearts subject to ischemic preconditioning. Journal of cardiovascular pharmacology. 2007;49(4):214–20. doi: 10.1097/FJC.0b013e3180325ad9. [DOI] [PubMed] [Google Scholar]
- 27.Shimizu M, Tropak M, Diaz RJ, Suto F, Surendra H, Kuzmin E, Li J, Gross G, Wilson GJ, Callahan J, Redington AN. Transient limb ischaemia remotely preconditions through a humoral mechanism acting directly on the myocardium: evidence suggesting cross-species protection. Clinical science. 2009;117(5):191–200. doi: 10.1042/CS20080523. [DOI] [PubMed] [Google Scholar]
- 28.Abu-Amara M, Yang SY, Quaglia A, Rowley P, de Mel A, Tapuria N, Seifalian A, Davidson B, Fuller B. Nitric oxide is an essential mediator of the protective effects of remote ischaemic preconditioning in a mouse model of liver ischaemia/reperfusion injury. Clinical science. 2011;121(6):257–66. doi: 10.1042/CS20100598. [DOI] [PubMed] [Google Scholar]
- 29.Bolli R. The late phase of preconditioning. Circulation research. 2000;87(11):972–83. doi: 10.1161/01.res.87.11.972. [DOI] [PubMed] [Google Scholar]
- 30.Rassaf T, Totzeck M, Hendgen-Cotta UB, Shiva S, Heusch G, Kelm M. Circulating Nitrite Contributes to Cardioprotection by Remote Ischemic Preconditioning. Circulation research. 2014 doi: 10.1161/CIRCRESAHA.114.303822. [DOI] [PubMed] [Google Scholar]
- 31.Sparacino-Watkins CE, Tejero J, Sun B, Gauthier MC, Thomas J, Ragireddy V, Merchant BA, Wang J, Azarov I, Basu P, Gladwin MT. Nitrite reductase and NO synthase activity of the mitochondrial molybdopterin enzymes mARC1 and mARC2. The Journal of biological chemistry. 2014 doi: 10.1074/jbc.M114.555177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tejero J, Gladwin MT. The globin superfamily: functions in nitric oxide formation and decay. Biological chemistry. 2014 doi: 10.1515/hsz-2013-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shiva S, Sack MN, Greer JJ, Duranski M, Ringwood LA, Burwell L, Wang X, MacArthur PH, Shoja A, Raghavachari N, Calvert JW, Brookes PS, Lefer DJ, Gladwin MT. Nitrite augments tolerance to ischemia/reperfusion injury via the modulation of mitochondrial electron transfer. The Journal of experimental medicine. 2007;204(9):2089–102. doi: 10.1084/jem.20070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chouchani ET, Methner C, Nadtochiy SM, Logan A, Pell VR, Ding S, James AM, Cocheme HM, Reinhold J, Lilley KS, Partridge L, Fearnley IM, Robinson AJ, Hartley RC, Smith RA, Krieg T, Brookes PS, Murphy MP. Cardioprotection by S-nitrosation of a cysteine switch on mitochondrial complex I. Nature medicine. 2013;19(6):753–9. doi: 10.1038/nm.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shiva S, Huang Z, Grubina R, Sun J, Ringwood LA, MacArthur PH, Xu X, Murphy E, Darley-Usmar VM, Gladwin MT. Deoxymyoglobin is a nitrite reductase that generates nitric oxide and regulates mitochondrial respiration. Circulation research. 2007;100(5):654–61. doi: 10.1161/01.RES.0000260171.52224.6b. [DOI] [PubMed] [Google Scholar]
- 36.Rassaf T, Flogel U, Drexhage C, Hendgen-Cotta U, Kelm M, Schrader J. Nitrite reductase function of deoxymyoglobin: oxygen sensor and regulator of cardiac energetics and function. Circulation research. 2007;100(12):1749–54. doi: 10.1161/CIRCRESAHA.107.152488. [DOI] [PubMed] [Google Scholar]
- 37.Hendgen-Cotta UB, Merx MW, Shiva S, Schmitz J, Becher S, Klare JP, Steinhoff HJ, Goedecke A, Schrader J, Gladwin MT, Kelm M, Rassaf T. Nitrite reductase activity of myoglobin regulates respiration and cellular viability in myocardial ischemia-reperfusion injury. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(29):10256–61. doi: 10.1073/pnas.0801336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siddiqi N, Neil C, Bruce M, Maclennan G, Cotton S, Papadopoulou S, Feelisch M, Bunce N, Lim PO, Hildick-Smith D, Horowitz J, Madhani M, Boon N, Dawson D, Kaski JC, Frenneaux M. Intravenous sodium nitrite in acute ST-elevation myocardial infarction: a randomized controlled trial (NIAMI) European heart journal. 2014 doi: 10.1093/eurheartj/ehu096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bueno M, Wang J, Mora AL, Gladwin MT. Nitrite signaling in pulmonary hypertension: mechanisms of bioactivation, signaling, and therapeutics. Antioxidants & redox signaling. 2013;18(14):1797–809. doi: 10.1089/ars.2012.4833. [DOI] [PMC free article] [PubMed] [Google Scholar]


